Abstract
Cystic fibrosis (CF) results from mutations in the CF transmembrane conductance regulator (CFTR) gene, which codes for a chloride/bicarbonate channel in the apical epithelial membranes. CFTR dysfunction results in a multisystem disease including the development of life limiting lung disease. The possibility of a cure for CF by replacing defective CFTR has led to different approaches for CF gene therapy; all of which ultimately have to be tested in preclinical model systems. Primary human nasal epithelial cultures (HNECs) derived from nasal turbinate brushing were used to test the efficiency of a helper-dependent adenoviral (HD-Ad) vector expressing CFTR. HD-Ad-CFTR transduction resulted in functional expression of CFTR at the apical membrane in nasal epithelial cells obtained from CF patients. These results suggest that HNECs can be used for preclinical testing of gene therapy vectors in CF.
Introduction
Tremendous improvement has occurred in the care of cystic fibrosis (CF) over the last 50 years. This is best illustrated by improved life expectancy. However people with CF still die prematurely, mainly due to respiratory failure.1 Organ dysfunction in CF is ultimately caused by mutations in the gene coding for CFTR, a membrane protein that helps regulate ion movement across epithelial barriers. When CFTR was identified as cause of CF in 1989, there was much hope that this would result in the development of a cure. Although to date a curative therapy remains elusive, much research has been done in the field of gene therapy.
Gene therapy directed toward CF lung disease aims to efficiently and safely express CFTR by the delivery of CFTR cDNA to the airway epithelium through the use of a viral or nonviral vectors. Replacing defective CFTR with a functional gene should prevent CF disease pathology. The lung has been a primary target for these approaches as lung disease is the main cause of death in individuals with CF, and the airway epithelium is relatively accessible.
Adenovirus (Ad), containing CFTR cDNA, was the first vector used in human CF gene therapy studies.2 These vectors were tested in human epithelial cells in vitro and then delivered to mice in vivo. Ad-mediated gene transfer has also been tested in humans with CF.3,4 These in vivo studies, in both mice and humans, resulted in inefficient gene transfer and thus did not achieve CFTR rescue. Issues included cell factors that limited Ad attachment and uptake, and host factors that led to an immune response targeting the Ad vector. Although this initial work was not encouraging, since then, refinements have been made in viral vectors in order to maximize transduction efficiency, while reducing host immune response.5–7
Strategies to improve the efficiency of gene therapy for the treatment of CF lung disease require methods to increase the attachment and internalization of vectors into well-differentiated respiratory epithelium.8 A key tool in this improvement process has been the use of well-differentiated human epithelial cell cultures.9 However, human lung epithelial cells used for in vitro models typically come from postmortem lungs or require invasive procedures such as bronchoscopies in order to be obtained; this limits cell access for investigational studies. Nasal brushing of turbinates offers an attractive alternative source of human airway cells. As shown previously well-differentiated primary human nasal epithelial cultures (HNECs) can be generated from brushings of nasal turbinates.10 In this study, we use HNECs generated from CF patients to test expression and function of transduced CFTR with a helper-dependent (HD)-Ad-CFTR vector.
Results
ALI culture results in a well-differentiated airway epithelium
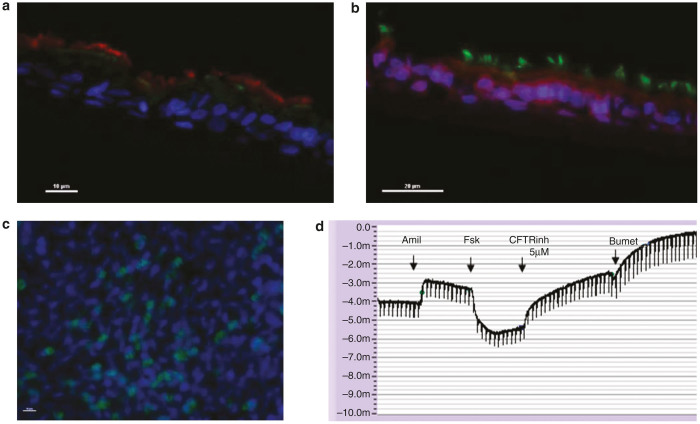
After 3 weeks of air liquid interface (ALI) culture, the apical surface of the cells was consistently dry and transepithelial resistance across the cell monolayer averaged 568 ± 118 (mean ± SD) Ohm per cm2. Ciliary motion was visualized by light microscopy. Examination of cells from non-CF control subject by immunofluorescence demonstrates a pseudostratified morphology, apical CFTR expression, and tight junctions (Figure 1a–c). Non-CF control cells displayed CFTR function as evidenced by cAMP-mediated epithelial currents that were sensitive to CFTRInh-172 (Figure 1d). Together, these characteristics demonstrate the successful generation of a well-differentiated respiratory epithelium.
