Abstract
Background
Immune-measurements that distinguish SOT recipients who control CMV infection from those who progress to CMV-disease (CMV-dz) may be clinically useful in guiding tailored prevention strategies. We previously reported that elevated plasma levels of the immune-modulator IL-10 are associated with late CMV-dz. Here we evaluate whether IL-10 levels measured soon after prophylaxis discontinuation are predictive of CMV-dz risk.
Methods
Plasma IL-10 levels were quantitatively measured by ELISA kit in 40 D+/R− SOT patients. All 40 D+/R− high-risk patients were prospectively followed for at least 12 months post-SOT: 13 subjects developed CMV-dz, all within 6 months of prophylaxis discontinuation.
Results
IL-10 was detectable at the first post-prophylaxis measurement for 11 of 13 subjects who developed CMV-dz. In contrast, IL-10 was detectable in only 6 of 27 CMV asymptomatic patients. Monitoring IL-10 plasma levels within 1 month prophylaxis suspension appeared to have clinically useful level of 85% sensitivity and 78% specificity.
Conclusions
The exact role of IL-10 with its multiple immunoregulatory effects during CMV infection is not clear. Moreover, IL-10 production can be influenced by pathological and infectious contexts, and/or anti-rejection immunosuppressant therapy. Despite mechanisms of IL-10 dysregulation may substantially differ among SOT patients, our findings suggest that measurable plasma IL-10 soon after prophylaxis discontinuation may be an adequate indicator of subsequent CMV-dz. If a similar prognostic performance is confirmed in a larger D+/R− cohort, IL-10 plasma levels could be used to guide the length of prophylaxis, providing a clinically useful means to reduce the incidence of CMV-dz in high risk patients.
Introduction
Cytomegalovirus (CMV) is the single most important viral infection in solid organ transplant (SOT) patients, both directly and indirectly affecting allograft rejection, decreased graft function, patient survival, and predisposition to opportunistic infections and malignancies[7,18,19]. Despite universal antiviral prophylaxis with the orally available agent valganciclovir (VALGAN) a CMV seronegative patient receiving an organ from a CMV seropositive donor (D+R−) remains at high risk for CMV complications[25]. After discontinuation of antiviral prophylaxis, CMV can replicate in the absence of adequate immunity during primary infection, predisposing D+R− SOT patient to late-onset CMV-dz[12]. The host immune response is critical for successful control of CMV infection after SOT, and it has been reported that negative immune-modulators, such as PD-1 and IL10 are elevated in SOT recipients who do not control CMV infection and develop clinical symptoms after discontinuation of antiviral prophylaxis[11,20,21,32]. In particular, high levels (>20 pg/ml) of plasma IL-10, an immune regulatory cytokine have been associated with suppression of CMV-specific cellular immune responses, uncontrolled viremia and clinical disease in D+R− SOT recipients[20,22]. A strong inverse association was found between levels of plasma IL-10 and CMV-specific CD8 and/or CD4 T cells in D+R− SOT recipients[20,22]. Primary CMV-specific T cell responses were detected early post-transplant only in D+R− SOT recipients whose levels of plasma IL-10 were low. Additionally, IL-10 levels were significantly higher at viraemic time points compared to time points at which CMV viraemia was undetectable in D+R− SOT recipients [22].
We hypothesized that plasma IL-10 levels, measured after discontinuation of antiviral prophylaxis might identify SOT patients at increased risk for CMV-dz. Optimally, the length of prophylaxis could be tailored to IL-10 levels in an individual patient. Such information may be clinically relevant in guiding prevention and could be useful to decide whether to prolong or to resume VALGAN prophylaxis, to prevent progression to CMV-dz[1,18]. With the aim of evaluating the potential for an individualized antiviral prophylaxis guided by IL-10 levels, we have measured plasma IL-10 levels in D+R− transplant recipients who progressed to CMV-dz and were treated with intravenous ganciclovir (GCV), as well as in CMV asymptomatic patients who did not receive any CMV antiviral treatment after prophylaxis discontinuation. The primary objective was to assess whether CMV-dz could be predicted to either warrant prophylaxis prolongation or its resumption for patients at enhanced risk for CMV-dz. A panel of analyses was performed to address when this immunological marker can identify patients at risk for progressing to CMV-dz.
