Abstract
Objective
We previously reported the interim effects in a per protocol analysis of a randomized controlled trial of an innovative neuroscience-informed computerized cognitive training approach in schizophrenia. Here we report the effects of training on behavioral outcome measures in our final sample using an intent-to-treat analysis. We also report the effects on serum brain-derived neurotrophic factor (BDNF).
Method
Eighty-seven clinically stable participants with schizophrenia were randomly assigned to either targeted auditory training (AT, N=46) or a computer games control condition (CG, N=41). Participants were assessed on neurocognition, symptoms and functional outcome at baseline and after 50 hours of intervention delivered over 10 weeks. Serum BDNF was assessed at baseline, at 2 weeks, and at 10 weeks.
Results
After the intervention, AT participants showed significant gains in global cognition, speed of processing, verbal learning, and verbal memory, relative to CG participants, with no changes in symptoms or functioning. At baseline, schizophrenia participants had significantly lower-than-normal serum BDNF. AT participants showed a significant increase in serum BDNF compared to CG participants, and “normalized” levels by post training.
Conclusions
Participants with chronic schizophrenia made significant cognitive gains after 50 hours of intensive computerized training delivered as a stand-alone treatment, but no improvement in symptoms or functioning. Serum BDNF levels were significantly increased, and may serve as a peripheral biomarker for the effects of training. Future research must focus on: 1) Methods of integrating cognitive training with psychosocial treatments; 2) A deeper understanding of underlying neurophysiology in order to enhance critical mechanisms of action.
Keywords: Schizophrenia, Cognitive remediation, Neuroplasticity, BDNF
1. Introduction
We previously reported our interim findings from a randomized clinical trial of targeted cognitive training of auditory processing and auditory/verbal working memory in schizophrenia. Using a per protocol analysis on data from 55 participants, we found significant cognitive gains after 50 hours of auditory cognitive training as a stand-alone treatment relative to an active control condition of computer games (Fisher et al., 2009), and a significant increase in serum brain-derived neurotrophic factor (BDNF) relative to the control condition (Vinogradov et al., 2009). Here, in our final sample of 87 participants, we report the behavioral effects of the training using an intent-to-treat analysis, as well as the effects on serum BDNF.
The study of cognitive remediation in schizophrenia has grown substantially over the past 20 years. Meta-analytic studies indicate that a vast array of cognitive training approaches have a small to medium effect on cognition, on functioning, and on durability of effects at follow-up, and a small but non-durable effect on symptoms (McGurk et al., 2007, Wykes et al., 2011). Wykes et al. (2011) found a mean global cognition effect size of 0.45, with heterogeneity of effect sizes in global cognition, speed of processing, and reasoning and problem solving; however, the meta-analysis did not find that type of training, participant characteristics, or trial quality could account for this heterogeneity in cognitive outcomes. It does appear that a wide range of approaches providing various forms of cognitive stimulation for variable amounts of time and treatment intensity all have a modest beneficial effect in schizophrenia. However, it is difficult to draw too many definitive conclusions from the currently available data from previous cognitive remediation studies, given the wide disparity in assessment measures, study designs, patient samples, cognitive remediation methodologies, and control groups used. Further, differences in methodology and design – including trials that combine cognitive remediation with other treatments – make it difficult to discern which components of improved cognition or functioning are the result of the cognitive training per se and which may be due to other study features, such as increased staff contact, computer exposure, or the provision of strategy coaching or other forms of skills training.
In the present study, we performed a double-blind randomized controlled trial of an innovative, neuroscience-informed approach to cognitive training in schizophrenia, delivered as a stand-alone treatment relative to a computer games control condition. The primary goal of the computerized exercises is to train the individual to become more efficient in the early processing of auditory and verbal information, and to increase auditory working memory capacity. This approach is based on the large body of research that demonstrates impairments in early sensory processing as well as associated frontally-mediated cognitions in schizophrenia (e.g., Adcock et al., 2009, Kasai et al., 2002, Ragland et al., 2004, Ragland et al., 2007). We posed two questions: 1) Using an intent-to-treat analysis in our final sample of 87 participants, do we replicate our earlier interim findings on the significant cognitive effects of 50 hours of training? 2) What is the effect of training on serum BDNF in our final sample?
Brain-derived neurotrophic factor (BDNF) plays a critical role in neurodevelopment, neuronal function, and neural plasticity. Schizophrenia may be related in part to decreases in normal BDNF functioning (Buckley et al., 2007, Carlino et al., 2012). Meta-analyses indicate that blood BDNF levels are significantly lower in schizophrenia relative to healthy controls (Green et al., 2011), and that peripheral BDNF and cognition are positively associated in schizophrenia (Ahmed et al., 2015, Carlino et al., 2012), however there is considerable heterogeneity across studies.
While the relationship between peripheral and central BDNF remains speculative, peripheral BDNF is hypothesized to reflect central BDNF based on evidence that BDNF crosses the blood-brain barrier (Pan et al., 1998), and based on findings of significant associations between peripheral and central BDNF levels. For example, Karege et al., 2002) found a strong correlation between serum and cortical BDNF levels in rats. In healthy individuals, BDNF serum concentration showed an association with in vivo level of cerebral N-asetylaspartate – a marker of neuronal integrity (Lang et al., 2007). In a study of drug-naïve patients with first-episode psychosis, plasma BDNF level and BDNF level in cerebrospinal fluid were significantly associated (Pillai et al., 2010).
