Abstract
Humanitarian emergencies may result in breakdown of regular health services including routine vaccination programs. Displaced populations including refugees and internally displaced persons are particularly susceptible to outbreaks of communicable diseases such as vaccine-preventable diseases (VPDs). Common VPDs encountered in humanitarian emergencies include measles, polio, and depending on geographical location, meningococcal meningitis, yellow fever, hepatitis A, and cholera. We conducted a review of 50 published articles from 2000 to 2015 concerning VPDs in humanitarian emergencies. This article provides an update on the available literature regarding vaccinations among this highly vulnerable population and describes the unique challenges of VPDs during humanitarian emergencies. Humanitarian emergencies place affected populations at risk for elevated morbidity and mortality from VPDs due to creation or exacerbation of factors associated with disease transmission such as mass population movements, overcrowding, malnutrition, and poor water and sanitation conditions. Vaccination is one of the most basic and critical health interventions for protecting vulnerable populations during emergencies. Growing insecurity, as seen in the increasing number of targeted attacks on health workers in recent years, as well as destruction of cold chain and infrastructure for transportation of supplies, are creating new challenges in provision of life saving vaccines in conflict settings. Population displacement can also threaten global VPD eradication and elimination efforts. While highly effective vaccines and guidelines to combat VPDs are available, the trend of increasing number of humanitarian emergencies globally poses new and emerging challenges in providing vaccination among displaced populations.
Keywords: civil conflicts, displacement, humanitarian emergency, immunizations, internally displaced persons, outbreaks, refugees, vaccines
Introduction
Humanitarian emergencies may result in breakdown of regular health services including routine vaccination programs.1 According to the United Nations High Commissioner for Refugees (UNHCR), there were 51.2 million people displaced by the end of 2013 due to conflict situations.2 In 2014, there were 4 Level 3 large-scale emergencies which led to the displacement of 5.5 million people in the Central African Republic, Iraq, South Sudan and Syria; this was in addition to a number of other crises in the Democratic Republic of the Congo (DRC), Libya, Nigeria, Somalia, and Ukraine.2 Displaced populations may include refugees who flee to escape a crisis by crossing recognized international borders and internally displaced persons (IDPs) who flee but remain within the borders of their own country. Security issues and logistic challenges associated with emergency settings can hamper the ability of affected populations to access routine health services and receive a complete series of recommended vaccinations. In certain emergencies, health services may be destroyed all together. Disruption of immunization services increases the number of susceptible individuals and the likelihood of outbreaks of vaccine-preventable diseases (VPDs).3
VPDs reported in the literature during humanitarian emergencies include measles, polio, and depending on geographical location, meningococcal meningitis, yellow fever, hepatitis A, and cholera. Camp settings, especially informal ones, increase a population's susceptibility to VPD's due to over-crowding, suboptimal living conditions, poor nutrition, scarcity of safe water and sanitation, and poor nutritional status.3,4 Furthermore, the forced migration of populations may exacerbate pre-existing health conditions. Risk factors for outbreaks are inextricably linked to excess risk of morbidity and mortality from VPDs, the reduction of which is the aim of public-health interventions during humanitarian crises.
The 2013 publication by the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) on vaccination in acute humanitarian emergencies was the first WHO document to provide a framework on decision making regarding vaccination strategies in emergencies.5 This review article aims to build on the WHO SAGE framework by providing a more current review of the available literature on VPDs among refugees and IDPs. The objectives of this article are to describe the risk factors for outbreaks of VPDs in emergency settings, key issues and challenges related to vaccination activities, and possible solutions to crisis-related impacts on vaccination efforts among displaced populations.
Methods
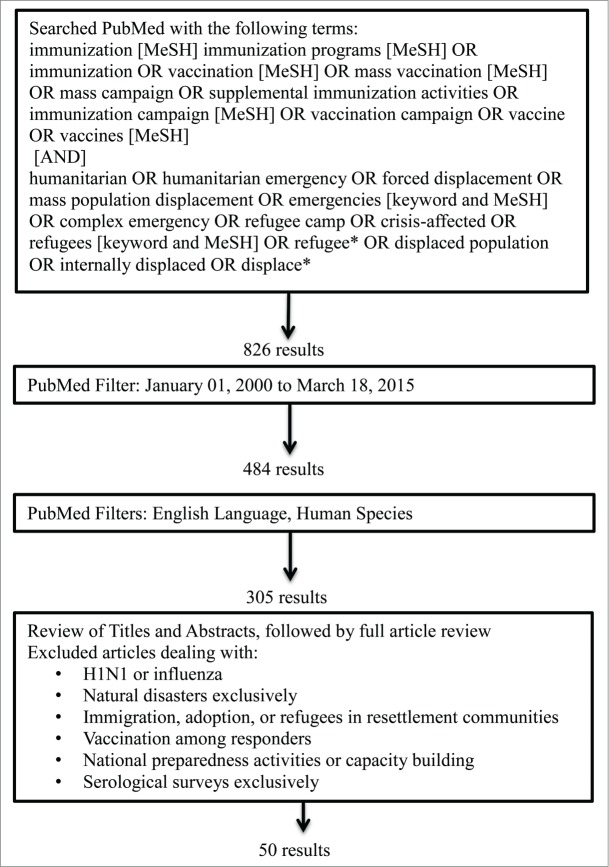
We searched the PubMed database for articles published from January 1st, 2000 to March 18th, 2015, providing a 15-year overview of VPDs among refugees and IDPs. Figure 1 provides the search terms used and the selection process of articles. The initial search term results with restriction to the 15 year time frame yielded 484 results, which were further reduced to 305 articles when accounting for articles only in English and related to human subjects. Review of the articles and abstracts further narrowed the search to 50 original articles, literature reviews, and published perspective pieces such as commentaries and letters to the editor.
Figure 1.
Literature search inclusion and exclusion criteria.
We defined a humanitarian crisis as a socio-political conflict or instability that threatened a population's safety and well-being by increasing morbidity and mortality above baseline and resulting in significant breakdown of national authority. We selected articles that addressed refugees or IDPs in the acute emergency phase as well as in the long-term phase recognizing that some crises may become protracted for years. Articles addressing influenza or H1N1 pandemic situations were excluded from this review as influenza vaccines are not often recommended in emergency situations. We excluded articles focused on natural disasters because the response to these events may differ from that of humanitarian emergencies. Articles focused on refugee resettlement were excluded because health issues and resources available to migrants may differ from those of refugees and IDPs. Articles addressing vaccinations among responders, national preparedness activities, capacity building, and serological surveys were also excluded.
