Abstract
Elevated interleukin-4 (IL-4) levels are associated with cardiac fibrosis in hypertension and heart failure in both patients and experimental animals. We hypothesized that chronically elevated IL-4 induces cardiac fibrosis, resulting in a predisposition of the heart to angiotensin II–induced damage. Wild-type Balb/c (WT, high circulating IL-4) and IL-4–deficient Balb/c mice (IL-4−/−) were used. WT mice exhibited cardiac fibrosis (evidenced by an increase in expression of procollagen genes/interstitial collagen fraction), enlarged left ventricle chamber, and declined cardiac function associated with a greater number of mast cells and macrophages in the heart compared with IL-4−/−. In contrast, IL-4−/− mice had normal cardiac architecture/function while showing a 57.9% reduction in heart interstitial collagen compared with WT, despite elevated proinflammatory cytokines in heart tissue. In response to angiotensin II administration, IL-4−/− had reduced interstitial myocardial fibrosis and were protected from developing dilated cardiomyopathy, which was seen in WT mice. This was associated with increased macrophage infiltration into the hearts of WT mice, despite a similar degree of hypertension and increased cardiac transforming growth factor-β1 in both groups. In vitro data demonstrated that IL-4 upregulates procollagen genes and stimulates collagen production in mouse cardiac fibroblasts. This process is mediated by signal transducer and activator of transcription 6 signaling pathway via IL-4 receptor alpha. This study not only establishes a causal relationship between IL-4 and cardiac fibrosis/dysfunction, but also reveals a critical role for IL-4 in angiotensin II–induced cardiac damage. IL-4 could serve as an additional target for the treatment of cardiac fibrosis.
Keywords: angiotensin II, balb/c mice, fibrosis, heart, interleukin-4
Progressive cardiac fibrosis is a major maladaptive response to hemodynamic stress and various profibrotic stimuli. It leads to a reduction in myocardial compliance and eventually to cardiac failure.1 Cytokine interleukin (IL)-4 has been implicated in this process.2–5
IL-4 is produced by immune cells, including CD4+ T-helper (Th) lymphocytes6 and mast cells.7 Among its multiple biological effects, IL-4 promotes tissue fibrotic remodeling in diseases involving lung,8 skin,9 and liver.10 Recent studies have shown a positive correlation between systemic IL-4 levels and cardiac fibrotic remodeling in both patients2,3 and experimental animals.4,5 Furthermore, previous studies have demonstrated that Balb/c mice, which are characterized by high levels of circulating IL-4, exhibited increased cardiac collagen deposition, left ventricular enlargement, and depressed cardiac function (fibrotic cardiomyopathy).11,12 When these mice were challenged with angiotensin II (Ang II), severe fibrosis and dilated cardiomyopathy (DCM) developed.12 Although these observations demonstrate a putative profibrotic role for IL-4 in the heart, the direct evidence linking high levels of IL-4 to cardiac fibrosis is lacking.
The role of IL-4 in cardiac fibrosis has recently been determined in a loss-of-function study showing that administration of an anti-IL-4 neutralizing antibody significantly blunted cardiac fibrotic remodeling in C57BL/6 mice with aortic coarctation.13 This study established a causal relationship between IL-4 and cardiac fibrosis in hypertension. However, it remains unknown whether (1) chronically elevated IL-4, as seen in primary hypertension5 and aging,4 is sufficient to initiate fibrotic response, leading to cardiac fibrosis and dysfunction and (2) stress challenges, such as Ang II–induced hypertension, exacerbate IL-4–induced fibrotic cardiomyopathy, leading to heart failure.
In this report, we used wild-type (WT) and IL-4−/− Balb/c mice to delineate the functional significance of chronic elevations in IL-4 levels during the development of cardiac fibrosis and dysfunction, further clarifying their causal relationship. We also demonstrated that IL-4 represents a critical determinant of progression to DCM (evidenced by severe cardiac fibrosis, dilated left ventricular chamber, and declined cardiac function) in Ang II–induced hypertension. Furthermore, we examined the effects of IL-4 on cardiac fibroblasts, the primary cellular source of collagens in the heart, and found that IL-4 not only significantly increased procollagen mRNA but also promoted collagen production through the signal transducer and activator of transcription 6 (STAT6) signaling pathway via IL-4 receptor alpha (IL-4Rα).
Methods
Detailed description of methods is available in the online-only Data Supplement.