We previously found a significant increase in serum BDNF at two weeks and 10 weeks of training relative to the computer games control condition (Vinogradov et al., 2009). In the active condition, participants’ BDNF level increased to that of a healthy comparison sample by post-training, whereas the control group showed no change. A deeper understanding of the role of BDNF in cognitive enhancement in schizophrenia will likely provide important insights for the design of future treatments.
2. Methods
2.1. Participants
We describe below our final sample of 87 participants who participated in the trial (ClinicalTrials.gov Identifier: NCT00312962). Clinically stable, chronically ill, volunteer schizophrenia participants were recruited from mental health treatment settings in the community. All participants gave written informed consent and underwent a series of baseline clinical and cognitive assessments. Participants were stratified by age, education, gender, and symptom severity, and randomly assigned to either the neuroplasticity-based auditory cognitive training (AT) condition or a control condition of engaging commercial computer games (CG). Participants were receiving case management in the community but were not enrolled in any psychiatric rehabilitation program, and reported no prior cognitive remediation treatment. Participants remained on stable doses of medications during the study, defined as no change in dosage greater than 10%. All participants received nominal payment for each successful day and week of participation, which was contingent on attendance only.
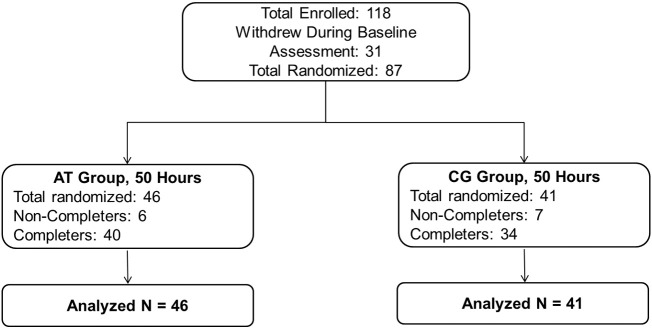
A CONSORT diagram of enrollment and allocation is shown in Fig. 1. Demographic characteristics and medication regimens of the participant groups are presented in Table 1, Table 2.
Fig. 1.
CONSORT diagram of auditory training participants (AT) and computer games control participants (CG).
Table 1.
Demographics of auditory training participants (AT) and computer games control participants (CG).
| AT (N=46) |
CG (N=41) |
||
|---|---|---|---|
| Mean (SD) | Mean (SD) | T-test (p-value) | |
| Male/Femalea | 34/12 | 29/12 | 0.11 (0.74) |
| Age | 40.70 (11.81) | 43.20 (10.62) | 1.03 (0.31) |
| Education | 13.24 (2.25) | 13.32 (1.93) | 0.17 (0.86) |
| WASI IQ | 101.11 (16.69) | 103.58 (16.24) | 0.69 (0.49) |
| PANSS Totalb | 73.41 (20.59) | 73.95 (15.85) | 0.14 (0.89) |
| QLS Average Item Ratingc | 3.10 (1.17) | 2.93 (0.95) | − 0.74 (0.46) |
| Hours of Training | 44.13 (12.34) | 39.37 (15.06) | − 1.62 (0.11) |
chi-Square results.
Positive and Negative Syndrome Scale.
Quality of Life Scale-Abbreviated.
Table 2.
Medication regimens of study participants.
| AT (N=46) | CG (N=41) | Total | Test Statistic | p value | |
|---|---|---|---|---|---|
| Antipsychotic Medicationa | |||||
| 1st Generation (N) | 2 | 5 | 7 | X2(1) = 1.80 | 0.18 |
| 2nd Generation (N) | 33 | 29 | 62 | X2(1) = 0.01 | 0.92 |
| Multiple (N) | 5 | 5 | 10 | X2(1) = 0.04 | 0.85 |
| No Antipsychotic (N) | 6 | 2 | 8 | X2(1) = 1.73 | 0.19 |
| Other Psychiatric Medication | |||||
| Antidepressants or | 24 | 20 | 44 | X2(1) = 1.00 | 0.75 |
| Mood Stabilizers (N) | |||||
| Benzodiazepines (N) | 9 | 12 | 21 | X2(1) = 1.12 | 0.29 |
| Other Medication Measures | |||||
| Chlorpromazine Equivalentsb | 346.20 (249.47) | 397.90 (297.49) | t(85) = 0.88 | 0.38 |
AT, auditory training group; CG, computer games control group.
1st Generation Antipsychotic medication = chlorpromazine, haloperidol, perphenazine, thioridazine, thiothixene.
2nd Generation Antipsychotic medication = aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone.
Mean and SD of Chlorpromazine Equivalents (Andreasen et al., 2010).
