Abstract
Socioeconomic status (SES) has been linked to functioning across a variety of neurocognitive domains including language, memory, executive functioning, and social-emotional processing. We review these findings and discuss the ways in which socioeconomic context may shape neural processes such that these skills are supported by different neurobiological pathways in children from lower versus high SES backgrounds. Moreover we consider the mechanisms by which SES may be related to specific neurocognitive functions. Specifically, we focus on linguistic exposure and stress as two main pathways through which SES could influence neurocognitive processes and shape relations between the neural and behavioral levels of functioning. Finally, suggestions for conceptualizing and measuring SES in future work are offered.
Keywords: Socioeconomic Status, Brain Development, Neurocognitive Function, Stress, Language
Introduction
Extensive research has documented socioeconomic disparities in academic performance (Sirin, 2005). In order to more clearly understand the cognitive disparities that may underlie these performance differences, studies have begun investigating specific neurocognitive systems that may be differentially associated with socioeconomic status (SES) (Noble & Farah, 2013). These studies have largely demonstrated that socioeconomically disadvantaged children exhibit poorer behavioral performance in the domains of language, memory, executive functioning, and social-emotional processing relative to their higher SES peers, with some evidence pointing to underlying neural differences. For example, differences have been reported in left hemisphere regions including the left superior temporal gyrus, left inferior frontal gyrus, and left fusiform which support various aspects of language development, (Noble et al., 2015a; Noble, Wolmetz, Ochs, Farah, & McCandliss, 2006; Noble, Houston, Kan, & Sowell, 2012; Raizada, Richards, Meltzoff, & Kuhl, 2008); the hippocampus, which supports memory, (Hanson, Chandra, Wolfe, & Pollak, 2011; Jednoróg et al., 2012; Luby et al., 2013; Noble et al., 2012; Noble et al., 2015a; Noble et al., 2012); the prefrontal cortex which supports executive functioning, (Gianaros et al., 2007; Noble et al., 2015a); and the amygdala which supports social-emotional processing, (Gianaros et al., 2008; Luby et al., 2013; Noble et al., 2012). As these findings have previously been extensively reviewed (Brito & Noble, 2014; Hackman & Farah, 2009; Perkins, Finegood, & Swain, 2013; Raizada & Kishiyama, 2010; Tomalski et al., 2013), we highlight key themes and results and then focus on mechanisms by which these processes may occur. Although disparities in brain function may be tied to neuroanatomical differences, functional deficits may also occur independently of structural differences, and few studies have simultaneously utilized both functional and structural imaging methods. As such, we focus this review on the way in which SES shapes brain function rather than structure and refer the reader to a recent review for information on neuroanatomical differences associated with SES (Brito & Noble, 2014). Specifically, we examine the ways in which SES may shape relations of neurobiology to cognitive skills such that age-appropriate cognitive development may be attained through different neurobiological mechanisms for children developing in different socioeconomic contexts.
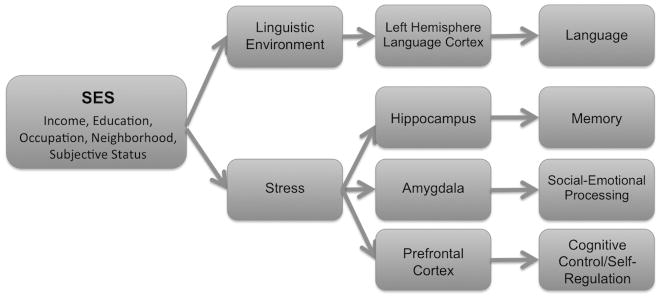
The mechanisms underlying SES differences in specific neurocognitive functions at both the behavioral and neurobiological levels have yet to be fully elucidated. Both theory and empirical evidence suggest that multiple pathways exist, with the links between SES and specific neurocognitive functions possibly mediated by different mechanisms. In this review, we draw on a conceptual model (Figure 1) that posits two main pathways by which SES may be related to functioning of specific brain areas and in turn to neurocognitive performance, namely linguistic stimulation and children’s experience of stress, recognizing that these pathways may not be completely independent, and that these may not be the only pathways operating to link SES to neural and cognitive outcomes. This model provides a conceptual organization of the empirical literature examining the relations of SES to neural and behavioral functioning and joins two separate literatures, which have largely independently theorized and examined the importance of the mechanistic pathways of stress and linguistic stimulation. We discuss ways in which these pathways may lead to neurocognitive differences such that SES may influence cognition on both neural and behavioral levels or may shape relations between these two levels of functioning. Additionally, we highlight ways in which these pathways of linguistic stimulation and stress may interact in the context of the developing child.
Figure 1.
Hypothesized mechanisms by which SES operates to influence neurocognitive functioning. Figure as originally published in Brito, N. H., & Noble, K. G. (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 1–12. doi:10.3389/fnins.2014.00276
To date, research on SES and the brain has largely progressed without a consensus as to how exactly SES should be conceptualized or operationalized. Traditional indicators of SES include income, education, and occupational status, but subjective indicators of social status have also recently been developed. Moreover, issues concerning the use of composite versus individual indicators, as well as how to capture dynamic aspects of SES are unresolved. We conclude our review with a discussion of these issues regarding SES measurement, and we offer some suggestions that can serve as a starting point for researchers who aim to incorporate SES into their research questions.
Neurocognitive Development in Socioeconomic Context
SES and Language
Extensive work has demonstrated SES disparities in children’s language and literacy abilities (Perkins et al., 2013). Lower SES has been associated with worse performance on many types of language skill, including vocabulary, phonological awareness, single-word decoding, reading comprehension, and grammar (Bowey, 1995; Noble, Tottenham, & Casey, 2005). SES has also been shown to be an important factor in predicting who, among those with poorer pre-literacy skills, will have reading difficulties: Among lower SES children, the relation between phonological awareness and reading ability is amplified, whereas high SES may serve as a buffer against reading disability among children with low phonological awareness (Noble, Farah, & McCandliss, 2006).
Consistent with these behavioral findings, SES disparities have also been found in studies of the neurobiology of language. Recordings of baseline EEG activity in 6 to 9 month olds showed that lower SES infants had lower frontal gamma power, which may potentially indicate early risk for language problems (Tomalski et al., 2013). Lower SES children have also been shown to exhibit less specialization in the left inferior frontal gyrus during a phonological awareness task (Raizada et al., 2008). The effects of childhood SES may carry over into adulthood as shown in one study which found that in response to syntactic violations, adults who grew up in lower SES environments exhibited smaller negative event-related potential (ERP) responses in left anterior sites at both 100–300msec and 300–700msec time windows than did those who had grown up in higher SES environments (Pakulak & Neville, 2010). This effect was independent of adult education level. Interestingly, the effect of SES on ERPs was also independent of behavioral performance, which may suggest that SES moderates the relation between neurobiology and behavioral performance, although the authors did not find support for this hypothesis, perhaps because of the high correlation between SES and behavioral performance.