Material and Methods
Patient population
This is a cumulative analysis encompassing two prospective studies[22,23], that were approved by the Institutional Review Boards (IRB) of the University of Washington Medical Center (UWMC, IRB 24704) and the City of Hope Comprehensive Cancer Center (COH, IRB 04024). All enrolled D+R− patients received an SOT at UWMC. Accessible plasma specimens from thirty-nine consecutive (UPN 1–39) D+R− liver (L) or kidney (K) transplant patients were analyzed for IL-10 levels up to 12 months post-SOT. UPN 17 and 18 were not included in the analysis because they were found to be CMV seropositive at time of transplant[22]. Data from UPN 31–39 have not been published. Additionally, prospective specimens from three D+R− patients from a previous study who developed CMV-dz[23] were evaluated, to better assess sensitivity. The full set of study subjects consisted of 13 D+R− patients who progressed to CMV-dz, and 27 D+R− patients who remained asymptomatic during the 12 month observation period (Table 1). The reason for transplant and the duration of prophylaxis are indicated in Table 1. Patients received induction therapy with daclizumab or antilymphocyte antibodies and maintenance immunosuppression with prednisone, tacrolimus, or cyclosporine and either azathioprine or mycophenolate mofetil. Rejection (Table 1) was treated with steroids or/and antithymocyte globulin, as detailed elsewhere[24]. Further clinical details of these cohorts have been previously published[22,23].
Table 1.
| UPN | Reason for SOT | Months of VALGAN prophylaxis |
Antirejection treatmenta |
Month of CMV disease |
|---|---|---|---|---|
| 1 K | Retransplant/Graft Failure | 0–3.5 | 3 | NONE |
| 2 L | Postnectrotic Type C Cirrhosis | 0–3.5 | Never | NONE |
| 3 K | Alport’s Syndrome | 0–3.5 | Never | NONE |
| 4 K | Diabetes Mellitus - Type II | 0–3 | Never | NONE |
| 5 K | IgA Nephropathy | 0–6 | Never | NONE |
| 6 K | IgA Nephropathy | 0–3.5 | Never | NONE |
| 7 L | Postnectrotic Type C Cirrhosis | 0–3 | Never | NONE |
| 8 L | Postnectrotic Type C Cirrhosis | 0–3.5 | Never | NONE |
| 9 L | Postnectrotic Type C Cirrhosis | 0–3 | Never | NONE |
| 10 L | Postnectrotic Type C Cirrhosis/Hepatocellular Carcinoma | 0–3.5 | 2 | 7 |
| 11 L | Hepatocellular Carcinoma | 0–3 | Never | NONE |
| 12 K | ESRD secondary to Hypertension | 0–3.5 | Never | 4.5 |
| 13 K | Congenital Obstructive Uropathty | 0–6 | Never | NONE |
| 14 L | Ulcerative Colitis | 0–3 | Never | NONE |
| 15 K | Hypertensive Nephrosclerosis | 0–6 | Never | NONE |
| 16 L | Postnectrotic Type C Cirrhosis | 0–3 | Never | NONE |
| 19 K | ESRD secondary to hyperpara-thyroidism | 0–3 | Never | 4.5 |
| 20 K | Lithium Toxicity | 0–3.5 | Never | NONE |
| 21 L | Alcoholic Cirrhosis with Hepatitis C | 0–3 | 2 | 4.5 |
| 22 K | Calcineurin Inhibitor Nephrotoxicity | 0–6 | Never | NONE |
| 23 K | Chronic Glomerulo-nephritis | 0–3 | Never | 4.5 |
| 24 L | Postnectrotic Type C Cirrhosis | 0–3 | Never | NONE |
| 25 L | Postnectrotic Type B Cirrhosis | 0–3 | Never | NONE |
| 26 K | Focal Glomerular Sclerosis | 0–3.5 | 2 | 5 |
| 27 L | Postnectrotic Type C Cirrhosis | 0–3 | Never | NONE |
| 28 K | Diabetes Mellitus - Type II | 0–6 | Never | NONE |
| 29 K | Diabetes Mellitus -Type II | 0–4 | 2 | 6.5 |
| 30 K | Diabetes Mellitus - Type II | 0–3 | 4 | 3.5 |
| 31 K | Diabetes Mellitus - Type II | 0–6 | Never | NONE |
| 32 L | Alcoholic Cirrhosis | 0–3 | Never | NONE |
| 33 K | Chronic Glomerulonephritis | 0–6 | 7, 8 | NONE |
| 34K | Chronic Glomerulo-nephritis | 0–6 | Never | 8 |
| 35 K | Crescentic Glomerulonephritis | 0–6 | Never | NONE |
| 36 K | Chronic Glomerulonephritis | 0–6 | Never | NONE |
| 37 L | Postnecrotic Type C Cirrhosis | 0–2 | Never | 6.5 |
| 38 K | Diabetes Mellitus - Type II | 0–3.5 | 5 | NONE |
| 39 L | Postnectrotic Type C Cirrhosis | 0–3 | Never | NONE |
| 3* L | Idiopathic/ Criptogenic Cirrhosis | 0–3 | 3, 4.5, 6 | 4 |
| 8* L | Primary Biliary Cirrhosis | 0–3 | Never | 5.5 |
| 15* L | Alcoholic Cirrhosis with Hepatitis C | 0–3 | Never | 4 |
Month of prednisone and antithymocyte globulin treatments for acute rejection.
