Abstract
Inherent to their pivotal roles in controlling all aspects of the liver cell life cycle, hepatocellular gap junctions are frequently disrupted upon impairment of the homeostatic balance, as occurs during liver toxicity. Hepatic gap junctions, which are mainly built up by connexin32, are specifically targeted by tumor promoters and epigenetic carcinogens. This renders inhibition of gap junction functionality a suitable indicator for the in vitro detection of nongenotoxic hepatocarcinogenicity. The establishment of a reliable liver gap junction inhibition assay for routine in vitro testing purposes requires a cellular system in which gap junctions are expressed at an in vivo-like level as well as an appropriate technique to probe gap junction activity. Both these models and methods are discussed in the current paper, thereby focusing on connexin32-based gap junctions.
Keywords: gap junction, connexin, liver, in vitro model, nongenotoxic carcinogen, intercellular communication
1. Introduction
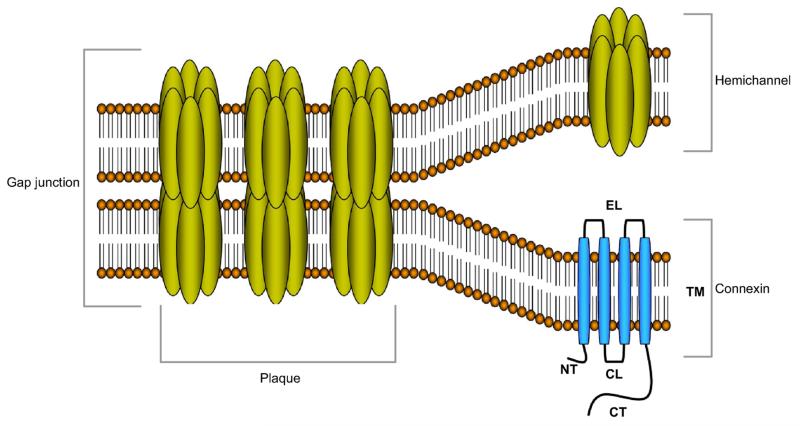
As in all other multicellular systems and organs, cellular communication is an absolute conditio sine qua non for the maintenance of liver homeostasis. Direct intercellular signaling is mediated by gap junctions. These communicating cell junctions arise from the interaction of two hemichannels of neighboring cells that on their turn are hexamers of connexin (Cx) proteins. More than twenty different connexin species have been identified, yet they all share a similar structure consisting of four transmembrane domains, two extracellular loops, one cytoplasmic loop, one cytoplasmic carboxytail and one cytoplasmic aminotail (Figure 1) (Maes et al., 2014; Vinken et al., 2008; Vinken et al., 2009; Vinken et al., 2011). The predominant connexin species in the liver is Cx32, which is abundantly expressed by hepatocytes and to a lesser extent by sinusoidal endothelial cells. The latter cells as well as stellate cells also produce small quantities of Cx26 (Fischer et al., 2005), while Cx43 is detectable in Kupffer cells, stellate cells, sinusoidal endothelial cells and cholangiocytes (Berthoud et al., 1992; Bode et al., 2002; Fischer et al., 2005). However, the presence of functional gap junctions has only been demonstrated in hepatocytes and stellate cells (Fischer et al., 2005). Gap junctional intercellular communication (GJIC) includes the passive exchange of small and hydrophilic substances, such as second messengers, between adjacent cells (Alexander and Goldberg, 2003; Wang et al., 2013), and is regulated by a vast array of mechanisms, including connexin phosphorylation (Solan and Lampe, 2009). As such, hepatic GJIC, in particular between hepatocytes, has been shown to drive a number of essential liver-specific processes, namely xenobiotic biotransformation (Neveu et al., 1994a; Shoda et al., 2000), bile secretion (Nathanson et al., 1999; Temme et al., 2001) albumin secretion (Yang et al., 2003a), glycogenolysis (Nelles et al., 1996; Stumpel et al., 1998) and ammonia detoxification (Yang et al., 2003a). Moreover, gap junctions are also key players in liver development (Vinken et al., 2012a), liver cell growth (Vinken et al., 2011) and liver cell death (Decrock et al., 2009; Vinken et al., 2010; Vinken et al., 2012b).
Figure 1. Molecular architecture of gap junctions.
Gap junctions are grouped in plaques at the cell plasma membrane surface of two apposed cells and are composed of twelve connexin proteins, organized as two hexameric hemichannels. The connexin protein is organized as four transmembrane domains (TM), two extracellular loops (EL), one cytoplasmic loop (CL), one cytoplasmic aminotail (NT) and one cytoplasmic carboxytail (CT).
Because of their critical roles in supporting liver homeostasis, it is not surprising that these structures are affected in disease. Indeed, hepatic GJIC typically deteriorates in liver cancer, cholestasis, liver fibrosis and cirrhosis, hepatitis and systemic inflammation, and hepatic liver ischemia and reperfusion injury (Vinken, 2012; Vinken et al., 2012a). In addition, gap junctions are also involved in liver toxicity. In this respect, a plethora of chemical and biological toxic compounds are known to suppress hepatocellular GJIC, including environmental pollutants, biological toxins, organic solvents, pesticides, pharmaceuticals, peroxides, metals and phthalates (Table 1). This is usually associated with the gradual disappearance of Cx32, whilst Cx43 production is enhanced in these pathological circumstances (Vinken et al., 2009). The deleterious outcome of these compounds on gap junction production and functioning is frequently manifested in a species-specific and tissue-specific manner. Such specificity in performing detrimental cellular actions as well as the lack of causing direct DNA damage are typical hallmarks of nongenotoxic carcinogenicity. In fact, many of the chemical and biological compounds that suppress hepatic GJIC are tumor promoters or epigenetic carcinogens. Hence, inhibition of GJIC may represent an interesting biomarker for the detection of nongenotoxic carcinogens in general (Budunova and Williams, 1994; Combes, 2000; Cowles et al., 2007; Mally and Chipman, 2002; Mesnil et al., 1995; Ruch and Klaunig, 1986). This may be challenging from an in vitro toxicologist’s perspective, since no validated in vitro assays are currently available for the testing of nongenotoxic carcinogenicity. When developing such in vitro screens, care should be taken while selecting the cellular system, which actually defines the scope of the present paper. Besides an overview of in vitro systems that appropriately maintain liver gap junctions at an in vivo-like level, in particular primary hepatocyte culture models, methods to probe hepatic GJIC are discussed.
Table 1. Agents that negatively affect hepatic gap junctions (Vinken et al., 2009).
| Environmental pollutants |
| Polycyclic aromatic hydrocarbons |
| Polychlorinated dibenzodioxins |
| Polychlorinated biphenyls |
| Biological toxins |
| Phorbol esters |
| Lipopolysaccharide |
| Ochratoxin A |
| Patulin |
| Gossypol |
| Organic solvents |
| Ethanol |
| Carbon tetrachloride |
| Trichloroethylene |
| Pesticides |
| Organophosphorous pesticides |
| Cyclodiene organochlorine pesticides |
| Dichlorodiphenyltrichloroethane |
| Lindane |
| Hexachlorobenzene |
| Pentachlorophenol |
| Pharmaceuticals |
| Hypolipidemic drugs |
| Phenobarbital |
| Methapyrilene |
| Miscellaneous compounds |
| Peroxides |
| Metals |
| Phthalates |
2. In vitro models to study of liver gap junctions
Cell lines are frequently used tools for studying gap junctions in vitro. However, cell lines not always provide an appropriate reflection of the in vivo situation. The human hepatoma-derived HepG2 cell line, routinely used in experimental liver research, lacks many liver-specific traits, including functional expression of biotransformation capacity (Wilkening et al., 2003). In addition, HepG2 cells do not exhibit physiological cell junction patterns. In these cells, production of Cx26 is downregulated, whereas Cx32 displays aberrant localization (Yano et al., 2001; Yano and Yamasaki, 2001). Likewise, nontumorigenic rat liver epithelial WB-F344 cells highly express Cx43, but not Cx32 (Neveu et al., 1994b; Rae et al., 1998). The former also holds true for immortal BRL-3A rat liver cells (Wang et al., 2015). As a result, hepatic cell lines can not be used to address specific toxicological issues, such as effects of strain, species, age and gender of nongenotoxic carcinogens on hepatocellular GJIC (Kamendulis et al., 2002; Klaunig and Ruch, 1987). For this reason, the use of primary hepatocyte systems is considered to be a more suitable alternative. Nevertheless, these in vitro models suffer from a number of fundamental disadvantages, which mainly result from events that occur during hepatocyte isolation and subsequent cultivation (Elaut et al., 2006; Fraczek et al., 2013). This topic as well as the attempts to overcome this hurdle are discussed in the following sections, with emphasis on the re-establishment of normal liver gap junction patterns in vitro.
2.1. Effects of hepatocyte isolation on gap junctions
The two-step collagenase perfusion technique is the most commonly used procedure to isolate hepatocytes from the livers of animals and humans (Lecluyse and Alexandre, 2010; Lee et al., 2013; Papeleu et al., 2006). One of the fundamental principles of this procedure is based on the notion that calcium ions are indispensable for cellular adhesion. Initially, the freshly isolated liver is perfused with a calcium-free medium, often supplemented with a calcium chelator, in order to abandon calcium-dependent cell-cell contacts (i.e. adherens junctions). In the second step, the liver is perfused with a collagenase-containing buffer to disrupt cell-extracellular matrix (ECM) interactions (Alpini et al., 1994; Berry et al., 1997; Seglen, 1976). Limited perfusion of the isolated liver with collagenase yields hepatocyte doublets (Gautam et al., 1987), which better retain gap junctions (Coleman et al., 1995; Roma et al., 1997; Yoshizawa et al., 1997). However, in the conventional procedure, gap junctions are fully disrupted by mechanically dispersing the digested liver (Berry et al., 1997). Finally, connective tissue is removed by filtering and further centrifugation separates viable hepatocytes from both dead hepatocytes and nonparenchymal cells (Alpini et al., 1994; Berry et al., 1997; Seglen, 1976). Subsequent cultivation elicits an adaptive response in hepatocytes, as they need to accustom to their new artificial environment. This is associated with a progressive loss of differentiated capacities, the acquisition of a more flattened fibroblast-like morphology and a concomitant return towards a more fetal-like status (Tuschl et al., 2009; Tuschl and Mueller, 2006). Several changes in gene expression profiles accompany this dedifferentiation event. Generally, proliferation-enhancing and survival-promoting genes are upregulated, whereas the expression of differentiation-related genes is lost (Baker et al., 2001). Not surprisingly, drastic modifications also occur at the level of gap junctions. Indeed, Cx43, a connexin species typically found in fetal hepatocytes, re-appears in cultured adult hepatocytes (Kojima et al., 1995a; Stutenkemper et al., 1992; Vinken et al., 2006a). The functional relevance of this process is unclear, but has been linked to the onset of spontaneous cell death in primary hepatocyte cultures (Vinken et al., 2012b). Possibly, de novo Cx43 expression results from altered cis/trans regulation of its gene expression. Upon isolation of hepatocytes, c-fos and cjun are induced (Etienne et al., 1988; Loyer et al., 1996). These proto-oncogenes dimerize to form the transcription factor activator protein-1, which is known to control Cx43 expression (Echetebu et al., 1999). In rat myometrium, activator protein-1 has been shown to induce Cx43 expression under stress conditions (Lefebvre et al., 1995). Although no solid scientific data are presently available, this scenario might also take place in isolated primary hepatocytes in culture.