Figure 1.
Primary culture of nasal epithelial cells results in a well-differentiated phenotype. Nasal cells were obtained by nasal brushing from non-cystic fibrosis (CF) human donor as described in methods. Epithelial cells were expanded in submerged cultures and then grown in air-liquid interface for 3 weeks. Cells imaged with confocal microscope. (a) Cross sectional view of human nasal epithelial cells demonstrating apical CFTR. Blue stain, DAPI (4’, 6-diamidino-2-phenylindole); red stain, β4 tubulin (cilia); green stain, CFTR protein. Bar indicates 10 µm. (b) Cross sectional view of human nasal epithelial cells demonstrating tight junctions. Blue stain, DAPI (looks purple); green stain, β4 tubulin (cilia); red stain, ZO1 (tight junctions). Bar indicates 20 µm. (c) Apical view of human nasal epithelial cells demonstrating cilia. Blue stain, DAPI; green stain, β4 tubulin (cilia). Bar indicates 10 µm. (d) Original Ussing trace from non-CF nasal cells displaying adequate bioelectric properties (Vte = −3.17 mV, Rte= 597 Ω cm2, Ieq = −5.31 µA/cm2) as well as functional expression of ENaC- and CFTR-mediated ion transport. CFTR, CF transmembrane conductance regulator; ENaC, epithelial sodium channel.
HNECs allow efficient expression of HD-Ad-GFP
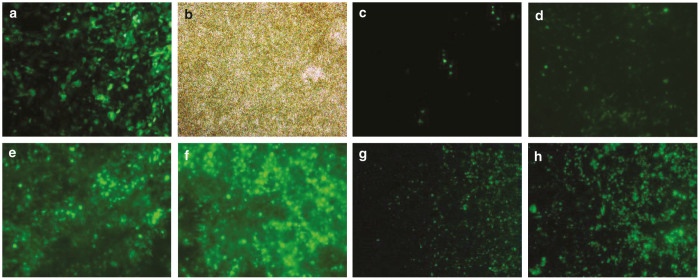
We followed vector transgene expression after transduction with HD-Ad-GFP. GFP was seen in cells after 24 days of ALI culture when the vector was added to cells at the time of cell seeding on the Transwell insert at a dose of 250 viral particles per cell (Figure 2a). This demonstrated that the vector was capable of transducing primary cells from a healthy non-CF subject prior to the formation of a monolayer with tight junctions.
Figure 2.
Well-differentiated primary nasal epithelial cultures can be transduced with adenovirus expressing GFP. (a) GFP and (b) bright-field view of well-differentiated primary human nasal epithelial cells transduced at time of seeding onto semipermeable inserts with HD-Ad-GFP. Image was taken after 24 days of air-liquid interface culture. Panel (c) to (h) demonstrates GFP signal in WD-PNECs transduced with HD-Ad-GFP after 21 days in ALI culture. GFP expression was examined day 3 post-transduction. (c) Hundred viral particles per cell were used without any pretreatment. (d to f) Cells were pretreated with apical 6 mmol/l ethylene glycol tetraacetic acid (EGTA) prior to addition of (d) 100, (e) 1,000, and (f) 2,500 viral particles HD-Ad-GFP per cell. (g) and (h) were parallel experiments. Cells were pretreated with (g) 0.01% lysophosphatidylcholine or (h) 6 mmol/l EGTA. ALI, air liquid interface; HD-Ad-GFP, helper-dependent adenovirus expressing green fluorescent protein.