Results
Systolic Blood Pressure, Cardiac Remodeling, and Function
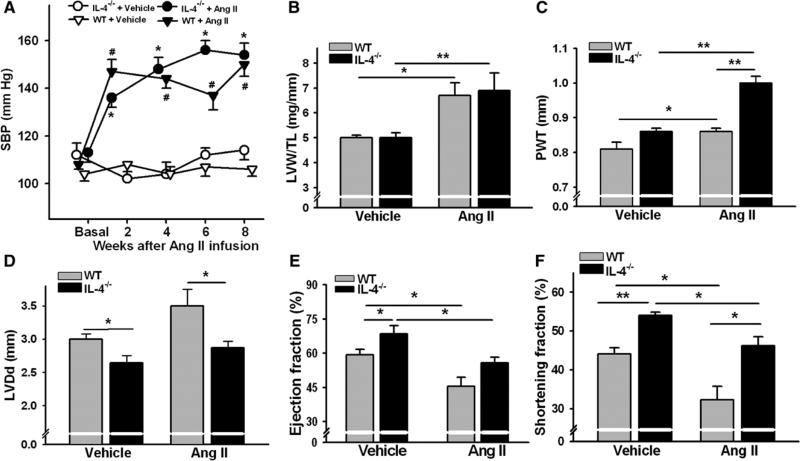
Basal systolic blood pressure (SBP) was similar between WT and IL-4−/− mice (Figure 1A). Ang II administration resulted in a significant increase in SBP within 2 weeks of exposure to Ang II and stayed elevated until the end of the experiment (8 weeks). There were no differences in the degree of induced hypertension between the groups at any time point measured (Figure 1A). Ang II also induced comparable cardiac hypertrophy in WT and IL-4−/− mice as indicated by significantly increased left ventricle (LV) weights after 8 weeks of Ang II infusion (Figure 1B).
Figure 1.
Systolic blood pressure (SBP) and cardiac remodeling in wild-type (WT) and interleukin-4 (IL-4)−/− mice at the steady-state condition and angiotensin (Ang) II–induced hypertension. A, SBP data. Results represent mean±SEM. #P<0.005 vs WT+vehicle; *P<0.005 vs IL-4−/−+vehicle, a Student's t test with a Hochberg correction for multiple testing. Cardiac hypertrophy/remodeling and function assessed by left ventricle weight (LVW) to tibia length (TL) ratio (B) or echocardiography as the sum of diastolic posterior wall thickness (PWT; C), left ventricular diastolic dimension (LVDd; D), ejection fraction (E), and shortening fraction (F) after 8 weeks of Ang II treatment in WT and IL-4−/− mice. The bars represent mean±SEM. *P<0.05, **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=6 to 14 per group.
Cardiac geometry and function are shown in Figures 1C–1F. At 16 to 20 weeks of age, WT mice showed significantly enlarged LV chamber and declined cardiac function compared with IL-4−/−. WT mice exhibited increased LV weight, severe LV chamber dilatation, wall thinning, and dramatically decreased ejection fraction and shortening fraction after 8 weeks of Ang II administration. In contrast, IL-4−/− mice had significantly increased LV weight to tibia length ratio and diastolic posterior wall thickness in response to Ang II and increased SBP. In Ang II–treated IL-4−/− mice, ejection fraction and shortening fraction were decreased compared with vehicle-treated mice.
Cardiac Fibrotic Remodeling
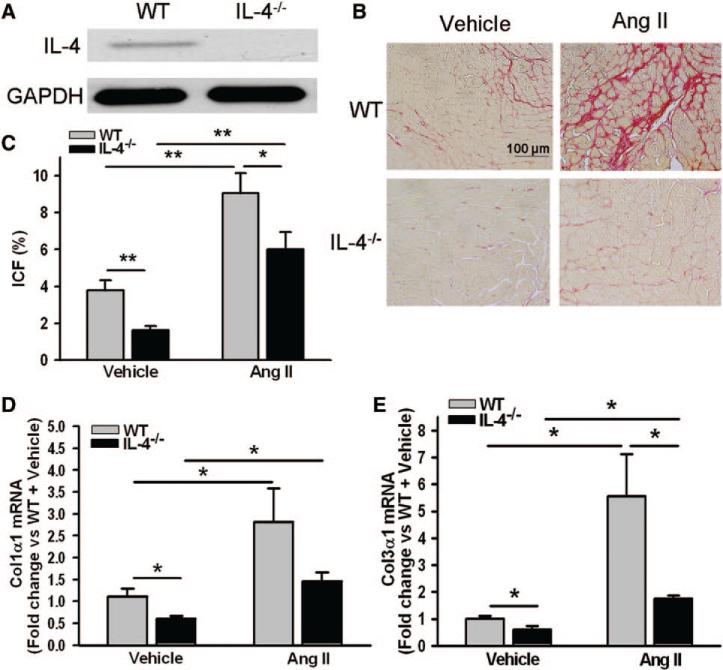
As detected by immunoblotting, IL-4 was expressed in the hearts of WT but was absent in IL-4−/− mice (Figure 2A). The fibrosis shown in WT mice with Ang II–induced hypertension was characterized as a dense crisscrossing meshwork of collagen fibers encircling muscle fibers and accumulated collagen extending outward from perivascular/pericapillary space (Figure 2B). Cardiac fibrosis seen in WT mice showed a significantly higher interstitial collagen fraction in the LV myocardium when compared with mice with genetic IL-4 deletion (Figure 2C). IL-4 deletion attenuated cardiac fibrosis by 57.9% (3.85%±0.53% in WT versus 1.62%±0.22% in IL-4−/−, P<0.005). Ang II infusion for 8 weeks resulted in a significant increase in interstitial collagen fraction in both WT and IL-4−/− mice with significantly higher interstitial collagen fraction in WT compared with IL-4−/− mice (Figure 2C). In addition, significantly higher mRNA levels of procollagen type-I alpha 1 (Col1α1) and procollagen type-III alpha 1 (Col3α1) were found in the hearts of WT mice compared with that in IL-4−/− (Figure 2D and 2E). Ang II induced significant upregulation of these 2 procollagen genes in the hearts of both WT and IL-4−/− mice with significantly higher mRNA level of Col3α1 in WT+Ang II than in the IL-4−/− mice+Ang II group (Figure 2D and 2E).