When seeded under conventional culture conditions, viable hepatocytes adhere to the plastic surface within four hours and aggregate in groups of two to ten cells, thus re-establishing intercellular contacts. After twelve hours of cultivation, hepatocytes start to regain their typical polyhedral morphology, yet this phenotype very rapidly deteriorates (Wanson et al., 1977). In order to counteract this dedifferentiation process and thus to maintain gap junctions, a number of strategies have been followed, which typically aim at mimicking the in vivo hepatocyte micro-environment in vitro, including (i) the addition of differentiation-promoting soluble molecules to the cell culture medium producing improved monolayer cultures, (ii) the direct restoration of homotypic and heterotypic intercellular contacts yielding co-cultures and (iii) the re-establishment of cell-ECM interactions resulting in tridimensional cultures (Fraczek et al., 2013; Godoy et al., 2013; Hewitt et al., 2007; LeCluyse et al., 1996; Vanhaecke and Rogiers, 2006).
2.2. Improved monolayer cultures
The formulation of the cell culture medium is a crucial parameter for maintaining the hepatocyte-specific phenotype in vitro. Historically, hepatocytes have been cultivated on plastic culture dishes covered with serum-supplemented standard media (LeCluyse et al., 1996). Although serum improves cell attachment and survival (Bissell and Guzelian, 1980), many deleterious effects have been reported. Indeed, serum decreases liver-specific functionality, such as phase I xenobiotic biotransformation activity (Enat et al., 1984; Paine and Andreakos, 2004). In addition, it promotes cellular depolarization by inhibiting the re-establishment of gap junctions (Lee et al., 1993; Spray et al., 1987). Therefore, a number of serum-free chemically defined culture media have been developed, including Dulbecco’s modified Eagle’s medium, William’s medium E and Leibovitz’s L15 medium. However, when cultivated in these media as such, hepatocytes rapidly undergo functional and morphological deterioration (LeCluyse et al., 1996). Thus, Cx26 and Cx32 levels decrease by 1.6-fold to 2.9-fold, respectively, within two days, regardless of the commercial medium used (Kwiatkowski et al., 1994). For this reason, efforts have been focused on the enrichment of these standard media with both physiological and nonphysiological soluble factors in order to improve the maintenance of the hepatocyte-specific functionality and morphology, all which affect gap junctions.
2.2.1. Dimethylsulfoxide
The beneficial effects of dimethylsulfoxide (DMSO) on the differentiated status of primary cultured hepatocytes have been extensively described. In general, 2% v/v DMSO is used and promotes liver-specific functionality, such as albumin secretion (Arterburn et al., 1995; Isom et al., 1987). This effect is associated with the long-term (i.e. more than four weeks) maintenance of an in vivo-like morphology, including the presence of gap junctions. The latter has been shown to result from enhanced expression of Cx26 and Cx32, which thereby promotes GJIC (Kojima et al., 1995a; Kojima et al., 1996a; Stoehr and Isom, 2003; Yoshizawa et al., 1997). Moreover, DMSO inhibits the re-appearance of Cx43 (Kojima et al., 1995a; Stoehr and Isom, 2003). The mechanisms by which DMSO exerts its differentiation-inducing effects are not clear. It has been proposed that DMSO is an oxygen radical scavenger (Kojima et al., 1996b). Alternatively, DMSO can alter intracellular calcium levels and phosphorylation events (Mizuguchi et al., 1998), which might directly affect gap junctions (Arterburn et al., 1995).
2.2.2. Corticosteroids
Glucocorticosteroids are known to retard dedifferentiation in primary cultures of hepatocytes. This has been attributed to their positive effects on cellular functionality (LeCluyse et al., 1996). Glucocorticosteroids, such as dexamethasone, also stabilize the in vitro hepatocyte morphology by improving cytoskeleton arrangement and cell-cell contacts (LeCluyse et al., 1996; Ren et al., 1994). In particular, the expression of Cx26 and Cx32 are promoted, resulting in enhanced GJIC (Kwiatkowski et al., 1994; Ren et al., 1994; Siddiqui et al., 1999). In general, glucocorticosteroids affect gene expression by acting at the level of gene transcription, a mechanism that is mediated by the glucocorticoid response element. However, neither Cx26 nor Cx32 contain such a cis-acting element within their gene structure (Hennemann et al., 1992). As the presence of adherens junctions is known to be a prerequisite for gap junction formation (Hernandez-Blazquez et al., 2001; Laird, 1996; Lampe et al., 1998) and since the E-cadherin gene is known to contain a glucocorticoid response element (Ringwald et al., 1991), it is thought that the glucocorticosteroid-mediated induction of connexin expression is an indirect result of its positive effects on E-cadherin gene expression (Kwiatkowski et al., 1994).
2.2.3. Nicotinamide and derivatives
Primary cultured hepatocytes rapidly lose their intracellular nicotinamide adenosine dinucleotide (NAD) content. Nevertheless, the presence of this factor is of utmost importance to maintain differentiated features, as NAD serves as a cellular co-enzyme for a number of biochemical reactions (Mitaka et al., 1998). Bearing this in mind, several research groups have explored the possibility of preventing NAD depletion by adding NAD precursors, such as the vitamin nicotinamide and its derivate 3-acetylpyridine, to the cell culture medium of primary hepatocytes. These molecules were shown to stabilize albumin secretion over the cultivation time course (Inoue et al., 1989; Sato et al., 1999) and to preserve Cx26 and Cx32 production for more than two weeks (Higaki et al., 2001; Sato et al., 1999). Nicotinamide not only serves as a NAD precursor, but also inhibits polyadenosine diphosphate ribose polymerase activity, an enzyme that negatively correlates with cellular differentiation (Mitaka et al., 1998).
2.2.4. Cyclic adenosine monophosphate derivatives and modulating agents
Cyclic adenosine monophosphate (cAMP) is a well-known inducer of GJIC in primary cultures of hepatocytes. Upon addition of 8-bromo-cAMP, a membrane-permeant cAMP derivative, to the cell culture medium, the disappearance of GJIC, which generally occurs soon after the hepatocyte isolation procedure, is delayed, concomitant with a well-preserved cellular morphology. This is thought to be due to positive effects on Cx32 mRNA stability and/or phosphorylation (Saez et al., 1986). Agents known to increase the intracellular cAMP amount in hepatocytes, such as irsogladine (Nakashima et al., 2000) and glucagon (Kojima et al., 1995b; Siddiqui et al., 1999), also enhance the expression of Cx26 and Cx32, and therefore GJIC. Paradoxically, insulin decreases the intracellular cAMP content by stimulating cAMP phosphodiesterase activity (Saez et al., 1986), while inducing connexin production in primary cultures of hepatocytes (Kojima et al., 1995b; Siddiqui et al., 1999). The mechanism behind this observation remains to be elucidated.
2.2.5. S-adenosylmethionine
S-adenosylmethionine is a precursor of glutathione and polyamine synthesis, and acts as a methyldonor in cellular transmethylation reactions in the liver. The deteriorative process taking place in cultures of primary hepatocytes, whereby a Cx32-to-Cx43 switch occurs, is associated with decreased S-adenosylmethionine levels. Supplementation of the hepatocyte culture medium with this compound results in elevated Cx32 levels and counteracts the appearance of Cx43. The latter is due to reduced accumulation of nuclear β-catenin, which in turn negatively affects Wnt signaling-dependent gene transcription of Cx43 (Yamaji et al., 2011).
2.2.6. Histone deacetylase inhibitors
Chromatin structure undergoes several changes in various physiological situations, such as during cellular differentiation. These effects are partly mediated by histone acetyltransferases and histone deacetylases (HDACs). The former catalyze the acetylation of histones, thereby disrupting DNA-histone interactions, whereas the latter promote the inverse reaction. In general, HDAC activities are associated with gene silencing. Several natural and synthetic molecules are known to inhibit HDAC activity, thereby drastically altering gene expression. This generally results in major modifications in the homeostatic balance in favor of the differentiated status (Papeleu et al., 2005; Vanhaecke et al., 2004; Zhang and Zhong, 2014). Thus, upon addition of sodium butyrate, a naturally occurring HDAC inhibitor, to primary cultured hepatocytes, both functionality and morphology are better maintained in comparison with nontreated cells. The morphological enhancement induced by sodium butyrate is linked to the presence of gap junctions at a level comparable to the in vivo situation (Engelmann et al., 1987; Gladhaug et al., 1988; Iwai et al., 2002; Staecker et al., 1988). In agreement with these findings, trichostatin A, a hydroxamate HDAC inhibitor, to the culture medium of primary rat hepatocytes, increases Cx32 expression and GJIC, yet it also enhances Cx43 production and negatively affects Cx26. All these effects are greatly enhanced when trichostatin A treatment is already initiated during the hepatocyte isolation procedure (Vinken et al., 2006a). 4-Me2N BAVAH, a structural analogue of trichostatin A with a more beneficial metabolic profile, also promotes Cx32 production in primary hepatocyte cultures and suppresses the expression of both Cx26 and Cx43 (Vinken et al., 2007).
2.2.7. Miscellaneous culture medium additives
Isolation and cultivation of primary hepatocytes is associated with oxidative stress (Elaut et al., 2006), which is deleterious for the stability of gap junctions (Morsi et al., 2003; Schmelz et al., 2001). The addition of oxygen radical scavengers was therefore thought to be an efficient strategy to maintain gap junction integrity in vitro. Indeed, upon addition of anti-oxidants, such as vitamin C derivatives (Tateno and Yoshizato, 1999), melatonin (Kojima et al., 1997) and taurine (Fukuda et al., 2000), to the medium of primary cultured hepatocytes, gap junctions are better preserved. Growth factors, like epidermal growth factor, are often used as medium additives, because they increase cell survival (LeCluyse et al., 1996). However, epidermal growth factor negatively affects xenobiotic phase I biotransformation activity (De Smet et al., 2001) and decreases the number of gap junctions in hepatocyte cultures (Berthiaume et al., 1996). Similarly, biotransformation enzyme inducers, such as phenobarbital, are known to retard dedifferentiation of primary cultured hepatocytes (LeCluyse et al., 1996), although they have been shown to inhibit GJIC in these in vitro models (Ren and Ruch, 1996).
