Abstract
The goal of the present study is to explore the physiological effects of injected human immunoglobulin on patients with severe bronchiolitis before and after treatment. 86 young children with severe bronchiolitis were randomly divided into the observation group (43 cases) and the treatment group (43 cases). On the basis of conventional therapy, the children in the treatment group were given human immunoglobulin (400 mg/kg, 1–3 times) via intravenous injection. 60 healthy young children, as determined by a physical examination given at the Zhumadian Central Hospital, were enrolled as the control group. The T lymphocytes, cytokines, IgA, IgG, and IgM immunoglobulins in the peripheral blood of all 3 groups were measured. The clinical efficacy of the immunoglobulins to mitigate the effects of bronchiolitis and the amount of time for the reduction of symptoms to occur were observed. The serum Ca, Fe, and Zn levels of children with severe bronchiolitis were significantly lower than those of the healthy control group (p < 0.05). As such, the CD8, IgA, IgG, IgM and IFN-γ levels were also significantly lower in the children with severe bronchiolitis than in the children in the healthy control group (p < 0.05). Furthermore, the CD4, IgE, IL-4, and IL-4/IFN-γ levels and CD4/CD8 ratio were dramatically higher than in the healthy control group (p < 0.05). Serum levels of the aforementioned indicators either increased or decreased after IVIG treatment. The amount of time required for coughing, wheezing, and pulmonary rales to seize, and the duration of illness for the children with the severe bronchiolitis children was significantly shorter for those in the treatment group than for those in the observation group. Human immunoglobulin via intravenous injection showed active therapeutical effects on trace elements, T lymphocytes, and cytokines in patients with severe bronchiolitis.
Keywords: clinical symptoms and signs, immunoglobulin, IVIG, severe bronchiolitis, trace element, T cell subsets
Abbreviations
- IVIG
Intravenous immunoglobulin
- Ig
immunoglobulin
- IL
interleukin
- IFN
interferon
- TNF
tumor necrosis factor
- NK
natural killer
- MC
mast cell
- DPV
duck plague virus
- PICU
Pediatric Intensive Care Unit
- CD
Cluster of Differentiation
- ELISA
enzyme linked immunosorbent assay
Introduction
Severe bronchiolitis is a lower respiratory tract infectious disease, a common clinical acute and critical case in pediatrics, and the leading cause of death among children, and it is more common in children less than 2 y old. The main symptoms are recurrent wheezing, difficulty breathing, thus causing hypoxia, endothelial damage, and platelet activation leading to a hypercoagulable state. Studies done on broncholitis are rarely reported, making it particularly important to conduct new studies on this topic.
Intravenous immunoglobulin (IVIG) contains plenty of antibodies with high potency. It possesses dual therapy effect of immune replacement and immune regulation. It is an immunoglobulin G (IgG) antibody with broad-spectrum anti-virus, bacteria or other pathogenic function. By inhibiting Th2 and activating Th1, IVIG can restore the imbalance of Th1/Th2 where bronchiolitis exists and can prevent the occurrence of airway inflammation. By directly neutralizing immunoglobulin E (IgE) and inhibiting interleukin-4 (IL-4) production of interferon-γ (IFN-γ), IVIG lowers levels of IgE indirectly, prohibiting the type I allergy of severe bronchiolitis and reducing the release of inflammatory mediators. Additionally, IVIG can also neutralize inflammatory mediators and cytokines, causing a decrease in their concentrations as well as mitigating the harm to the body caused by inflammatory mediator cytokines. This leads to a restoration of the tracheal epithelium as soon as possible and shortening of the course of disease.1 Research also shows that large doses of IVIG have certain effects on preventing severe bronchitis from developing into asthma.2 IVIG can reduce the abnormal activation of immunization, reduce levels of cytokines, and improve clinical symptoms.3 The exact pathogenesis of bronchiolitis is uncertain. The vast majority of current research considers the disease to be an immune dysfunction disease stimulated by infection, with a variety of immune cells and cytokines involved in the pathogenesis. The specific pathogenesis has not been clear. By detecting and analyzing the trace elements and T-cell subset levels in the serum of young children with severe bronchiolitis, this study aims to investigate the internal relations between immunology and the pathogens of severe bronchiolitis. The ultimate goal is to provide an experimental basis for exploring the pathogenesis of bronchiolitis and to develop new therapeutic methods and early intervention protocols.