2.2. Auditory cognitive training exercises
Auditory training (AT) was provided by software developed by PositScience, Inc. Participants were driven to make progressively more accurate distinctions about the spectro-temporal fine-structure of auditory stimuli and speech under conditions of increasing working memory load (i.e. increasing number of stimuli, and decreasing inter-stimulus intervals and duration of stimulus presentation). Stimuli across the exercises spanned the acoustic and organizational structure of speech, from very simple acoustic stimuli and tasks (e.g., time order judgments of rapidly successive frequency modulated sweeps) to the complex manipulations of continuous speech (e.g., narrative memory). The exercises were continuously adaptive in that they first established the precise parameters within each stimulus set required for an individual participant to maintain 80% correct performance; once that threshold was determined, task difficulty increased or decreased systematically and parametrically as performance improved or declined. In all exercises, correct performance was heavily rewarded in a game-like fashion: each correct response was followed by novel and amusing visual and auditory embellishments as well as the accumulation of points. After several correct responses, a longer and more elaborate animation was provided.
2.3. Computer games control condition
The computer games (CG) condition was designed to control for the effects of computer exposure, contact with research personnel, and monetary payments. Participants in the CG condition came to the lab five days a week, one hour per day, and were monitored by staff in the same manner as AT participants. CG participants rotated through a series of 16 different enjoyable commercially available computer games (e.g., visuospatial puzzle games, clue-gathering mystery games) playing 4–5 games on any given day.
2.4. Assessments
The Positive and Negative Syndrome Scale (PANSS, Kay et al., 1987), an abbreviated version of the Quality of Life Scale (QLS, Bilker et al., 2003, Heinrichs et al., 1984), and MATRICS recommended cognitive measures (Nuechterlein and Green, 2006) were administered at baseline and after training. For problem solving, the BACS Tower of London (Keefe et al., 2004) was used in place of the NAB Mazes. At the time this study was initiated, the MCCB battery was not yet available but the list of recommended measures for the MCCB Beta Version were available on the MATRICS website (http://www.matrics.ucla.edu). We obtained the MATRICS recommended measures from test publishers, and converted raw scores to z-scores using normative data, stratified by age, published by the test authors. All measures were distinct and independent from tasks practiced during training. Alternate forms of tests were administered and counterbalanced for tests sensitive to practice effects.
At study entry, each participant received a standardized diagnostic and clinical evaluation performed by research personnel trained in research diagnostic techniques. Evaluations included the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 2002), as well as review of clinical records and interview with patient informants (e.g., psychiatrists, therapists, social workers). All participants in this study had a diagnosis of schizophrenia or schizoaffective disorder. Research staff who conducted neurocognitive testing or PANSS and QLS interviews first completed extensive training on testing/interviewing and scoring criteria of individual items (e.g., scoring videotaped sessions, observation of sessions conducted by experienced staff, and participating in mock sessions). In our laboratory, intra-class correlation coefficients (ICC) are greater than 0.85 for the PANSS and QLS Total and subscale scores. Participants and assessment personnel were blind to group assignment. All neurocognitive tests were scored and re-scored by a second staff member blind to the first scoring.
A subset of participants rated their level of interest/enjoyment in the computer games and cognitive training exercises using the 7-item subscale of Interest/Enjoyment from the Intrinsic Motivation Inventory – a 1–7 Likert scale, with higher scores corresponding to greater interest/enjoyment (Deci EL et al., 1994, Ryan et al., 1991). CG participants rated the computer games as slightly more enjoyable relative to AT participants (AT M = 4.44, SD = 1.61, CG M = 5.38, SD = 1.10, t(35) = 2.06, p = 0.05).
2.5. Measurement of serum BDNF levels
Blood samples were drawn at baseline, after 10 hours of training (2 weeks), and at the end of the intervention in 70 study participants (AT N=37, CG N=33). Blood samples were drawn at baseline only in a sample of healthy comparison participants matched for age, sex, body mass index (BMI), smoking history, and education. All samples were drawn in the early afternoon (~ 1 PM + 1 hour). Complete BDNF analytic procedures are described in Vinogradov et al. (2009).
2.6. Statistical analyses
All variables were screened and normally distributed after winsorising of outlying values. In order to answer Question 1, an intent-to-treat analysis was conducted with last-observation-carried-forward. Repeated Measures ANOVA was used to compare the participant groups on the change in the PANSS and QLS Total and subscale scores and in 8 cognitive domains (listed in Table 3). Composite and domain scores were computed as the average z-score across all measures defining the composite or cognitive domain. Effect sizes (Cohen’s d) were calculated using the AT and CG change scores and the pooled standard deviation.
Table 3.
Intent to treat analysis of cognitive and clinical outcomes in auditory training participants (AT) and computer games control participants (CG).