2.3. Co-cultures
In liver, hepatocytes are in direct contact with each other by means of gap junctions. They also form heterotypic contacts with the surrounding nonparenchymal cells. The presence of both cellular interactions is a prerequisite for normal liver-specific functioning (Bhatia et al., 1999; LeCluyse et al., 2012; Maher and Friedman, 1993). Therefore, the restoration and/or boosting of these contacts was considered an evident strategy to improve liver-specific functionality in vitro (LeCluyse et al., 1996). Several research groups have explored co-cultivation of hepatocytes with another cell type. Both hepatic nonparenchymal and nonhepatic cells have been used for this purpose (Coecke et al., 1999; LeCluyse et al., 1996), but the best results have been obtained by co-cultivating hepatocytes with rat liver epithelial cells of primary biliary origin (Corlu et al., 1997; Guguen-Guillouzo and Guillouzo, 1983). In general, co-cultivated hepatocytes display liver-specific functions, including xenobiotic biotransformation capacity and albumin secretion, for long periods (Coecke et al., 1999; LeCluyse et al., 1996). Simultaneously, hepatocyte morphological traits are well preserved and stable Cx32-based gap junctions are present (Bhatia et al., 1999; Mesnil et al., 1993). However, the exact nature of the cellular interaction between hepatocytes and their cultivation partners remains elusive. It has been suggested that heterologous GJIC could account for the improvement of the hepatocyte phenotype. Nevertheless, no gap junction-mediated communication has been observed between hepatocytes and rat liver epithelial cells (Diener et al., 1994; Mesnil et al., 1987; Novikoff et al., 1991). Likewise, heterologous GJIC is absent in co-culture systems consisting of hepatocytes and fibroblasts (Sugimachi et al., 2004) as well as of hepatocytes and stellate cells (Fischer et al., 2005). Hepatocytes and their cultivation partners might be in paracrine contact with each other. A candidate mediator for such communication is epimorphin, a protein that is produced by a number of hepatic cells, such as stellate cells, but not by hepatocytes. Epimorphin plays a role in liver differentiation and its expression pattern, as observed during liver regeneration, is very similar to that of Cx32 in hepatocytes (Spray et al., 1987).
Another strategy to retard dedifferentiation in vitro includes the boosting of homotypic hepatocyte interactions. This can be achieved by continuously rotating hepatocytes in suspension or by using cell-repelling substrata. Both methods result in the formation of multicellular hepatocyte aggregates called spheroids (LeCluyse et al., 1996). Within these structures, hepatocytes are in intimate contact with each other. This is associated with the abundant presence of gap junctions (Abu-Absi et al., 2002; Hou et al., 2001; Koide et al., 1990; Yang et al., 2003a) and results in the long-term maintenance of liver-specific functionality. This approach has been further optimized by including nonparenchymal liver cells in the hepatocyte aggregation process. Such heterospheroids retain global cell-cell contacts and thus closely resemble the in vivo situation (LeCluyse et al., 1996).
2.4. Tridimensional cultures
In normal liver, hepatocytes are in contact with a broad range of ECM proteins, including collagens, glycoproteins, proteoglycans and glycosaminoglycans (Amenta and Harrison, 1997; Iredale and Arthur, 1994; LeCluyse et al., 2012; Maher and Bissell, 1993). These ECM components control hepatocellular homeostasis via integrin-mediated signaling (LeCluyse et al., 1996; Mousa, 1998). Based on this knowledge, many studies have focused on the re-introduction of a natural scaffold in hepatocyte cultures in order to regain the in vivo-like hepatocyte phenotype (Depreter et al., 2002; Hamilton et al., 2001; LeCluyse, 2001). In general, the presence of ECM proteins promotes liver-specific functionality, which is associated with enhanced gap junction formation (Berthiaume et al., 1996; Iredale and Arthur, 1994; Mooney et al., 1992). In this respect, collagens, glycoproteins, proteoglycans and glycosaminoglycans were all shown to induce the expression of connexin proteins and GJIC activity (Fujita et al., 1986; Fujita et al., 1987; Spray et al., 1987). However, seeding cells on a layer of ECM proteins provides in vitro systems that retain liver-specific functions for about five days (Knop et al., 1995; Maher and Bissell, 1993), which is only two days more in comparison with conventional monolayer cultures (Vanhaecke and Rogiers, 2006). Improvements of this strategy rely on the application of a second ECM layer (i.e. the sandwich technique) (Dunn et al., 1991; Koebe et al., 1994) or the entrapment of hepatocytes in a collagen gel (i.e. the immobilization method) (Koebe et al., 1994) in which hepatocyte functionality, such as xenobiotic biotransformation capacity and albumin secretion, can be kept for more than two months. This stabilized functionality is also reflected at the level of cell morphology. Indeed, an in vivo-like morphology, including the presence of gap junctions, is re-established in these in vitro systems (Berthiaume et al., 1996; Hamilton et al., 2001; LeCluyse, 2001).
Nonphysiological and synthetic ECM scaffolds have also been used to create organotypical hepatocyte cultures. Thus, Matrigel®, a laminin-rich extract from Engelbreth-Holm-Swarm mouse tumor, has been proposed as a cultivation substratum for hepatocytes (Iredale and Arthur, 1994; Maher and Bissell, 1993). Upon cultivation in this nonphysiological matrix, hepatocytes express liver-specific functions for more than four weeks associated with the abundant presence of Cx32-based gap junctions (Hamilton et al., 2001; LeCluyse, 2001). Several laboratories have tested synthetic compounds as hepatocyte scaffolds, such as poly(lactide-co-glycolide) acid (Hasirci et al., 2001) polyvinyl formal resin (Yang et al., 2001) and polyurethane foam (Pahernik et al., 2001). The use of synthetic ECM scaffolds is frequently combined with spheroid formation, yielding hepatocyte aggregates that highly express Cx32 (Seo et al., 2004).
3. In vitro methods to study liver gap junctions
Several methods are currently available to test GJIC in cultured cells. They can be divided into three classes, namely (i) metabolic coupling assays, (ii) electrical coupling assays and (iii) dye coupling assays.
3.1. Metabolic coupling assays
The metabolic co-operation approach is based upon the monitoring of the transfer of endogenous and biologically relevant compounds. For this procedure, fluorescently marked donor cells are incubated in the presence of radiolabelled precursors, like nucleotides or glucose, and then co-cultured with unlabeled recipient cells. Subsequently, donor cells are separated from receiver cells through fluorescence-activated cell sorting and the amount of the radio-isotope in the receipt cell population is assessed by chromatography and/or quantitative autoradiography (Goldberg et al., 1999; Goldberg et al., 1998). A more indirect method includes the tracking of calcium waves, which correlates with the presence of functional gap junctions. In this technique, cells are loaded with a calcium-sensitive fluorescent dye and are stimulated electrically, mechanically or chemically in order to generate inositol triphosphate, which triggers the actual calcium wave. A more sophisticated approach is the local liberation of inositol triphosphate from a caged precursor by flash photolysis, which allows the stimulation of single cells (Decrock et al., 2015; Leybaert and Sanderson, 2001). Metabolic coupling assays, in particular transfer of labelled nucleotides, have been used to demonstrate the inhibitory effects of 12-O-tetradecanoylphorbol-13-acetate on GJIC in co-cultures of primary chick embryo hepatocytes and Chinese hamster V79 lung fibroblasts (van der Zandt et al., 1990).
3.2. Electrical coupling assays
The dual voltage patch clamp technique envisages the recording of gap junctional electrical conductance, whereby originally two separate micro-electrodes were introduced in each cell of a cell pair, one for current injection and another one for voltage control (Spray et al., 1979, 1981). This technique was later modified to a double whole cell voltage clamp technique, using only one patch pipet per cell, which is a very sensitive method that allows the recording of a single gap junction channel (Hamill and Sakmann, 1981; Neyton and Trautmann, 1985). In a more recent method, GJIC is measured using a combination of single cell electrophysiology, large-scale optical recordings and a sensor of plasma membrane potential (Ceriani and Mammano, 2013). Analysis of gap junctional electrical conductance, however, is a labor-intensive, expensive and rather slow technique that requires appropriate expertise and technical skills (Abbaci et al., 2008; Yamasaki, 1997). Electrical coupling assays, in particular voltage patch clamping, have been used to demonstrate the inhibitory effects of carbon tetrachloride on GJIC in cultures of primary rat hepatocytes (Saez et al., 1987).
3.3. Dye coupling assays
Dye coupling methods are by far the most frequently used ones, mainly because of their ease of use. This kind of assays relies on the introduction of small dyes into living cells that are traced in their intercellular movement. A wide variety of tracers, mostly fluorescent, are used (Abbaci et al., 2008; Meda, 2000), and there are several ways to introduce these reporter dyes into cells, including micro-injection (Kanno and Loewenstein, 1964), mechanical loading by scraping (el-Fouly et al., 1987) and electroporation (De Vuyst et al., 2008; Decrock et al., 2015; Raptis et al., 1994). Recently, a high-throughput GJIC measurement system based on robotic micro-injection has been described (Liu et al., 2015). In addition, a number of noninvasive dye coupling protocols have been established. In fluorescence recovery after photobleaching (FRAP) analysis, cells are loaded with a lipophilic cell plasma membrane permeable dye, such as calcein acetoxymethyl ester. Upon cellular uptake, this dye is hydrolized by cytoplasmic esterases, producing calcein, which is a fluorescent and membrane-impermeable molecule. Fluorescence in a single cell is then irreversibly photobleached using a high-powered laser beam and subsequent transfer of fluorescent dye from neighboring cells into the target cell is monitored (Abbaci et al., 2007; Wade et al., 1986). FRAP can be applied to monolayer culture systems as well as to tridimensional in vitro models (Kuzma-Kuzniarska et al., 2014).