Results
All articles from the final search results were grouped by disease and refugee or IDP status for review. A summary of the articles categorized by VPD and displacement status is presented in Table 1 and a reference list of articles by VPD, authors, and title in Table 2. The following provides a summary of the literature review according to various VPD groupings from the search term results.
Table 1.
Summary of articles by vaccine-preventable diseases and population type
| By disease | Refugees | IDPs | Both |
|---|---|---|---|
| Polio | 4 | 1 | 0 |
| Measles | 7 | 5 | 4 |
| Rubella | 1 | 0 | 0 |
| Mumps | 0 | 1 | 0 |
| Meningitis | 1 | 2 | 1 |
| Yellow Fever | 1 | 1 | 0 |
| Hepatitis A | 1 | 0 | 0 |
| Hepatitis B | 0 | 1 | 0 |
| Hepatitis E | 0 | 2 | 1 |
| Rotavirus | 1 | 0 | 0 |
| Cholera | 4 | 1 | 0 |
| Tetanus | 1 | 0 | 0 |
| Pneumococcus | 1 | 0 | 0 |
| Varicella | 1 | 0 | 0 |
| Total* | 25 | 17 | 8 |
Total reflects number of articles; some articles addressed multiple antigens
Abbreviations: IDPs = internally displaced persons.
Table 2.
Reference list of articles by vaccine-preventable disease, authors, and title
| Author | Year Published | Name of Article | Vaccine-Preventable Disease |
|---|---|---|---|
| Valente, F., et al. | 2000 | Massive outbreak of poliomyelitis caused by type-3 wild poliovirus in Angola in 1999. | Polio |
| Ahmad, K. | 2001 | Fears that Afghan exodus threatens polio eradication | Polio |
| Mohammadi, D. | 2013 | Middle Eastern countries scramble to stop spread of polio | Polio |
| Sheikh, M. A., et al. | 2014 | Combined use of inactivated and oral poliovirus vaccines in a large-scale campaign in refugee camps and host communities | Polio |
| Bonn, D. | 2001 | Infectious diseases threaten refugees entering Pakistan. | Polio, Measles |
| Kamugisha, C., et al. | 2003 | An outbreak of measles in Tanzanian refugee camps | Measles |
| Talley L, Salama P. | 2003 | Short report: assessing field vaccine efficacy for measles in famine-affected rural Ethiopia. | Measles |
| WHO WER | 2004 | Prevention of measles deaths in Darfur, Sudan. | Measles |
| CDC MMWR | 2004 | Emergency measles control activities–Darfur, Sudan | Measles |
| Mupere, E., et al. | 2005 | Impact of emergency mass immunisations on measles control in displaced populations in Gulu district, northern Uganda. | Measles |
| Guerrier, G., et al. | 2009 | Malnutrition and mortality patterns among internally displaced and non-displaced population living in a camp, a village or a town in Eastern Chad. | Measles |
| Kouadio, I. K., et al. | 2010 | Measles outbreaks in displaced populations: a review of transmission, morbidity and mortality associated factors | Measles |
| Kamadjeu, R., et al. | 2011 | Measles control and elimination in Somalia: the good, the bad, and the ugly. | Measles |
| Grais, R. F., et al. | 2011 | Measles vaccination in humanitarian emergencies: a review of recent practice | Measles |
| WHO WER | 2012 | Measles–Horn of Africa | Measles |
| Polonsky, J. A., et al. | 2013 | High levels of mortality, malnutrition, and measles, among recently-displaced Somali refugees in Dagahaley camp, Dadaab refugee camp complex, Kenya, 2011. | Measles |
| Mahamud, A., et al. | 2013 | Risk factors for measles mortality among hospitalized Somali refugees displaced by famine, Kenya, 2011. | Measles |
| Navarro-Colorado, C., et al. | 2014 | Measles outbreak response among adolescent and adult Somali refugees displaced by famine in Kenya and Ethiopia, 2011. | Measles |
| Kaiser, R. | 2014 | Emergency settings: be prepared to vaccinate persons aged 15 and over against measles | Measles |
| Kouadio, I. K., et al. | 2009 | Outbreak of measles and rubella in refugee transit camps. | Measles, Rubella |
| Hindiyeh, M. Y., et al. | 2009 | Characterization of large mumps outbreak among vaccinated Palestinian refugees | Mumps |
| Santaniello-Newton, A., Hunter, P. R. | 2000 | Management of an outbreak of meningococcal meningitis in a Sudanese refugee camp in Northern Uganda. | Meningitis |
| Gaspar, M., et al. | 2001 | Epidemiology of meningococcal meningitis in Angola, 1994–2000 | Meningitis |
| Iriso, R., et al. | 2008 | Bacterial meningitis following introduction of Hib conjugate vaccine in northern Uganda. | Meningitis |
| WHO WER | 2014 | Meningococcal disease control in countries of the African meningitis belt, 2013. | Meningitis |
| Nathan, N. | 2001 | Shortage of vaccines during a yellow fever outbreak in Guinea. | Yellow fever |
| Huhn, G. D., et al. | 2006 | Vaccination coverage survey versus administrative data in the assessment of mass yellow fever immunization in internally displaced persons–Liberia, 2004. | Yellow fever |
| Kaic, B., et al. | 2001 | Hepatitis A control in a refugee camp by active immunization. | Hepatitis A |
| Alavian, S. M., et al. | 2007 | The changing epidemiology of viral hepatitis B in Iran. | Hepatitis B |
| Boccia, D., et al. | 2006 | High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. | Hepatitis E |
| CDC MMWR | 2013 | Investigation of hepatitis E outbreak among refugees - Upper Nile, South Sudan, 2012–2013 | Hepatitis E |
| Ope, M., et al. | 2014 | Rotavirus enteritis in Dadaab refugee camps: implications for immunization programs in Kenya and Resettlement Countries. | Rotavirus |
| Chaignat, C. L., Monti, V. | 2007 | Use of oral cholera vaccine in complex emergencies: what next? Summary report of an expert meeting and recommendations of WHO. | Cholera |
| Verma, R., et al. | 2012 | Cholera vaccine: new preventive tool for endemic countries. | Cholera |
| Porta, M. I., et al. | 2014 | Feasibility of a preventive mass vaccination campaign with 2 doses of oral cholera vaccine during a humanitarian emergency in South Sudan | Cholera |
| Martin, S., et al. | 2014 | Post-licensure deployment of oral cholera vaccines: a systematic review. | Cholera |
| WHO WER | 2014 | Oral cholera vaccine campaign among internally displaced persons in South Sudan. | Cholera |
| Howard, N., et al. | 2011 | Reproductive health for refugees by refugees in Guinea III: maternal health. | Tetanus |
| Moszynski, P. | 2013 | Refugees in South Sudan to receive pneumococcal vaccine after delays over price. | Pneumococcus |
| Shimakawa, Y., et al. | 2010 | Outbreak of chickenpox in a refugee camp of northern Thailand. | Varicella |
| Morris, K. | 2000 | Agency warns of crisis in beleaguered Democratic Republic of Congo. | General |
| Koop, D. G., et al. | 2001 | Results of the expanded program on immunization in the Macedonian refugee camps. | General |
| Leus, X., et al. | 2001 | Internally displaced persons | General |
| Bhatia, S., et al. | 2002 | A social and demographic study of Tibetan refugees in India. | General |
| Connolly, M. A., et al. | 2004 | Communicable diseases in complex emergencies: impact and challenges | General |
| Avogo, W. A., Agadjanian, V. | 2010 | Forced migration and child health and mortality in Angola. | General |
| Moodley, K., et al. | 2013 | Ethical considerations for vaccination programmes in acute humanitarian emergencies. | General |
| Caplan, A. L., Curry, D. R. | 2015 | Refugees, humanitarian aid and the right to decline vaccinations | General |
| Devi, S. | 2015 | Long-term planning needed for Iraq's displaced. | General |
Abbreviations: CDC, Centers for Disease Control and Prevention; MMWR, Morbidity and Mortality Weekly Report; WHO, World Health Organization; WER, Weekly Epidemiological Report.