Results
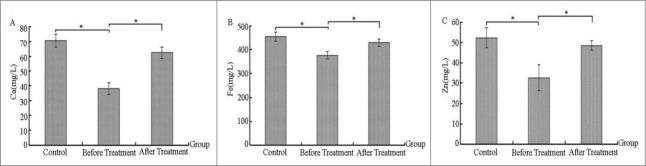
Comparing the levels of trace elements before and after treatment in the severe bronchiolitis group and control group
As observed in Figure 1, the serum levels of Ca, Fe, and Zn in the severe bronchiolitis group were significantly lower than those in the healthy control group, reaching a level of significant difference (p < 0.05, Fig. 1A, B, and C). After IVIG treatment, the Ca, Fe, and Zn levels in the severe bronchiolitis group all increased to some extent, and they also reached a level of significant difference (p < 0.05, Fig. 1A, B, and C) compared with levels observed before treatment.
Figure 1.
Comparing the levels of trace elements before and after treatment in the severe bronchiolitis group and control group (mg/L).
Comparing the levels of T cell subsets before and after treatment in the severe bronchiolitis group and control group
As can be seen in Figure 2, CD3 and CD8 levels in serum of the severe bronchiolitis group declined to a certain degree when compared with those of the control group, but the reduction level was not significant (Fig. 2A). However, the CD4 level was significantly higher in the severe bronchiolitis group than in the control group (p < 0.05, Fig. 2A). After IVIG treatment, the level of T-cell subsets, compared with that before treatment, increased or decreased in varying degrees without significant difference (Fig. 2A). The ratio of CD4/CD8 is an important indicator of the body's immune system homeostasis, the imbalance of which will lead to a variety of immune diseases. The CD4/CD8 ratio of the bronchiolitis group was greatly higher that of the control group before treatment (Fig. 2B), indicating that changes may exist in T-cell subsets in the bronchiolitis group and that an excessive T-cell differentiation may occur to CD4 in the bronchiolitis group. This would lead to the imbalance of CD4/CD8 ratio and the abnormal immunological mechanisms involved in the pathogenesis of bronchiolitis. The ratio of CD4/CD8 reduced dramatically post-treatment, and the difference from the pre-treatment ratio was significant (p < 0.05, Fig. 2B).
Figure 2.
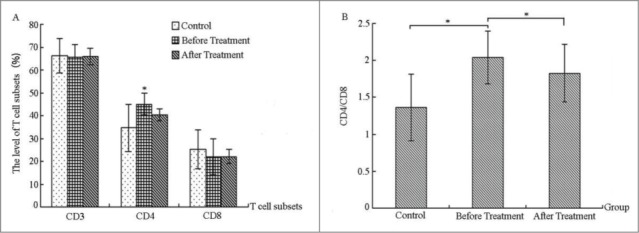
Comparing the levels of T-cell subsets before and after treatment in the severe bronchiolitis group and control group (%).