Both the preloading assay and the parachute technique also require cell loading with cell plasma membrane-permeable dyes. In the former, loaded cells are suspended together with unloaded counterparts and are then allowed to form a confluent monolayer (Goldberg et al., 1995), whereas in the latter, loaded cells in suspension adhere to a monolayer of unloaded cells (Ziambaras et al., 1998). In both cases, the spread of the dye from donor cells to receiver cells is studied by fluorescence microscopy and is a measure for GJIC. In the local activation of molecular fluorescent probe (LAMP) method, a new generation of caged coumarin-like fluorophores is used. Like in FRAP, these dyes are processed by intracellular esterases, but they only become fluorescent upon subsequent local illumination with a small dose of ultraviolet light. The latter is unlikely to cause photodamage, in contrast to the high-powered laser beam used in the FRAP approach (Dakin et al., 2005). An improvement to the LAMP method has been described, the so-called infrared-LAMP assay, which allows examination of cell-cell coupling in three dimensions (Yang and Li, 2009). Dye coupling assays, such as based on microinjected Lucifer Yellow, have been used to demonstrate the inhibitory effects of the nongenotoxic peroxisome proliferating drug nafenopin in cultures of primary rat hepatocytes (Elcock et al., 1998).
4. Conclusions and perspectives
Because of its unique localization and function in the organism, the liver is a primary target for systemic toxicity. For this reason, a lot of attention has been paid, and it still being paid, to the establishment of liver-based models for in vitro toxicity testing purposes. Among those, cultures of primary hepatocytes are considered as the gold standard, as they provide an appropriate reflection of the hepatic in vivo situation (Fraczek et al., 2013; Godoy et al., 2013; Lin et al., 2015). In these experimental systems, gap junctions and concomitant physiological connexin expression can be maintained by applying a number of techniques that intend to create an in vivo-like environment for hepatocytes (Vinken et al., 2006b). Although promising, these techniques are not able to completely counteract the dedifferentiation process that is triggered during hepatocyte isolation. A major reason for this shortcoming is that these culture configurations act on the consequences of this deteriorative process. In recent years, a number of innovative methodologies has been introduced that are targeted towards the actual cause of dedifferentiation, such as by directly interfering with the gene transcription of liver-specific proteins (Fraczek et al., 2013; Vinken et al., 2012c). It is conceivable to assume that gap junctions are equally positively affected by these novel strategies, yet this remains to be experimentally confirmed. Furthermore, in the last decade, the field of in vitro toxicology has witnessed the introduction of sophisticated liver-based systems in which the in vivo functional phenotype can be kept for extended periods of time, such as microfluidic liver bioreactors (Khetani et al., 2015; LeCluyse et al., 2012; Lin et al., 2015). In parallel, stem cells have entered the in vitro toxicology area along with several strategies for their differentiation into hepatocyte-like cells (Kia et al., 2013; Sauer et al., 2014). In this context, liver progenitor cells or oval cells mainly express Cx43 (Zhang and Thorgeirsson, 1994), which also holds true for many liver cell lines. As there is increasing evidence that alteration of liver progenitor cells and oval cells by toxic chemicals plays an important role in the development of different chronic liver diseases (Canovas-Jorda et al., 2014), it should be stressed that liver cell lines may still be of great use to study alterations of GJIC by chemicals in toxicologically and pathologically relevant Cx43-expressing cell types, thereby providing information complementary to chemical effects on Cx32-dependent GJIC in differentiated hepatocytes. Obviously, these developments may open new perspectives for the establishment of cutting-edge in vitro systems to test hepatic GJIC. The latter is a goalkeeper of liver homeostasis and hence a key determinant of hepatotoxicity. In particular, unlike their genotoxic counterparts (Ruch, 1994; Yamasaki and Naus, 1996), nongenotoxic carcinogens typically inhibit GJIC, in casu in liver (Budunova and Williams, 1994; Oyamada et al., 1990; Trosko et al., 1994; Vinken et al., 2009; Yamasaki, 1995). A number of assays are nowadays used for testing gap junction functionality, with the dye-based methods being the most commonly used ones. These assays are featured by many advantages (Abbaci et al., 2008), yet they may not reflect actual GJIC per se. Indeed, the reporter dyes used in these methods substantially differ from the natural gap junction permeants. In this regard, the biophysical properties of a given gap junction highly depend on the connexin species that compose the channel. Thus, Cx26-based gap junctions are known to favor cation transfer, whereas gap junctions consisting of Cx32 rather promote anion passage (Bukauskas et al., 1995). In a similar way, adenosine triphosphate is conveyed about three hundred times better through gap junctions formed by Cx43 compared with Cx32-based channels (Goldberg et al., 2002). In the upcoming years, efforts should be focused on the further optimization of gap junction methods that allow (patho)physiologically relevant assessment of GJIC. When combined with appropriate cellular systems, it can be expected that a valuable in vitro tool will be generated eligible for the evaluation of the nongenotoxic carcinogenic potential of chemical compounds during the process of risk assessment.
Acknowledgements
This work was financially supported by the grants of Agency for Innovation by Science and Technology in Flanders (IWT), the University Hospital of the Vrije Universiteit Brussel-Belgium (Willy Gepts Fonds UZ-VUB), the Fund for Scientific Research-Flanders (FWO grants G009514N and G010214N), the European Research Council (ERC Starting Grant 335476), the University of São Paulo-Brazil and the Foundation for Research Support of the State of São Paulo (FAPESP SPEC grant 2013/50420-6).
Abbreviations
- cAMP
cyclic adenosine monophosphate
- Cx
connexin
- DMSO
dimethylsulfoxide
- ECM
extracellular matrix
- FRAP
fluorescence recovery after photobleaching
- GJIC
gap junctional intercellular communication
- HDAC(s)
histone deacetylase(s)
- LAMP
local activation of molecular fluorescent probe
- NAD
nicotinamide adenosine dinucleotide
Footnotes
All authors have read the journal’s policy on conflicts of interest and have no conflicts of interest to declare. All authors have read the journal’s authorship agreement.
References
- Abbaci M, Barberi-Heyob M, Blondel W, Guillemin F, Didelon J. Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques. 2008;45:33–52. 56–62. doi: 10.2144/000112810. [DOI] [PubMed] [Google Scholar]
- Abbaci M, Barberi-Heyob M, Stines JR, Blondel W, Dumas D, Guillemin F, Didelon J. Gap junctional intercellular communication capacity by gap-FRAP technique: a comparative study. Biotechnol J. 2007;2:50–61. doi: 10.1002/biot.200600092. [DOI] [PubMed] [Google Scholar]
- Abu-Absi SF, Friend JR, Hansen LK, Hu WS. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res. 2002;274:56–67. doi: 10.1006/excr.2001.5467. [DOI] [PubMed] [Google Scholar]
- Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- Alpini G, Phillips JO, Vroman B, LaRusso NF. Recent advances in the isolation of liver cells. Hepatology. 1994;20:494–514. [PubMed] [Google Scholar]
- Amenta PS, Harrison D. Expression and potential role of the extracellular matrix in hepatic ontogenesis: a review. Microsc Res Tech. 1997;39:372–386. doi: 10.1002/(SICI)1097-0029(19971115)39:4<372::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Arterburn LM, Zurlo J, Yager JD, Overton RM, Heifetz AH. A morphological study of differentiated hepatocytes in vitro. Hepatology. 1995;22:175–187. [PubMed] [Google Scholar]
- Baker TK, Carfagna MA, Gao H, Dow ER, Li Q, Searfoss GH, Ryan TP. Temporal gene expression analysis of monolayer cultured rat hepatocytes. Chem Res Toxicol. 2001;14:1218–1231. doi: 10.1021/tx015518a. [DOI] [PubMed] [Google Scholar]
- Berry MN, Grivell AR, Grivell MB, Phillips JW. Isolated hepatocytes: past, present and future. Cell Biol Toxicol. 1997;13:223–233. doi: 10.1023/a:1007402505482. [DOI] [PubMed] [Google Scholar]
- Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Iwanij V, Garcia AM, Sáez JC. Connexins and glucagon receptors during development of rat hepatic acinus. Am J Physiol. 1992;263:G650–658. doi: 10.1152/ajpgi.1992.263.5.G650. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- Bissell DM, Guzelian PS. Phenotypic stability of adult rat hepatocytes in primary monolayer culture. Ann N Y Acad Sci. 1980;349:85–98. doi: 10.1111/j.1749-6632.1980.tb29518.x. [DOI] [PubMed] [Google Scholar]
- Bode HP, Wang L, Cassio D, Leite MF, St-Pierre MV, Hirata K, Okazaki K, Sears ML, Meda P, Nathanson MH, Dufour JF. Expression and regulation of gap junctions in rat cholangiocytes. Hepatology. 2002;36:631–640. doi: 10.1053/jhep.2002.35274. [DOI] [PubMed] [Google Scholar]
- Budunova IV, Williams GM. Cell culture assays for chemicals with tumor-promoting or tumor-inhibiting activity based on the modulation of intercellular communication. Cell Biol Toxicol. 1994;10:71–116. doi: 10.1007/BF00756491. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Elfgang C, Willecke K, Weingart R. Heterotypic gap junction channels (connexin26-connexin32) violate the paradigm of unitary conductance. Pflugers Arch. 1995;429:870–872. doi: 10.1007/BF00374812. [DOI] [PubMed] [Google Scholar]