| Outcome Measuresa |
AT (N=46) |
CG (N=41) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Post |
Baseline |
Post |
Effect |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Fb | p | Size | |
| Global Cognition | − 1.10 | 0.73 | − 0.84 | 0.77 | − 0.88 | 0.77 | − 0.89 | 0.75 | 9.83 | < 0.01 | 0.74 |
| Speed of Processing | − 0.73 | 0.69 | − 0.50 | 0.64 | − 0.60 | 0.80 | − 0.64 | 0.86 | 5.01 | 0.03 | 0.51 |
| Working Memory | − 0.74 | 1.03 | − 0.49 | 1.07 | − 0.61 | 1.12 | − 0.46 | 1.14 | 0.67 | 0.42 | 0.16 |
| Verbal Learningc | − 2.39 | 1.26 | − 2.01 | 1.32 | − 1.96 | 1.10 | − 2.30 | 1.20 | 10.58 | < 0.01 | 0.73 |
| Verbal Memoryc | − 2.23 | 1.29 | − 1.66 | 1.40 | − 1.73 | 1.35 | − 2.14 | 1.35 | 13.84 | < 0.01 | 0.85 |
| Visual Learning | − 1.50 | 1.24 | − 1.15 | 1.45 | − 1.12 | 1.39 | − 1.03 | 1.47 | 0.59 | 0.45 | 0.21 |
| Visual Memory | − 1.14 | 1.62 | − 1.09 | 1.66 | − 0.90 | 1.72 | − 0.67 | 1.68 | 0.99 | 0.32 | − 0.14 |
| Problem Solving | − 0.09 | 0.95 | 0.01 | 0.76 | − 0.02 | 0.84 | 0.10 | 0.97 | 0.46 | 0.50 | − 0.09 |
| PANSS Totald | 73.41 | 20.59 | 73.15 | 19.36 | 73.95 | 15.85 | 71.76 | 16.50 | 1.08 | 0.30 | 0.18 |
| QLS Average Item Rating e | 3.10 | 1.17 | 3.16 | 1.03 | 2.93 | 0.95 | 3.17 | 0.91 | 1.86 | 0.18 | − 0.25 |
Speed of processing (symbol coding; category fluency; trails a); working memory (letter-number span; WMS-III spatial span); verbal learning (HVLT trials 1–3), verbal memory (HVLT delayed recall); visual learning (BVMT trials 1–3), visual memory (BVMT delayed recall); problem solving (BACS tower of London); and global cognition (composite score of all measures).
Repeated measures ANCOVA, condition-by-time interaction, controlling for hours of training.
Group differences remained significant with baseline entered as a covariate (p<0.01).
Positive and negative syndrome scale.
Quality of life scale-abbreviated.
Independent Samples t-test tested for group differences in baseline BDNF between the healthy comparison group and participants with schizophrenia. Repeated Measures ANOVA was used to compare the AT and CG groups on the change in BDNF. Post hoc comparisons tested for group differences in the change in BDNF from baseline to 2 weeks and from baseline to post training. Pearson correlations tested if the change in BDNF was associated with the change in cognitive outcomes or QLS measures.
3. Results
3.1. Demographic variables and medication regimens
There were no significant group differences in demographic variables (Table 1). There was a non-significant group difference in total hours of training (p=0.11, Table 1). All group differences in medication regimens were non-significant (Table 2).
3.2. Cognitive outcomes
All group differences in baseline cognitive performance were non-significant. Group differences in baseline verbal learning and verbal memory were at trend level. The analyses were conducted with and without co-varying for hours of training and baseline verbal learning and verbal memory. Repeated Measures ANCOVA omnibus F-tests showed significant condition-by-time interactions for global cognition, speed of processing, verbal learning, and verbal memory (Table 3). Effect sizes were medium to large for global cognition, verbal learning, and verbal memory (0.72 < d < .86) and in the medium range for speed of processing (d = .51) (Table 3). All main effects of time were non-significant and the results were the same with and without co-varying for hours of training and baseline verbal learning and verbal memory.
3.3. Clinical and functional outcomes
There were no significant differences between the groups in PANSS and QLS ratings at baseline (Table 1). Repeated Measures ANCOVA omnibus F-tests revealed no significant condition-by-time interactions for the PANSS and QLS Total (Table 3) or the PANSS and QLS subscales. Main effects of time were not significant with hours of training entered as a covariate. Without co-varying for hours of training, main effects of time were evident on the PANSS Positive Symptom subscale (F (1,85) = 6.04, p = 0.02), QLS Intrapsychic Foundations (F (1,85) = 4.32, p = 0.04), QLS Environmental Engagements (F (1,85) = 4.60, p = 0.04), and the QLS Total (F (1,85) = 4.38, p = 0.04).
3.4. Serum BDNF levels over the course of cognitive training
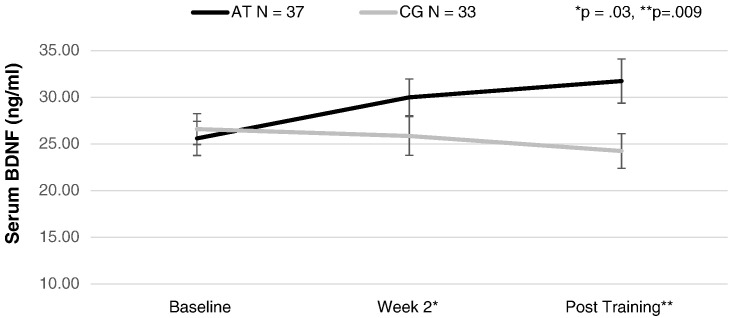
Repeated-measures ANCOVA revealed a significant difference between the AT and CG schizophrenia groups in BDNF change from baseline, to Week 2, to post training, (F (2,66) = 4.01, p = .02) (Fig. 2). Post hoc contrasts revealed that the AT and CG groups differed significantly in BDNF serum level from baseline to Week 2, (F(1,67) = 5.06, p = .03), and from baseline to post training, (F(1,67) = 7.28, p = .009). Effect sizes of the change in BDNF were in the medium range from baseline to Week 2 (d = 0.55) and from baseline to post training (d = .64). The results were the same without co-varying for hours of training.
