Abstract
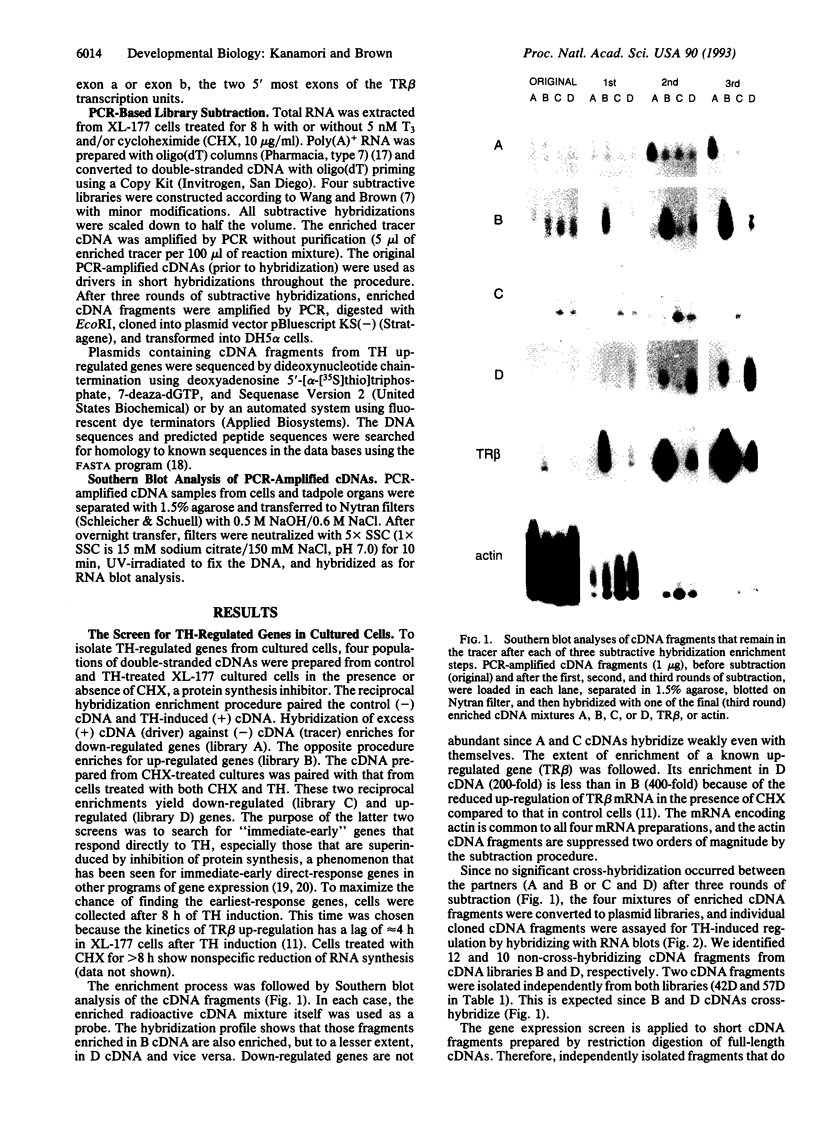
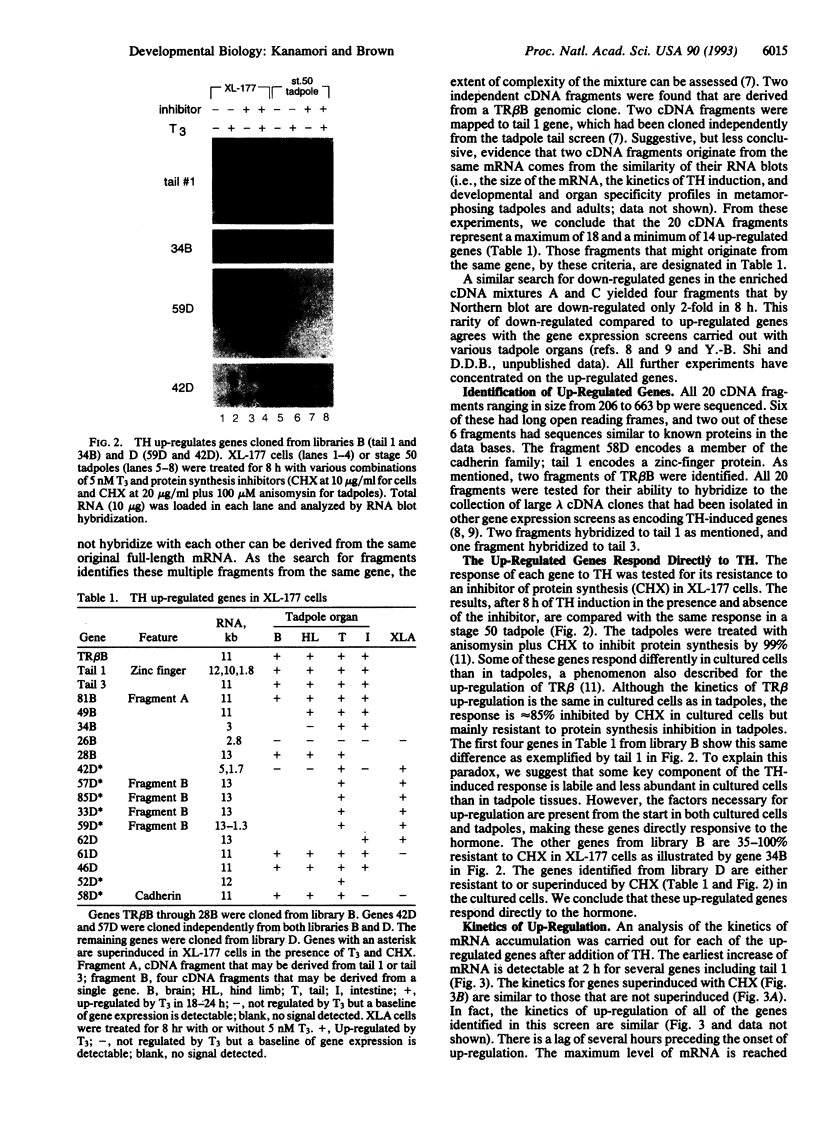
Gene expression screens have been applied to a cultured cell line of Xenopus laevis, XL-177, to isolate genes that are up- and down-regulated in the first 8 h after thyroid hormone (TH) induction. At least 14 up-regulated genes were isolated from TH-induced cells grown in the presence or absence of cycloheximide, an inhibitor of protein synthesis. These genes respond directly to TH as demonstrated by the resistance of up-regulation to protein synthesis inhibition in the cultured cells or in tadpoles. Kinetics of mRNA accumulation after TH induction is similar for these genes, including those that are superinduced by cycloheximide. Their mRNAs start to be up-regulated several hours after TH treatment and reach maximum levels between 8 and 16 h. These genes show up-regulation in one or more tadpole organs in response to exogenous TH. Only a few minimally down-regulated genes were identified. Fourteen of the 20 genes that were found to be up-regulated by TH in tadpole tail are also up-regulated in XL-177 cells. Their up-regulation falls into the same two kinetic patterns in the cultured cells as it does in tadpole tail. Another cell line of X. laevis, XLA, is greatly reduced in its ability to up-regulate the same genes isolated from XL-177 cells and tadpole tails in response to TH. Thus these cell lines make up a model system to examine the interactions of gene expression triggered by TH during amphibian metamorphosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckbinder L., Brown D. D. Thyroid hormone-induced gene expression changes in the developing frog limb. J Biol Chem. 1992 Dec 25;267(36):25786–25791. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Ellison T. R., Mathisen P. M., Miller L. Developmental changes in keratin patterns during epidermal maturation. Dev Biol. 1985 Dec;112(2):329–337. doi: 10.1016/0012-1606(85)90403-8. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Kanamori A., Brown D. D. The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J Biol Chem. 1992 Jan 15;267(2):739–745. [PubMed] [Google Scholar]
- Machuca I., Tata J. R. Autoinduction of thyroid hormone receptor during metamorphosis is reproduced in Xenopus XTC-2 cells. Mol Cell Endocrinol. 1992 Sep;87(1-3):105–113. doi: 10.1016/0303-7207(92)90238-2. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Wang Z., Brown D. D. A gene expression screen. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11505–11509. doi: 10.1073/pnas.88.24.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Brown D. D. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990 Nov;4(11):1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Shi Y. B., Brown D. D. Xenopus laevis alpha and beta thyroid hormone receptors. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7090–7094. doi: 10.1073/pnas.87.18.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]