- Canovas-Jorda D, Louisse J, Pistollato F, Zagoura D, Bremer S. Regenerative toxicology: the role of stem cells in the development of chronic toxicities. Expert Opin Drug Metab Toxicol. 2014;10:39–50. doi: 10.1517/17425255.2013.844228. [DOI] [PubMed] [Google Scholar]
- Ceriani F, Mammano F. A rapid and sensitive assay of intercellular coupling by voltage imaging of gap junction networks. Cell Commun Signal. 2013;11:78. doi: 10.1186/1478-811X-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coecke S, Rogiers V, Bayliss M, Castell J, Doehmer J, Fabre G, Fry J, Kern A, Westmoreland C. The Use of Long-term Hepatocyte Cultures for Detecting Induction of Drug Metabolising Enzymes: The Current Status. Altern Lab Anim. 1999;27:579–638. doi: 10.1177/026119299902700408. [DOI] [PubMed] [Google Scholar]
- Coleman R, Wilton JC, Stone V, Chipman JK. Hepatobiliary function and toxicity in vitro using isolated hepatocyte couplets. Gen Pharmacol. 1995;26:1445–1453. doi: 10.1016/0306-3623(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Combes RD. The use of structure-activity relationships and markers of cell toxicity to detect non-genotoxic carcinogens. Toxicol In Vitro. 2000;14:387–399. doi: 10.1016/s0887-2333(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Corlu A, Ilyin G, Cariou S, Lamy I, Loyer P, Guguen-Guillouzo C. The coculture: a system for studying the regulation of liver differentiation/proliferation activity and its control. Cell Biol Toxicol. 1997;13:235–242. doi: 10.1023/a:1007475122321. [DOI] [PubMed] [Google Scholar]
- Cowles C, Mally A, Chipman JK. Different mechanisms of modulation of gap junction communication by non-genotoxic carcinogens in rat liver in vivo. Toxicology. 2007;238:49–59. doi: 10.1016/j.tox.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Dakin K, Zhao Y, Li WH. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat Methods. 2005;2:55–62. doi: 10.1038/nmeth730. [DOI] [PubMed] [Google Scholar]
- De Smet K, Loyer P, Gilot D, Vercruysse A, Rogiers V, Guguen-Guillouzo C. Effects of epidermal growth factor on CYP inducibility by xenobiotics, DNA replication, and caspase activations in collagen I gel sandwich cultures of rat hepatocytes. Biochem Pharmacol. 2001;61:1293–1303. doi: 10.1016/s0006-2952(01)00612-8. [DOI] [PubMed] [Google Scholar]
- De Vuyst E, De Bock M, Decrock E, Van Moorhem M, Naus C, Mabilde C, Leybaert L. In situ bipolar electroporation for localized cell loading with reporter dyes and investigating gap junctional coupling. Biophys J. 2008;94:469–479. doi: 10.1529/biophysj.107.109470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decrock E, De Bock M, Wang N, Bol M, Gadicherla AK, Leybaert L. Flash photolysis of caged IP3 to trigger intercellular Ca2+ waves. Cold Spring Harb Protoc. 2015;2015:289–292. doi: 10.1101/pdb.prot076570. [DOI] [PubMed] [Google Scholar]
- Decrock E, Vinken M, De Vuyst E, Krysko DV, D’Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16:524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- Depreter M, Walker T, De Smet K, Beken S, Kerckaert I, Rogiers V, Roels F. Hepatocyte polarity and the peroxisomal compartment: a comparative study. Histochem J. 2002;34:139–151. doi: 10.1023/a:1020990414190. [DOI] [PubMed] [Google Scholar]
- Diener B, Beer N, Dürk H, Traiser M, Utesch D, Wieser RJ, Oesch F. Gap junctional intercellular communication of cultured rat liver parenchymal cells is stabilized by epithelial cells and their isolated plasma membranes. Experientia. 1994;50:124–126. doi: 10.1007/BF01984948. [DOI] [PubMed] [Google Scholar]
- Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- Echetebu CO, Ali M, Izban MG, MacKay L, Garfield RE. Localization of regulatory protein binding sites in the proximal region of human myometrial connexin 43 gene. Mol Hum Reprod. 1999;5:757–766. doi: 10.1093/molehr/5.8.757. [DOI] [PubMed] [Google Scholar]
- el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Elaut G, Henkens T, Papeleu P, Snykers S, Vinken M, Vanhaecke T, Rogiers V. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab. 2006;7:629–660. doi: 10.2174/138920006778017759. [DOI] [PubMed] [Google Scholar]
- Elcock FJ, Chipman JK, Roberts RA. The rodent nongenotoxic hepatocarcinogen and peroxisome proliferator nafenopin inhibits intercellular communication in rat but not guinea-pig hepatocytes, perturbing S-phase but not apoptosis. Arch Toxicol. 1998;72:439–444. doi: 10.1007/s002040050524. [DOI] [PubMed] [Google Scholar]
- Enat R, Jefferson DM, Ruiz-Opazo N, Gatmaitan Z, Leinwand LA, Reid LM. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984;81:1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann GL, Staecker JL, Richardson AG. Effect of sodium butyrate on primary cultures of adult rat hepatocytes. In Vitro Cell Dev Biol. 1987;23:86–92. doi: 10.1007/BF02623587. [DOI] [PubMed] [Google Scholar]
- Etienne PL, Baffet G, Desvergne B, Boisnard-Rissel M, Glaise D, Guguen-Guillouzo C. Transient expression of c-fos and constant expression of c-myc in freshly isolated and cultured normal adult rat hepatocytes. Oncogene Res. 1988;3:255–262. [PubMed] [Google Scholar]
- Fischer R, Reinehr R, Lu TP, Schonicke A, Warskulat U, Dienes HP, Haussinger D. Intercellular communication via gap junctions in activated rat hepatic stellate cells. Gastroenterology. 2005;128:433–448. doi: 10.1053/j.gastro.2004.11.065. [DOI] [PubMed] [Google Scholar]
- Fraczek J, Bolleyn J, Vanhaecke T, Rogiers V, Vinken M. Primary hepatocyte cultures for pharmaco-toxicological studies: at the busy crossroad of various anti-dedifferentiation strategies. Arch Toxicol. 2013;87:577–610. doi: 10.1007/s00204-012-0983-3. [DOI] [PubMed] [Google Scholar]
- Fujita M, Spray DC, Choi H, Saez J, Jefferson DM, Hertzberg E, Rosenberg LC, Reid LM. Extracellular matrix regulation of cell-cell communication and tissue-specific gene expression in primary liver cultures. Prog Clin Biol Res. 1986;226:333–360. [PubMed] [Google Scholar]
- Fujita M, Spray DC, Choi H, Saez JC, Watanabe T, Rosenberg LC, Hertzberg EL, Reid LM. Glycosaminoglycans and proteoglycans induce gap junction expression and restore transcription of tissue-specific mRNAs in primary liver cultures. Hepatology. 1987;7:1S–9S. doi: 10.1002/hep.1840070702. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ikejima K, Hirose M, Takei Y, Watanabe S, Sato N. Taurine preserves gap junctional intercellular communication in rat hepatocytes under oxidative stress. J Gastroenterol. 2000;35:361–368. doi: 10.1007/s005350050361. [DOI] [PubMed] [Google Scholar]
- Gautam A, Ng OC, Boyer JL. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987;7:216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Gladhaug IP, Refsnes M, Sand TE, Christoffersen T. Effects of butyrate on epidermal growth factor receptor binding, morphology, and DNA synthesis in cultured rat hepatocytes. Cancer Res. 1988;48:6560–6564. [PubMed] [Google Scholar]
- Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J, Braeuning A, Budinsky RA, Burkhardt B, Cameron NR, Camussi G, Cho CS, Choi YJ, Craig Rowlands J, Dahmen U, Damm G, Dirsch O, Donato MT, Dong J, Dooley S, Drasdo D, Eakins R, Ferreira KS, Fonsato V, Fraczek J, Gebhardt R, Gibson A, Glanemann M, Goldring CE, Gómez-Lechón MJ, Groothuis GM, Gustavsson L, Guyot C, Hallifax D, Hammad S, Hayward A, Häussinger D, Hellerbrand C, Hewitt P, Hoehme S, Holzhütter HG, Houston JB, Hrach J, Ito K, Jaeschke H, Keitel V, Kelm JM, Kevin Park B, Kordes C, Kullak-Ublick GA, LeCluyse EL, Lu P, Luebke-Wheeler J, Lutz A, Maltman DJ, Matz-Soja M, McMullen P, Merfort I, Messner S, Meyer C, Mwinyi J, Naisbitt DJ, Nussler AK, Olinga P, Pampaloni F, Pi J, Pluta L, Przyborski SA, Ramachandran A, Rogiers V, Rowe C, Schelcher C, Schmich K, Schwarz M, Singh B, Stelzer EH, Stieger B, Stöber R, Sugiyama Y, Tetta C, Thasler WE, Vanhaecke T, Vinken M, Weiss TS, Widera A, Woods CG, Xu JJ, Yarborough KM, Hengstler JG. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg GS, Bechberger JF, Naus CC. A pre-loading method of evaluating gap junctional communication by fluorescent dye transfer. Biotechniques. 1995;18:490–497. [PubMed] [Google Scholar]
- Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Lampe PD, Sheedy D, Stewart CC, Nicholson BJ, Naus CC. Direct isolation and analysis of endogenous transjunctional ADP from Cx43 transfected C6 glioma cells. Exp Cell Res. 1998;239:82–92. doi: 10.1006/excr.1997.3872. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C, Guillouzo A. Modulation of functional activities in cultured rat hepatocytes. Mol Cell Biochem. 1983;53-54:35–56. doi: 10.1007/BF00225245. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981;294:462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Hamilton GA, Jolley SL, Gilbert D, Coon DJ, Barros S, LeCluyse EL. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell Tissue Res. 2001;306:85–99. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- Hasirci V, Berthiaume F, Bondre SP, Gresser JD, Trantolo DJ, Toner M, Wise DL. Expression of liver-specific functions by rat hepatocytes seeded in treated poly(lactic-coglycolic) acid biodegradable foams. Tissue Eng. 2001;7:385–394. doi: 10.1089/10763270152436445. [DOI] [PubMed] [Google Scholar]
- Hennemann H, Kozjek G, Dahl E, Nicholson B, Willecke K. Molecular cloning of mouse connexins26 and -32: similar genomic organization but distinct promoter sequences of two gap junction genes. Eur J Cell Biol. 1992;58:81–89. [PubMed] [Google Scholar]
- Hernandez-Blazquez FJ, Joazeiro PP, Omori Y, Yamasaki H. Control of intracellular movement of connexins by E-cadherin in murine skin papilloma cells. Exp Cell Res. 2001;270:235–247. doi: 10.1006/excr.2001.5342. [DOI] [PubMed] [Google Scholar]