Preliminary evidence in support of the hypothesis that SES may moderate relations between neural processes and language skill did come from a study of adult readers with a history of childhood reading disabilities (Shaywitz et al., 2003). Participants were imaged while performing a reading task. Individuals who had improved in accuracy as adults, as compared to those who remained poor readers in adulthood, had lower activation in left perisylvian regions, but greater activation in right perisylvian and superior frontal cortices, which may suggest that the accuracy-improved readers were using the latter brain regions to compensate for deficits (see Grady, 2008 for an explanation of neural compensation versus inefficiency). SES played a role in that the persistently poor readers were more likely to have come from a lower quality school, and did not show this compensatory pattern. More direct evidence for SES moderation was found in a study of children with below average reading abilities, in which SES moderated the relation of brain activation to phonological skill (Noble et al., 2006). Specifically, among lower SES struggling readers, phonological skill differences were associated with large differences in brain activation during a reading task, primarily in the left fusiform gyrus region, an area of the brain that has been shown to be important for visual-orthographic aspects of reading. This brain-behavior relationship weakened, however, as SES increased. One possible interpretation is that higher SES children who struggled with reading were less likely to engage this area that is involved in typical reading, and more likely to engage other areas while reading. As such, these results suggest that there could be etiological heterogeneity in reading struggles such that that the prototypical cause of reading problems is systematically different between children from higher versus lower SES families because of differences in their environmental risk factors. Moreover, a trend-level interaction indicated that higher SES struggling readers tended to show associations between phonological awareness and activation in right superior temporal gyrus and bilateral superior frontal gyri during the reading task, the very areas of the brain that had been shown by Shaywitz et al (2003) to be activated by adults who had overcome childhood reading impairment. Taken together, these results suggest that socioeconomic advantage may act as a buffer among those who are at risk for reading difficulties such that children from higher SES families may recruit alternate, possibly compensatory, neural networks to support phonological skills which may allow them to develop better reading skills despite atypical activation in systems that are classically important for reading development (Noble et al., 2006). Furthermore, these studies demonstrate the importance of considering the role of SES when examining relations between brain activity and language skills. Without considering SES, important individual differences in the brain systems underlying reading would have remained obscured.
SES and Memory
SES disparities have also been found in the neurocognitive domain of memory. Behaviorally, several studies have shown that lower SES is associated with poorer memory in adulthood (see (Herrmann & Guadagno, 1997) for a review; (Singh-Manoux, Richards, & Marmot, 2005). Studies in children have found similar results with lower SES children performing more poorly on measures of incidental (Farah et al., 2006; Noble, McCandliss, & Farah, 2007) and episodic (Akshoomoff et al., 2014) memory. Few studies have investigated socioeconomic disparities in the neural correlates of memory performance. One study found that maternal reports of higher subjective social status were related to greater hippocampal activation in children during a relational memory task, but subjective social status was unrelated to behavioral performance (Sheridan, How, Araujo, Schamberg, & Nelson, 2013). High SES may also buffer some of the memory decline typically associated with aging. In a task of recency judgments, higher SES older adults performed similarly to younger participants, whereas lower SES older adults performed worse (Czernochowski, Fabiani, & Friedman, 2008). Further, higher SES older adults appear to recruit additional neural resources as evidenced by a larger long-duration frontal negativity ERP for recency versus recognition trials (Czernochowski et al., 2008). Thus, as in the domain of language, lower SES tends to be associated with worse performance on memory tasks, and individuals of higher SES are reported to recruit additional neural resources, which may buffer age-related decline. Such findings support the theory of cognitive reserve, which states that, because of differences in lifetime experience, higher SES individuals may be better able to call upon other neurocognitive resources and/or alter their neurocognitive processing such that brain pathology does not result in otherwise expected cognitive deficits (Stern, 2009). Without examining the role of SES, the lack of memory decline and corresponding compensatory brain processes utilized by the higher SES older adults may have been masked by the deficits shown in the overall aging population.
SES and Executive Function
Several studies have demonstrated that children from lower SES families tend to perform worse on most aspects of executive functioning, including working memory, inhibitory control, and attention shifting (Blair et al., 2011; Farah et al., 2006; Noble et al., 2007; Sarsour et al., 2011). Longitudinal work has also demonstrated that greater chronic exposure to childhood poverty is associated with poorer executive function (EF) in early childhood (Raver, Blair, & Willoughby, 2013) and poorer working memory in young adulthood (Evans & Schamberg, 2009). In line with these behavioral findings, several studies have reported neurobiological evidence of SES-related disparities in EF using both functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) methods. In one study that used a complex stimulus-response learning task, which elicits prefrontal activation in adults, lower SES children performed more poorly than their higher SES counterparts. FMRI analyses indicated that lower SES children were also more likely to activate the right medial frontal gyrus as compared to higher SES children, which may reflect an inefficiency of recruitment of neural resources during the task because this increased brain activation was not associated with behavioral improvements (Sheridan, Sarsour, Jutte, D’Esposito, & Boyce, 2012). In adults, a study of functional connectivity of corticostriatal brain systems during a reward processing task found that lower parental education was associated with reduced functional connectivity of perigenual anterior cingulate cortex (pACC) and orbitofrontal cortex (OFC) regions to the dorsomedial prefrontal cortex (dMPFC) and ventral striatum, even after controlling for participants’ own (adult) level of education (Gianaros et al., 2011). This reduced functional connectivity indicates that the brain regions involved in reward processing and decision making are less correlated in their activation which may have consequences for integrating information during decision making as well as for executing top-down control in the face of riskier decision making (Gianaros et al., 2011). Without explicitly examining the role of SES, these main effect differences in brain and behavioral aspects of EF would have simply been left as unexplained error.
Electrophysiological studies have also reported SES disparities in EF-related processes. The lower frontal gamma power that was observed in EEG recordings of 6 to 9 month olds may indicate early risk for attention problems, in addition to risk for language problems (Tomalski et al., 2013). Relatedly, several studies have also demonstrated SES differences in ERP measures of selective attention (D’Angiulli, Herdman, Stapells, & Hertzman, 2008; Kishiyama, Boyce, Jimenez, Perry, & Knight, 2009; Stevens, Lauinger, & Neville, 2009). Using a visual attention task on which there were no SES differences in performance, Kishiyama et al (Kishiyama et al., 2009) found that SES predicted prefrontal cortex responsivity in 7 to 12 year old children. Specifically, lower SES children exhibited a pattern of reduced prefrontal-dependent, early extrastriate (P1 and N1) and fronto-central novelty-related (N2) ERP components, which was similar to the response pattern seen in patients with prefrontal damage. Several studies have also demonstrated SES differences in ERP measures of selective auditory attention (D’Angiulli et al., 2008; Stevens et al., 2009). One study used a selective auditory attention task in which children had to respond to a certain type of tone while ignoring others. While no behavioral differences were found between SES groups, there was differential ERP activity as a function of SES. Children from higher SES families exhibited a greater difference in mid-frontal cortical response to attended vs. unattended tones than did children from lower SES families, which suggested that lower SES children allotted attention more equally to both unattended and attended tones (D’Angiulli et al., 2008). Additionally, lower SES children had greater event-related frontal midline theta power when hearing unattended vs. attended tones whereas higher-SES children showed very small or no differences. Importantly, low and high SES groups performed behaviorally similarly, although they exhibited different neural responses. Thus, by examining the relation of ERP response to behavior without considering the role of SES, important information would have been lost. Similarly, in a sample of 3 to 8 year old children, higher SES was associated with a greater anterior ERP response to attended vs. unattended auditory information, which peaked around 150 msec after probe onset. The differential response was much weaker for lower SES children, despite similar behavioral performance on comprehension questions following the task (Stevens et al., 2009). Additional analyses demonstrated that these SES differences were driven by reduced suppression of distractor information among the lower SES children. Across both studies, findings suggest that lower SES children may be less likely to suppress irrelevant information. This pattern of results may indicate that lower SES children may use an alternate or compensatory strategy to perform the task as indicated by the greater frontal midline theta power exhibited by the lower SES children (D’Angiulli et al., 2008). Alternatively, it is possible that the behavioral task was too easy to reveal SES differences and that a more difficult task would show SES disparities in behavioral performance (Stevens et al., 2009).