Figure 2.
Cardiac fibrotic remodeling in the hearts of wild-type (WT) and interleukin-4 (IL-4)−/− mice. A, Left ventricle lysates were analyzed for IL-4 by immunoblotting with an antibody against mouse IL-4. Representative images of interstitial fibrillar collagen (red) in the hearts of mice treated with either vehicle or angiotensin (Ang) II for 8 weeks (B) and quantification of interstitial collagen fraction (ICF) of the study animals (C). Procollagen type-I alpha 1 (Col1α1; D) mRNA and procollagen type-III alpha 1 (Col3α1; E) mRNA in the myocardium of mice treated with either vehicle or Ang II for 8 weeks. The bars represent mean±SEM, *P<0.05 and **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=6 to 14 per group.
Mast Cells and CD68+ Macrophages in the Heart
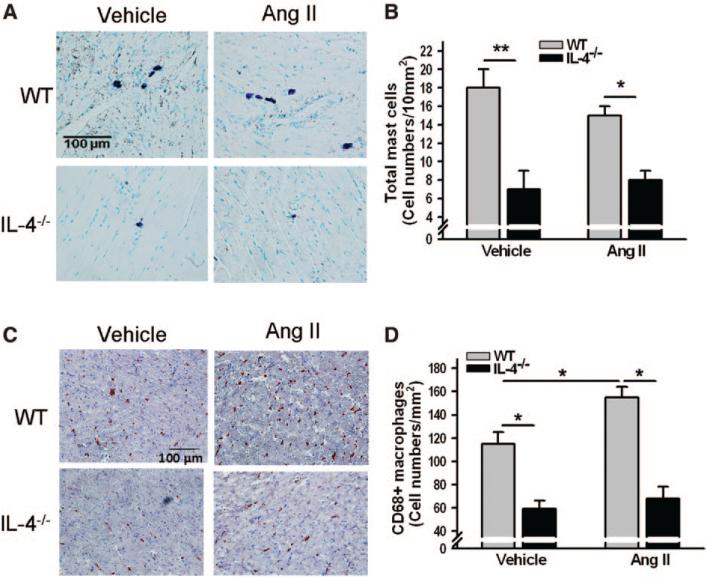
Mast cells were primarily observed surrounding the vessels and the pericardium (Figure 3A). A more than 2-fold increase in total mast cells was seen in the hearts of WT mice compared with IL-4−/−; however, Ang II infusion for 8 weeks did not change the numbers of mast cells in the hearts (Figure 3B). A similar pattern was observed with CD68+ macrophages in the hearts of vehicle-treated WT and IL-4−/− mice. Chronic Ang II administration significantly increased the numbers of macrophages in the hearts of WT but not in IL-4−/− mice (Figure 3C and 3D). Noticeable accumulation of macrophages was also seen around the vessels.
Figure 3.
Mast cells and CD68+ macrophages in the hearts of wild-type (WT) and interleukin-4 (IL-4)−/− mice. Representative images of mast cells (purple; A) and CD68+ macrophages (red-brown; C) in the myocardium of mice treated with either vehicle or angiotensin (Ang) II for 8 weeks and quantification of the numbers of mast cells (B) and CD68+ macrophages (D). The bars represent mean±SEM, *P<0.05, **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=5 to 9 per group.
Cardiac Cytokines and Monocyte Chemoattractant Protein-1 in the Heart
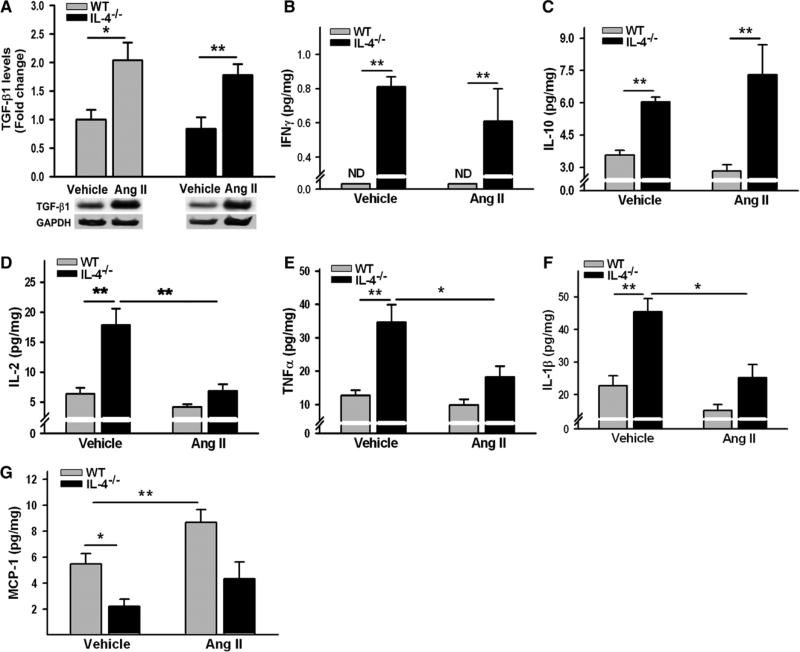
There were no significant differences in cardiac transforming growth factor-β1 (TGF-β1) levels between WT and IL-4−/− mice. Ang II administration for 8 weeks significantly increased TGF-β1 levels in the hearts of both groups (Figure 4A). Values of IL-4 and IL-5 in the mouse hearts were below the lowest levels of detection. Interferon gamma (IFNγ) in the hearts of WT mice was too low to detect with the used kit; however, IL-4 deletion induced robust IFNγ production (Figure 4B). Significant increases in IL-10, IL-1β, IL-2, and tumor necrosis factor-α were observed in the hearts of IL-4−/− mice compared with WT mice (Figure 4C–4F). Interestingly, chronic Ang II administration for 4 weeks resulted in a significant reduction in cardiac IL-1β, IL-2, and tumor necrosis factor-α only in IL-4–deficient mice. Cardiac monocyte chemoattractant protein-1 (MCP-1) levels in WT mice were >2× greater than those in IL-4−/− mice. Ang II infusion for 4 weeks induced increases in MCP-1 in the hearts, which was significant in WT mice (Figure 4G).