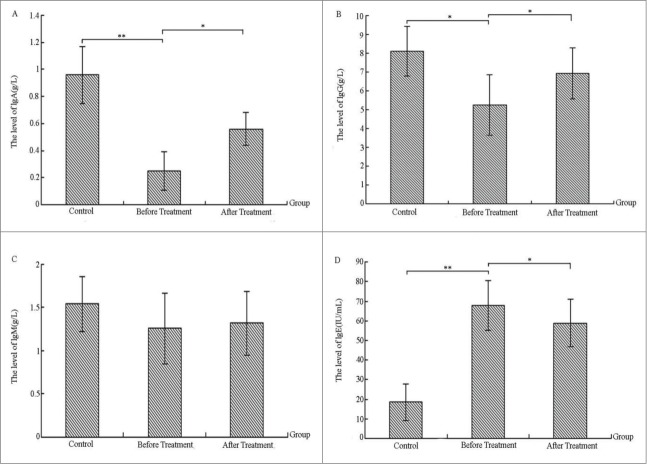
Comparing the levels of serum immunoglobulin before and after treatment in the severe bronchiolitis group and control group
Figure 3 shows that serum immunoglobulin A (IgA), IgG, and immunoglobulin M (IgM) levels in children with severe bronchiolitis were significantly lower than those in the control group. IgG was significantly different (p < 0.05, Fig. 3B) between groups, and IgA levels showed extremely significant differences (p < 0.01, Fig. 3A) between groups. Compared with the previous controls, the IgE level was significantly higher (p < 0.01, Fig. 3D). IgA, IgG, and IgM levels of patients were higher after IVIG than before treatment, with IgA and IgG being significantly different (p < 0.05, Fig. 3A, B). IgE levels also achieved a significant decrease after treatment (p < 0.05, Fig. 3D), illustrating that immunoglobulin levels in children with severe bronchiolitis are abnormally expressed. After IVIG treatment, content of serum immunoglobulins had improved to some extent.
Figure 3.
Comparing the levels of serum immunoglobulin before and after treatment in the severe bronchiolitis group and control group (g/L).
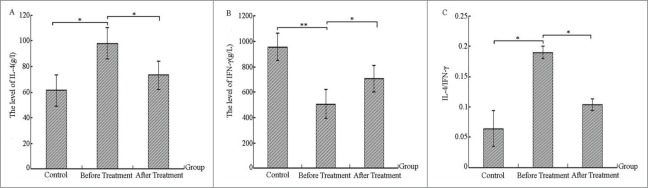
Comparing the levels of serum inflammatory cytokines before and after treatment in the severe bronchiolitis group and control group
Figure 4 demonstrates that serum IFN-γ level of children with severe bronchiolitis showed a highly significant decrease in comparison with controls (p < 0.01, Fig. 4B), and IL-4 levels and the IL-4/IFN-γ ratio were significantly higher than the controls (p < 0.05, Fig. 4A, C). After IVIG treatment, IL-4, IFN-γ, and IL-4/IFN-γ levels decreased or increased in varying degrees, reaching a significant difference (p < 0.05, Fig. 4A, B and C). This suggests a Th1/Th2 imbalance. Th1 function declined in patients prior to treatment, but inflammatory cytokines in the serum had been somewhat recovered and conditions had improved after treatment.
Figure 4.
Comparing the levels of serum inflammatory cytokines before and after treatment in the severe bronchiolitis group and control group (g/L).
Comparing the clinical symptoms and signs disappeared time between the severe bronchiolitis group and control group
Table 1 shows that the disappeared time of cough, wheezing, and pulmonary rales of children with severe bronchiolitis and length of stay were significantly shorter than those in the observation group (p < 0.01).
Table 1.
Comparing the disappeared time of clinical symptoms and signs
| Group | Cases | Cough (h) | Wheezing (d) | Pulmonary rales (d) | Length of stay (d) |
|---|---|---|---|---|---|
| Observation group | 43 | 6.47±1.39 | 4.67±1.48 | 5.49±1.61 | 11.13±2.32 |
| Treatment group | 43 | 5.21±1.42** | 3.72±1.23** | 4.31±1.27** | 8.72±2.13** |
| t value | 6.15 | 2.81 | 4.47 | 6.11 |
Discussion
Severe bronchiolitis seriously affects the respiratory function of infants and young children. If not treated promptly, more than 30% of them will have cases that develop into asthma.4 The pathogenesis of bronchiolitis is related to many factors; in addition to the airway mucosal injury caused by a viral infection, the virus can induce a relevant immune response, resulting in imbalance and damage of immune function5,6 and a production of a large number of cytokines and chemokines. Studies have shown that severe bronchiolitis is a risk factor for the occurrence of bronchial asthma. Children with severe bronchiolitis present deficiencies in certain aspects of immune function and regulatory function, which is often accompanied by an onset of symptoms such as breathing and heart failure.7 This also contributes to the frequent occurrence of immune sensitization, repeated wheezing, and bronchial asthma.8 Moreover, many clinical symptoms of bronchiolitis are associated with the bronchial hyper-responsiveness.9 Data increasingly support the notion that bronchiolitis is a complex clinical syndrome that is subject to an interaction between infectious inflammation and allergic inflammation. The pathogenesis of bronchiolitis is closely related to the damage of immunological function.