SES and Social-emotional Processing
SES differences have been reported in social-emotional functioning, another essential aspect of neurocognitive development. Poverty has been linked to reductions in children’s psychological wellbeing, as reported by both parents and the children themselves (Evans & English, 2002). These results were extended in a longitudinal investigation that found effects in adolescence of income on learned helplessness, self-report of psychological distress, and teacher ratings of self-regulatory behavior, even when controlling for earlier measures of each construct (Evans, Gonnella, Marcynyszyn, Gentile, & Salpekar, 2005). Interestingly, early experiences may play a particularly important role as parental education has been found to be related to impulsive decision making even after taking adult SES into account (Sweitzer, Donny, Dierker, Flory, & Manuck, 2008).
Neuroimaging studies have extended these results to examine the ways in which SES may be associated with neural functions that underlie specific aspects of social-emotional processing. Lower perceived parental social standing has been associated with greater amygdala reactivity to angry faces in a sample of undergraduate students (Gianaros et al., 2008). Similarly, adults who experienced greater poverty at age 9 had more difficulty suppressing amygdala activation and had reduced prefrontal cortex activity during a task in which they had to use cognitive reappraisal to regulate their emotional responses to negative stimuli (Kim et al., 2013). Interestingly, these associations were specific to childhood income as adult income was not associated with brain activity. Other evidence from a study of middle-aged adults demonstrated that lower parental education was related to activation in and connectivity among corticostriatal brain systems that are important for reward processing, even after controlling for participants’ own levels of education and household income (Gianaros et al., 2011). Thus, although the investigation of SES disparities in social-emotional brain functioning is only beginning, evidence to date suggests that SES shapes behavioral and neural functioning in ways that lead to differences in the processing of emotionally salient stimuli. Here as in many of the studies reviewed above, early SES appears to play a particularly important role, and failing to include it may lead researchers to ignore the important role of environmental context. Moreover, by including both childhood and adult SES, researchers may be able to begin to tease apart the mechanisms by which SES is associated with neurocognitive functioning. Specifically, early childhood socioeconomic conditions versus those experienced later may have different implications for the pathways through which SES operates.
Mechanisms of SES Disparities in Neurocognitive Processes
As shown in Figure 1, SES is hypothesized to affect neurocognitive functioning on both neural and behavioral levels through two major pathways: language exposure and experience of stress (Brito & Noble, 2014; Noble et al., 2012). Although these two paths may operate to some extent independently, it is also likely that they have complex and interacting effects on neurocognitive functioning (Perkins et al., 2013). In describing these pathways, we highlight the ways in which associated changes in cognitive functioning, although not necessarily optimal or desirable by mainstream standards, may in fact be adaptive for dealing with the circumstances faced by children in disadvantaged homes.
Linguistic Exposure
Extensive research has demonstrated that SES is related to cognitive and linguistic stimulation in the home, and that differences in exposure to language are predictive of differences in children’s language abilities. In a seminal study of SES disparities in linguistic exposure, Hart and Risley (1995) found that SES was predictive of the number of words and the complexity of language that children were exposed to in the home. These characteristics of the home language environment were in turn associated with differences in children’s vocabulary growth. Several other studies have replicated and extended these findings (Perkins et al., 2013), demonstrating, for example, that the proportion of multiclause sentences in maternal language mediates the relation between SES and children’s use of multiclause sentences (Huttenlocher, Vasilyeva, Cymerman, & Levine, 2002), and that mean length of maternal utterances mediates the relation between SES and children’s vocabulary growth (Hoff, 2003). Although maternal language is often measured in terms of quantity of words such as through mean length of utterance, it is important to remember that quantity measures and quality measures are often related as parents who speak longer sentences are more likely to use a more diverse vocabulary and more complex syntactical structures (Hoff, 2003). Moreover, quality related aspects of maternal language including verbal responsiveness and provision of verbal input based on following rather than redirecting the child’s attentional focus are also related to children’s vocabulary development (see Hoff, 2006 for a review). The importance of quality versus quantity is also shown through a study of low SES infants in which the amount of child-directed speech, but not the amount of speech simply overheard, was related to vocabulary size at 24 months of age (Weisleder & Fernald, 2013). Early language development is also influenced by parents’ use of gestures (Rowe & Goldin-Meadow, 2009). Higher SES children have been found to use more gestures when interacting with their caregivers at 18 months of age, and this relation was mediated by their parents’ own use of gestures. Moreover, children’s use of gestures at 18 months of age in turn predicted children’s vocabulary development at 42 months of age, thereby mediating the SES differences in vocabulary development.
Other work has taken a more global approach to measuring language exposure by looking at the home literacy (or learning) environment (Perkins et al., 2013). As measured by the Home Observation and Measurement of the Environment (HOME) inventory, the home learning environment captures materials and practices such as the nature of play materials and environmental organization, in addition to aspects of maternal language. Parental SES has been repeatedly associated with ratings on the home learning environment subscale of the HOME (Caldwell & Bradley, 1984). Home learning environments in turn predict language skills in early (Noble et al., 2015b; Rodriguez & Tamis-LeMonda, 2011; Son & Morrison, 2010) and middle childhood (Farah et al., 2008), and are most beneficial for child development when they are rich and remain stable across childhood (Rodriguez & Tamis-LeMonda, 2011). Research suggests that interactions with parents around stimulating materials and activities are likely important ways by which high SES children gain exposure to more complex language. Moreover, it is possible that this greater exposure to language experienced by higher SES children in general could explain why high SES children are sometimes less likely to develop reading impairments even when they have early impairments in phonological skill. That is, among children who struggle with learning to read, higher SES children are likely to have greater exposure to more diverse and complex language and literacy activities. This increased exposure to, and practice with, language may promote the development of compensatory neural networks, which may help to prevent children with below-average phonological skills from developing or maintaining reading impairments.
Linguistic exposure has important effects on brain functioning. For example, following repeated exposure to non-native speech sounds, English-speaking infants show differential ERP responses to deviant versus standard sounds in the non-native language (Conboy & Kuhl, 2011). In adult native English speakers who were taught Chinese words, fast learning of the words was associated with a left-lateralized increase in N170 amplitude and an increased anteriorly-distributed N400 amplitude (Yum, Midgley, Holcomb, & Grainger, 2014). Slower learners, however, exhibited increases in posterior positive-going waveforms. Additional evidence has demonstrated that whereas younger first language learners (14 month olds) show similar ERP N200–N400 amplitudes to known words and to phonetically similar nonsense words, as compared with phonetically dissimilar nonsense words, 20-month-olds exhibit larger N200–N400 amplitudes only to known words (Mills et al., 2004). However, the extent to which SES disparities in language exposure lead to differential ERP response to native language sounds is unclear. One study reported that lower academic stimulation and encouragement in the home was related to larger ERP amplitudes in response to speech sounds in 3 year olds, interpreted by the authors as possibly indicating that these children exerted greater effort to process speech (Molfese & Molfese, 2002). In sum, although there is significant behavioral evidence that linguistic exposure plays a role in socioeconomic disparities in multiple aspects of language, more work is needed to understand the neurobiological mechanisms by which parents’ language may affect children’s development of brain areas important for language reception and expression (Perkins et al., 2013). Differential exposure to language early on may strengthen different neural pathways to support language development, and thus even when there is evidence of equivalent behavioral competencies between children from different backgrounds, the neural pathways supporting these competencies may differ.