Figure 4.
Cytokine and monocyte chemoattractant protein-1 (MCP-1) expression in the hearts of wild-type (WT) and interleukin (IL)-4−/− mice. Left ventricle lysates from WT and IL-4−/− mice treated with either vehicle or angiotensin (Ang) II were processed by Western blot for transforming growth factor-β1 (TGF-β1; A) by a cytokine bead array for interferon gamma (IFNγ), IL-10, IL-2, IL-1β, and tumor necrosis factor-α (TNFα; B-F) and by ELISA for MCP-1 (G). The bars represent mean±SEM, *P<0.05, **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=4 to 5 per group.
IL-4 Signaling and Collagen Production in Cardiac Fibroblasts
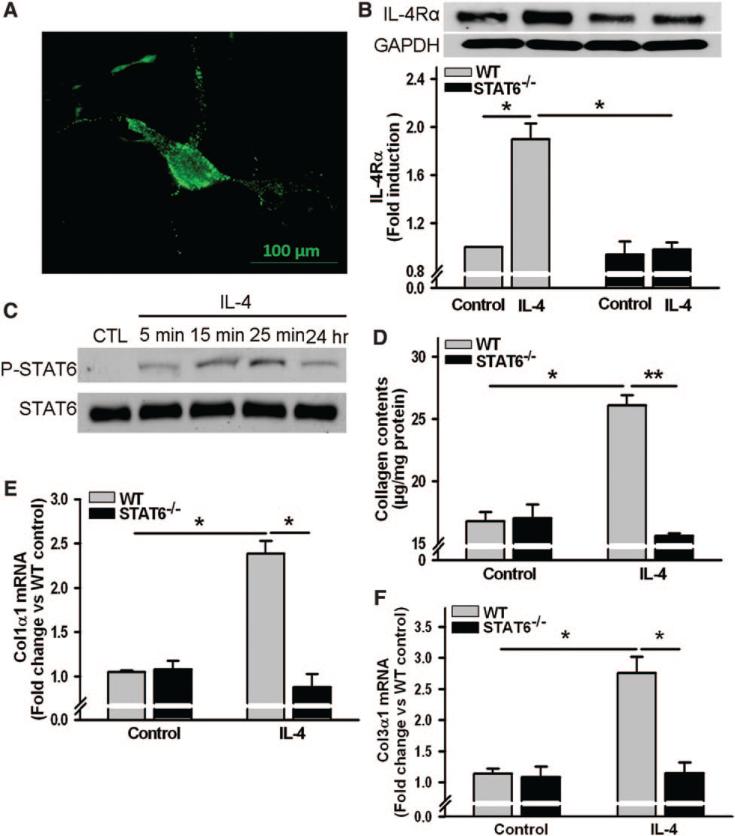
Immunocytochemistry results showed constitutive expression of functional IL-4Rα on the cell-surface of mouse cardiac fibroblasts (Figure 5A). Western blot analysis revealed that IL-4Rα expression was doubled after cells were incubated with IL-4 for 24 hours in a STAT6-dependent fashion (Figure 5B). IL-4 rapidly induced phosphorylation of STAT6 (within minutes), with the phosphorylation lasting >24 hours (Figure 5C). Importantly, IL-4 markedly increased collagen production (Figure 5D) and significantly induced Col1α1 and Col3α1 mRNA expression by cardiac fibroblasts (Figure 5E and 5F); these effects were STAT6-dependent.
Figure 5.
Effects of interleukin-4 (IL-4) on cultured mouse cardiac fibroblasts. A, Representative immunofluorescent image of cell-surface IL-4 receptor alpha (IL-4Rα) on mouse cardiac fibroblasts (green). Primary cardiac fibroblasts were prepared from wild-type (WT) or signal transducer, and activator of transcription 6 (STAT6)−/− mice, IL-4Rα expression (B), and IL-4–induced phosphorylated STAT6 (P-STAT6; C) were analyzed by Western blot. A and C, Results from 3 independent experiments. D, Collagen contents in conditioned media of cardiac fibroblasts treated with IL-4 (10 ng/mL) for 48 hours. mRNA abundance of procollagen type-I α1 (Col1α1; E) and procollagen type-III α1 (Col3α1; F) after cells were incubated with IL-4 (10 ng/mL) for 6 hours. The bars represent mean±SEM, *P<0.05, **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=4 per group.