Measles, mumps, and rubella
Measles is a well-documented epidemic-prone VPD in the emergency context. An assessment of risk factors for measles mortality in a 2011 outbreak among Somali refugees in Kenya found that gastrointestinal and respiratory complications of measles were common, and increased mortality was associated with malnutrition and neurologic complications.6 This was compounded by factors related to severe famine and drought and an intensified civil war resulting in mass population displacement from Somalia to neighboring countries. Case fatality rates (CFR) of measles cases among displaced populations are often higher than those observed in stable populations.3,4,6 Recommendations for case management include vitamin A supplementation, enrollment in feeding programs if necessary, as well as oral rehydration and/or antibacterial treatment as needed.4,7 However, diagnosis and management of severe measles complications in refugee camps and other resource-limited settings can be extremely difficult; therefore every effort should be made to achieve >95% 2-dose measles vaccination coverage to prevent outbreaks as an effective alternative.6
We reviewed articles related to measles outbreaks among displaced populations in Tanzania, Ivory Coast, Darfur, Ethiopia, Somalia and Kenya.8-11 Movement of new arrivals into a refugee camp may introduce measles transmission. For example, initial cases in concurrent measles outbreaks in the Dollo Ado refugee camp in Ethiopia and the Dadaab refugee camp in Kenya during 2010 and 2011 were likely new arrivals from Somalia, a country that was also experiencing an ongoing measles outbreak.11,12 A 2000–2001 outbreak of measles in 4 established Burundi refugee camps in Tanzania was an extension of an outbreak in Burundi and precipitated by a large influx and inadequate vaccination of new arrivals.9 Therefore, priority should be placed on disease surveillance to camp areas with the newest refugee arrivals where timely and reliable disease reporting is likely to be weakest.6
Although camp conditions are common risk factors for measles transmission, movement between camp and host populations also contributes to measles outbreaks.9-11,13 Close interactions between Liberian refugees and Ivorian host community in the urban setting of Abidjan contributed to an outbreak of measles and rubella in 2003 and 2004 in transit camps within Ivory Coast.10 When both the displaced and host communities have apparent immunity gaps for VPDs, it is therefore recommended to vaccinate both groups of susceptibles, as was done in the mass measles vaccination efforts of both refugee and non-displaced populations in Abidjan.10 Although not always possible due to political and security reasons, establishment of transit and reception centers at crossing points can ensure timely screening of those with measles and help provide appropriate case management during mass population movement.6
Various target age-groups were reported for measles vaccination campaigns in the available literature within the African region.11,14,15 Over the past 15 years, increasing consideration in examining the local epidemiology and context of measles virus transmission in various settings are reflected in the evoluation of recommendations and different target age groups in outbreak response.16
Collaboration with key agency and community partners are also crucial in outbreak response vaccination strategies. In 2004, grassroots community organizations and mass media were engaged to social mobilize IDP camps of West and North Darfur for a measles vaccination campaign targeting those aged 9 months to 15 years.8 Given the insecurity in Western Darfur and limited access due to the rainy season, this large-scale vaccination campaign was made possible through stakeholder cooperation including negotiations with opposition forces which allowed for vaccinations to take place in hard-to-reach areas.8,15
Outbreaks of rubella and mumps have also been reported among displaced populations. In the 2003–2004 measles and rubella outbreak in Ivory Coast transit camps, incidence of rubella was highest among children aged 5–15 years old. Cases among relatively older children may have resulted from limited exposure to infection when living in remote areas.10 This study also highlights the importance of testing for both measles and rubella in febrile rash outbreaks in refugee settings. In addition, index cases among refugees were not vaccinated in transit camps on arrival; these situations highlight the importance of early vaccination upon arrival at camps according to existing guidelines.17 From 2003 to 2005, a large mumps outbreak among 6 to 15 year olds was reported in Palestinian refugee camps. While mumps remained endemic in the Palestinian West Bank, cases among individuals born after 1994 with historical vaccination coverage over 85% suggested inadequate protection against mumps with only one dose of measles-mumps-rubella (MMR) vaccination. As a result of this mumps outbreak, a campaign using MMR vaccine took place in May of 2005 targeting grades 1–12 and college students.18
Polio
Despite significant progress in the global polio eradication efforts, outbreaks of poliomyelitis can take place in displaced populations until poliovirus transmission is stopped in all countries globally. Due to political instability, inadequate sanitation, and displacement of unvaccinated individuals in highly dense urban centers, a polio outbreak took place in Luanga province of Angola.19 In addition, the presence of landmines and collapsed infrastructure resulted in reduced access to health services in many districts of the country. This outbreak highlights the impact of war on access to routine immunizations and vaccination campaigns for all children during periods of conflict and displacement. In 2015, only 3 countries continue to have endemic circulation of wild polio virus: Afghanistan, Pakistan, and Nigeria, and the last reported WPV case in Nigeria was in July 2014. After airstrikes in Afghanistan in 2001, Afghani refugees were expected to enter Pakistan thereby raising concerns of increasing transmission of polio between the 2 countries.20,21 As a result, National Immunization Days were conducted in Pakistan and Afghanistan in September of 2001 vaccinating over 30 million children under the age of 5 years. Moreover, all incoming refugee children were preemptively vaccinated against polio and measles in Pakistan.21
After more than 10 years without polio, laboratory-confirmed cases reemerged in Syria in 2013 due to recent conflicts, following confirmation of poliovirus in the sewers of Egypt, Israel, and West Bank, Gaza. 22 Outbreaks can provide opportunities to sensitize populations at risk and strengthen eradication efforts. In addition to the local outbreak response immunization efforts in Syria, regional vaccination responses were launched in surrounding countries of Lebanon, Jordan, Turkey, Palestine, and Egypt. Another example of a post-outbreak opportunity to eradicate poliomyelitis was the response to a 2013 outbreak of wild polio virus that took place in the Horn of Africa.23 As part of the vaccination response, a combined inactivated poliovirus (IPV) and oral poliovirus vaccine (OPV) campaign targeting children under 5 years of age took place in refugee camps and surrounding communities near the Kenya-Somalia border.23 The addition of IPV in the response was to boost population immunity among children who have received OPV to ensure interruption of any residual polio virus transmission and prevent any future outbreaks from new importations.