Stress
The second major pathway by which SES is hypothesized to affect neurocognitive functioning is through exposure to stress, which may particularly influence areas of the brain such as the prefrontal cortex, hippocampus, and amygdala, which contain high concentrations of glucocorticoid receptors. Socioeconomic disadvantage can cause stress through many pathways including both physical and social characteristics of the environment (Evans, 2004). Lower SES homes are often characterized by poorer parenting, crowding, noise, chaotic schedules, a lack of routines, and a generally higher level of unpredictability, all of which can contribute to an increase in stress (Adler & Snibbe, 2003; Combs-Orme & Cain, 2006; Evans et al., 2005). These and other stressors are expected to induce physiological stress responses in children. It is this physiologic response to stress, rather than the preceding stressors, that we propose as the proximal mechanism underlying SES disparities in certain neurocognitive functions. Although physiological stress responses can manifest in many ways such as vagal tone (see Propper & Holochwost, 2013 for a review) and allostatic load (McEwen, 1998), for the purposes of this manuscript, we limit our definition of stress to focus on neuroendocrine activity, primarily in the HPA axis.
The stress response is coordinated through both the sympathetic nervous system (SNS) and through the hypothalamic-pituitary-adrenal (HPA) axis. The SNS is a fast responding, “fight or flight” system that regulates heart rate through the release of catecholamines including norepinephrine. The HPA axis mounts a slower response to stress, resulting in the release of cortisol, which has both fast and slow effects on neurocognitive systems. In part because of the relative ease of collecting salivary cortisol, significant research focus has been placed on understanding the HPA axis as one important component of both short- and long-term stress responses. Cortisol levels in the body follow a diurnal rhythm characterized by a rapid increase for about 30 minutes after awakening, followed by a decline throughout the rest of the day with lowest levels being reached in the late evening (Kirschbaum & Hellhammer, 1989). Salivary cortisol levels also show marked increases about 20 minutes following an acute stressor, with a subsequent decline to baseline levels.
Several studies have demonstrated that greater socioeconomic disadvantage is associated with a pattern of hyper-cortisolism as evidenced by higher resting cortisol levels (Blair et al., 2011), higher basal morning cortisol levels (Lupien, King, Meaney, & McEwen, 2000; Lupien, King, Meaney, & McEwen, 2001), higher overnight cortisol levels (Evans & English, 2002), greater increases in daily cortisol output over a two year period (Chen, Cohen, & Miller, 2010), and greater reactivity to and recovery from a laboratory stress paradigm (Hackman, Betancourt, Brodsky, Hurt, & Farah, 2012). Other studies, however, have found evidence for a pattern of hypo-cortisolism in the face of socioeconomic disadvantage, as evidenced by lower basal cortisol (Badanes, Watamura, & Hankin, 2011; Chen & Paterson, 2006; Kliewer, Reid-Quiñones, Shields, & Foutz, 2009), and, in response to stress paradigms, lower cortisol levels (Kraft & Luecken, 2009), and attenuated reactivity (Badanes et al., 2011). Possible explanations for these discrepancies include the moderating roles of participant characteristics such as age (Ursache, Noble, & Blair, in press) and gender, as well as differences in the levels of adversity experienced. Thus, while the exact relation between SES and cortisol production in children is not completely clear, the literature is clear that socioeconomic disadvantage tends to be related to some form of dysregulation of the HPA axis.
On a neurobiological level, a dysregulation in stress physiology, whether manifest as hypo- or hyper-activation, can have consequences for neurocognitive functioning (Blair, 2010). On a broader conceptual level, such a notion is consistent with the Yerkes-Dodson (Yerkes & Dodson, 1908) principle, which demonstrates that complex cognition is supported by moderate levels of arousal, whereas at very high or very low levels of arousal, higher-level cognitive processes are impaired. The prefrontal cortex, hippocampus, and amygdala all have high concentrations of corticosteroid receptors, and as such influence and are influenced by activation of the HPA axis and resulting cortisol output (Lupien & Lepage, 2001). Corticosteroid receptors include both glucocorticoid receptors (GR) as well as mineralocorticoid receptors (MR), which have a much higher affinity for cortisol than do GRs. As such, when both receptor types are present in a given brain structure, MRs become occupied first, with GRs becoming occupied only at moderate to high concentrations of corticosteroids.
The importance of this balance between MR and GR occupation in supporting or impairing neurocognitive functioning has been highly investigated with regard to hippocampal functioning, as this area of the brain contains both receptor types (de Kloet, Oitzl, & Joëls, 1999; Lupien & Lepage, 2001). Specifically, this balance between MRs and GRs is important for understanding the ways in which hippocampal functioning follows an inverted-U curve in response to stress. As cortisol levels increase, MRs become occupied first, followed by occupation of GRs. When levels of GR occupation are moderate and MR occupation levels are high, long-term potentiation and learning is supported. However, with very high levels of GR occupation, long-term depression is activated (de Kloet et al., 1999; Lupien & Lepage, 2001). Interestingly, long-term potentiation is also impaired when corticosteroid levels are very low and neither MRs nor GRs are occupied (Lupien & Lepage, 2001). As such, mild stress may actually enhance hippocampal function with impairments not typically seen until higher levels of stress are reached (Arnsten, 2009). Moreover, long-term exposure to high levels of corticosteroids can be detrimental in that they can lead to hippocampal atrophy, which may impact memory functioning (Lupien & Lepage, 2001). However, one study in humans found that, although subjective social status was associated both with higher baseline cortisol and with greater hippocampal activation during a memory task, baseline cortisol was unrelated to hippocampal function (Sheridan et al., 2013).
Whereas high stress impairs the hippocampus, even mild stress can inhibit prefrontal cortex (PFC) functioning, making it arguably the brain region most sensitive to stress (Arnsten, 2009). Whereas limbic brain structures including the hippocampus and amygdala contain both types of corticosteroid receptors, the prefrontal cortex (PFC) almost exclusively contains GR receptors (Lupien & Lepage, 2001). Because of this lower concentration of MRs, sensitivity of GRs in the PFC is thought to be heightened (Lupien & Lepage, 2001). Moreover, the PFC contains a high number of catecholamine receptors, thus making it additionally sensitive to the faster acting SNS stress response described above (Arnsten, 2009). As such, several studies in humans and animals have shown how neuromodulators associated with stress impact PFC function (Arnsten & Li, 2005; Arnsten, 2009). At very low levels of arousal, such as under conditions of fatigue, the PFC cannot be appropriately activated to support EF. When arousal increases to moderate levels, norepinephrine levels increase and bind to receptors in the PFC, and PFC activity increases to support EF processes including effortful regulation of attention, emotion, and action (Arnsten, 2009). At a certain point, however, catecholamine receptors in the PFC become saturated, PFC activity is inhibited, and activity increases in limbic brain areas that support more automatic or reflexive responses to stimuli (Arnsten, 2000). For this reason, with increasing levels of stress, it becomes more difficult to ignore distractions while trying to complete a task.
Some work in children has provided evidence that higher levels of stress can impair EF. Blair and colleagues (Blair et al., 2011) found that, when parents exhibited fewer positive parenting behaviors, children tended to have higher basal cortisol levels, which were in turn associated with lower EF. Moreover, Kim et al. (2013) found that chronic stress mediated links between lower family income in childhood and reduced PFC activation during an emotion regulation task in adulthood. Importantly, however, better regulation of response to stress is associated with better EF. For example, preschoolers who exhibited a profile of moderate cortisol reactivity and recovery in response to a mild stressor had higher levels of EF compared to children who did not exhibit reactivity to this stressor (Blair, Granger, & Peters Razza, 2005). This lack of reactivity was likely indicative of dysregulation in children’s stress physiology which can occur for many potential reasons such as burn out of the system following repeated activation in the context of continuous exposure to stressors. Further, moderate activation of the HPA axis in response to the challenge of participating in a neuroimaging study was related both to higher SES and to lower PFC activation during an EF task, perhaps reflecting more efficient neural recruitment (Sheridan et al., 2012). Although this study measured all of the elements necessary to test the full pathway depicted in Figure 1, the small sample size precluded formal testing of mediating mechanisms.