Fig. 2.
Serum brain-derived Neurotrophic factor (BDNF) levels (ng/ml) at baseline, 2 weeks of training, and post training in auditory training participants (AT) and computer games control participants (CG).
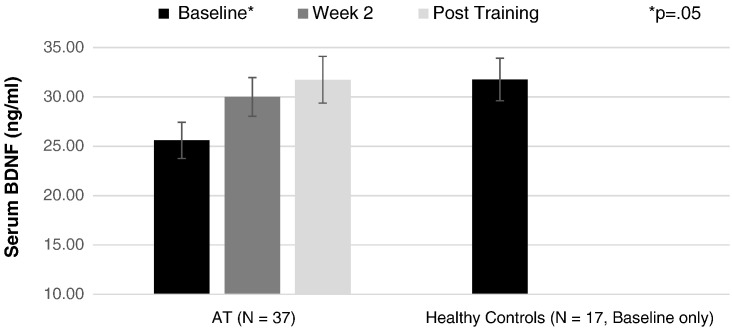
Participants with schizophrenia and healthy control participants showed a significant difference in serum BDNF level at baseline (t (85) = 2.10, p = 0.04). AT and healthy control participants also differed significantly in baseline serum BDNF level (t (52) = 2.01, p = 0.05). By post-training, serum BDNF level of AT participants was comparable to the healthy control sample (Fig. 3). There was no significant association between change in BDNF and change in any of the cognitive outcomes or QLS measures (Supplemental Table 1).
Fig. 3.
Serum brain-derived Neurotrophic factor (BDNF) levels (ng/ml) at baseline, 2 weeks of training, and post training in auditory training participants (AT) and at baseline in healthy control participants.
4. Discussion
4.1. Cognitive outcomes
Using an intent-to-treat analysis, our results indicate that 50 hours of neuroscience-informed cognitive training exercises that target early auditory perceptual processes drive medium to large gains in global cognition, speed of processing, verbal learning, and verbal memory. These positive effects are consistent with our per protocol interim report, (Fisher et al., 2009), with one exception: in our final ITT analysis, AT participants also show significant gains in speed of processing relative to CG participants. Our results are also similar to the results from our trial in participants with recent-onset schizophrenia (Fisher et al., 2015): AT participants showed significant gains in global cognition, verbal memory, and problem solving relative to a computer games control condition.
The medium to large effect sizes in verbal learning and verbal memory are also strikingly similar to two other studies that have tested the effects of this auditory training module in schizophrenia. In a multi-site feasibility study, a moderate to large effect size in verbal learning was evident after 20 hours of auditory training and weekly “bridging groups” relative to a computer games control condition and weekly healthy lifestyles groups (Keefe et al., 2012). By 40 hours of training, the effect size was in the small to medium range (d~.4), however the between-group difference was no longer significant. The authors note that 9 out of the 25 participants did not complete all 40 hours of training which may account for the reduced efficacy at post-training. Popov et al. (2011) also found medium to large effect sizes on verbal cognitive measures after 20 hours of auditory training among inpatients with schizophrenia relative to patients who completed a similar number of hours of Cogpack exercises (see Fig. 3 in Popov et al., 2011). The robust effects on verbal learning measures provide confirmatory evidence of the targeted nature of the training on auditory and verbal processing systems (Adcock et al., 2009, Dale et al., 2010).
Two studies of the same auditory training module did not show significant positive training effects. In a single arm, multi-site trial of individuals with schizophrenia, Murthy et al. (2012) did not find improvements on the CogState battery; this battery was administered 3 times prior to training, with the third set of battery measures used as “baseline”. Interestingly, in a post-hoc analysis, a subgroup of participants who showed improvement in Cogstate performance across all 3 baseline timepoints (called “learners”) also showed gains in auditory processing speed as a result of training, as well as significant improvement in the CogState cognitive composite score at the end of training compared to baseline. “Non-learners” showed a slight improvement on Cogstate performance between the first and second baseline assessments, but no improvement on the third baseline Cogstate, no improvement in auditory processing speed after training, and no gain in cognition. These data suggest that there may be moderators (such as “learning potential” or the ability to engage the auditory training target) that mediate the response to this form of treatment. Piskulic et al. (2015) recently examined the effects of the training versus a computer games control condition in 32 individuals at clinical high risk for psychosis and found no significant group differences in cognition, symptoms, or functioning at post training. Participants were asked to complete 40 hours of training over a 10–12 week period, however only 7 of the 18 participants completed 20–40 hours of the auditory training, and there was a discrepancy in the mean number of sessions completed in each group (16 auditory training sessions compared to 24 computer game sessions). The authors note that the study was underpowered due to a large attrition rate: 61% of the auditory training participants and 50% of the control group participants discontinued.