Meningitis
Meningitis outbreaks can have significant economic and psychological impacts on households and communities, particularly when the majority of cases are among adults and adolescents. In endemic situations, meningococcal meningitis mostly affects young children; however, persons aged 15 to 29 years old were the most affected in the outbreaks reported among displaced populations in Angola. The greater number of cases in this older age group is not unusual in epidemics within areas of high density such as refugees and IDP camps. The International Coordinating Group on Vaccine Provision for Epidemic Meningitis Control is tasked with maintaining an emergency vaccine stockpile to respond to meningitis outbreaks globally.24
In the Yambala area in Angola, insecurity constrained outbreak response immunization activities and disease transmission in inaccessible areas stopped only with the onset of the rainy season. Population movement across borders also raised concerns about the spread of disease outside of the meningitis belt. Six meningococcal meningitis outbreaks in Angola affected displaced populations during the civil conflict.25 Mass population movements due to civil war, rampant urbanization, and formation of informal camps and settlements contribute to meningococcal disease transmission. Economic decline and the collapse of public health and health service infrastructure in Angola highlighted the crisis-related impacts of implementing surveillance and outbreak response to communicable diseases.
After a large influx of Sudanese refugees into camps in Northern Uganda in 1994, there were 2 consecutive outbreaks of group A meningococcal meningitis.26 Maintaining effective surveillance system in refugee settings was proved difficult with competing health care priorities including concurrent epidemics of measles and malnutrition. As part of the response, mass vaccination of 1 to 30 year olds was successfully conducted to control the initial outbreak. The decision to extend the upper limit of the target age-group to 30 years was based on analysis of age-specific attack rates. The sudden influx of refugees overwhelmed screening at registration resulting in 11,000 refugees not being screened or vaccinated upon arrival. This may have led to the second consecutive meningitis outbreak that required a catch-up vaccination campaign in 1995. However, this outbreak demonstrated the effectiveness of rapid mass vaccination campaigns with high coverage in controlling outbreaks of serogroup A meningococcal meningitis among refugee population.
Cholera and rotavirus
Diarrheal diseases are one of the leading causes of death among children under 5 years of age in emergency settings. Rotavirus has not been well studied among displaced populations. WHO recommends introduction of rotavirus vaccine into national immunization programs for countries where diarrheal deaths account for ≥10% of mortality among children under 5 years of age. In 2011, a diarrheal disease surveillance system implemented in the Dadaab refugee camp in Kenya found rotavirus circulating year-round with 23% of children under 5 years of age presenting with diarrhea in a health facility to be infected with rotavirus.27 This study supports the use of rotavirus vaccination in refugee camps; however, additional evaluation in other refugee settings is needed to provide further evidence of its efficacy among refugee populations.
Several oral cholera vaccines (OCV) have been developed as a public health intervention for vulnerable populations in emergency settings.28,29 Mass vaccinations with OCV acquired from the global stockpile have been used in several emergency contexts to prevent outbreaks of cholera in camp situations.28-32 A meeting among WHO experts noted that OCV could be used as a preventive intervention in humanitarian emergencies.29 Further studies are needed to determine the possible cost-benefit of OCV, number of doses needed for adequate protection, and best practices for cold chain implementation in the emergency context.29
In 2012–2014 the first use of the global OCV stockpile was conducted in OCV campaigns in IDP camps and surrounding communities in South Sudan during a humanitarian crisis. The OCV campaigns were completed through fixed outreach posts and mobile door-to-door teams targeting individuals >1 year of age, excluding pregnant women.31,33 Distribution of vaccination cards were used to track immunization status, and soap as an incentive to receive the second OCV dose.31,32 However, despite the success of campaigns, there were security, logistical, and financial challenges related to the 2 dose administration schedule as well as weakened cold chain capacity due to the ongoing conflict.31 Furthermore, additional costs were required to transport OCV via charter flights due to insecurity. Curfew and access constraints limited staff movement and resulted in restricted and delayed operations. This study showed that mass OCV campaigns in emergency settings is possible but not without additional human resources, cold-chain capacity, logistical and communications support from all relevant partners.31
Yellow fever
The mosquito vector Aedes aegypti for yellow fever thrives in densely-populated areas such as urban areas and camps. In 2004, 2 yellow fever mass vaccination campaigns were launched in IDP camps and surrounding communities in Liberia after reports of 4 lab-confirmed cases.33 Due to the 14 years of civil war, much of Liberia's healthcare infrastructure, public health disease surveillance, and immunization programs were severely disrupted. Survey assessment of vaccination coverage reported 90% by self-report and 80% by proof of vaccination card. The success of the mass vaccination efforts among IDPs required intensive social mobilization, efficient logistical networks, well-trained health staff, and safe vaccine delivery.33
During a time Liberian and Sierra Leonean refugees and IDPs were in Guinea, a yellow fever outbreak was reported with 688 cases and 225 deaths (CFR 33%) in 2000.34 Movement through the forested areas of Guinea where yellow fever is endemic into densely-populated camps and urban areas may have led to increased transmission. In addition, this outbreak highlighted at that time the global shortage of available yellow fever vaccines, which ultimately resulted in the creation of the UNICEF stockpile of 2 million doses of yellow fever vaccine to be used in response to future outbreaks.