As more complex EF and memory processes are inhibited by high levels of stress, more automatic processes of reactive learning that rely on limbic structures, such as fear conditioning, are improved (Arnsten, 2009; Blair, 2010). This relation is consistent with a less discussed aspect of the Yerkes-Dodson model (Yerkes & Dodson, 1908), which describes a linear relation between arousal and learning in more automatic systems. Thus, while high levels of stress impair functioning in aspects of higher-level cognitive control, they enhance functioning in areas of the brain that carry out more automatic responses. The amygdala is one important area for social-emotional processing which also has a high density of glucocorticoid receptors and is thus highly susceptible to the effects of stress (see Tottenham & Sheridan, 2010 for a review). In contrast to the hippocampus and the prefrontal cortex, which are involved in feedback-controlled down-regulation of the HPA axis stress response, the amygdala plays a facilitative role in activating the HPA axis, which can potentiate stress responses (Tottenham & Sheridan, 2010). The amygdala also plays a similar facilitative role in activating the release of catecholamines in response to stress which in turn decrease PFC activation and increase amygdala function (Arnsten, 2009). Moreover, chronic exposure to stress appears to up-regulate amygdala activity such that the threshold for reacting to emotional events is decreased. Thus, as high stress impairs PFC and hippocampal functioning, it increases activity in the amygdala in ways that lead the individual to appraise and respond to socio-emotional stimuli in faster, more automatic and stimulus driven -- and thus less thoughtful or task relevant -- ways.
From a life course and developmental systems perspective, this increase in fast, automatic processing, along with the corresponding decrease in slow, thoughtful processing, may be adaptive for dealing with the demands of daily stress that are so prevalent in lower SES environments. However, these changes in neurocognitive functioning may not be optimal for school and later life health outcomes (Blair, 2010; Blair & Raver, 2012). That is, growing up in contexts with high levels of unpredictability and stress may lead to greater engagement of brain areas that are important for vigilance and automatic processing, allowing the individual to quickly deal with threats that may arise at any time. This tuning of neural processes toward more automatic ways of assessing and reacting to the environment, however, may have detrimental consequences for higher-level cognitive functioning. Indeed, although not yet tested, it is possible that putting more neural resources into some tasks, for example attending to irrelevant stimuli (Stevens, et al., 2009) and up-regulating the amygdala in order to respond to emotionally salient stimuli (Gianaros et al., 2008), may make it more difficult to recruit extra neural resources to compensate for age- or skill-related performance deficits in other neurocognitive domains. Similarly, the constant activation of physiological stress responses and the heightened vigilance that it promotes can have detrimental effects on health in the long run by leading to alterations in metabolic and cardiovascular functioning. Thus, although these adaptations toward vigilance may be adaptive for children in the short run, they can have negative consequences for health and neurocognitive functioning.
Stress and Language – A Developmental Perspective on Intersecting Pathways
Although language exposure and stress represent distinct hypothesized pathways by which SES may affect different aspects of neurocognitive functioning, it is also likely that these pathways are not completely independent. For example, stress in the home may have effects on language development by decreasing the time and mental resources that parents have to engage in verbal communication and book-reading with their children. Moreover, children’s cortisol levels might influence which neural regions are effectively available to process language stimuli in the environment such that high cortisol levels may make it more difficult to process complex syntactical structures but may lead to faster processing of fear laden content. Conversely, exposure to and practice with more diverse forms of language may provide a rich opportunity to practice EF skills as more complex language requires greater use of working memory resources (Noble, Norman, & Farah, 2005; Perkins et al., 2013). Consistent with this notion, family language complexity, but not child’s own language use, has been found to be related to accuracy on an EF task and to activation in the right medial frontal gyrus, an area of the brain in which evidence for SES differences during the task had been found (Sheridan et al., 2012). Thus although the language exposure and stress pathways provide a useful framework for organizing prior research and future investigations, they are likely to be at least somewhat interdependent.
Moreover, from a developmental perspective, it is important to recognize that a child’s relationship with adult caregivers is one of the most prominent developmental contexts in early childhood and this context of parenting plays an important role in both the language exposure and stress pathways linking SES to neurocognitive functioning. The role of parenting in language exposure is readily apparent, as parents are one of the main sources of linguistic input for children. Moreover, facets of parenting such as maternal affect during interactions with children (Estrada, Arsenio, Hess, & Holloway, 1987), vocal responsivity, maternal responsivity to distress (Coates & Lewis, 1984), maternal responsiveness to children’s vocalizations (Tamis-LeMonda, Bornstein, Baumwell, & Melstein Damast, 1996), and maternal interaction style (Murray & Hornbaker, 1997) predict children’s language skills. Additionally, maternal sensitivity has been shown to mediate relations of SES to children’s receptive and expressive language skills at age 3 (Raviv, Kessenich, & Morrison, 2004). Thus, parent-child interactions provide a context for language exposure and shape children’s language development.
The stresses of poverty may lead to poorer parenting behaviors, which may in turn be a primary cause of stress for children (Blair, 2010; Blair & Raver, 2012). From a developmental perspective, infants and young children learn about their ability to exert control through the contingent and responsive interactions of supportive parents. Poor parenting behaviors can be stressful for children because inconsistent, unpredictable, and non responsive parenting behaviors may lead children to feel a lack of control over their physical, social, and emotional needs. For example, when a mother comforts her child who is crying because he sees a stranger, that child will learn that the mother can help him to regulate his emotional needs. When caregivers do not respond in a consistent manner, however, over time the child will experience a lack of control over his distress and will have more difficulty regulating his emotional needs. In the context of socioeconomic resources, when parents have to worry about a lack of physical resources, they may have less time and energy to devote to supportive parenting behaviors (Mani, Mullainathan, Shafir, & Zhao, 2013). Consistent with this hypothesis, several studies have demonstrated that poverty is associated with less supportive parenting behaviors (Blair et al., 2011; Brody & Flor, 1998; Jackson, Brooks-Gunn, Huang, & Glassman, 2000). These less supportive parenting behaviors have in turn been associated with dysregulation of children’s stress physiology as manifest by higher basal levels of cortisol (Blair et al., 2011) as well as attenuated stress reactivity (Hackman et al., 2013). Moreover, parenting behaviors have been shown to mediate relations between SES and certain neurocognitive functions. For example, in a large longitudinal study of primarily low-income, rural families, lower household income was associated with less positive parenting during a structured mother-child interaction when children were 7, 15, and 24 months of age. A lack of positive parenting was in turn related to deficits in EF in early childhood and mediated links between income and EF (Blair et al., 2011). Although much work has focused on early childhood, parenting continues to remain important in middle childhood. For example, in one study of school-age children, parental responsivity and family companionship mediated relations between SES and EF skills, including inhibitory control and working memory (Sarsour et al., 2011).