Utilizing a vector dose of 250 viral particles per cell at day 21 of ALI culture after cells had formed a monolayer with tight junctions did result in GFP expression with HD-Ad-GFP (Figure 2c). However, expression was much reduced compared to adding the vector to cells in suspension prior to seeding on the insert. This may be explained by the fact that uptake of adenovirus occurs through CAR (the coxsackievirus and adenovirus receptor) located on the basolateral side of the epithelium. Thus, we used pretreatment to transiently disrupt the epithelial barrier, as previously described11 to enhance transduction and thus GFP expression in HNECs (Figure 2d). Other strategies to improve transgene expression include increasing the dose of the vector. A higher dose of HD-Ad GFP in combination with ethylene glycol tetraacetic acid (EGTA) pretreatment increased the GFP signal (Figure 2e,f). A dose of 2500 viral particles per cell in combination with EGTA pretreatment resulted in a strong GFP signal. There are additional methods to disrupt epithelial barriers, which may thus influence transgene expression. We tried 0.01% lysophosphatidylcholine to improve transduction, but the GFP signal was less as compared to EGTA (Figure 2g,h).
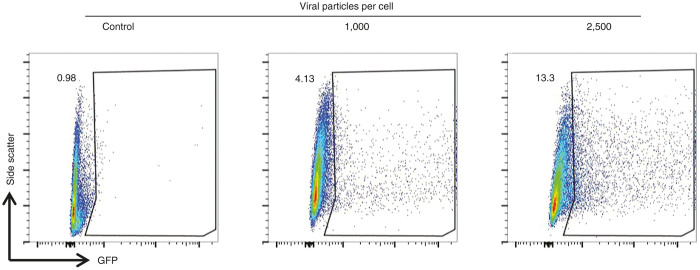
These initial experiments confirmed that HNECs can be transduced with HD-Ad vectors. In order to better quantify the efficiency of HD-Ad transduction in this system, cells were apically transduced on day 21 of ALI culture after pretreatment with EGTA and then dissociated and analyzed by flow cytometry for GFP. We found that 4.13% of the cells were GFP positive when transduced with 1,000 HD-Ad viral particles per cell. This increased to 13.3% of cells when we used a higher dose of 2,500 viral particles per cells (Figure 3).
Figure 3.
Number of GFP expressing cells is related to dose of adenovirus vector. Representative scatter plots demonstrating gating of human nasal epithelial cells analyzed by flow cytometry after transduction. Side scatter and GFP signal as indicated. For these experiments, cells were apically transduced on day 21 of air liquid interface culture after pretreatment with ethylene glycol tetraacetic acid and studied on day 3 post-transduction. The three graphs show untransduced cells (control); cells transduced with 1,000 and 2,500 viral particles per cells as indicated. GFP-positive cells increase with higher vector dose.
HNECs allow efficient transduction of CFTR
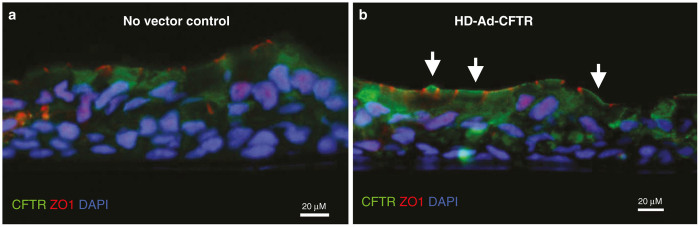
After demonstrating the feasibility of transduction of GFP in HNECs, we then studied nasal cells obtained from subjects with CF using a HD-Ad-CFTR vector.11 HD-Ad-CFTR was added on day 21 at a dose of 2,500 viral particles per cell following EGTA pretreatment. HNECs were studied 3 days later. Immunohistochemical examination demonstrated significant CFTR protein expression at the apical surface of cells when compared to untransduced cells (Figure 4).
Figure 4.
Apical expression of CFTR in cystic fibrosis (CF) cells postadenoviral transduction. (a) Human nasal epithelial cells from p.F508del/p.F508del CF patient in the absence of transduction and (b) post-transduction using HD-Ad-CFTR vector (2,500 viral particles per cell). Vector was added apically to cells after 21 days of air liquid interface culture and pretreatment with ethylene glycol tetraacetic acid. CFTR staining (green) is seen on the apical membrane of cells (white arrows). CFTR, CF transmembrane conductance regulator; DAPI, 4’, 6-diamidino-2-phenylindole.