Hepatitis A and E
Several types of viral hepatitis were noted among displaced populations in our review. In 1999, a hepatitis A outbreak among Kosovar refugees in Croatia was primarily a result of poor sanitation and housing.35 Outbreak response included sanitation improvement, health education, screening of children 1–15 years of age and vaccination for seronegative children. Serological studies conducted 4 weeks post-vaccination reported 97% seroconversion. The authors suggested active vaccination was a successful component in disrupting hepatitis A transmission in this refugee population.35
Hepatitis E virus (HEV) infection is generally a self-limiting condition with low fatality to the general population, although there is high case-fatality among pregnant women. In 2004, a HEV outbreak in Mornay IDP Camp, Darfur, reported a CFR of 33% among pregnant women highlighting the need for rapid interventions to prevent mortality in this special population.36 A 2012–2013 outbreak of HEV in the refugee camps of Maban County in South Sudan had a CFR of 10% among pregnant women.37 HEV transmission control often includes improvements to water and sanitation, clinical care, and surveillance. Although there is currently no HEV vaccine available globally, recent developments of a hepatitis E vaccine in China may have utility in controlling outbreaks among displaced populations.38 The vaccine has yet to achieve WHO prequalification and requires further studies regarding safety in children and pregnant women. Additional research is needed to assess appropriate dosing regimen and efficacy of HEV vaccine to control outbreaks among refugees and IDPs.
Varicella
Varicella is not included in most national routine vaccination programs, though outbreaks may be of particular concern among displaced populations in tropical settings. While the majority of individuals in temperate climates develop natural immunity from previous infection before adolescence, high levels of seronegativity are typically still observed among adults in tropical regions. Adults infected with varicella may suffer from increased disease severity and greater complications. Moving from rural areas of low population density to densely populated refugee camps may also increase transmission. In 2008, a varicella outbreak in refugee settings was documented among Lao Hmong refugees in Thailand.39 The affected population was between 3 months and 53 years old in this outbreak with 14% of cases ≥15 years old or older. Moreover, hospitalizations occurred exclusively among adults aged ≥15 years. Preventive varicella vaccination may be warranted in refugee camps if epidemiological evidence suggests increased risk of an outbreak with high levels of morbidity.
Routine immunizations
Establishment of routine immunization services is not well studied in the emergency context. Three articles discussed integration of routine vaccination schedules of host country national expanded program on immunization (EPI) program for refugee populations.40-42 Tibetan refugees in India were provided routine childhood immunizations according to the national EPI schedule of the India Ministry of Health.40 The program reported challenges in delivery of vaccine to the target population, with less than half of the Tibetan refugee children reported as fully vaccinated.40 As a solution to improve routine immunization coverage among displaced populations, the Macedonian Ministry of Health delivered vaccines to Albanian Kosovar refugees living in camps and the surrounding communities through weekly mobile immunization clinics.42 In Guinea, more than 90% of Liberian and Sierra Leonean refugee mothers knew about tetanus vaccination during pregnancy, though only 11–42% utilized the free antenatal care in government facilities sponsored by UNHCR.41
EPI programs for refugees may differ between that of host nations and their countries of origin thereby creating both ethical and logistical challenges.43,44 Ethical concerns have also been raised regarding the ability of the host nations to deny humanitarian assistance to refugees refusing vaccinations during emergency situations. Such was the case in 2014 during the response of the governments of Lebanon, to resistance from Syrian refugees, and Pakistan, to Afghan refugees, in receiving polio vaccination upon entrance into camps.45 The authors argued that host governments and supporting agencies have the responsibility to require such vaccinations in humanitarian emergencies where the risk of VPD outbreaks can endanger both host and displaced communities.
EPI programs can often become non-functional during humanitarian emergencies.46-48, To address this, multiple opportunities for vaccination, including child health days and catch-up vaccination campaigns, can be used to boost population immunity.48 Protracted emergencies reinforce the importance of thinking beyond the emergency mindset and including long-term strategies in basic health service provisions.48,49 WHO and other UN agencies can play a critical role in coordinating the establishment of regular health services, including routine immunizations, in situations where the national government is unwilling or unable to provide the necessary aid to IDPs.50
In regards to financing and use of newer vaccines among displaced populations, 2 articles mentioned the support of the Global Alliance for Vaccines and Immunization (GAVI) in acquiring Haemophilus influenza type b (Hib) and pneumococcal conjugate vaccine (PCV) in emergencies.51,52 GAVI has helped fund the provision of pentavalent vaccines for IDPs in Uganda from 2002 to 2006 and PCV for Yida refugee camp in South Sudan in 2013.51,52
Discussion
Mass population movement can increase risk of VPDs among IDPs, refugees, and host communities. Displaced populations can introduce endemic pathogens to a new place as well as make contact with new pathogens either on their journey or within the host community. The same conditions that increase transmission of communicable diseases—high prevalence of malnutrition, unsanitary conditions, population displacement, overcrowding, and lack of clean water—can result in VPD outbreaks and high rates of morbidity and mortality. The primary objective of vaccination in an acute humanitarian emergency is to rapidly reduce the risk of disease to protect a population during periods of extreme vulnerability.5
While the goals of vaccination during an acute phase of an emergency focus on limiting the number of preventable deaths, the goals of routine vaccination programs aim to ensure long-term protection against a given disease through progressive increase of population immunity. In particular, protracted emergencies need to consider reestablishing regular immunization services as soon as possible given the rapid accumulation of susceptible populations with missed opportunities of routine vaccinations. According to UNHCR, protracted camps with 25,000 or more displaced persons should implement routine services equivalent to host country immunization policies with occasional supplementary vaccination campaigns.11 Mass vaccination campaigns in camps may consider administering multiple antigens with the aim of reducing administrative and logistical costs.8,15,33
Forced migration may result in incomplete vaccinations of routine immunizations placing both the displaced and the host population at an elevated risk for contracting disease. In situations where the displaced population is unstable or there is frequent population movement, mobile clinics have been used to effectively achieve high vaccination coverage.23,33,34,42 Vaccination schedules in the country of origin may not always align with that of the host country, thereby creating challenges in providing interventions that are well understood by the displaced population. Intensive social mobilization is needed in situations where the perceived importance of vaccination is low.41 Opportunities for defaulter tracing activities and catch up campaigns should also be in place for those who may have missed routine vaccinations during the emergency.