Trace elements constitute the material basis of human life, and their contents in the human body can directly or indirectly affect human health. Therefore, calcium, iron and zinc play an important role in maintaining the body's vital movement. The results by Miaoling Cai (Cai et al) indicated that the amount of zinc deficiency comes first among children under 1 y old, lead comes second and the amount of calcium deficiency comes third.10 By studying serum trace elements and state of immune function in recurrent aphthous children patients during their morbidity, Minhua Qu found that the contents of Fe and Zn in patients were significantly higher than those of the controls.11 A study conducted by Guiqin Mu showed that contents of calcium, iron, and zinc in children with severe bronchiolitis were significantly lower than those in the control group.12 This study indicated that serum Ca, Fe, and Zn levels in the severe bronchiolitis group were significantly lower than those in the control group (p < 0.05), which was in line with the results of Mu's study. After IVIG treatment, Ca, Fe, and Zn levels improved (p < 0.05), indicating that severe bronchiolitis had some impact on the levels of these trace elements in children.
The great majority of studies have found that the pathogenesis and clinical manifestations of bronchiolitis and asthma are very similar,13 so there are many studies on immune function of patients with bronchiolitis.14,15 There are a variety of immune and inflammatory cells, such as T lymphocytes and eosinophils that play key roles in the development and progression of bronchiolitis. By secreting cytokines in the immune network, CD4 T-cells can regulate the biological functions of other cells to induce and assist cellular immunity and humoral immunity. A main function of CD8 T-cells is to kill the cells infected by virus and other pathogens and to provide conditions for the body to clear infected cells. CD8 cells have cytotoxic effects, and they can also play a role in inhibiting cellular and humoral immunity. Studies have shown that CD4 and CD8 T-cells play an important role in clearing respiratory syncytial virus (RSV) infection caused bronchiolitis, especially the CD8 cells.16 Ping Gao, by the application of Siqikang in bronchiolitis, found that expressions of CD4 and CD8 T-cells reduced and CD4/CD8 decreased after treatment, indicating that Siqikang had a certain role in mitigating bronchiolitis.17 This study showed that serum CD3 and CD8 levels of the children in the severe bronchiolitis group were lower than those of the control group; CD4 levels were significantly higher than that of the control group (p < 0.05). After IVIG treatment, all serum T-cell subset levels increased to a certain degree in comparison with levels before treatment (p < 0.05). The CD4/CD8 ratio in the bronchiolitis group was significantly higher than that in the controls (p < 0.05). After treatment, the CD4/CD8 ratio decreased, indicating a ration imbalance between the T-cell subsets in patients, thus resulting in B-cell proliferation and differentiation, ultimately inducing specific IgA and IgE increases and subsequent immune damage. Under the IVIG treatment, however, the proportion of T-cell subsets improved. The results of this study were consistent with Gao's.