Discussion
This is the first study to establish a causal relationship between chronically elevated IL-4 and cardiac fibrotic remodeling and dysfunction. Not only does high IL-4 induce LV fibrosis and dysfunction, but its deletion markedly attenuates this effect as demonstrated in Balb/c hearts and IL-4−/− Balb/c hearts, respectively. The present study also showed that in contrast to WT, IL-4−/− mice were protected from fibrotic lesions and did not develop DCM in Ang II–induced hypertension.
The role for IL-4 in the regulation of blood pressure has not been adequately addressed previously. We did not observe significant differences in SBP between WT and IL-4−/− mice at both basal condition and Ang II–induced hypertension. This finding is in line with a previous study showing that systemic administration of an anti-IL-4 neutralizing antibody did not affect the increased blood pressure in C57BL/6 mice with aortic coarctation.13 Vascular endothelial cells express IL-4Rα and respond to IL-4.14 In the lungs, IL-4 activates pulmonary endothelial cells and induces endothelin-1 production, resulting in the development of pulmonary hypertension.15 Therefore, we cannot exclude the possibility that IL-4 may play a role in the development of hypertension over time, especially under the conditions combined with other activated signaling pathways, such as Ang II signaling.
An increase in content or a transformed structure of cardiac fibrillar collagen can impede muscular contraction and relaxation.16 Cardiac fibrosis may cause systolic dysfunction through several distinct mechanisms, including impaired force generation by myocytes,17 disrupted normal coordination of myocardial excitation–contraction coupling,18 and an asynchronous contraction of the myocardium.19 These proposed mechanisms may be involved in cardiac fibrosis–induced decline in the cardiac function observed in Balb/c mice. The fact that this decline in cardiac function disappeared when cardiac fibrosis was absent in IL-4−/− further supports the notion of cardiac fibrosis–induced systolic dysfunction.
In the present study, we found positive correlation between IL-4 and Col1α1/Col3α1 gene expression/collagen production in the hearts of mice, as well as in cultured cardiac fibroblasts. In cultured cells, upregulation of Col1α1/Col3α1 gene was mediated through STAT6 signaling via IL-4Rα. These findings are consistent with the results of in vivo20 and in vitro21–23 studies using noncardiac tissue and fibroblasts, respectively. The important finding of our study was an increase in IL-4Rα expression on cardiac fibroblasts in response to IL-4, which was mediated via STAT6 signaling. This phenomenon was also observed in T and B cells from Balb/c mice.24 Increased IL-4 receptor expression by IL-4 provides an important mechanism for amplification of IL-4–dependent activation of STAT6 signaling in cardiac fibroblasts. Therefore, IL-4–induced collagen production in fibroblasts significantly contributes to cardiac fibrosis and dysfunction.
We observed that IL-4 was associated with an increase in the number of mast cells in the heart. This finding could have been because of IL-4–induced mast cell proliferation because mast cells express IL-4Rα in vivo.25 In disease states, mast cells can be activated by cytokines, such as IL-4, leading to either secretion of mediators by degranulation or release of distinct mediators without overt degranulation.13,26 The positive association between total mast cells and cardiac fibrosis shown in our study is consistent with reported results from clinical studies27 and in experimental animal models.28 Notably, a study using mast cell–deficient mice29 confirms a profibrotic role for mast cells in the evolution of congestive heart failure. Here, we provide evidence for the first time that mast cells, which are known to secrete IL-4,30 might participate in mediating IL-4–induced fibrotic cardiomyopathy. Thus, IL-4 produced by mast cell may function in an autocrine manner, leading to further mast cell proliferation and IL-4 production. We propose that mast cells could be an important cellular source of cardiac IL-4, which is significantly elevated in Balb/c mice.
Our data indicate that IL-4 contributes to an increased number of macrophages in the fibrotic heart. This finding may be as a result of IL-4–induced upregulation of MCP-1 expression because deletion of IL-4 was associated with a markedly decreased number of macrophages and MCP-1 in the myocardium. The mechanisms underlying IL-4–induced accumulation of macrophages in the heart could be because of (1) IL-4–induced recruitment of the cells via upregulation of expressions of adhesion molecules and MCP-1 in endothelial cells31–33 and (2) IL-4–stimulated proliferation of resident and recruited macrophages.34 In an IL-4 rich environment, macrophages polarize toward an M2 phenotype (alternative activation),35 which has been shown to significantly contribute to cardiac fibrosis.36
Although it has been reported that IL-4 might mediate fibrosis in part by increasing the expression of the TGF-β mRNA in fibroblasts,37 we observed neither a significant difference in TGF-β1 levels between WT and IL-4–deficient mouse hearts nor an induction of TGF-β1 production in cultured rat cardiac fibroblasts stimulated with IL-4 (Figure S3 in the online-only Data Supplement). Our data are consistent with reported findings showing that IL-4–induced cardiac fibrosis in hypertension was independent of TGF-β1.13 IL-4 not only promotes Th2 differentiation, but at the same time suppresses Th1 differentiation.38 Not surprisingly, genetic deletion of IL-4 in Balb/c mice results in the skew from Th2 differentiation to Th1 as evidenced by markedly increased IFNγ in the myocardium of IL-4−/− mice. Our finding that cardiac fibrosis was abrogated along with a marked induction of IFNγ and IL-10 in IL-4–deficient Balb/c mice is consistent with reports indicating antifibrotic role of IFNγ in the heart39 and IL-10's efficacy in the treatment of fibrosis in disease models.40,41
Enhanced Th1 responses, as a result of a striking induction of IFNγ, were observed in the hearts of IL-4−/− mice as evidenced by significantly increased cardiac IL-1β, IL-2, and tumor necrosis factor-α. These cytokines have been shown to have negative inotropic effects on cardiac myocytes.42,43 Despite the inhibitory effects of these cytokines on cardiomyocytes, cardiac functions in IL-4−/− mice were preserved up to at least 16 to 20 weeks of age. However, it is possible that cardiac function of IL-4−/− mice could gradually decline with age.