Destruction of cold chain equipment and infrastructure for transportation provide significant challenges to vaccination during humanitarian conflicts. Growing insecurities, as seen in the increasing number of targeted attacks on health workers in recent years during polio eradication efforts in Pakistan, can dramatically limit the ability of humanitarian players to provide the necessary vaccinations.53-55 These issues may be even more pronounced in IDP camps, where operations are within the borders of a country in conflict and the status of protection and assistance from the international community may not apply.50 Collaboration with defacto local authorities to deliver vaccination to chronically insecure areas are warranted at times.48
Other major challenges in addition to insecurity, logistical, and access issues encountered during emergencies may include insufficiently trained staff, insufficient supplies and equipment, communication issues and perception within the host community, and limited camp capacity after massive population influx.4,33 For example, observation of polio vaccination teams in 2013 in Kenya noted errors in the injection technique of IPV in the field and cold chain issues during the OPV and IPV combined campaign; thereby stressing the need for appropriate training of vaccinators and supervisors.23 In a 2014 mass OCV vaccination campaign in South Sudan, there was lower participation from men who believed vaccination was for women and children.33 There may be local resistance to the idea of expanding refugee or IDP camps to accommodate new arrivals that can lead to unplanned transition camps.11,56 In addition, new waves of displaced persons can come suddenly and overwhelm existing capacity.
Guidelines to protect populations against VPDs in conflict settings include the UNHCR handbook for emergencies, the UNICEF's code of conduct, the WHO SAGE framework document, and the Sphere handbook which includes internationally recognized principles and universal minimum standards for response to humanitarian emergencies.5,17,57,58 Recent WHO position papers also provide guidance on vaccinations during emergencies, including development of new OCVs and their safety, effectiveness, and efficacy profiles.59. WHO also released a new Meningococcal position paper in 2015 addressing the conjugate vaccine safety, schedule, and efficacy based on preventive mass campaigns, along with updated recommendations that are particularly relevant in the African meningitis belt.60 In 2012, polio was declared a global public health emergency; the WHO position paper on polio vaccination recommends the inclusion of one dose of IPV for routine immunization programs in countries using only OPV.61
Limitations of this review include possible omission of articles, as this review was restricted to articles written in English and available on PubMed. The articles were also restricted by publication date from 2000 to 2015. However, this review provides an update on the available literature regarding vaccinations among a highly vulnerable population and describes the unique challenges of VPDs during humanitarian emergencies. Further operational research is needed to evaluate the prioritization of public health interventions such as vaccinations, timing of implementation during the various phases of emergencies, and the use of newer vaccines including PCV, OCV, Hib, rotavirus, and pentavalent vaccines.
Epidemic-prone VPDs are of particular concern during acute emergencies. Displaced populations are at an increased risk for outbreaks of VPDs due to creation or exacerbation of factors associated with disease transmission such as mass population movements, overcrowding, malnutrition, and poor water and sanitation conditions. Vaccination is one of the most basic and critical health interventions for protecting vulnerable populations during emergencies. While highly effective vaccines and guidelines to combat VPDs are available, the trend of increasing number of humanitarian emergencies globally poses new and emerging challenges in ensuring all susceptible individuals have access to life saving vaccines.
Disclosure of Potential Conflicts of Interest
None of the authors have a commercial or other financial interest associated with the information presented in this article. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Acknowledgments
We would like to thank Jennifer Head, MPHc, and Anyie Li, MPHc, from the Rollins School of Public Health, Emory University, for their editorial assistance and help in review of the current literature.
References
- 1.Morris K. Agency warns of crisis in beleaguered Democratic Republic of Congo. Lancet 2000; 355:210; PMID:10675131; http://dx.doi.org/ 10.1016/S0140-6736(05)72089-4 [DOI] [PubMed] [Google Scholar]
- 2.UNHCR Global Appeal 2015 Update Geneva, Switzerland: United Nations, 2015. [Google Scholar]
- 3.Connolly MA, Gayer M, Ryan MJ, Salama P, Spiegel P, Heymann DL. Communicable diseases in complex emergencies: impact and challenges. Lancet 2004; 364:1974-83; PMID:15567014; http://dx.doi.org/ 10.1016/S0140-6736(04)17481-3 [DOI] [PubMed] [Google Scholar]
- 4.Kouadio IK, Kamigaki T, Oshitani H. Measles outbreaks in displaced populations: a review of transmission, morbidity and mortality associated factors. BMC Int Health Human Rights 2010; 10:5; PMID:20298611; http://dx.doi.org/ 10.1186/1472-698X-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SAGE Vaccination in acute humanitarian emergencies: a framework for decision making In: Immunization VaBI, ed. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 6.Mahamud A, Burton A, Hassan M, Ahmed JA, Wagacha JB, Spiegel P, Haskew C, Eidex RB, Shetty S, Cookson S, et al.. Risk factors for measles mortality among hospitalized Somali refugees displaced by famine, Kenya, 2011. Clin Infect Dis 2013; 57:e160-6; PMID:23821730; http://dx.doi.org/ 10.1093/cid/cit442 [DOI] [PubMed] [Google Scholar]
- 7.Grais RF, Strebel P, Mala P, Watson J, Nandy R, Gayer M. Measles vaccination in humanitarian emergencies: a review of recent practice. Conflict Health 2011; 5:21; PMID:21942984; http://dx.doi.org/ 10.1186/1752-1505-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevention of measles deaths in Darfur, Sudan. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2004; 79:344-8; PMID:15707083 [PubMed] [Google Scholar]