Activating CD4 T lymphocytes can lead to the secretion of a variety of cytokines. Secreted cytokines will be divided into 2 subpopulations: Th1 and Th2. Th1 cells mainly secrete IFN-γ, interleukin-2 (IL-2), and tumor necrosis factor β (TNF-β), while Th2 cells mainly secrete IL-4, interleukin-5 (IL-5), and interleukin-10 (IL-10). IL-4 can further promote the differentiation of CD4+ T-cells into Th2 cells, and Th2 cells secrete more IL-4. IFN-γ is a micro-molecule polypeptide having a regulatory role on cell function, and it is mainly generated by T-cells and natural killer (NK) cells. It can promote the differentiation of Th1 cells and inhibit Th2 cell responses.18 In many of the cytokines, IL-4 and IFN-γ are a mutually-antagonistic pair of cytokines, which are often used to reflect the balance of Th1/Th2. Studies have shown that in addition to their mutual inhibition, IL-4 and IFN-γ are also the positive and negative regulatory factors of IgE, the ratio imbalance of which is the main reason of too much IgE synthesis in asthma patients.19 Jian Chang's study showed that Th1 cells may not be involved in the pathogenesis of bronchiolitis and that IL-2 was significantly increased. He proposed that in the course of infection, the release of different cytokines may be relevant to gene polymorphisms. Many studies still need to be conducted to explore whether the Th1 cellular immune response in bronchiolitis exists or not.20 In this study, in comparison to the control group: IL-4 levels increased, IFN-γ levels decreased, the IL-4/IFN-γ ratio was significantly reduced, the CD4 level was elevated, CD8 decreased, and the CD4/CD8 ratio increased of children with severe bronchiolitis, indicating that the Th1 cytokines were inhibited and Th2 cells experienced hyperfunction. This led to the production of large amounts of IL-4 and IL-5, so it could be argued that the T-cell subsets in body were in an unbalanced state. Additionally, there was a Th1 and Th2 function imbalance. High levels of IL-4 promoted the proliferation of B lymphocytes and produced large amounts of IgE. IgE was binded with mast cell (MC) membrane surface. When the allergen entered into the body once again, the MC released inflammatory mediators by degranulation, causing inflammation and hyperresponsiveness and resulting in breathing episodes. After IVIG treatment, the indicators above all increased or decreased in varying degrees and had a tendency to convert to the normal indicators, thus improving the condition.
Generally, IgM and IgG are the main antibodies of humoral immunity in the immune system. The increase of Th2 cells in the body will promote B cell proliferation, inducing a large amount of IgA, IgE, IgG and other antibodies and inhibiting the cell effector of Th1.21 Research from Xuefeng Qi indicated that IgM played an important role in the early stage of duck plague virus (DPV) infection. With the decline in concentration of IgM, IgG antibodies increased and maintained for a long time, thus playing its role of immune relay, whereas IgA appeared in the serum late. In this study, serum IgA, IgG, and IgM levels of children with severe bronchiolitis were lower than those of the controls. After IVIG treatment, IgA, IgG, and IgM levels of patients were higher than those before treatment (p < 0.05). IgE levels also achieved a significant decrease after treatment (p < 0.05), suggesting that IgA, IgG, and IgM were involved in decreased levels caused by the antigen-antibody reaction in the pathogenesis of inflammation. This also explained the existence of immune dysfunction in severe bronchiolitis patients. This result was consistent with Chuansheng Liao's results.22 After IVIG treatment, IgA and IgG levels increased, indicating that IVIG had a certain role in the recovery of severe bronchiolitis.
The study also revealed that after IVIG treatment, the disappeared time of cough, wheezing, pulmonary rales, and length of stay of children with severe bronchiolitis clearly decreased in comparison with the observation group. This indicates that IVIG had certain effect on improving the clinical symptoms of severe bronchiolitis, likely by activating Th1 through the inhibition of Th2. Consequently, the imbalance of Th1/Th2 was restored, the airway inflammation was prevented, and direct neutralization of IgE and inhibition of IL-4, the secretion of IFN-γ increased and the generation of IgE was indirectly reduced. Thereby, the occurrence of bronchiolitis was blocked.
Conclusions
Through a series of reactions of T lymphocytes and cytokines in the body, severe bronchiolitis plays its role. Therefore, for patients with severe bronchiolitis, detecting the related indicators of peripheral blood upon entering the intensive care unit can allow for prediction of disease severity and prognosis at an early stage. This could help doctors provide the right interventions and evaluate the efficacies in order to effectively adjust the appropriate therapeutic measures, providing a theoretical basis for the development of immunological therapy for diseases and the prevention of repeated wheezing of severe bronchiolitis.