In addition to highlighting the important role of parenting in stress and language stimulation, a developmental perspective also suggests that early childhood may be a particularly sensitive time in which these mechanisms exert their effects. Income poverty early in childhood has been shown to be more closely related to achievement than is family income in adolescence (Duncan & Magnuson, 2003). Moreover, the effect of income seems to be largest for children who spend more time in poverty and who live in families that are at or below 50% of the poverty threshold (Brooks-Gunn & Duncan, 1997). Similarly, income effects are larger for children at or below the poverty line than for children in middle-class families (Duncan & Magnuson, 2003), suggesting that the stresses of material deprivation play a strong role. One possible reason for these findings is that early childhood is a time of rapid brain development and structural plasticity. For this reason, early exposures may be able to alter development on a neural level more easily and to a greater degree than later experiences can. A second possible reason for the greater importance of early childhood may have to do with the possibility for cascading effects such that early deficits may set the stage for a cascading accumulation of deficits throughout life (Masten & Cicchetti, 2010). From this perspective, early deficits in one domain will not only grow as children get older, but they will transfer to deficits in other domains. For example, early life stress that causes executive functioning deficits early on may make it more difficult for children to understand complex sentences or to follow complex sets of directions as they transition to middle school. Similarly, early language deficits have been shown to have consequences for social-emotional development (McCabe & Meller, 2004). Thus, deficits that occur early in life may be exacerbated and transferred to other domains of functioning such that improvements in SES later in life cannot compensate for or reverse the developmental trajectories.
Measuring Socioeconomic Status
Although social scientists have long been careful to consider individual components of SES separately, neuroscientists are just beginning to recognize these nuances. Traditional objective indicators of SES include income, education, and occupational status. Income is typically measured as total monthly or annual household income. Rather than ask participants to report an exact figure, some studies have asked for categorical reporting of income level. To more precisely characterize how family income relates to need, many researchers use the income-to-needs ratio (ITN), or the level of household income divided by the poverty threshold for a family of that size. Using this measure, participants are sometimes divided into groups depending on whether they are poor (ITN < 1), near-poor (ITN < 2) or non-poor (ITN >2). Education is usually coded either as the highest level of completion (e.g., high school, college) or as the total number of years completed. Occupational status can be informative; however, quantification of occupational prestige can be difficult, as it is dependent on historical time and place. In the United States, many researchers use the Hollingshead (Hollingshead, 1975) categories to classify occupations, despite the fact that this instrument is widely considered to be outdated (Duncan & Magnuson, 2003). Sociologists and the U.S. Census Bureau have developed more detailed classification systems for coding and assigning prestige scores to occupations (Entwisle & Astone, 1994). Another important objective indicator of SES that is less commonly measured is wealth, which summarizes the net worth of both liquid and illiquid financial assets that would be available after paying off any debts (Duncan & Magnuson, 2012).
Whether or not to aggregate these indicators of SES is a common question for researchers. As mentioned above, many studies have used the Hollingshead scale, which aggregates the occupation and education of parents (in the Two-Factor Index) and can also take into account marital status and employment (using the Four-Factor Index). Other common routes to creating aggregate measures are to standardize and average across indicators of income, education, and occupation or to generate a composite based on the factor loadings of those indicators. Duncan and Magnuson (Duncan & Magnuson, 2003), however, have argued that creating composite scores of SES is not well motivated as these constructs are theoretically distinct and have differential links to children’s experiences and development. In general, parental education and income seem to be more robustly associated with child development than occupational status. Parental education has been associated with both children’s academic and behavioral outcomes, whereas income has been more strongly associated with academic outcomes (Duncan & Magnuson, 2003). Moreover, from a policy perspective, these different aspects of SES may be sensitive to different interventions. The extent to which these different aspects of SES may be differentially associated with specific neurocognitive outcomes, however, is an open question for future research.
More recently, measures of subjective indicators of SES have been introduced as well. The Macarthur Scale of Subjective Social Status (Adler, Epel, Castellazzo, & Ickovics, 2000) is one popular measure in which participants are given a drawing of a ladder and asked to mark where they think they would stand in relation to others of a particular group. In one version of the ladder, participants are asked to think about money, education, and jobs and to rank themselves compared to others in the United States. In a second version of the ladder, participants are asked to rank themselves in their community, however they choose to define it. Different hypotheses have been set forth in terms of understanding what these ladders are capturing and why they may account for additional variance when controlling for objective indicators of SES. In one interpretation, participants are thought to be providing a more global indicator of their SES by taking into account factors such as wealth, standard of living, and financial security (Singh-Manoux, Adler, & Marmot, 2003; Singh-Manoux, Marmot, & Adler, 2005). In a second interpretation, participants are thought to be indicating independent information about their psychological perceptions of relative inequality (Adler et al., 2000). Both of these aspects may be important for understanding the ways in which lower SES translates to higher stress and differential neurocognitive outcomes. Other subjective indicators of SES including measures of perceived economic insufficiency may be particularly beneficial for assessing whether lower-income families’ feel they can provide for basic needs (Blair, Raver, Granger, Mills-Koonce, & Hibel, 2011).
Thus, at a minimum, we recommend that researchers aim to include measures of income, education, and occupation, which can be completed in just a few minutes’ time. If 5 to 10 minutes of time can be spent, however, a broader range of measures including aspects of subjective social status can be collected to give more in-depth information about participants’ SES (Duncan & Magnuson, 2003). The MacArthur Research Network on SES and Health (2000) provides a sociodemographic questionnaire that assesses multiple aspects of both subjective and objective facets of SES, which could be a productive starting point for researchers who wish to include measurement of SES. The full measure contains 11 items, but an 8 item version is also available. One word of caution regarding the income question, however, is that researchers may need to add more categories to distinguish among participants who earn above $100,000. Alternatively, having individuals report their exact income figure would alleviate this problem. Collecting multiple measures of SES and reporting significant as well as non-significant associations will also aid in comparing findings across studies. Being able to draw on findings from multiple studies will in turn greatly enhance our understanding of whether and how specific aspects of SES are related to neurobiological and behavioral aspects of neurocognitive processes and will allow us to build more detailed theoretical models of the mechanisms and the pathways by which they operate.
In addition to the question of how to measure SES, there is the important question of when to measure SES. While cross sectional research captures SES at only one point in time, SES is not a static characteristic of American families and there is good reason to believe that understanding the dynamic nature of SES is important (Duncan & Magnuson, 2003; Raver, Roy, & Pressler, 2015). In particular, income is the most volatile of the three indicators, and household income can show dramatic changes across childhood (Duncan, Yeung, Brooks-Gunn, & Smith, 1998). Differences in duration of exposure to poverty as well as in differences in the number of moves into and out of poverty can have important consequences. Evidence suggests that early exposure to poverty may have the most detrimental effects in that family income in early childhood is more predictive of achievement than is family income in adolescence (Duncan & Magnuson, 2003). Moreover, other work suggests that it is important to capture the specific amount of time that children have spent in poverty in order to better predict their neurocognitive outcomes (Evans & English, 2002; Raver et al., 2013). The dynamic nature of moves into and out of poverty may also affect neurocognitive development in ways that may not necessarily be expected. On the one hand, increases in income can lead to greater resources and better developmental outcomes. However, increases in income to levels just above the poverty level may also make families ineligible for certain services, which could possibly negate any beneficial effects of increased income. Moreover, the uncertainty faced by families with fluctuating incomes may be an important stressor that has independent effects on neurocognitive outcomes (Raver et al., 2015).