- Hewitt NJ, Lechón MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, Guillouzo A, Tuschl G, Li AP, LeCluyse E, Groothuis GM, Hengstler JG. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- Higaki N, Mitaka T, Sato F, Hirata K, Mochizuki Y. Maintenance of connexin 32 and 26 expression in primary cultured rat hepatocytes treated with 3-acetylpyridine. J Gastroenterol Hepatol. 2001;16:806–815. doi: 10.1046/j.1440-1746.2001.02529.x. [DOI] [PubMed] [Google Scholar]
- Hou DX, Arimura M, Fukuda M, Oka T, Fujii M. Expression of cell adhesion molecule and albumin genes in primary culture of rat hepatocytes. Cell Biol Int. 2001;25:239–244. doi: 10.1006/cbir.2000.0596. [DOI] [PubMed] [Google Scholar]
- Inoue C, Yamamoto H, Nakamura T, Ichihara A, Okamoto H. Nicotinamide prolongs survival of primary cultured hepatocytes without involving loss of hepatocyte-specific functions. J Biol Chem. 1989;264:4747–4750. [PubMed] [Google Scholar]
- Iredale JP, Arthur MJ. Hepatocyte-matrix interactions. Gut. 1994;35:729–732. doi: 10.1136/gut.35.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom I, Georgoff I, Salditt-Georgieff M, Darnell JE. Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J Cell Biol. 1987;105:2877–2885. doi: 10.1083/jcb.105.6.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Tanaka S, Mori T, Harada Y, Muramatsu A, Morikawa T, Kashima K, Fushiki S. Investigation of parenchymal cell differentiation in organotypic slice culture of mouse fetal liver under administration of sodium butyrate. Cell Biol Toxicol. 2002;18:147–156. doi: 10.1023/a:1015500219572. [DOI] [PubMed] [Google Scholar]
- Kamendulis LM, Isenberg JS, Smith JH, Pugh G, Lington AW, Klaunig JE. Comparative effects of phthalate monoesters on gap junctional intercellular communication and peroxisome proliferation in rodent and primate hepatocytes. J Toxicol Environ Health A. 2002;65:569–588. doi: 10.1080/152873902317349736. [DOI] [PubMed] [Google Scholar]
- Kanno Y, Loewenstein WR. Low-Resistance Coupling between Gland Cells. Some Observations on Intercellular Contact Membranes and Intercellular Space. Nature. 1964;201:194–195. doi: 10.1038/201194a0. [DOI] [PubMed] [Google Scholar]
- Khetani SR, Berger DR, Ballinger KR, Davidson MD, Lin C, Ware BR. Microengineered Liver Tissues for Drug Testing. J Lab Autom. 2015;20:216–250. doi: 10.1177/2211068214566939. [DOI] [PubMed] [Google Scholar]
- Kia R, Sison RL, Heslop J, Kitteringham NR, Hanley N, Mills JS, Park BK, Goldring CE. Stem cell-derived hepatocytes as a predictive model for drug-induced liver injury: are we there yet? Br J Clin Pharmacol. 2013;75:885–896. doi: 10.1111/j.1365-2125.2012.04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Ruch RJ. Strain and species effects on the inhibition of hepatocyte intercellular communication by liver tumor promoters. Cancer Lett. 1987;36:161–168. doi: 10.1016/0304-3835(87)90087-5. [DOI] [PubMed] [Google Scholar]
- Knop E, Bader A, Böker K, Pichlmayr R, Sewing KF. Ultrastructural and functional differentiation of hepatocytes under long-term culture conditions. Anat Rec. 1995;242:337–349. doi: 10.1002/ar.1092420307. [DOI] [PubMed] [Google Scholar]
- Koebe HG, Wick M, Cramer U, Lange V, Schildberg FW. Collagen gel immobilisation provides a suitable cell matrix for long term human hepatocyte cultures in hybrid reactors. Int J Artif Organs. 1994;17:95–106. [PubMed] [Google Scholar]
- Koide N, Sakaguchi K, Koide Y, Asano K, Kawaguchi M, Matsushima H, Takenami T, Shinji T, Mori M, Tsuji T. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp Cell Res. 1990;186:227–235. doi: 10.1016/0014-4827(90)90300-y. [DOI] [PubMed] [Google Scholar]
- Kojima T, Mitaka T, Paul DL, Mori M, Mochizuki Y. Reappearance and long-term maintenance of connexin32 in proliferated adult rat hepatocytes: use of serum-free L-15 medium supplemented with EGF and DMSO. J Cell Sci. 1995a;108:1347–1357. doi: 10.1242/jcs.108.4.1347. [DOI] [PubMed] [Google Scholar]
- Kojima T, Mitaka T, Shibata Y, Mochizuki Y. Induction and regulation of connexin26 by glucagon in primary cultures of adult rat hepatocytes. J Cell Sci. 1995b;108:2771–2780. doi: 10.1242/jcs.108.8.2771. [DOI] [PubMed] [Google Scholar]
- Kojima T, Mochizuki C, Mitaka T, Mochizuki Y. Effects of melatonin on proliferation, oxidative stress and Cx32 gap junction protein expression in primary cultures of adult rat hepatocytes. Cell Struct Funct. 1997;22:347–356. doi: 10.1247/csf.22.347. [DOI] [PubMed] [Google Scholar]
- Kojima T, Yamamoto M, Tobioka H, Mizuguchi T, Mitaka T, Mochizuki Y. Changes in cellular distribution of connexins 32 and 26 during formation of gap junctions in primary cultures of rat hepatocytes. Exp Cell Res. 1996a;223:314–326. doi: 10.1006/excr.1996.0087. [DOI] [PubMed] [Google Scholar]
- Kojima T, Mitaka T, Mizuguchi T, Mochizuki Y. Effects of oxygen radical scavengers on connexins 32 and 26 expression in primary cultures of adult rat hepatocytes. Carcinogenesis. 1996b;17:537–544. doi: 10.1093/carcin/17.3.537. [DOI] [PubMed] [Google Scholar]
- Kuzma-Kuzniarska M, Yapp C, Pearson-Jones TW, Jones AK, Hulley PA. Functional assessment of gap junctions in monolayer and three-dimensional cultures of human tendon cells using fluorescence recovery after photobleaching. J Biomed Opt. 2014;19:15001. doi: 10.1117/1.JBO.19.1.015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski AP, Baker TK, Klaunig JE. Comparison of glucocorticoid-mediated changes in the expression and function of rat hepatocyte gap junctional proteins. Carcinogenesis. 1994;15:1753–1757. doi: 10.1093/carcin/15.8.1753. [DOI] [PubMed] [Google Scholar]
- Laird DW. The life cycle of a connexin: gap junction formation, removal, and degradation. J Bioenerg Biomembr. 1996;28:311–318. doi: 10.1007/BF02110107. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Nguyen BP, Gil S, Usui M, Olerud J, Takada Y, Carter WG. Cellular interaction of integrin alpha3beta1 with laminin 5 promotes gap junctional communication. J Cell Biol. 1998;143:1735–1747. doi: 10.1083/jcb.143.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–368. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- Lecluyse EL, Alexandre E. Isolation and culture of primary hepatocytes from resected human liver tissue. Methods Mol Biol. 2010;640:57–82. doi: 10.1007/978-1-60761-688-7_3. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Bullock PL, Parkinson A. Strategies for restoration and maintenance of normal hepatic structure and function in long-term cultures of rat hepatocytes. Adv Drug Deliv Rev. 1996;22:133–186. [Google Scholar]
- LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol. 2012;42:501–548. doi: 10.3109/10408444.2012.682115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Bradley G, Zhang JT, Ling V. Differential expression of P-glycoprotein genes in primary rat hepatocyte culture. J Cell Physiol. 1993;157:392–402. doi: 10.1002/jcp.1041570223. [DOI] [PubMed] [Google Scholar]
- Lee SM, Schelcher C, Demmel M, Hauner M, Thasler WE. Isolation of human hepatocytes by a two-step collagenase perfusion procedure. (79).J Vis Exp. 2013 doi: 10.3791/50615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre DL, Piersanti M, Bai XH, Chen ZQ, Lye SJ. Myometrial transcriptional regulation of the gap junction gene, connexin-43. Reprod Fertil Dev. 1995;7:603–611. doi: 10.1071/rd9950603. [DOI] [PubMed] [Google Scholar]
- Leybaert L, Sanderson MJ. Intercellular calcium signaling and flash photolysis of caged compounds. A sensitive method to evaluate gap junctional coupling. Methods Mol Biol. 2001;154:407–430. doi: 10.1385/1-59259-043-8:407. [DOI] [PubMed] [Google Scholar]
- Lin C, Ballinger KR, Khetani SR. The application of engineered liver tissues for novel drug discovery. Expert Opin Drug Discov. 2015;10:519–540. doi: 10.1517/17460441.2015.1032241. [DOI] [PubMed] [Google Scholar]
- Liu J, Siragam V, Gong Z, Chen J, Fridman MD, Leung C, Lu Z, Ru C, Xie S, Luo J, Hamilton RM, Sun Y. Robotic adherent cell injection for characterizing cell-cell communication. IEEE Trans Biomed Eng. 2015;62:119–125. doi: 10.1109/TBME.2014.2342036. [DOI] [PubMed] [Google Scholar]
- Loyer P, Cariou S, Glaise D, Bilodeau M, Baffet G, Guguen-Guillouzo C. Growth factor dependence of progression through G1 and S phases of adult rat hepatocytes in vitro. Evidence of a mitogen restriction point in mid-late G1. J Biol Chem. 1996;271:11484–11492. doi: 10.1074/jbc.271.19.11484. [DOI] [PubMed] [Google Scholar]
- Maes M, Decrock E, Cogliati B, Oliveira AG, Marques PE, Dagli ML, Menezes GB, Mennecier G, Leybaert L, Vanhaecke T, Rogiers V, Vinken M. Connexin and pannexin (hemi)channels in the liver. Front Physiol. 2014;4:405. doi: 10.3389/fphys.2013.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JJ, Bissell DM. Cell-matrix interactions in liver. Semin Cell Biol. 1993;4:189–201. doi: 10.1006/scel.1993.1023. [DOI] [PubMed] [Google Scholar]
- Maher JJ, Friedman SL. Parenchymal and nonparenchymal cell interactions in the liver. Semin Liver Dis. 1993;13:13–20. doi: 10.1055/s-2007-1007334. [DOI] [PubMed] [Google Scholar]
- Mally A, Chipman JK. Non-genotoxic carcinogens: early effects on gap junctions, cell proliferation and apoptosis in the rat. Toxicology. 2002;180:233–248. doi: 10.1016/s0300-483x(02)00393-1. [DOI] [PubMed] [Google Scholar]