Methods and Materials
General information
86 severe bronchiolitis infants treated at the Pediatric Intensive Care Unit (PICU) ward of Zhumadian Central Hospital from January 2011 to January 2014 were selected, 47 males and 39 females, ranging in age from 1 to 13 months with an average age of 9.62 (±1.15) months. There were 43 cases in both the observation group and treatment group. All of them had been diagnosed as having severe bronchiolitis by X-rays and blood oxygen saturation, and infants with congenital asthma were excluded. 60 healthy infants who received physical examination in the hospital at the same period were chosen as the control group, 35 males and 25 females, aged from 1 to 13 months, with an average age of 10.14 (±1.27) months. Differences in gender, age, and other general information was not statistically significant (p < 0.05) between the 2 groups.
The inclusion and exclusion criteria
Inclusion criteria: less than 2 y old, the first onset of the disease, clinical diagnosis of fever, cough, severe wheezing, shortness of breath, and other symptoms, the lungs mainly displaying signs of stridor, the total number and distribution of white blood cells and C-reactive protein were to be within the normal range, varying degrees of obstructive pulmonary emphysemas and spot film shadows on the chest were allowed for inclusion.
Exclusion criteria: mild wheezing, congenital heart disease, bronchopulmonary dysplasia, whooping cough, rickets, moderate and severe anemia, foreign bodies in the bronchus, tuberculosis infection, congenital laryngeal stridor, and other diseases that may manifest.
Diagnostic criteria
We referred to the Zhu Futang Textbook of Pediatrics diagnostic criteria to diagnose patients as having severe bronchiolitis.23
Methods
The two groups of patients were given conventional and comprehensive treatment, including anti-infection, spasmolysis and anti-asthma, atomized inhalation (Ventolin + Pulmicort), suction, oxygen supply, etc. In addition to these, the treatment group was given an early application of IVIG (1.25 g/branch, Hualan Biological Engineering Inc.., Xinxiang, Henan, P.R. China; National Medicine Permission No. S10970031) at a dose of 400 mg/kg, 1 to 3 times.
Detection of trace elements
Trace elements are necessary for human body. The excessive or deficient intake, imbalance or a shortage of them will cause human physiological abnormalities in different degrees or even disease. Calcium, iron, and zinc have strong ties to the body's normal physiological activities. These trace elements have the ability to affect immune function. If the content of these elements is reduced, it will lead to a decline in body's resistance to the incidence of diseases.24 Zinc is one of the necessary trace elements. It plays an extremely important role in the physiological process such as body growth and development, reproduction and genetics, immune and endocrine. Previous data reports that zinc deficiency can significantly reduce T-cell function, thus weakening the body's defenses. Iron also has a role in promoting immunization; a deficiency in this key element also leads to a decrease in immunity. Calcium is required to maintain and regulate many biochemical processes within the body; it maintains the acid-base balance, maintains the integrity and permeability of the cell membrane, reduces capillary permeability, prevents exudation, and controls inflammation and edema.
40 μL of peripheral blood of infants was collected and thoroughly dissolved into 1.2 mL of cytolysis fluid. The BH-5100 atomic absorption spectrometer (Beijing Bohui Innovation Technology Co., Ltd., Beijing, China) was used for detection. All operations were in strict accordance with the instructions.