Ang II significantly increased TGF-β1 levels in the hearts of both WT and IL-4−/− mice. In fibroblasts, TGF-β1 induces a sustained increase in Col1α1 mRNA expression and collagen type-I secretion, but does not do the same with Col3 mRNA and collagen type-III.44,45 This phenomenon may explain why only Col3α1 mRNA levels, but not Col1α1, were significantly different between Ang II–treated WT and IL-4−/− mice in our study. This finding stems from the fact that significant increases in Col1α1 mRNA in both WT and IL-4−/− mice diminish the difference in Col1α1 mRNA expression induced by IL-4. TGF-β1 also induces other profibrotic factors, including connective tissue growth factor,44 which might upregulate both Col1α1 and Col3α1 mRNAs in both WT and IL-4−/− mice treated with Ang II. This results in significantly higher Col1α1/Col3α1 mRNA levels compared with its vehicle-treated group. An interesting finding of the current study was the reduction of IL-1β, IL-2, and tumor necrosis factor-α in the hearts of IL-4−/− mice treated with Ang II. The mechanisms by which these cytokines were reduced in mice with Ang II exposure are unknown. Recent studies have provided evidence of an important role for MCP-1 in the recruitment of bone marrow–derived fibroblast precursors in Ang II–induced cardiac fibrosis.46,47 In addition, it has been shown that IL-4 induces MCP-1 expression in endothelial cells.33,48 The cardiac MCP-1 levels in our WT mice were significantly higher than that in IL-4−/−. Therefore, robust MCP-1 induction by IL-4 and Ang II results in a strong recruitment of monocytes (then M2 macrophage differentiation) and bone marrow–derived fibroblast precursors. This may explain the dense collagen extending outward from the perivascular/pericapillary space to the myocardium in our Ang II–treated WT mice. The pathophysiologic effects of Ang II administration over imbalanced Th1/Th2 cytokine expression on the heart are complex and involve many variables. Our data suggest that in the presence of high levels of IL-4 and Ang II–induced high systolic afterload and stimulation, cardiac fibrosis is a major cause for impaired cardiac function in mice. This could be the outcomes of synergic effects of IL-4 and Ang II on cardiac resident cells and infiltrated immune cells.
Perspectives
The present study establishes the causal relationship between chronically elevated IL-4 and fibrotic cardiomyopathy by using WT and IL-4−/− Balb/c mice. In addition, by infusing Ang II, we develop a hypertension-induced DCM mouse model in Balb/c mice. This is likely the result of IL-4 accumulation over time because IL-4–deficient mice were protected from exacerbated fibrotic cardiac remodeling and DCM. Our in vitro study proposes a mechanism by which IL-4–enhanced collagen production in cardiac fibroblasts is mediated through the STAT6 signaling pathway. Of note, fibrogenesis has been recognized as a major cause of morbidity and mortality in many chronic diseases,49 but for which no specific therapies are available yet. The discovery of IL-4 upregulating fibrogensis would be of great clinical interest and would allow IL-4 to serve as a potential target for future therapeutic intervention.
Supplementary Material
Novelty and Significance.
What Is New?
This study shows that chronically elevated interleukin-4, a cytokine produced by immune cells, is sufficient to induce cardiac fibrosis/dysfunction, resulting in the heart being susceptible to angiotensin II–induced cardiac damage.
What Is Relevant?
Cardiac fibrosis represents a critical component of chronic heart diseases and hypertension-related end-organ damage.
Fibrogenesis represents a common pathophysiological change in many chronic diseases, becoming a major cause of morbidity and mortality, but no specific therapies are currently available to halt or reverse fibrosis.
Summary
Our findings reveal that interleukin-4 is a key profibrotic factor in the heart, which implicates interleukin-4 as an additional diagnostic test for the diseases and a promising new therapeutic target.
Acknowledgments
We thank Gulser Gurocak for her excellent technical assistance.
Sources of Funding
This study was supported by American Heart Association 14GRNT20460148 (H. Peng) and Henry Ford Hospital Institutional Fund (N.E. Rhaleb).
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.115.05627/-/DC1
Disclosures
None.