- Meda P. Probing the function of connexin channels in primary tissues. Methods. 2000;20:232–244. doi: 10.1006/meth.1999.0940. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Fraslin JM, Piccoli C, Yamasaki H, Guguen-Guillouzo C. Cell contact but not junctional communication (dye coupling) with biliary epithelial cells is required for hepatocytes to maintain differentiated functions. Exp Cell Res. 1987;173:524–533. doi: 10.1016/0014-4827(87)90292-8. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Krutovskikh V, Omori Y, Yamasaki H. Role of blocked gap junctional intercellular communication in non-genotoxic carcinogenesis. Toxicol Lett. 1995;82-83:701–706. doi: 10.1016/0378-4274(95)03588-5. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Piccoli C, Yamasaki H. An improved long-term culture of rat hepatocytes to detect liver tumour-promoting agents: results with phenobarbital. Eur J Pharmacol. 1993;248:59–66. doi: 10.1016/0926-6917(93)90025-l. [DOI] [PubMed] [Google Scholar]
- Mitaka T, Mizuguchi T, Sato F, Mochizuki C, Mochizuki Y. Growth and maturation of small hepatocytes. J Gastroenterol Hepatol. 1998;13(Suppl):S70–77. doi: 10.1111/jgh.1998.13.s1.70. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Mitaka T, Hirata K, Oda H, Mochizuki Y. Alteration of expression of liver-enriched transcription factors in the transition between growth and differentiation of primary cultured rat hepatocytes. J Cell Physiol. 1998;174:273–284. doi: 10.1002/(SICI)1097-4652(199803)174:3<273::AID-JCP1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151:497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- Morsi AS, Godfrey RE, Chipman JK, Minchin SD. Characterisation of the connexin32 promoter and changes in response element complexes in rat liver and hepatocytes during culture associated with oxidative stress. Toxicol In Vitro. 2003;17:191–199. doi: 10.1016/s0887-2333(03)00003-1. [DOI] [PubMed] [Google Scholar]
- Mousa SA. Cell adhesion molecules and extracellular matrix proteins: potential therapeutic applications. Expert Opin Investig Drugs. 1998;7:1159–1171. doi: 10.1517/13543784.7.7.1159. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Kohno H, EL-Assal ON, Dhar DK, Ueda F, Nagasue N. Irsogladine upregulates expressions of connexin32 and connexin26 in the rat liver. Hepatol Res. 2000;18:29–42. doi: 10.1016/s1386-6346(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Nathanson MH, Rios-Velez L, Burgstahler AD, Mennone A. Communication via gap junctions modulates bile secretion in the isolated perfused rat liver. Gastroenterology. 1999;116:1176–1183. doi: 10.1016/s0016-5085(99)70021-1. [DOI] [PubMed] [Google Scholar]
- Nelles E, Butzler C, Jung D, Temme A, Gabriel HD, Dahl U, Traub O, Stumpel F, Jungermann K, Zielasek J, Toyka KV, Dermietzel R, Willecke K. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc Natl Acad Sci U S A. 1996 doi: 10.1073/pnas.93.18.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu MJ, Babcock KL, Hertzberg EL, Paul DL, Nicholson BJ, Pitot HC. Colocalized alterations in connexin32 and cytochrome P450IIB1/2 by phenobarbital and related liver tumor promoters. Cancer Res. 1994a;54:3145–3152. [PubMed] [Google Scholar]
- Neveu MJ, Sattler CA, Sattler GL, Hully JR, Hertzberg EL, Paul DL, Nicholson BJ, Pitot HC. Differences in the expression of connexin genes in rat hepatomas in vivo and in vitro. Mol Carcinog. 1994b;11:145–154. doi: 10.1002/mc.2940110305. [DOI] [PubMed] [Google Scholar]
- Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature. 1985;317:331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Novikoff PM, Ikeda T, Hixson DC, Yam A. Characterizations of and interactions between bile ductule cells and hepatocytes in early stages of rat hepatocarcinogenesis induced by ethionine. Am J Pathol. 1991;139:1351–1368. [PMC free article] [PubMed] [Google Scholar]
- Oyamada M, Krutovskikh VA, Mesnil M, Partensky C, Berger F, Yamasaki H. Aberrant expression of gap junction gene in primary human hepatocellular carcinomas: increased expression of cardiac-type gap junction gene connexin 43. Mol Carcinog. 1990;3:273–278. doi: 10.1002/mc.2940030507. [DOI] [PubMed] [Google Scholar]
- Pahernik SA, Thasler WE, Doser M, Gomez-Lechon MJ, Castell MJ, Planck H, Koebe HG. High density culturing of porcine hepatocytes immobilized on nonwoven polyurethane-based biomatrices. Cells Tissues Organs. 2001;168:170–177. doi: 10.1159/000047832. [DOI] [PubMed] [Google Scholar]
- Paine AJ, Andreakos E. Activation of signalling pathways during hepatocyte isolation: relevance to toxicology in vitro. Toxicol In Vitro. 2004;18:187–193. doi: 10.1016/s0887-2333(03)00146-2. [DOI] [PubMed] [Google Scholar]
- Papeleu P, Vanhaecke T, Elaut G, Vinken M, Henkens T, Snykers S, Rogiers V. Differential effects of histone deacetylase inhibitors in tumor and normal cells-what is the toxicological relevance? Crit Rev Toxicol. 2005;35:363–378. doi: 10.1080/10408440590935639. [DOI] [PubMed] [Google Scholar]
- Papeleu P, Vanhaecke T, Henkens T, Elaut G, Vinken M, Snykers S, Rogiers V. Isolation of rat hepatocytes. Methods Mol Biol. 2006;320:229–237. doi: 10.1385/1-59259-998-2:229. [DOI] [PubMed] [Google Scholar]
- Rae RS, Mehta PP, Chang CC, Trosko JE, Ruch RJ. Neoplastic phenotype of gap-junctional intercellular communication-deficient WB rat liver epithelial cells and its reversal by forced expression of connexin 32. Mol Carcinog. 1998;22:120–127. doi: 10.1002/(sici)1098-2744(199806)22:2<120::aid-mc7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Raptis LH, Brownell HL, Firth KL, Mackenzie LW. A novel technique for the study of intercellular, junctional communication: electroporation of adherent cells on a partly conductive slide. DNA Cell Biol. 1994;13:963–975. doi: 10.1089/dna.1994.13.963. [DOI] [PubMed] [Google Scholar]
- Ren P, de Feijter AW, Paul DL, Ruch RJ. Enhancement of liver cell gap junction protein expression by glucocorticoids. Carcinogenesis. 1994;15:1807–1813. doi: 10.1093/carcin/15.9.1807. [DOI] [PubMed] [Google Scholar]
- Ren P, Ruch RJ. Inhibition of gap junctional intercellular communication by barbiturates in long-term primary cultured rat hepatocytes is correlated with liver tumour promoting activity. Carcinogenesis. 1996;17:2119–2124. doi: 10.1093/carcin/17.10.2119. [DOI] [PubMed] [Google Scholar]
- Ringwald M, Baribault H, Schmidt C, Kemler R. The structure of the gene coding for the mouse cell adhesion molecule uvomorulin. Nucleic Acids Res. 1991;19:6533–6539. doi: 10.1093/nar/19.23.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma MG, Orsler DJ, Coleman R. Canalicular retention as an in vitro assay of tight junctional permeability in isolated hepatocyte couplets: effects of protein kinase modulation and cholestatic agents. Fundam Appl Toxicol. 1997;37:71–81. doi: 10.1006/faat.1997.2309. [DOI] [PubMed] [Google Scholar]
- Ruch RJ. The role of gap junctional intercellular communication in neoplasia. Ann Clin Lab Sci. 1994;24:216–231. [PubMed] [Google Scholar]
- Ruch RJ, Klaunig JE. Effects of tumor promoters, genotoxic carcinogens and hepatocytotoxins on mouse hepatocyte intercellular communication. Cell Biol Toxicol. 1986;2:469–483. doi: 10.1007/BF00117849. [DOI] [PubMed] [Google Scholar]
- Saez JC, Spray DC, Nairn AC, Hertzberg E, Greengard P, Bennett MV. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986;83:2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Bennett MV, Spray DC. Carbon tetrachloride at hepatotoxic levels blocks reversibly gap junctions between rat hepatocytes. Science. 1987;236:967–969. doi: 10.1126/science.3576214. [DOI] [PubMed] [Google Scholar]
- Sato F, Mitaka T, Mizuguchi T, Mochizuki Y, Hirata K. Effects of nicotinamide-related agents on the growth of primary rat hepatocytes and formation of small hepatocyte colonies. Liver. 1999;19:481–488. doi: 10.1111/j.1478-3231.1999.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Sauer V, Roy-Chowdhury N, Guha C, Roy-Chowdhury J. Induced pluripotent stem cells as a source of hepatocytes. Curr Pathobiol Rep. 2014;2:11–20. doi: 10.1007/s40139-013-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmid VJ, Parrish AR. Selective disruption of cadherin/catenin complexes by oxidative stress in precision-cut mouse liver slices. Toxicol Sci. 2001;61:389–394. doi: 10.1093/toxsci/61.2.389. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seo SJ, Park IK, Yoo MK, Shirakawa M, Akaike T, Cho CS. Xyloglucan as a synthetic extracellular matrix for hepatocyte attachment. J Biomater Sci Polym Ed. 2004;15:1375–1387. doi: 10.1163/1568562042368059. [DOI] [PubMed] [Google Scholar]
- Shoda T, Mitsumori K, Onodera H, Toyoda K, Uneyama C, Takada K, Hirose M. Liver tumor-promoting effect of beta-naphthoflavone, a strong CYP 1A1/2 inducer, and the relationship between CYP 1A1/2 induction and Cx32 decrease in its hepatocarcinogenesis in the rat. Toxicol Pathol. 2000;28:540–547. doi: 10.1177/019262330002800406. [DOI] [PubMed] [Google Scholar]
- Siddiqui MU, Benatmane S, Zachayus JL, Plas C. Gap junctional communication and regulation of the glycogenic response to insulin by cell density and glucocorticoids in cultured fetal rat hepatocytes. Hepatology. 1999;29:1147–1155. doi: 10.1002/hep.510290443. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Fujita M, Saez JC, Choi H, Watanabe T, Hertzberg E, Rosenberg LC, Reid LM. Proteoglycans and glycosaminoglycans induce gap junction synthesis and function in primary liver cultures. J Cell Biol. 1987;105:541–551. doi: 10.1083/jcb.105.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Voltage dependence of junctional conductance in early amphibian embryos. Science. 1979;204:432–434. doi: 10.1126/science.312530. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Staecker JL, Sattler CA, Pitot HC. Sodium butyrate preserves aspects of the differentiated phenotype of normal adult rat hepatocytes in culture. J Cell Physiol. 1988;135:367–376. doi: 10.1002/jcp.1041350303. [DOI] [PubMed] [Google Scholar]
- Stoehr SA, Isom HC. Gap junction-mediated intercellular communication in a long-term primary mouse hepatocyte culture system. Hepatology. 2003;38:1125–1135. doi: 10.1053/jhep.2003.50418. [DOI] [PubMed] [Google Scholar]