Detection of immunoglobulin and T lymphocyte subsets levels
T lymphocytes are the main immune cells, but they are also an important indicator for the evaluation of cellular immune function. IgM plays a key role in the early phase of anti-infection and it is a vanward antibody against pathogenic microorganisms. IgG is the major antibody against infection, and it has a high level in the blood during infection. IgA is a first line of defense for mucosa resisting pathogenic microorganism and harmful substances. It is the main effector molecule of humoral immunity.25-27
Two mL of fasting venous blood was taken. The Roche P module automatic biochemical analyzer and the matching reagents were utilized to conduct the immunoturbidimetric immunoglobulin assay. Levels of Cluster of Differentiation 4 (CD4) and Cluster of Differentiation 8 (CD8) were detected, as these are surface markers for T-cell subsets. The anti-human T cells monoclonal antibody APAAP bridge-linked enzyme linked immunosorbent assay (ELISA) was used to detect T lymphocyte subsets (provided by Wuhan Institute of Biological Products Co., Ltd.).
Detection of the serum inflammatory factors' levels
Double-antibody sandwich ELISA was used for quantitative detection of human IL-4, IFN-γ, and IgE. The ELISA kits were all provided by Beijing SinoSec MicroTest Sci. & Tech. Co. Ltd. The Qtat-fax-2100 microplate reader produced by US AWARENESS Company was used.
Follow-up
We recorded age, gender, incidence season, pathogen present, severity, family history of asthma and eczema, among others of the 86 children with bronchiolitis. Every patient was asked to receive a follow-up every 6 months (clinical or telephone follow-up).
Statistical methods
SPSS17.0 software was used for statistical analysis of data. Comparison of data between groups was performed using a t-test.* indicated that the difference was significant (p < 0.05). ** indicated that the difference was extremely significant (p < 0.01).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I thank all authors who have contributed to this paper for advice and comments. Moreover, the authors would like to thank Zhumadian Central Hospital for providing the experimental base.
References
- 1.Sibéril S, Elluru SR, Negi VS, Ephrem A, Misra N, Delignat S, Bayary J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Intravenous immunoglobulin in autoimmune and inflammatory diseases: More than mere transfer of antibodies. Transfus Apher Sci 2007; 37:103-107; http://dx.doi.org/ 10.1016/j.transci.2007.01.012 [DOI] [PubMed] [Google Scholar]
- 2.Li H, Wang YT. Clinical study on large dose intravenous immunoglobulin in decreasing incidence of asthma following bronchiolitis. J Appl Clin Pediatr 2006; 21:1265-1266. [Google Scholar]
- 3.Du ZD, Zhang YL, Zhao D, Du JB, Lu S, Yi JM, Hou AC, Zhou ZS, Ding GF, Lin Y, Liu C. Retreatment and risk factors of IVIG nonresponsiveness. Chin J Pediatr 2006; 21:738-741. [Google Scholar]
- 4.Gao WH, Li CR. A follow-up analysis of infants with acute bronchiolitis. Chin J Contemp Pediatr 2003; 5:357-358. [Google Scholar]
- 5.Yan HM. Curative effect of interferon and phentolamine on children with bronchiolitis. J Xinxiang Med Coll 2008; 25:603. [Google Scholar]
- 6.Ye XB, Tian HZ, Luo W, Luo W, Chen GQ, Luan HL, Zou GQ. Curative effect observation of budesonide combined with ventolin aerosol inhalation therapy of bronchiolitis. J Clin Med Pract 2008; 12:44. [Google Scholar]
- 7.Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, Espinola JA, Camargo CA Jr. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med 2012; 166:700-706; PMID:22473882; http://dx.doi.org/ 10.1001/archpediatrics.2011.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HD, Chen HZ, Zhang ZJ, Zhang WK, Wang CH, Qian Y, Zhang T, Li JY. A 5-year follow-up study on 463 cases of epidemic asthmatic pneumonia. J Clin Pediatr 2006; 24:40-42. [Google Scholar]