References
- 1.Vasan RS, Benjamin EJ. Diastolic heart failure–no time to relax. N Engl J Med. 2001;344:56–59. doi: 10.1056/NEJM200101043440111. doi: 10.1056/NEJM200101043440111. [DOI] [PubMed] [Google Scholar]
- 2.Roselló-Lletí E, Rivera M, Bertomeu V, Cortés R, Jordán A, González-Molina A. [Interleukin-4 and cardiac fibrosis in patients with heart failure]. Rev Esp Cardiol. 2007;60:777–780. [PubMed] [Google Scholar]
- 3.Catapano G, Pedone C, Nunziata E, Zizzo A, Passantino A, Incalzi RA. Nutrient intake and serum cytokine pattern in elderly people with heart failure. Eur J Heart Fail. 2008;10:428–434. doi: 10.1016/j.ejheart.2008.02.016. doi: 10.1016/j.ejheart.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol. 2011;50:248–256. doi: 10.1016/j.yjmcc.2010.10.019. doi: 10.1016/j.yjmcc.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hyper-tensive rat heart. Hypertension. 2009;53:1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 6.Yagi R, Suzuki W, Seki N, Kohyama M, Inoue T, Arai T, Kubo M. The IL-4 production capability of different strains of naive CD4(+) T cells controls the direction of the T(h) cell response. Int Immunol. 2002;14:1–11. doi: 10.1093/intimm/14.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Bradding P, Feather IH, Howarth PH, Mueller R, Roberts JA, Britten K, Bews JP, Hunt TC, Okayama Y, Heusser CH. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992;176:1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walford HH, Doherty TA. STAT6 and lung inflammation. JAKSTAT. 2013;2:e25301. doi: 10.4161/jkst.25301. doi: 10.4161/jkst.25301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuber JP, Spertini F. Immunological basis of systemic sclerosis. Rheumatology (Oxford) 2006;45(suppl 3):iii23–iii25. doi: 10.1093/rheumatology/kel285. [DOI] [PubMed] [Google Scholar]
- 10.Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A. 1997;94:10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Q, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension. 2006;48:98–104. doi: 10.1161/01.HYP.0000227247.27111.b2. doi: 10.1161/01.HYP.0000227247.27111.b2. [DOI] [PubMed] [Google Scholar]
- 12.Peng H, Yang XP, Carretero OA, Nakagawa P, D'Ambrosio M, Leung P, Xu J, Peterson EL, González GE, Harding P, Rhaleb NE. Angiotensin II-induced dilated cardiomyopathy in Balb/c but not C57BL/6J mice. Exp Physiol. 2011;96:756–764. doi: 10.1113/expphysiol.2011.057612. doi: 10.1113/expphysiol.2011.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanellakis P, Ditiatkovski M, Kostolias G, Bobik A. A pro-fibrotic role for interleukin-4 in cardiac pressure overload. Cardiovasc Res. 2012;95:77–85. doi: 10.1093/cvr/cvs142. doi: 10.1093/cvr/cvs142. [DOI] [PubMed] [Google Scholar]
- 14.Kotowicz K, Callard RE, Friedrich K, Matthews DJ, Klein N. Biological activity of IL-4 and IL-13 on human endothelial cells: functional evidence that both cytokines act through the same receptor. Int Immunol. 1996;8:1915–1925. doi: 10.1093/intimm/8.12.1915. [DOI] [PubMed] [Google Scholar]
- 15.Yamaji-Kegan K, Takimoto E, Zhang A, Weiner NC, Meuchel LW, Berger AE, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (FIZZ1/ RELMα) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1090–L1103. doi: 10.1152/ajplung.00279.2013. doi: 10.1152/ajplung.00279.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brower GL, Gardner JD, Forman MF, Murray DB, Voloshenyuk T, Levick SP, Janicki JS. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg. 2006;30:604–610. doi: 10.1016/j.ejcts.2006.07.006. doi: 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Bartosová D, Chvapil M, Korecký B, Poupa O, Rakusan K, Turek Z, Vízek M. The growth of the muscular and collagenous parts of the rat heart in various forms of cardiomegaly. J Physiol. 1969;200:285–295. doi: 10.1113/jphysiol.1969.sp008693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibro-blast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 19.Winegrad S, Robinson TF. Force generation among cells in the relaxing heart. Eur J Cardiol. 1978;7(suppl):63–70. [PubMed] [Google Scholar]
- 20.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 21.Büttner C, Skupin A, Rieber EP. Transcriptional activation of the type I collagen genes COL1A1 and COL1A2 in fibroblasts by interleukin-4: analysis of the functional collagen promoter sequences. J Cell Physiol. 2004;198:248–258. doi: 10.1002/jcp.10395. doi: 10.1002/jcp.10395. [DOI] [PubMed] [Google Scholar]
- 22.McGaha TL, Le M, Kodera T, Stoica C, Zhu J, Paul WE, Bona CA. Molecular mechanisms of interleukin-4-induced up-regulation of type I collagen gene expression in murine fibroblasts. Arthritis Rheum. 2003;48:2275–2284. doi: 10.1002/art.11089. doi: 10.1002/art.11089. [DOI] [PubMed] [Google Scholar]
- 23.Aoudjehane L, Pissaia A, Jr, Scatton O, Podevin P, Massault PP, Chouzenoux S, Soubrane O, Calmus Y, Conti F. Interleukin-4 induces the activation and collagen production of cultured human intrahepatic fibroblasts via the STAT-6 pathway. Lab Invest. 2008;88:973–985. doi: 10.1038/labinvest.2008.61. doi: 10.1038/labinvest.2008.61. [DOI] [PubMed] [Google Scholar]