Similar to our prior reports, participants in the computer games control condition show a decrease in verbal learning and memory. We hypothesize that the nonspecific visuospatial processing required by 10 weeks of an intensive schedule of visually engaging computer games resulted in competitive interference for limited neural resources, causing worse performance on the HVLT at the second time point. These findings require replication and further investigation, as they have important implications for individuals with schizophrenia. Surprisingly few studies have examined the effects of video game exposure on verbal memory in either healthy or cognitively impaired individuals. In a sobering study with healthy school-age children, Dworak et al. (2007) found a significant reduction in verbal memory after a single day of exposure to voluntary excessive computer game playing and television. For individuals with impaired or developmentally anomalous neural systems, the impact of exercising certain networks or functions (such as those involved in visual perception and attention, visual working memory, and visuo-motor processing) may have compensatory consequences on other neural systems, as suggested by the basic science literature (Mao et al., 2011).
4.2. Clinical and functional outcomes
Also consistent with our prior report, we found no significant group differences in the change in symptom and functional outcome ratings immediately after training. A subset of these participants completed a 6 month follow-up assessment, and we found significant associations between gains in cognition and gains in functioning at the follow-up in the training group, however the gains in symptoms and functioning at the group level were non-significant (Fisher et al., 2010). We posit that the treatment of cognitive deficits may not have an immediate direct effect on real-world functioning, but rather, may enhance one’s capacity to benefit from vocational, psychosocial, and other positive environmental learning experiences (Green et al., 2004). This hypothesis is supported by our imaging studies which indicate a significant positive relationship between training-induced enhancements of prefrontal cortical activity and better ratings on social and occupational domains of the Quality of Life Scale 6 months later (Subramaniam et al., 2012, 2014). The recent report by McGurk et al. (2015) on better occupational functioning over an 18 month period after a combination of supported employment and cognitive remediation in individuals with serious mental illness (vs. supported employment alone) also provides support for this model.
4.3. Serum brain-derived neurotrophic factor
Consistent with our interim findings (Vinogradov et al., 2009), baseline serum BDNF was significantly lower in our sample of schizophrenia subjects compared with healthy subjects matched for age, sex, smoking history, BMI, and education. Cognitive training participants showed a significant increase in serum BDNF compared with carefully matched control subjects who engaged in computer games (Fig. 2). A significant group difference was observed after only 2 weeks of training, and after 50 hours, the AT participants had achieved mean serum BDNF levels comparable to healthy subjects (Fig. 3). In contrast, control subjects who played computer games for the same amount of time showed no change in BDNF levels from baseline at either time point. These data indicate that processes related to the specific demands of the active cognitive training condition induced a sustained increase in serum BDNF, separate from the general factors of computer exposure, payment for study participation, contact with laboratory personnel, or engagement with enjoyable games.
To our knowledge, no other studies of cognitive training in schizophrenia have investigated the change in serum BDNF, while two studies have examined these effects in other patient populations. A later version of the auditory training program was tested in heart failure patients with memory loss. The participant group, who performed 40 hours of training over 8 weeks, showed a significant increase in BDNF and working memory relative to a 1-hour per week health education control condition, who showed a decrease in BDNF (Pressler et al., 2015). In a study of individuals with Parkinson’s Disease, participants completed twelve 45-minute sessions of paper and pencil exercises over one month to improve set-shifting with increasing levels of difficulty, and showed significant gains in serum BDNF and in planning, relative to a control condition of the same frequency and duration of breathing exercises and simple tasks of attention and language abilities that did not vary in difficulty across sessions (Angelucci et al., 2015). Taken together, these early data suggest that different forms of cognitive training induce neurobiological changes that may be consistent with increased BDNF signaling (with the caveat that the relationship between central and peripheral BDNF remains highly speculative). A wealth of animal research demonstrates that BDNF-signaling plays a key role in experience-dependent plasticity (e.g., Anomal et al., 2013).
Despite the increased serum BDNF levels observed in active training participants in our study, there was no relationship between change in BDNF and improved cognition – consistent with, Angelucci et al. (2015). (Pressler et al., did not test this association.) Also, the association between change in BDNF and change in functioning reported in our interim analysis was no longer significant in this final sample. Thus, the relationship of increased BDNF in relation to treatment success – at least as measured by cognitive or functional change scores – is unclear. It may be that increases in peripheral BDNF reflect a general process that occurs as a result of neurocognitive training but is not linearly associated with the outcome of training; this would be analogous to the increase in VO2 max that occurs during physical fitness conditioning but that shows no linear relationship to fitness performance. The next research step is to deepen our understanding of these plasticity-related neurophysiological processes in order to improve cognitive training success. For example, aerobic exercise has a beneficial effect on central BDNF signaling and neuroplasticity responses, and is highly likely to be a useful adjunct for cognitive training (Kimhy et al., 2015), with studies underway in this area; in addition, innovative BDNF delivery methods via transgene expression are under study in hearing and vision loss and may eventually provide insights for schizophrenia (Khalin et al., 2015).
4.4. Study limitations and conclusions
One limitation of this study is that we only have 6 month follow-up data on a subset of participants (reported in Fisher et al., 2010), and thus the durability of the effects on cognition is unknown in this larger and final sample. A second limitation is that the AT participant group rated the cognitive exercises as slightly less enjoyable relative to the CG group. While the difference between the groups was small, this has important implications for the design of cognitive training exercises for real-world treatment settings (as reflected in the high attrition rate reported by Piskulic et al. 2015). Third, there were no significant group differences in the change in symptom ratings or functional outcome, as noted earlier. Clearly, cognitive training methods will serve as but one potential treatment tool in the array of services that must be offered to people with schizophrenia.