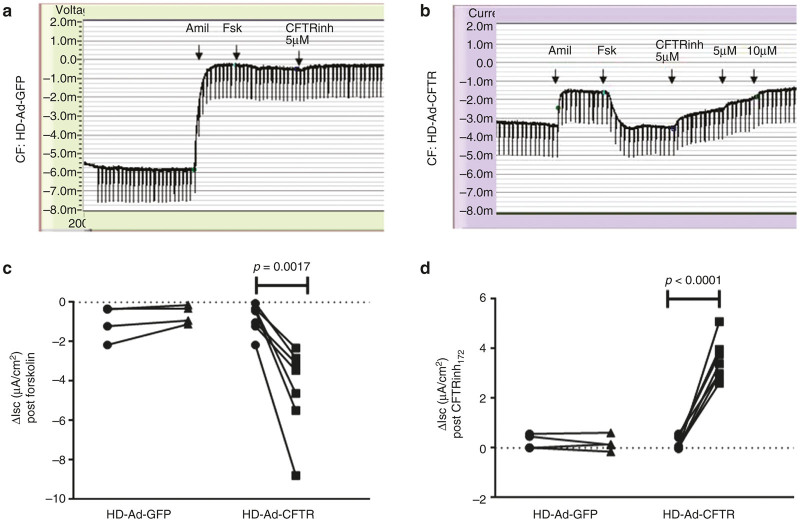
Next, we wanted to confirm whether transduced CFTR resulted in functional expression of CFTR. In nasal cells obtained from CF patients transduced with HD-Ad GFP without CFTR, no CFTR function was detected (Figure 5a,c,d). Specifically, the baseline mean forskolin activatable current was −1.01 μA/cm2 and did not change significantly with HD-Ad GFP transduction (mean −0.62 μA/cm2, P = 0.45, Figure 5c). The same was true for the mean CFTRinh172 inhibitable current (mean 0.26 μA/cm2 versus 0.19 μA/cm2, P = 0.74, Figure 5d). By contrast, cells transduced with HD-Ad-CFTR did demonstrate a significant change in forskolin-activatable currents (baseline mean −0.79 μA/cm2 versus transduced mean −4.37 μA/cm2, P = 0.002) that were sensitive to inhibition with CFTRinh172 (baseline mean −0.22 μA/cm2 versus transduced mean −3.54 μA/cm2, P < 0.0001, Figure 5d,e). Viral transduction did not affect the mean transepithelial resistance in transduced versus nontransduced cells (413.4 Ω cm2 versus 460.6 Ω cm2, P = 0.52, data not shown).
Figure 5.
Functional expression of CFTR in human nasal epithelial cells (HNECs) following treatment HD-Ad-CFTR. Functional CFTR expression in HNEC measured in Ussing chamber studies. Original HNEC Ussing tracings from a CF patient (G480S/A559T) 72 hours after treatment. (a) HD-Ad-GFP or (b) HD-Ad-GFP. For panels (c) and (d), circles represent nontransduced cells, squares represent HD-Ad-CFTR transduced cells, and triangles represent HD-Ad-GFP-transduced cells. (c) Summary of CFTR function measured as Forskolin-activated current (Ieq-FSK) in HNEC from different CF patients, showed no change in CFTR function following treatment with HD-Ad-GFP (n = 4), but significant Ieq-FsK response following HD-Ad-CFTR (n = 7, P = 0.0017). (d) Similarly, the summary of CFTR function expressed as CFTRInh172 sensitive currents (Ieq CFTRInh172) showed no change with HD-Ad-GFP (n = 4), but significant increase in Ieq CFTRInh172 following HD-Ad-CFTR (n = 7, P < 0.001). CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; HD-Ad-GFP, helper-dependent adenovirus expressing green fluorescent protein.
Discussion
Over the last 20 years, progress has been made in the field of CFTR gene therapy. Initial hurdles such as vector toxicity, immune responses to vectors and reduced gene expression over an extended time period12 have been recognized and progress has been made to address these issues.13,14
In order to further advance progress in gene therapy, relevant model systems are required to test and improve the efficacy of the gene therapy strategies. In this study, we demonstrate that HNECs can be transduced by a HD-Ad-CFTR vector resulting in significant functional expression of CFTR. These cells form a well-differentiated airway epithelium with characteristics of respiratory airway epithelium.15,16 The ALI interface reflects the anatomy of human airway epithelium in vivo including functional tight junctions that need to be overcome when attempting efficient cellular gene transfer. Specifically in current work, epithelial tight junctions, the presence of cilia and mucous all are thought to restrict adenovirus attachment and internalization.10 Strategies that are employed in vitro, such as EGTA pretreatment, may help to identify better means for efficient CFTR transduction in vivo.