- 24.Renz H, Domenico J, Gelfand EW. IL-4-dependent up-regulation of IL-4 receptor expression in murine T and B cells. J Immunol. 1991;146:3049–3055. [PubMed] [Google Scholar]
- 25.Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013;6:740–750. doi: 10.1038/mi.2012.112. doi: 10.1038/ mi.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 27.Kolck UW, Alfter K, Homann J, von Kügelgen I, Molderings GJ. Cardiac mast cells: implications for heart failure. J Am Coll Cardiol. 2007;49:1107. doi: 10.1016/j.jacc.2006.12.018. author reply 1107–1107; author reply 1108. doi: 10.1016/j.jacc.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Levick SP, Meléndez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2011;89:12–19. doi: 10.1093/cvr/cvq272. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara M, Ono K, Hwang MW, Iwasaki A, Okada M, Nakatani K, Sasayama S, Matsumori A. Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med. 2002;195:375–381. doi: 10.1084/jem.20002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339:64–67. doi: 10.1038/339064a0. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 31.Lee YW, Kühn H, Hennig B, Neish AS, Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J Mol Cell Cardiol. 2001;33:83–94. doi: 10.1006/jmcc.2000.1278. doi: 10.1006/jmcc.2000.1278. [DOI] [PubMed] [Google Scholar]
- 32.Khew-Goodall Y, Wadham C, Stein BN, Gamble JR, Vadas MA. Stat6 activation is essential for interleukin-4 induction of P-selectin transcription in human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:1421–1429. doi: 10.1161/01.atv.19.6.1421. [DOI] [PubMed] [Google Scholar]
- 33.Lee YW, Hennig B, Toborek M. Redox-regulated mechanisms of IL-4-induced MCP-1 expression in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H185–H192. doi: 10.1152/ajpheart.00524.2002. doi: 10.1152/ajpheart.00524.2002. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Shivshankar P, Halade GV, Calhoun C, Escobar GP, Mehr AJ, Jimenez F, Martinez C, Bhatnagar H, Mjaatvedt CH, Lindsey ML, Le Saux CJ. Caveolin-1 deletion exacerbates cardiac interstitial fibrosis by promoting M2 macrophage activation in mice after myocardial infarction. J Mol Cell Cardiol. 2014;76:84–93. doi: 10.1016/j.yjmcc.2014.07.020. doi: 10.1016/j.yjmcc.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kodera T, McGaha TL, Phelps R, Paul WE, Bona CA. Disrupting the IL-4 gene rescues mice homozygous for the tight-skin mutation from embryonic death and diminishes TGF-beta production by fibroblasts. Proc Natl Acad Sci U S A. 2002;99:3800–3805. doi: 10.1073/pnas.052709999. doi: 10.1073/pnas.052709999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng WP. ‘All things considered’: transcriptional regulation of T helper type 2 cell differentiation from precursor to effector activation. Immunology. 2013;140:31–38. doi: 10.1111/imm.12121. doi: 10.1111/imm.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arai T, Abe K, Matsuoka H, Yoshida M, Mori M, Goya S, Kida H, Nishino K, Osaki T, Tachibana I, Kaneda Y, Hayashi S. Introduction of the inter-leukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278:L914–L922. doi: 10.1152/ajplung.2000.278.5.L914. [DOI] [PubMed] [Google Scholar]
- 41.Demols A, Van Laethem JL, Quertinmont E, Degraef C, Delhaye M, Geerts A, Deviere J. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1105–G1112. doi: 10.1152/ajpgi.00431.2001. doi: 10.1152/ajpgi.00431.2001. [DOI] [PubMed] [Google Scholar]
- 42.Mehra VC, Ramgolam VS, Bender JR. Cytokines and cardiovascular disease. J Leukoc Biol. 2005;78:805–818. doi: 10.1189/jlb.0405182. doi: 10.1189/jlb.0405182. [DOI] [PubMed] [Google Scholar]
- 43.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95:1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 44.Rolfe KJ, Irvine LM, Grobbelaar AO, Linge C. Differential gene expression in response to transforming growth factor-beta1 by fetal and post-natal dermal fibroblasts. Wound Repair Regen. 2007;15:897–906. doi: 10.1111/j.1524-475X.2007.00314.x. doi: 10.1111/j.1524-475X.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 45.Lam S, Verhagen NA, Strutz F, van der Pijl JW, Daha MR, van Kooten C. Glucose-induced fibronectin and collagen type III expression in renal fibroblasts can occur independent of TGF-beta1. Kidney Int. 2003;63:878–888. doi: 10.1046/j.1523-1755.2003.00824.x. doi: 10.1046/j.1523-1755.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 46.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, Wang Y. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol. 2011;301:H538–H547. doi: 10.1152/ajpheart.01114.2010. doi: 10.1152/ajpheart.01114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rollins BJ, Pober JS. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991;138:1315–1319. [PMC free article] [PubMed] [Google Scholar]
- 49.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013;304:C216–C225. doi: 10.1152/ajpcell.00328.2012. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.