- 9.Shen YL, Zhang Y, Wang YJ. Detection and analysis of interleukin-12 and γ- interferon of bronchiolitis children. Chin J Pract Pediatr 2005; 20:498. [Google Scholar]
- 10.Cai ML. Trace elements in hair of infants aged 0–2. Guangdong Trace Elements Sci 2002; 9:35-37. [Google Scholar]
- 11.Qu MH, Weng ZQ. Analysis on serum trace elements and immunity of children with RAU. Guangdong Trace Elements Sci 1999; 6:37-39. [Google Scholar]
- 12.Mu GQ. Study on relationship between trace elements and immune function of children with severe bronchiolitis. Chin Community Doctors 2014; 30:129-131. [Google Scholar]
- 13.Wang SZ, Forsyth KD. Asthma and respiratory syncytial virus infection in infancy: is there a link? Clin Exp Allergy 1998; 28:927-935; PMID:9756195; http://dx.doi.org/ 10.1046/j.1365-2222.1998.00353.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neveu WA, Allard JL, Raymond DM, Bourassa LM, Burns SM, Bunn JY, Irvin CG, Kaminsky DA, Rincon M. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res 2010; 11:28; PMID:20205953; http://dx.doi.org/ 10.1186/1465-9921-11-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosmi L, Maggi L, Santrlasci V, Capone M, Cardilicchia E, Frosali F, Querci V, Angeli R, Matucci A, Fambrini M, Liotta F, Parronchi P, Maggi E, Romagnani S, Annunziato F. Indentification of a novel subset of human circulating memory CD4 (+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol 2010; 125:222-30; PMID:20109749; http://dx.doi.org/ 10.1016/j.jaci.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 16.Schwarze J, O'Donnell DR, Rohwedder A, Openshaw PJ. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med 2004; 169:801-5; PMID:14742302; http://dx.doi.org/ 10.1164/rccm.200308-1203OC [DOI] [PubMed] [Google Scholar]
- 17.Gao P, Zhao Y, Song M, Xu J, Zheng RF, Cong L. The effect of Siqikang on T cell subsets and cytokines in children with bronchiolitis. China Maternal Child Health Care 2005; 20:476-7. [Google Scholar]
- 18.Grünig G, Ford JG, Donaldson DD, Venkayya R, McArthur C, Hansell E, Kurup VA, Warnock M, Rennick D. Roles of interleuckin-13 and interferon-γ in lung inflammation. Chest 2002; 121:88S; PMID:11796436; http://dx.doi.org/ 10.1378/chest.121.1.88 [DOI] [PubMed] [Google Scholar]
- 19.Li MH, Yin KS, Zhu SL. Asthmology. 1st ed. Beijing: People's Medical Publishing House; 1998:45. [Google Scholar]
- 20.Chang J, Liang D, Chen YB, Lu JR, Li YM. The role of TH1 cell in respiratory syncytial virus bronchiolitis. Chin J Immunol 2006, 22:489-490. [Google Scholar]
- 21.Zhang M. Changes and significance of CD4+, CD8+ T lymphocytes and immunoglobulin in peripheral blood of children with henoch-schonlein purpura in acute phase Dalian: Dalian Medical University, 2014. [Google Scholar]
- 22.Liao CS, Chai WX. Detection of immune function in children with severe bronchiolitis and clinical research of treatment with IVIG. Asia-Pacific Trandition Med 2013; 9:139-141. [Google Scholar]
- 23.Hu YF, Jiang ZF. Zhu Futang Textbook of Pediatrics Beijing: People's Medical Publishing House, 2005. [Google Scholar]
- 24.Wang G, Liu DG. Effects of thymosin on immunologic function of patients with severe bronchiolitis. J Med Forum 2012; 33:70-71. [Google Scholar]
- 25.Kerr MA. Function of immunoglobulin A in immunity. Gut 2000; 47:751-752; PMID:11076871; http://dx.doi.org/ 10.1136/gut.47.6.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunogiobulin A in protection against reovirus entry into Murine Peyer's patches. J Virol 2001; 11:10870-10879; http://dx.doi.org/ 10.1128/JVI.75.22.10870-10879.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langford TD, Housley MP, Boes M, Chen JZ, Kagnoff MF, Gillin FD, Eckmann L. Central importance of immunoglobulin A in host defense against Giardia spp. Infect Immun 2002; 4:11-18; http://dx.doi.org/ 10.1128/IAI.70.1.11-18.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]