In order to generate human ALI cultures for these kinds of studies, cells from nasal polyps (postpolyp removal) or from whole sections of lung (explants taken from lung transplant patients or post mortem) have been used. Although many cells can be obtained for study by these methods, these resources are limited. An advantage of HNECs is that they are easily accessible from individual patients without the need for specialized training or anesthesia. This increases the availability of this model system for research purposes. Importantly, there is evidence that nasal derived ALI cultures are similar to cultures obtained from bronchial brushing in various models including studies of viral infection, inflammation, and chronic obstructive pulmonary disease.10,17,18 Further, these cells remain functional after HD-Ad transduction allowing an assessment of vector efficiency, which is a key outcome of interest in these vector studies.
Cell culture models by nature are simple and generally involve limited cell types. Animal models are thus important in the setting of CF gene therapy as they move beyond the simplicity of an in vitro system and add the elements of a functional lung and the immune system.14 A number of animal models have been used in the field of CF gene therapy including mice, larger rodents, and pigs. These models provide important insight into immune barriers to the vector used and tolerability or toxicity of the strategy needed to optimize transgene expression. However, there is evidence that species differences exist in airway cell biology. These differences lead to considerable species diversity, which may become relevant when testing potential gene therapy protocols.11,19 Thus, recombinant adeno-associated virus (rAAV) types 1, 2, and 5 show different in vitro transduction efficiencies depending on whether human, mouse, pig or ferret airway cells are studied. Some of these differences were related to differences in the affinity and location of the receptor for rAAV, but other reasons for species differences were related to the use of the proteosome to process rAAV in some species and the availability of sialic acid coreceptors which can vary by species. Thus before considering human delivery of a vector, in addition to in vivo animal studies, a human model system should be used to assess transgene expression.
There are some limitations with the use of HNECs in this setting. Nasal brushing provides a smaller population of cells to work with as compared to a whole section of lung post transplant. These cells have a limited expansion ability and so rebrushing may be required if many experiments are planned from the same source. Efforts are underway to improve cell survival including use the Rho-associated kinase inhibitor Y-27632 with fibroblast “feeder cells”.20 Although nasal cells behave the same as bronchial cells in many models, it is possible that there are subtle differences that result in altered gene expression between nasal derived cultures and bronchial derived cultures. This may relate to the expression of cell surface receptors, for example. Efforts are underway to assess transduction efficiency between bronchial and nasal cells from the same individual. Finally, as mentioned above, with this model, a key missing component is the host adaptive immune system which clearly can influence gene expression.
In summary, we provide evidence that HNECs are a suitable model system to test the efficiency of CFTR gene therapy vectors.
Materials and Methods
Patients
Nasal cell were obtained from seven CF patients with the following CFTR mutations; four were p.F508del/p.F508del, one was p.G480S/p.A559T, one was p.G542X/p.N1303K, and one was p.F508del/p.Y109GfsX4. In addition, we obtained nasal cells from non-CF healthy control subjects. All protocols had local Research Ethics Board approval and study subjects or parents of minor study subjects signed consent forms.
Nasal brushing
Nasal brushing was performed by a research nurse or medical doctor with procedural experience. A 3-mm diameter sterile cytology brush (MP Corporation, Camarillo, CA) was used. The inferior turbinate was visualized and the brush inserted into the nares and rotated to brush the turbinate. The brush was then placed in warm basal epithelial growth media (BEGM, Lonza, Walkersville, MD). Cells were dissociated with gentle agitation and seeded on a collagen-coated flask (P0 or passage number = 0). Cultures were maintained in BEGM with antibiotics in an incubator at 37 °C with a humidified 5% CO2 atmosphere. Cells were subsequently expanded into a larger flask and passaged once 70–80% confluence was achieved.