- 9.Kamugisha C, Cairns KL, Akim C. An outbreak of measles in Tanzanian refugee camps. J Infect Dis 2003; 187 Suppl 1:S58-62; PMID:12721892; http://dx.doi.org/ 10.1086/368057 [DOI] [PubMed] [Google Scholar]
- 10.Kouadio IK, Koffi AK, Attoh-Toure H, Kamigaki T, Oshitani H. Outbreak of measles and rubella in refugee transit camps. Epidemiol Infect 2009; 137:1593-601; PMID:19379539; http://dx.doi.org/ 10.1017/S0950268809002520 [DOI] [PubMed] [Google Scholar]
- 11.Navarro-Colorado C, Mahamud A, Burton A, Haskew C, Maina GK, Wagacha JB, Ahmed JA, Shetty S, Cookson S, Goodson JL, et al.. Measles outbreak response among adolescent and adult Somali refugees displaced by famine in Kenya and Ethiopia, 2011. J Infect Dis 2014; 210:1863-70; PMID:25117754; http://dx.doi.org/ 10.1093/infdis/jiu395 [DOI] [PubMed] [Google Scholar]
- 12.Measles–Horn of Africa, 2010–2011 MMWR Morbidity and mortality weekly report 2012; 61:678-84. [PubMed] [Google Scholar]
- 13.Guerrier G, Zounoun M, Delarosa O, Defourny I, Lacharite M, Brown V, Pedalino B. Malnutrition and mortality patterns among internally displaced and non-displaced population living in a camp, a village or a town in Eastern Chad. PloS one 2009; 4:e8077; PMID:19956627; http://dx.doi.org/ 10.1371/journal.pone.0008077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mupere E, Onek P, Babikako HM. Impact of emergency mass immunisations on measles control in displaced populations in Gulu district, northern Uganda. East Afr Med J 2005; 82:403-8; PMID:16261916 [DOI] [PubMed] [Google Scholar]
- 15.Emergency measles control activities–Darfur, Sudan, 2004 MMWR Morbidity and mortality weekly report 2004; 53:897-9. [PubMed] [Google Scholar]
- 16.Kaiser R. Emergency settings: be prepared to vaccinate persons aged 15 and over against measles. J Infect Dis 2014; 210:1857-9; PMID:25193980; http://dx.doi.org/ 10.1093/infdis/jiu463 [DOI] [PubMed] [Google Scholar]
- 17.The Sphere Handbook 2011 : Humanitarian Charter and Minimum Standards in Humanitarian Response. Bourton on Dunsmore, UK: Practical Action Publishing, 2011. [Google Scholar]
- 18.Hindiyeh MY, Aboudy Y, Wohoush M, Shulman LM, Ram D, Levin T, Frank T, Riccardo F, Khalili M, Sawalha ES, et al.. Characterization of large mumps outbreak among vaccinated Palestinian refugees. J Clin Microbiol 2009; 47:560-5; PMID:19144793; http://dx.doi.org/ 10.1128/JCM.01756-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valente F, Otten M, Balbina F, Van de Weerdt R, Chezzi C, Eriki P, Van-Dúnnen J, Bele JM. Massive outbreak of poliomyelitis caused by type-3 wild poliovirus in Angola in 1999. Bull World Health Organ 2000; 78:339-46; PMID:10812730 [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad K. Fears that Afghan exodus threatens polio eradication. Lancet 2001; 358:1161; PMID:11597676; http://dx.doi.org/ 10.1016/S0140-6736(01)06306-1 [DOI] [PubMed] [Google Scholar]
- 21.Bonn D. Infectious diseases threaten refugees entering Pakistan. Lancet Infect Dis 2001; 1:214; PMID:11871501; http://dx.doi.org/ 10.1016/S1473-3099(01)00102-5 [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi D. Middle Eastern countries scramble to stop spread of polio. Lancet 2013; 382:1621-2; PMID:24251324; http://dx.doi.org/ 10.1016/S0140-6736(13)62289-8 [DOI] [PubMed] [Google Scholar]
- 23.Sheikh MA, Makokha F, Hussein AM, Mohamed G, Mach O, Humayun K, Okiror S, Abrar L, Nasibov O, Burton J, et al.. Combined use of inactivated and oral poliovirus vaccines in refugee camps and surrounding communities - Kenya, December 2013. MMWR Morb Mortal Wkly Rep 2014; 63:237-41; PMID:24647400 [PMC free article] [PubMed] [Google Scholar]
- 24.Meningococcal disease control in countries of the African meningitis belt, 2013 Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2014; 89:206-14 [PubMed] [Google Scholar]
- 25.Gaspar M, Leite F, Brumana L, Felix B, Stella AA. Epidemiology of meningococcal meningitis in Angola, 1994–2000. Epidemiol Infect 2001; 127:421-4; PMID:11811874; http://dx.doi.org/ 10.1017/S0950268801006318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santaniello-Newton A, Hunter PR. Management of an outbreak of meningococcal meningitis in a Sudanese refugee camp in Northern Uganda. Epidemiol Infect 2000; 124:75-81; PMID:10722133; http://dx.doi.org/ 10.1017/S0950268899003398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ope M, Ochieng SB, Tabu C, Marano N. Rotavirus enteritis in Dadaab refugee camps: implications for immunization programs in Kenya and Resettlement Countries. Clin Infect Dis 2014; 59:v-vi; PMID:25551839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma R, Khanna P, Chawla S. Cholera vaccine: new preventive tool for endemic countries. Hum Vaccin Immunother 2012; 8:682-4; PMID:22634452; http://dx.doi.org/ 10.4161/hv.19083 [DOI] [PubMed] [Google Scholar]
- 29.Chaignat CL, Monti V. Use of oral cholera vaccine in complex emergencies: what next? Summary report of an expert meeting and recommendations of WHO. J Health Popul Nutr 2007; 25:244-61; PMID:17985828 [PMC free article] [PubMed] [Google Scholar]
- 30.Martin S, Lopez AL, Bellos A, Deen J, Ali M, Alberti K, Anh DD, Costa A, Grais RF, Legros D, et al.. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ 2014; 92:881-93; PMID:25552772; http://dx.doi.org/ 10.2471/BLT.14.139949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oral cholera vaccine campaign among internally displaced persons in South Sudan Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2014; 89:214-20 [PubMed] [Google Scholar]
- 32.Porta MI, Lenglet A, de Weerdt S, Crestani R, Sinke R, Frawley MJ, Van Herp M, Zachariah R. Feasibility of a preventive mass vaccination campaign with two doses of oral cholera vaccine during a humanitarian emergency in South Sudan. Trans R Soc Trop Med Hyg 2014; 108:810-5; PMID:25311798; http://dx.doi.org/ 10.1093/trstmh/tru153 [DOI] [PubMed] [Google Scholar]
- 33.Huhn GD, Brown J, Perea W, Berthe A, Otero H, LiBeau G, Maksha N, Sankoh M, Montgomery S, Marfin A, et al.. Vaccination coverage survey versus administrative data in the assessment of mass yellow fever immunization in internally displaced persons–Liberia, 2004. Vaccine 2006; 24:730-7; PMID:16182416; http://dx.doi.org/ 10.1016/j.vaccine.2005.08.077 [DOI] [PubMed] [Google Scholar]