While the evidence indicates that this intensive training approach provides cognitive benefit to patients with schizophrenia, several questions remain unanswered. Future research is needed to determine the optimal number of hours of training, and whether additional modules targeting other cognitive domains or the use of “booster sessions” are required. Finally, we must develop a deeper understanding of the underlying physiology, including the role of BDNF-associated systems and other plasticity-related mechanisms, in mediating optimal and enduring changes in cognition for people with schizophrenia.
The following is the supplementary data related to this article.
The association between change in BDNF, and changes in cognition and quality of life scale ratings.
Funding sources
The work reported here was supported by NIMH grant MH-068725, MH-68725-02, NIMH Small Business Technology Transfer grant R42 MH-073358, NIH/National Center for Research Resources University of California, San Francisco-Clinical Translational Science Institute grant UL1 RR024131, and the San Francisco VA Medical Center. Software was provided by Posit Science Corporation free of charge.
Acknowledgements
The authors gratefully acknowledge participants and their families, the support of M. Merzenich, H. Mahncke, S. Chan, P. Delahunt, and D. Tinker of Posit Science, Inc., and the assistance of Gina Poelke, Christine Holland, Alexander Genevsky, Coleman Garrett, Phillip Alexander, Virginia Powell and Rachel So.
Footnotes
ClinicalTrials.gov Identifier: NCT00312962.
References
- Adcock A., Dale C., Fisher M., Aldebot S., Genevsky A., Simpson G., Nagarajan S., Vinogradov S. When Top-down meets bottom-Up: Auditory training enhances verbal memory in schizophrenia. Schizophr. Bull. 2009;35(6):1132–1141. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.O., Mantini A.M., Fridberg D.J., Buckley P.F. Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: A meta-analysis. Psychiatry Res. 2015;226:1–13. doi: 10.1016/j.psychres.2014.12.069. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.-C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci F., Peppe A., Carlesimo G.A., Serafini F., Zabberoni S., Barban F., Shofany J., Caltagirone C., Costa A. A pilot study on the effect of cognitive training on BDNF serum levels in individuals with Parkinson's disease. Front. Hum. Neurosci. 2015;16(9):130. doi: 10.3389/fnhum.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anomal R., de Villers-Sidani E., Merzenich M.M., Panizzutti R. Manipulation of BDNF signaling modifies the experience-dependent plasticity induced by pure tone exposure during the critical period in the primary auditory cortex. PLoS One. 2013;21:8(5). doi: 10.1371/journal.pone.0064208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilker W.B., Brensinger C., Kurtz M.M., Kohler C., Gur R.C., Siegel S.J., Gur R.E. Development of an abbreviated Schizophrenia Quality of Life Scale using a new method. Neuropsychopharmacology. 2003;28:773–777. doi: 10.1038/sj.npp.1300093. [DOI] [PubMed] [Google Scholar]
- Buckley P.F., Mahadik S., Pillai A., Terry A., Jr. Neurotrophins and schizophrenia. Schizophr. Res. 2007;94:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Carlino D., De Vanna M., Tongiorgi E. Is altered BDNF biosynthesis a general feature in patients with cognitive dysfunctions? Neuroscientist. 2012;19(4):345–353. doi: 10.1177/1073858412469444. [DOI] [PubMed] [Google Scholar]
- Dale C.L., Findlay A.M., Adcock R.A., Vertinski M., Fisher M., Genevsky A., Aldebot S., Subramaniam K., Luks T.L., Simpson G.V., Nagarajan S.S., Vinogradov S. Timing is everything: Neural response dynamics during syllable processing and its relation to higher-order cognition in schizophrenia and healthy comparison subjects. Int. J. Psychophysiol. 2010;75(2):183–193. doi: 10.1016/j.ijpsycho.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci EL E.H., Patrick B.C., Leone D. Facilitating internalization: The self-determination theory perspective. J. Pers. 1994;62:119–142. doi: 10.1111/j.1467-6494.1994.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Dworak M., Schierl T., Bruns T. Impact of singular excessive computer game and television exposure on sleep patterns and memory performance of school-aged children. Pediatrics. 2007;120(5):978–985. doi: 10.1542/peds.2007-0476. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- Fisher M., Holland C., Merzenich M., Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am. J. Psychiatr. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., Holland C., Subramaniam K., Vinogradov S. Neuroplasticity-based cognitive training: What are the effects 6-months later? Schizophr. Bull. 2010;36(4):869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., Loewy R., Carter C., Lee A., Ragland J.D., Niendam T., Schlosser D., Pham L., Miskovich T., Vinogradov S. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr. Bull. 2015;41(1):250–258. doi: 10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Heaton R.K. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Green M.J., Matheson S.L., Shepherd A., Weickert C.S., Carr V.J. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol. Psychiatry. 