ALI culture
P2 cells were seeded on collagen coated Transwell inserts (6.5 mm diameter, 0.4 µm pore size, Corning, Corning, Tewksbury, MA) at a density of 105 cells per insert. Cells were maintained in BEGM but once confluent on the Transwell, the media was changed to ALI by removing the media at the apical side of the cells and changing to a basal differentiation media (PneumaCult, StemCell Tech, Vancouver, Canada). Basal media was changed every day for the first week and then every 2 days. The apical surface was washed weekly with phosphate-buffered saline (PBS). By 3 weeks, cells were well differentiated and had a ciliated phenotype. Transepithelial resistance was quantified with an ohmmeter (World Precision Instruments, Sarasota, FL) to evaluate epithelial barrier function prior to proceeding to Ussing chamber experiments.
Vector production
HD-Ad-GFP vector (helper-dependent adenovirus expressing green fluorescent protein) with a cytomegalovirus promoter and HD-Ad-CFTR with a K18 (keratin 18) promoter were produced as previously described.13,21 Briefly, HD-Ad vectors were amplified by serial passage in 116 cells, a human colon carcinoma cell line, with NG163 helper virus, and were purified by CsCl density gradient centrifugation. HD-Ad preparations were determined by real-time quantitative polymerase chain reaction to contain < 0.05% helper virus contamination. Ad particle numbers were calculated by absorbance at 260 nm.
Transduction
For transduction prior to ALI culture, HD-Ad vectors were added to cells in suspension; and then, cells were seeded onto transwells. For transduction after ALI culture, cells at day 21 of ALI culture were treated apically with 6 mmol/l EGTA or 0.01% lysophosphatidylcholine for 1 hour after which the apical liquid was gently aspirated. HD-Ad vectors were then added to the apical cell surface in 100 µl for 2 hours after which any apical liquid was gently aspirated. The concentration of HD-Ad vectors ranged from 100 to 2,500 viral particles per cell.
Measurement of CFTR activity
Nasal epithelial cells were studied in a nonperfused Ussing chamber (Physiologic Instruments, San Diego, CA). The buffer (126 mmol/l NaCl, 24 mmol/l NaHCO3, 2.13 mmol/l K2HPO4, 0.38 mmol/l KH2PO4, 1 mmol/l MgSO4, 1 mmol/l CaCl2, and 10 mmol/l glucose) was maintained at pH 7.4 and 37 °C and continuously gassed with a 5% CO2/95% O2 mixture. The transepithelial potential (Vte) was recorded and the baseline resistance (Rte) was measured following repeated, brief short-circuit current pulses (1 µA every 30 seconds). The results are presented as equivalent transepithelial current (Ieq), which was calculated using Ohm’s law. CFTR function was determined after inhibition of the epithelial sodium channel (ENaC) with amiloride (100 µmol/l, Spectrum Chemical, Gardena, CA) and cAMP activation with forskolin (10 µmol/l, Sigma-Aldrich). CFTR activity was calculated as Ieq difference following CFTR inhibition with CFTRInh-172 (5 µmol/l, EMD Millipore, Billerica, MA).
CFTR immunofluorescence
Culture membranes were fixed in 4% paraformaldehyde solution and embedded in optimal cutting temperature embedding compound (Tissue-Tek). Double immunofluorescent labeling was performed on cells with and without vector transduction. Cryosections were fixed in methanol for 5 minutes at −20 °C, soaked in 0.2% Triton X-100 in PBS for 5 minutes, blocked with 4% bovine serum albumin in PBS for 30 minutes, and then incubated for 2 hours with primary antibodies against CFTR (clone 24-1, R & D, Minneapolis, MN) or Zo-1 (Zymed, Burlington, Canada). Following washing with PBS five times, the cryosections were incubated with Alexa 488 or Alexa 594–conjugated secondary antibodies (Invitrogen, Burlington, Canada).
Flow cytometry
ALI cell cultures were trypsinized and suspended in PBS as a single cell suspension. Cells were fixed with 2% paraformaldehyde for 1 hour and washed. Cells were analyzed on a Becton Dickinson LSR II CFI (SickKids Flow Cytometry Facility).
Acknowledgments
This work was partially support by a Canadian Institute for Health Research (CIHR) grant (MOP 125882) to J.H. and CIHR/SickKids Foundation grant to T.J.M. The authors thank Jason Debley for discussions regarding primary cell culture and Valerie Waters for advice on antibiotics for cell culture.