- 34.Nathan N, Barry M, Van Herp M, Zeller H. Shortage of vaccines during a yellow fever outbreak in Guinea. Lancet 2001; 358:2129-30; PMID:11784630; http://dx.doi.org/ 10.1016/S0140-6736(01)07185-9 [DOI] [PubMed] [Google Scholar]
- 35.Kaic B, Borcic B, Ljubicic M, Brkic I, Mihaljevic I. Hepatitis A control in a refugee camp by active immunization. Vaccine 2001; 19:3615-9; PMID:11395194; http://dx.doi.org/ 10.1016/S0264-410X(01)00103-7 [DOI] [PubMed] [Google Scholar]
- 36.Boccia D, Guthmann JP, Klovstad H, Hamid N, Tatay M, Ciglenecki I, Nizou JY, Nicand E, Guerin PJ. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis 2006; 42:1679-84; PMID:16705571; http://dx.doi.org/ 10.1086/504322 [DOI] [PubMed] [Google Scholar]
- 37.Investigation of hepatitis E outbreak among refugees - Upper Nile, South Sudan, 2012–2013. MMWR Morb Mortal Wkly Rep 2013; 62:581-6; PMID:23884344 [PMC free article] [PubMed] [Google Scholar]
- 38.Teshale E, Ward JW. Making hepatitis E a vaccine-preventable disease. N Engl J Med 2015; 372:899-901; PMID:25738664; http://dx.doi.org/ 10.1056/NEJMp1415240 [DOI] [PubMed] [Google Scholar]
- 39.Shimakawa Y, Camelique O, Ariyoshi K. Outbreak of chickenpox in a refugee camp of northern Thailand. Confl Health 2010; 4:4; PMID:20175899; http://dx.doi.org/ 10.1186/1752-1505-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatia S, Dranyi T, Rowley D. A social and demographic study of Tibetan refugees in India. Soc Sci Med 2002; 54:411-22; http://dx.doi.org/ 10.1016/S0277-9536(01)00040-5 [DOI] [PubMed] [Google Scholar]
- 41.Howard N, Woodward A, Souare Y, Kollie S, Blankhart D, von Roenne A, Borchert M. Reproductive health for refugees by refugees in Guinea III: maternal health. Confl Health 2011; 5:5; PMID:21486433; http://dx.doi.org/ 10.1186/1752-1505-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koop DG, Jackson BM, Nestel G. Results of the expanded program on immunization in the Macedonian refugee camps. American journal of public health 2001; 91:1656-9; PMID:11574331; http://dx.doi.org/ 10.2105/AJPH.91.10.1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moodley K, Hardie K, Selgelid MJ, Waldman RJ, Strebel P, Rees H, Durrheim DN. Ethical considerations for vaccination programmes in acute humanitarian emergencies. Bull World Health Organ 2013; 91:290-7; PMID:23599553; http://dx.doi.org/ 10.2471/BLT.12.113480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alavian SM, Fallahian F, Lankarani KB. The changing epidemiology of viral hepatitis B in Iran. J Gastrointestin Liver Dis 2007; 16:403-6; PMID:18193122 [PubMed] [Google Scholar]
- 45.Caplan AL, Curry DR. Refugees, humanitarian aid and the right to decline vaccinations. J Med Ethics 2015; 41:276-7; PMID:25135799; http://dx.doi.org/ 10.1136/medethics-2014-102383 [DOI] [PubMed] [Google Scholar]
- 46.Avogo WA, Agadjanian V. Forced migration and child health and mortality in Angola. Soc Sci Med 2010; 70:53-60; http://dx.doi.org/ 10.1016/j.socscimed.2009.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talley L, Salama P. Short report: assessing field vaccine efficacy for measles in famine-affected rural Ethiopia. Am J Trop Med Hyg 2003; 68:545-6; PMID:12812341 [DOI] [PubMed] [Google Scholar]
- 48.Kamadjeu R, Assegid K, Naouri B, Mirza IR, Hirsi A, Mohammed A, Omer M, Dualle AH, Mulugeta A. Measles control and elimination in Somalia: the good, the bad, and the ugly. J Infect Dis 2011; 204 Suppl 1:S312-7; PMID:21666179; http://dx.doi.org/ 10.1093/infdis/jir066 [DOI] [PubMed] [Google Scholar]
- 49.Devi S. Long-term planning needed for Iraq's displaced. Lancet 2015; 385:594; PMID:25713855; http://dx.doi.org/ 10.1016/S0140-6736(15)60220-3 [DOI] [PubMed] [Google Scholar]
- 50.Leus X, Wallace J, Loretti A. Internally displaced persons. Prehosp Disaster Med 2001; 16:116-23; PMID:11875794 [DOI] [PubMed] [Google Scholar]
- 51.Iriso R, Ocakacon R, Acayo JA, Mawanda MA, Kisayke A. Bacterial meningitis following introduction of Hib conjugate vaccine in northern Uganda. Ann Trop Paediatr 2008; 28:211-6; PMID:18727850; http://dx.doi.org/ 10.1179/146532808X335660 [DOI] [PubMed] [Google Scholar]
- 52.Moszynski P. Refugees in South Sudan to receive pneumococcal vaccine after delays over price. BMJ 2013; 347:f5042; PMID:23935063 [DOI] [PubMed] [Google Scholar]
- 53.Alexander JP Jr., Zubair M, Khan M, Abid N, Durry E. Progress and peril: poliomyelitis eradication efforts in Pakistan, 1994–2013. J Infect Dis 2014; 210 Suppl 1:S152-61; PMID:25316830; http://dx.doi.org/ 10.1093/infdis/jiu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owais A, Khowaja AR, Ali SA, Zaidi AK. Pakistan's expanded programme on immunization: an overview in the context of polio eradication and strategies for improving coverage. Vaccine 2013; 31:3313-9; PMID:23707167; http://dx.doi.org/ 10.1016/j.vaccine.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 55.Riaz H, Rehman A. Polio vaccination workers gunned down in Pakistan. Lancet Infect Dis 2013; 13:120; PMID:23472249; http://dx.doi.org/ 10.1016/S1473-3099(12)70344-4 [DOI] [PubMed] [Google Scholar]
- 56.Polonsky JA, Ronsse A, Ciglenecki I, Rull M, Porten K. High levels of mortality, malnutrition, and measles, among recently-displaced Somali refugees in Dagahaley camp, Dadaab refugee camp complex, Kenya, 2011. Confl Health 2013; 7:1; PMID:23339463; http://dx.doi.org/ 10.1186/1752-1505-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.UNHCR Handbook for Emergencies. Geneva, Switzerland: Office of the United Nations High Commissioner for Refugees (UNHCR), 2007. [Google Scholar]
- 58.Watkins C, Gorostiaga A. Children's rights in policies and codes of conduct: A tool for companies. Geneva, Switzerland: United Nations Children's Fund (UNICEF) and Save the Children, 2013:32. [Google Scholar]
- 59.Cholera vaccines: WHO position paper. Wkly Epidemiol Rec 2010; 13:117-28 [PubMed] [Google Scholar]
- 60.Meningococcal A conjugate vaccine: updated guidance, February 2015. Wkly Epidemiol Rec 2015; 8:57-68 [PubMed] [Google Scholar]
- 61.Polio vaccines: WHO position paper, January 2014. Wkly Epidemiol Rec 2014; 9:73-93 [PubMed] [Google Scholar]