- Stumpel F, Ott T, Willecke K, Jungermann K. Connexin 32 gap junctions enhance stimulation of glucose output by glucagon and noradrenaline in mouse liver. Hepatology. 1998;28:1616–1620. doi: 10.1002/hep.510280622. [DOI] [PubMed] [Google Scholar]
- Stutenkemper R, Geisse S, Schwarz HJ, Look J, Traub O, Nicholson BJ, Willecke K. The hepatocyte-specific phenotype of murine liver cells correlates with high expression of connexin32 and connexin26 but very low expression of connexin43. Exp Cell Res. 1992;201:43–54. doi: 10.1016/0014-4827(92)90346-a. [DOI] [PubMed] [Google Scholar]
- Sugimachi K, Sosef MN, Baust JM, Fowler A, Tompkins RG, Toner M. Long-term function of cryopreserved rat hepatocytes in a coculture system. Cell Transplantation. 2004;13:187–195. doi: 10.3727/000000004773301799. [DOI] [PubMed] [Google Scholar]
- Tateno C, Yoshizato K. Growth potential and differentiation capacity of adult rat hepatocytes in vitro. Wound Repair Regen. 1999;7:36–44. doi: 10.1046/j.1524-475x.1999.00036.x. [DOI] [PubMed] [Google Scholar]
- Temme A, Stumpel F, Sohl G, Rieber EP, Jungermann K, Willecke K, Ott T. Dilated bile canaliculi and attenuated decrease of nerve-dependent bile secretion in connexin32-deficient mouse liver. Pflugers Arch. 2001;442:961–966. doi: 10.1007/s004240100623. [DOI] [PubMed] [Google Scholar]
- Trosko JE, Chang CC, Madhukar BV. The role of modulated gap junctional intercellular communication in epigenetic toxicology. Risk Anal. 1994;14:303–312. doi: 10.1111/j.1539-6924.1994.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Tuschl G, Hrach J, Walter Y, Hewitt PG, Mueller SO. Serum-free collagen sandwich cultures of adult rat hepatocytes maintain liver-like properties long term: a valuable model for in vitro toxicity and drug-drug interaction studies. Chem Biol Interact. 2009;181:124–137. doi: 10.1016/j.cbi.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Tuschl G, Mueller SO. Effects of cell culture conditions on primary rat hepatocytes-cell morphology and differential gene expression. Toxicology. 2006;218:205–215. doi: 10.1016/j.tox.2005.10.017. [DOI] [PubMed] [Google Scholar]
- van der Zandt PT, de Feijter AW, Homan EC, Spaaij C, de Haan LH, van Aelst AC, Jongen WM. Effects of cigarette smoke condensate and 12-O-tetradecanoylphorbol-13-acetate on gap junction structure and function in cultured cells. Carcinogenesis. 1990;11:883–888. doi: 10.1093/carcin/11.6.883. [DOI] [PubMed] [Google Scholar]
- Vanhaecke T, Papeleu P, Elaut G, Rogiers V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: toxicological point of view. Curr Med Chem. 2004;11:1629–1643. doi: 10.2174/0929867043365099. [DOI] [PubMed] [Google Scholar]
- Vanhaecke T, Rogiers V. Hepatocyte cultures in drug metabolism and toxicological research and testing. Methods Mol Biol. 2006;320:209–227. doi: 10.1385/1-59259-998-2:209. [DOI] [PubMed] [Google Scholar]
- Vinken M. Gap junctions and non-neoplastic liver disease. J Hepatol. 2012;57:655–662. doi: 10.1016/j.jhep.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Vinken M, De Kock J, Oliveira AG, Menezes GB, Cogliati B, Dagli ML, Vanhaecke T, Rogiers V. Modifications in connexin expression in liver development and cancer. Cell Commun Adhes. 2012a;19:55–62. doi: 10.3109/15419061.2012.712576. [DOI] [PubMed] [Google Scholar]
- Vinken M, Decrock E, De Vuyst E, De Bock M, Vandenbroucke RE, De Geest BG, Demeester J, Sanders NN, Vanhaecke T, Leybaert L, Rogiers V. Connexin32 hemichannels contribute to the apoptotic-to-necrotic transition during Fas-mediated hepatocyte cell death. Cell Mol Life Sci. 2010;67:907–918. doi: 10.1007/s00018-009-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M, Decrock E, De Vuyst E, Ponsaerts R, D’Hondt C, Bultynck G, Ceelen L, Vanhaecke T, Leybaert L, Rogiers V. Connexins: sensors and regulators of cell cycling. Biochim Biophys Acta. 2011;1815:13–25. doi: 10.1016/j.bbcan.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Vinken M, Decrock E, Vanhaecke T, Leybaert L, Rogiers V. Connexin43 signaling contributes to spontaneous apoptosis in cultures of primary hepatocytes. Toxicol Sci. 2012b;125:175–186. doi: 10.1093/toxsci/kfr277. [DOI] [PubMed] [Google Scholar]
- Vinken M, Doktorova T, Decrock E, Leybaert L, Vanhaecke T, Rogiers V. Gap junctional intercellular communication as a target for liver toxicity and carcinogenicity. Crit Rev Biochem Mol Biol. 2009;44:201–222. doi: 10.1080/10409230903061215. [DOI] [PubMed] [Google Scholar]
- Vinken M, Henkens T, De Rop E, Fraczek J, Vanhaecke T, Rogiers V. Biology and pathobiology of gap junctional channels in hepatocytes. Hepatology. 2008;47:1077–1088. doi: 10.1002/hep.22049. [DOI] [PubMed] [Google Scholar]
- Vinken M, Henkens T, Snykers S, Lukaszuk A, Tourwé D, Rogiers V, Vanhaecke T. The novel histone deacetylase inhibitor 4-Me2N-BAVAH differentially affects cell junctions between primary hepatocytes. Toxicology. 2007;236:92–102. doi: 10.1016/j.tox.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Vinken M, Henkens T, Vanhaecke T, Papeleu P, Geerts A, Van Rossen E, Chipman JK, Meda P, Rogiers V. Trichostatin a enhances gap junctional intercellular communication in primary cultures of adult rat hepatocytes. Toxicol Sci. 2006a;91:484–492. doi: 10.1093/toxsci/kfj152. [DOI] [PubMed] [Google Scholar]
- Vinken M, Papeleu P, Snykers S, De Rop E, Henkens T, Chipman JK, Rogiers V, Vanhaecke T. Involvement of cell junctions in hepatocyte culture functionality. Crit Rev Toxicol. 2006b;36:299–318. doi: 10.1080/10408440600599273. [DOI] [PubMed] [Google Scholar]
- Vinken M, Vanhaecke T, Rogiers V. Primary hepatocyte cultures as in vitro tools for toxicity testing: quo vadis? Toxicol In Vitro. 2012c;26:541–544. doi: 10.1016/j.tiv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta. 2013;1828:35–50. doi: 10.1016/j.bbamem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Huang F, Chen Z, Li S. Downregulation of connexin 32 attenuates hypoxia/reoxygenation injury in liver cells. J Biochem Mol Toxicol. 2015;29:189–197. doi: 10.1002/jbt.21684. [DOI] [PubMed] [Google Scholar]
- Wanson JC, Drochmans P, Mosselmans R, Ronveaux MF. Adult rat hepatocytes in primary monolayer culture. Ultrastructural characteristics of intercellular contacts and cell membrane differentiations. J Cell Biol. 1977;74:858–877. doi: 10.1083/jcb.74.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- Yamaji S, Droggiti A, Lu SC, Martinez-Chantar ML, Warner A, Varela-Rey M. S-Adenosylmethionine regulates connexins sub-types expressed by hepatocytes. Eur J Cell Biol. 2011;90:312–322. doi: 10.1016/j.ejcb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H. Non-genotoxic mechanisms of carcinogenesis: studies of cell transformation and gap junctional intercellular communication. Toxicol Lett. 1995;77:55–61. doi: 10.1016/0378-4274(95)03272-x. [DOI] [PubMed] [Google Scholar]
- Yamasaki H. Cellular and molecular methods to study the role of gap junctional intercellular communication in toxicology. Toxicol In Vitro. 1997;11:535–542. doi: 10.1016/s0887-2333(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Naus CC. Role of connexin genes in growth control. Carcinogenesis. 1996;17:1199–1213. doi: 10.1093/carcin/17.6.1199. [DOI] [PubMed] [Google Scholar]
- Yang J, Ichikawa A, Tsuchiya T. A novel function of connexin 32: marked enhancement of liver function in a hepatoma cell line. Biochem Biophys Res Commun. 2003a;307:80–85. doi: 10.1016/s0006-291x(03)01117-3. [DOI] [PubMed] [Google Scholar]
- Yang B, Guo M, Herman JG, Clark DP. Aberrant promoter methylation profiles of tumor suppressor genes in hepatocellular carcinoma. Am J Pathol. 2003b;163:1101–1107. doi: 10.1016/S0002-9440(10)63469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li WH. Assaying dynamic cell-cell junctional communication using noninvasive and quantitative fluorescence imaging techniques: LAMP and infrared-LAMP. Nat Protoc. 2009;4:94–101. doi: 10.1038/nprot.2008.219. [DOI] [PubMed] [Google Scholar]
- Yang TH, Miyoshi H, Ohshima N. Novel cell immobilization method utilizing centrifugal force to achieve high-density hepatocyte culture in porous scaffold. J Biomed Mater Res. 2001;55:379–386. doi: 10.1002/1097-4636(20010605)55:3<379::aid-jbm1026>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Yano T, Hernandez-Blazquez FJ, Omori Y, Yamasaki H. Reduction of malignant phenotype of HEPG2 cell is associated with the expression of connexin 26 but not connexin 32. Carcinogenesis. 2001;22:1593–1600. doi: 10.1093/carcin/22.10.1593. [DOI] [PubMed] [Google Scholar]
- Yano T, Yamasaki H. Regulation of cellular invasion and matrix metalloproteinase activity in HepG2 cell by connexin 26 transfection. Mol Carcinog. 2001;31:101–109. doi: 10.1002/mc.1045. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Watanabe S, Hirose M, Miyazaki A, Sato N. Dimethylsulfoxide maintains intercellular communication by preserving the gap junctional protein connexin32 in primary cultured hepatocyte doublets from rats. J Gastroenterol Hepatol. 1997;12:325–330. doi: 10.1111/j.1440-1746.1997.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhong Q. Histone deacetylase inhibitors and cell death. Cell Mol Life Sci. 2014;71:3885–3901. doi: 10.1007/s00018-014-1656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Thorgeirsson SS. Modulation of connexins during differentiation of oval cells into hepatocytes. Exp Cell Res. 1994;213:37–42. doi: 10.1006/excr.1994.1170. [DOI] [PubMed] [Google Scholar]
- Ziambaras K, Lecanda F, Steinberg TH, Civitelli R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J Bone Miner Res. 1998;13:218–228. doi: 10.1359/jbmr.1998.13.2.218. [DOI] [PubMed] [Google Scholar]