The authors declared no conflict of interest.
References
- Cystic Fibrosis Foundation Patient Registry: 2012. Annual Data Report to the Center Directors, Bethesda, MD, 2013.
- Grubb, BR, Pickles, RJ, Ye, H, Yankaskas, JR, Vick, RN, Engelhardt, JF et al. (1994). Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature 371: 802–806. [DOI] [PubMed] [Google Scholar]
- Zabner, J, Ramsey, BW, Meeker, DP, Aitken, ML, Balfour, RP, Gibson, RL et al. (1996). Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Invest 97: 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner, J, Couture, LA, Smith, AE and Welsh, MJ (1994). Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: efficiency of adenovirus-mediated gene transfer in vitro. Hum Gene Ther 5: 585–593. [DOI] [PubMed] [Google Scholar]
- Griesenbach, U and Alton, EW (2011). Current status and future directions of gene and cell therapy for cystic fibrosis. BioDrugs 25: 77–88. [DOI] [PubMed] [Google Scholar]
- Mueller, C and Flotte, TR (2008). Gene therapy for cystic fibrosis. Clin Rev Allergy Immunol 35: 164–178. [DOI] [PubMed] [Google Scholar]
- Flotte, TR, Ng, P, Dylla, DE, McCray, PB Jr, Wang, G, Kolls, JK et al. (2007). Viral vector-mediated and cell-based therapies for treatment of cystic fibrosis. Mol Ther 15: 229–241. [DOI] [PubMed] [Google Scholar]
- Pickles, RJ, McCarty, D, Matsui, H, Hart, PJ, Randell, SH and Boucher, RC (1998). Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol 72: 6014–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granio, O, Ashbourne Excoffon, KJ, Henning, P, Melin, P, Norez, C, Gonzalez, G et al. (2010). Adenovirus 5-fiber 35 chimeric vector mediates efficient apical correction of the cystic fibrosis transmembrane conductance regulator defect in cystic fibrosis primary airway epithelia. Hum Gene Ther 21: 251–269. [DOI] [PubMed] [Google Scholar]
- Guo-Parke, H, Canning, P, Douglas, I, Villenave, R, Heaney, LG, Coyle, PV et al. (2013). Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am J Respir Crit Care Med 188: 842–851. [DOI] [PubMed] [Google Scholar]
- Cao, H, Machuca, TN, Yeung, JC, Wu, J, Du, K, Duan, C et al. (2013). Efficient gene delivery to pig airway epithelia and submucosal glands using helper-dependent adenoviral vectors. Mol Ther Nucleic Acids 2: e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, H, Molday, RS and Hu, J (2011). Gene therapy: light is finally in the tunnel. Protein Cell 2: 973–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler, DR, Sajjan, U, Chow, YH, Martin, B, Kent, G, Tanswell, AK et al. (2003). Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proc Natl Acad Sci USA 100: 15364–15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Stewart ZA, Sinn PL, Olsen JC, Hu J,, McCray PB Jr and Engelhardt JF (2015). Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. Hum Gene Ther Clin Dev 26:38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay, MK, González, PA, León, MA, Céspedes, PF, Bueno, SM, Riedel, CA et al. (2013). Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response. Microbes Infect 15: 230–242. [DOI] [PubMed] [Google Scholar]
- Villenave, R, Thavagnanam, S, Sarlang, S, Parker, J, Douglas, I, Skibinski, G et al. (2012). In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc Natl Acad Sci USA 109: 5040–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRedmond, RE, Greene, CM, Dorscheid, DR, McElvaney, NG and O’Neill, SJ (2007). Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res 8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall, CM, Blaylock, MG, Douglas, JG, Brooker, RJ, Helms, PJ and Walsh, GM (2008). Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol 39: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X, Luo, M, Guo, C, Yan, Z, Wang, Y and Engelhardt, JF (2007). Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther 14: 1543–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horani, A, Nath, A, Wasserman, MG, Huang, T and Brody, SL (2013). Rho-associated protein kinase inhibition enhances airway epithelial Basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol 49: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, D and Ng, P (2003). Improved system for helper-dependent adenoviral vector production. Mol Ther 8: 846–852. [DOI] [PubMed] [Google Scholar]