Conclusion
SES disparities in neurocognitive functioning have been shown across the domains of language, EF, memory, and social-emotional processing on both the behavioral and neurobiological levels, and SES has been shown to shape the relation between these two levels of functioning. Socioeconomic disparities in linguistic exposure and stress may explain these associations. Although behavioral research has provided evidence for the pathway linking SES to language development, much more work is needed to understand the underlying neurobiological mechanisms. Research has examined the neurobiological mechanisms by which stress may influence the function of the hippocampus, prefrontal cortex, and amygdala, but the ways in which different aspects of SES may shape stress response systems as well as the ways in which different patterns of stress responding may differentially shape specific areas of the brain remain to be elucidated. Moreover, this review has focused on the HPA axis, but SES likely influences multiple stress response systems and how these multiple systems may interact to influence functioning on a neurobiological level is not well known. Finally, much research is needed to test and understand whether the links found among the two pathways can be drawn together to demonstrate complete pathways in which stress and language exposure mediate relations from SES to neurological, and subsequently to behavioral, neurocognitive functioning. Understanding these full pathways and the ways in which they may differ for children from different socioeconomic backgrounds will have far reaching implications for basic science, intervention research, and policy settings.
Acknowledgments
The authors are grateful for funding from the Neuro-epidemiology Training Program at Columbia University (NIH/NINDS T32-NS07153), the Irving Institute for Clinical and Translational Research, and the Annie E. Casey Foundation.
References
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, white women. Health Psychology. 2000;19(6):586–592. doi: 10.1037/0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Adler NE, Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Current Directions in Psychological Science. 2003;12(4):119–123. doi: 10.1111/1467-8721.01245. [DOI] [Google Scholar]
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, Frazier JA. The NIH toolbox cognition battery: Results from a large normative developmental sample (PING) Neuropsychology. 2014;28(1):1. doi: 10.1037/neu0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Through the looking glass: Differential noradenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7(1–2):133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Li BM. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology. 2011;23(3):881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. Stress and the development of self-regulation in context. Child Development Perspectives. 2010;4(3):181–188. doi: 10.1111/j.1750-8606.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D, Peters Razza R. Cortisol reactivity is positively related to executive function in preschool children attending head start. Child Development. 2005;76(3):554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT the FLP Investigators. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82(6):1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist. 2012;67(4):309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger DA, Mills-Koonce R, Hibel LC. Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology. 2011;23(3):845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowey JA. Socioeconomic status differences in preschool phonological sensitivity and first-grade reading achievement. Journal of Educational Psychology. 1995;87(3):476. [Google Scholar]
- Brito NH, Noble KG. Socioeconomic status and structural brain development. Frontiers in Neuroscience. 2014;8:1–12. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Flor DL. Maternal resources, parenting practices, and child competence in rural, single-parent african american families. Child Development. 1998;69(3):803–816. doi: 10.1111/j.1467-8624.1998.tb06244.x. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan GJ. The effects of poverty on children. The Future of Children. 1997;7(2):55–71. doi: 10.2307/1602387. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Home observation for measurement of the environment. University of Arkansas; Little Rock Little Rock: 1984. [Google Scholar]
- Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science. 2010;21(1):31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychology. 2006;25(6):704–714. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Coates DL, Lewis M. Early mother-infant interaction and infant cognitive status as predictors of school performance and cognitive behavior in six-year-olds. Child Development. 1984;55(4):1219–1230. doi: 10.2307/1129991. [DOI] [PubMed] [Google Scholar]
- Combs-Orme T, Cain DS. Poverty and the daily lives of infants. Journal of Children & Poverty. 2006;12(1):1–20. doi: 10.1080/10796120500502052. [DOI] [Google Scholar]
- Conboy BT, Kuhl PK. Impact of second-language experience in infancy: Brain measures of first- and second-language speech perception. Developmental Science. 2011;14(2):242–248. doi: 10.1111/j.1467-7687.2010.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernochowski D, Fabiani M, Friedman D. Use it or lose it? SES mitigates age-related decline in a recency/recognition task. Neurobiology of Aging. 2008;29(6):945–958. doi: 10.1016/j.neurobiolaging.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angiulli A, Herdman A, Stapells D, Hertzman C. Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22(3):293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22(10):422–426. doi: 10.1016/S0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Magnuson K. Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(3):377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Magnuson KA. Off with hollingshead: Socioeconomic resources, parenting, and child development. Socioeconomic Status, Parenting, and Child Development. 2003:83–106. [Google Scholar]
- Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How much does childhood poverty affect the life chances of children? American Sociological Review. 1998;63(3):406–423. doi: 10.2307/2657556. [DOI] [Google Scholar]
- Entwisle DR, Astone NM. Some practical guidelines for measuring youth’s race/ethnicity and socioeconomic status. Child Development. 1994;65(6):1521–1540. [Google Scholar]
- Estrada P, Arsenio WF, Hess RD, Holloway SD. Affective quality of the mother–child relationship: Longitudinal consequences for children’s school-relevant cognitive functioning. Developmental Psychology. 1987;23(2):210–215. doi: 10.1037/0012-1649.23.2.210. [DOI] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Gonnella C, Marcynyszyn LA, Gentile L, Salpekar N. The role of chaos in poverty and children’s socioemotional adjustment. Psychological Science. 2005;16(7):560–565. doi: 10.1111/j.0956-7976.2005.01575.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Environmental stimulation, parental nurturance and cognitive development in humans. Developmental Science. 2008;11(5):793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EKk, Hurt H. Childhood poverty: Specific associations with neurocognitive development. Brain Research. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, Critchley HD, Manuck SB, Hariri AR. Perigenual anterior cingulate morphology covaries with perceived social standing. Social Cognitive and Affective Neuroscience. 2007;2(3):161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein J, Hariri A, Sheu L, Manuck S, Matthews K, Cohen S. Potential neural embedding of parental social standing. Social Cognitive and Affective Neuroscience. 2008;3(2):91–6. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck S, Sheu L, Kuan DCH, Votruba Drzal E, Craig A, Hariri A. Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex. 2011;21(4):896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Frontiers in Human Neuroscience. 2012;6(277):1–11. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Kobrin L, Hurt H, Farah MJ. Selective impact of early parental responsivity on adolescent stress reactivity. PloS One. 2013;8(3):e58250–e58250. doi: 10.1371/journal.pone.0058250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PloS One. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley TR. Meaningful differences in the everyday experience of young american children. Baltimore, MD: ERIC; 1995. [Google Scholar]
- Herrmann D, Guadagno MA. Memory performance and socio-economic status. Applied Cognitive Psychology. 1997;11(2):113–120. doi: 10.1002/(SICI)1099-0720(199704)11:2<113::AID-ACP424>3.0.CO;2-F. [DOI] [Google Scholar]
- Hoff E. The specificity of environmental influence: Socioeconomic status affects early vocabulary development via maternal speech. Child Development. 2003;74(5):1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Hoff E. How social contexts support and shape language development. Developmental Review. 2006;26(1):55–88. doi: 10.1016/j.dr.2005.11.002. [DOI] [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975 Unpublished manuscript. [Google Scholar]
- Huttenlocher J, Vasilyeva M, Cymerman E, Levine S. Language input and child syntax. Cognitive Psychology. 2002;45(3):337–374. doi: 10.1016/S0010-0285(02)00500-5. [DOI] [PubMed] [Google Scholar]
- Jackson AP, Brooks-Gunn J, Huang C, Glassman M. Single mothers in Low-Wage jobs: Financial strain, parenting, and preschoolers’ outcomes. Child Development. 2000;71(5):1409–1423. doi: 10.1111/1467-8624.00236. [DOI] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, Ramus F. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8):e42486. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Liberzon I, Phan KL. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: A overview. Neuropsychobiology. 1989;22(3):150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience. 2009;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Kliewer W, Reid-Quiñones K, Shields BJ, Foutz L. Multiple risks, emotion regulation skill, and cortisol in low-income african american youth: A prospective study. Journal of Black Psychology. 2009;35(1):24–43. doi: 10.1177/0095798408323355. [DOI] [Google Scholar]
- Kraft AJ, Luecken LJ. Childhood parental divorce and cortisol in young adulthood: Evidence for mediation by family income. Psychoneuroendocrinology. 2009;34(9):1363–1369. doi: 10.1016/j.psyneuen.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D. The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry. 2000;48(10):976–980. doi: 10.1016/S0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13(3):653–676. doi: 10.1017/S0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behavioural Brain Research. 2001;127(1–2):137–158. doi: 10.1016/S0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- MacArthur Research Network on Socioeconomic Status and Health. John D. and Catherine T. MacArthur Research Network on Socioeconomic Status and Health Sociodemographic Questionnaire. 2000 Retrieved from http://www.macses.ucsf.edu/Research/SocialEnvironment/notebook/measure.html.
- Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341(6149):976–980. doi: 10.1126/science.1238041. [DOI] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and Psychopathology. 2010;22(03):491–495. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- McCabe PC, Meller PJ. The relationship between language and social competence: How language impairment affects social growth. Psychology in the Schools. 2004;41(3):313–321. doi: 10.1002/pits.10161. [DOI] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mills DL, Prat C, Zangl R, Stager CL, Neville HJ, Werker JF. Language experience and the organization of brain activity to phonetically similar words: ERP evidence from 14-and 20-month-olds. Journal of Cognitive Neuroscience. 2004;16(8):1452–1464. doi: 10.1162/0898929042304697. [DOI] [PubMed] [Google Scholar]
- Molfese VJ, Molfese DL. Environmental and social influences on reading skills as indexed by brain and behavioral responses. Annals of Dyslexia. 2002;52:121–137. doi: 10.1007/s11881-002-0009-6. [DOI] [Google Scholar]
- Murray AD, Hornbaker AV. Maternal directive and facilitative interaction styles: Associations with language and cognitive development of low risk and high risk toddlers. Development and Psychopathology. 1997;9(03):507–516. doi: 10.1017/S0954579497001272. [DOI] [PubMed] [Google Scholar]
- Noble KG, Farah MJ. Neurocognitive consequences of socioeconomic disparities: The intersection of cognitive neuroscience and public health. Developmental Science. 2013;16(5):639–640. doi: 10.1111/desc.12076. [DOI] [PubMed] [Google Scholar]
- Noble KG, Farah MJ, McCandliss BD. Socioeconomic background modulates cognition–achievement relationships in reading. Cognitive Development. 2006;21(3):349–368. doi: 10.1016/j.cogdev.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental Science. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Tottenham N, Casey B. Neuroscience perspectives on disparities in school readiness and cognitive achievement. The Future of Children. 2005;15(1):71–89. doi: 10.1353/foc.2005.0006. [DOI] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science. 2006;9(6):642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, Brickman AM. Hippocampal volume varies with educational attainment across the lifespan. Frontiers in Human Neuroscience. 2012;6(307) doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nature Neuroscience. 2015a doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Engelhardt LE, Brito NH, Mack LJ, Nail EJ, Angal J, Barr R, Fifer WP, Elliot AJ PASS Network. Socioeconomic disparities in neurocognitive development in the first two years of life. Developmental Psychobiology. 2015b doi: 10.1002/dev.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SC, Finegood ED, Swain JE. Poverty and language development: Roles of parenting and stress. Innovations in Clinical Neuroscience. 2013;10(4):10–19. [PMC free article] [PubMed] [Google Scholar]
- Propper CB, Holochwost SJ. The influence of proximal risk on the early development of the autonomic nervous system. Developmental Review. 2013;33(3):151–167. doi: 10.1016/j.dr.2013.05.001. [DOI] [Google Scholar]
- Raizada RDS, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Frontiers in Human Neuroscience. 2010;4(3):1–11. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada RDS, Richards TL, Meltzoff A, Kuhl PK. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuro Image. 2008;40(3):1392–1401. doi: 10.1016/j.neuroimage.2008.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Blair C, Willoughby MT. Poverty as a predictor of 4-year-olds’ executive function: New perspectives on models of differential susceptibility. Developmental Psychology. 2013;49(2):292–304. doi: 10.1037/a0028343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Roy AL, Pressler E. Struggling to stay afloat: Dynamic models of poverty-related adversity and child outcomes. In: Amato PR, Booth A, McHale SM, Van Hook J, editors. Families in an era of increasing inequality. Springer; 2015. pp. 201–212. [Google Scholar]
- Raviv T, Kessenich M, Morrison FJ. A mediational model of the association between socioeconomic status and three-year-old language abilities: The role of parenting factors. Early Childhood Research Quarterly. 2004;19(4):528–547. doi: 10.1016/j.ecresq.2004.10.007. [DOI] [Google Scholar]
- Rodriguez ET, Tamis-LeMonda CS. Trajectories of the home learning environment across the first 5 years: Associations with children’s vocabulary and literacy skills at prekindergarten. Child Development. 2011;82(4):1058–1075. doi: 10.1111/j.1467-8624.2011.01614.x. [DOI] [PubMed] [Google Scholar]
- Rowe ML, Goldin-Meadow S. Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science (New York, NY) 2009;323(5916):951–953. doi: 10.1126/science.1167025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, Boyce WT. Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. Journal of the International Neuropsychological Society. 2011;17(1):120–132. doi: 10.1017/S1355617710001335. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Fletcher JM. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54(1):25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Developmental Science. 2013;16(5):665–675. doi: 10.1111/desc.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Sarsour K, Jutte D, D’Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PloS One. 2012;7(4):e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: Its determinants and its association with measures of ill-health in the whitehall II study. Social Science & Medicine. 2003;56(6):1321–1333. doi: 10.1016/S0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine. 2005;67(6):855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Richards M, Marmot M. Socioeconomic position across the lifecourse: How does it relate to cognitive function in mid-life? Annals of Epidemiology. 2005;15(8):572–578. doi: 10.1016/j.annepidem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Sirin SR. Socioeconomic status and academic achievement: A meta-analytic review of research. Review of Educational Research. 2005;75:417–453. doi: 10.3102/00346543075003417. [DOI] [Google Scholar]
- Son S, Morrison FJ. The nature and impact of changes in home learning environment on development of language and academic skills in preschool children. Developmental Psychology. 2010;46(5):1103–1118. doi: 10.1037/a0020065. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Lauinger B, Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: An event-related brain potential study. Developmental Science. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: Association with the fagerstrom test for nicotine dependence but not cigarettes smoked per day. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2008;10(10):1571–1575. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, Baumwell L, Melstein Damast A. Responsive parenting in the second year: Specific influences on children’s language and play. Early Development and Parenting. 1996;5(4):173–183. doi: 10.1002/(SICI)1099-0917(199612)5:4<173::AID-EDP131>3.0.CO;2-V. [DOI] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, Johnson MH, Kushnerenko E. Socioeconomic status and functional brain development—Associations in early infancy. Developmental Science. 2013;16(5):676–687. doi: 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3(68):1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, Noble KG, Blair C. Socioeconomic status, subjective social status, and perceived stress: Associations with stress physiology and executive functioning. Behavioral Medicine. doi: 10.1080/08964289.2015.1024604. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18(5):459–482. doi: 10.1002/cne.920180503. [DOI] [Google Scholar]
- Yum YN, Midgley KJ, Holcomb PJ, Grainger J. An ERP study on initial second language vocabulary learning. Psychophysiology. 2014;51(4):364–373. doi: 10.1111/psyp.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]