2011;16:960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- Heinrichs D.W., Hanlon T.E., Carpenter W.T., Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr. Bull. 1984;10(3):388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Karege F., Schwald M., Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Kasai K., Nakagome K., Itoh K., Koshida I., Hata A., Iwanami A., Fukuda M., Kato N. Impaired cortical network for preattentive detection of change in speech sounds in schizophrenia: a high-resolution event-related potential study. Am. J. Psychiatry. 2002;159(4):546–553. doi: 10.1176/appi.ajp.159.4.546. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Goldberg T.E., Harvey P.D., Gold J.M., Poe M.P., Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004;68(2-3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E., Vinogradov S., Medalia A., Buckley P.F., Caroff S.N., D’Souza D.C., Harvey P.D., Graham K.A., Hamer R.M., Marder S.M., Miller D.D., Olson S.J., Patel J.K., Velligan D., Walker T.M., Haim A.J., Stroup T.S. Feasibility and pilot efficacy results from the multi-site Cognitive Remediation in the Schizophrenia Trials Network (CRSTN) study. J. Clin. Psychiatry. 2012;73:1016–1022. doi: 10.4088/JCP.11m07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalin I., Alyautdin R., Kocherga G., Bakar M.A. Targeted delivery of brain-derived neurotrophic factor for the treatment of blindness and deafness. Int. J. Nanomedicine. 2015;10:3245–3267. doi: 10.2147/IJN.S77480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D., Vakhrusheva J., Bartels M.N., Armstrong H.F., Ballon J.S., Khan S., Chang R.W., Hansen M.C., Ayanruoh L., Lister A., Castrén E., Smith E.E., Sloan R.P. The impact of aerobic exercise on brain-derived Neurotrophic factor and neurocognition in individuals with schizophrenia: A single-blind, randomized clinical trial. Schizophr. Bull. 2015;41(4):859–868. doi: 10.1093/schbul/sbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang U.E., Hellweg R., Seifert F., Schubert F., Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol. Psychiatry. 2007;62:530–535. doi: 10.1016/j.biopsych.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Mao Y.T., Hua T.M., Pallas S.L. Competition and convergence between auditory and cross-modal visual inputs to primary auditory cortical areas. Neurophysiology. 2011;105(4):1558–1573. doi: 10.1152/jn.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk S.R., Twamley E.W., Sitzer D.I., McHugo G.J., Mueser K.T. A meta-analysis of cognitive remediation in schizophrenia. Am. J. Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk S.R., Mueser K.T., Xie H., Welsh J., Kaiser S., Drake R.E., Becker D.R., Bailey E., Fraser G., Wolfe R., McHugo G.J. Cognitive enhancement treatment for people with mental illness Who Do Not respond to supported employment: A randomized controlled trial. Am. J. Psychiatry. 2015;172(9):852–861. doi: 10.1176/appi.ajp.2015.14030374. [DOI] [PubMed] [Google Scholar]
- Murthy N.V. Computerized cognitive remediation training for schizophrenia: an open label, multi-site, multinational methodology study. Schizophr. Res. 2012;139:87–91. doi: 10.1016/j.schres.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F. MATRICS Assessment, Inc.; Los Angeles: 2006. MATRICS Consensus Cognitive Battery Manual. [Google Scholar]
- Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Pillai A., Kale A., Joshi S., Naphade N., Raju M.S., Nasrallah H. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int. J. Neuropsychopharmacol. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- Piskulic D., Barbato M., Liu L., Addington J. Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. Psychiatry Res. 2015;225:93–98. doi: 10.1016/j.psychres.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Popov T., Jordanov T., Rockstroh B. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biol. Psychiatry. 2011;69(5):465–471. doi: 10.1016/j.biopsych.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Pressler S.J., Titler M., Koelling T., Riley P.L., Jung M., Hoyland-Domenico L., Ronis D.L., Smith D.G., Bleske B.E., Dorsey S.G., Giordani B. Nurse-enhanced computerized cognitive training increases serum brain-derived Neurotrophic factor levels and improves working memory in heart failure. J. Card. Fail. 2015;8:630–641. doi: 10.1016/j.cardfail.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Ragland J.D., Gur R.C., Valdez J., Turetsky B.I., Elliott M., Kohler C., Siegel S., Kanes S., Gur R.E. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am. J. Psychiatry. 2004;161(6):1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J.D., Moelter S.T., Bhati M.T., Valdez J.N., Kohler C.G., Siegel S.J., Gur R.C., Gur R.E. Effect of retrieval effort and switching demand on fMRI activation during semantic word generation in schizophrenia. Schizophr. Res. 2007;99(1-3):312–323. doi: 10.1016/j.schres.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R., Koestner R., Deci E.L. Ego-involved persistence: When free-choice behavior is not intrinsically motivated. Motiv. Emot. 1991;15:185–205. [Google Scholar]
- Subramaniam K., Luks T., Fisher M., Simpson G.V., Nagarajan S., Vinogrdov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S., Fisher M., Holland C., Shelly W., Wolkowitz O., Mellon S.H. Is serum BDNF a biomarker for cognitive enhancement in schizophrenia? Biol. Psychiatry. 2009;66(6):549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T., Huddy V., Cellard C. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The association between change in BDNF, and changes in cognition and quality of life scale ratings.