Shaded rows indicate the 13 patients who developed CMV-dz;
identifies patient from Ref. [12] study;
K= kidney recipient; L= liver recipient
Blood specimen collection and logistics
Blood specimens were collected up to 12 months post-transplant, in heparinized Vacutainer® blood collection tubes according to the United States Public Health Service guidelines and Helsinki doctrine, either at UWMC or at the patient’s Primary Care Provider (PCP)[22,23]. Most of the accessible specimens (N=268 out of a total of 306 specimens obtained) for IL-10 measurements were sampled at biweekly intervals over the first four months after prophylaxis discontinuation (as detailed in Figure 1). With the exception of asymptomatic UPN 20 (first accessible specimen: 60 days after prophylaxis discontinuation), 39 evaluated patients had specimens tested for IL-10 levels within 1 month after prophylaxis discontinuation (Figure 1). CMV viral load measurements were performed at the same time points as IL-10. All specimens were shipped overnight to COH and to UWMC with identical shipping procedures[22,23]. Clinical and diagnostic assays, including CMV viremia were done at UWMC, IL-10 monitoring was performed at COH. Plasma was recovered from blood specimens by centrifugation and stored in centrally monitored −20 °C freezers, until analyzed.
Figure 1.
IL-10 timeline for each of the 40 UPN (y axes). Times of IL-10 measurements (months after prophylaxis discontinuation, x axes) are shown as vertical tick marks with the line to the right indicating the respective IL-10 measurement. The red lines indicate undetectable IL-10 levels (<2 pg/ml, below the assay sensitivity, see Materials and Methods). Black lines indicate detectable IL-10, with width proportional to the logarithm of IL-10 value. Black diamonds indicate the time of CMV-dz onset, in the 13 patients who developed CMV-dz. The top 13 lines show the 13 patients who developed CMV-dz. Vertical red tick marks indicate time points in which CMV DNA was detected. The first 3 lines show patients selected retrospectively after observing CMV-dz. (* symbol identifies patients from Ref. [12] study) All other patients were followed 12 months post-transplant without developing CMV-dz.
Viremia
Quantitative TaqMan real time polymerase chain reaction (PCR, sensitivity 100 copies/ml) to assess CMV viral load (DNAemia) was performed in plasma as previously described [4,23]. Viral load testing was performed as batch testing at the end of the study for research purposes only, and therefore it was not available “real-time” for treatment decisions. CMV PCR assays in this study were done prior to the time of introduction of the NIST and WHO standards. We have subsequently performed calibration studies and concluded that the approximate conversion between copies/mL and IU/mL (WHO standard) is approximately: 250 CMV DNA copies/mL = 1 CMV IU/mL.
IL-10 measurements
Levels of IL-10 were quantitatively measured in the plasma of D+R− patients by “Human IL-10 ELISA Ready-SET-GO!” (assay sensitivity: ≥2 pg/ml; standard curve range: 2 pg/mL - 300 pg/mL; eBioscience, San Diego, USA), following manufacturer’s procedure.
Plasma samples were tested in duplicate and means were calculated. Samples were re-tested if differences between duplicate values were ≥25%.
Statistical analysis
Sensitivity and specificity are estimated from D+R− patients developing CMV-dz or remaining asymptomatic during the 12 month observation period, respectively. The focus on detectable levels of IL-10 in the immediate post-prophylaxis period is based on examination of the data, as well as on practical and biological considerations, so future validation data is called for. Confidence intervals (CI) are exact, based on the binomial distribution using a 95% confidence level. A log-rank test comparing the hazard of CMV-dz is the only use of formal hypotheses testing, as both good and poor predictive performance occurs far from the null hypothesis situation. Statistical computing was done using R version 3.1.1, with the survival and ROCR packages. Scatter plots were obtained using GraphPad Prism-V5.02 (San Diego, CA).
Results
Overview of IL-10 profiles in the SOT population
To analyze dynamics and kinetics of plasma IL-10 on a single patient basis, we measured plasma IL-10 levels in 40 D+R− recipients, who received VALGAN prophylaxis. Timing of the longitudinal plasma IL-10 monitoring is shown in Figure 1 for each of the UPN analyzed. All subjects were prospectively monitored for CMV disease for one year post-SOT. All CMV-dz patients required hospitalization and were treated with intravenous GCV (Table 1, shaded rows), while asymptomatic patients did not receive any CMV antiviral treatment after prophylaxis discontinuation (Table 1, clear rows). In accordance with results from similar transplant settings, CMV-dz developed between 3.5 and 8 months after SOT[20,22,24,25]. All 13 CMV-dz events in our study occurred two to five months after discontinuation of prophylaxis (Figure 1 and Table 1). IL-10 measurements were taken at bi-weekly intervals, when possible, focusing on the period of highest risk for CMV-dz [20,22,24,25].
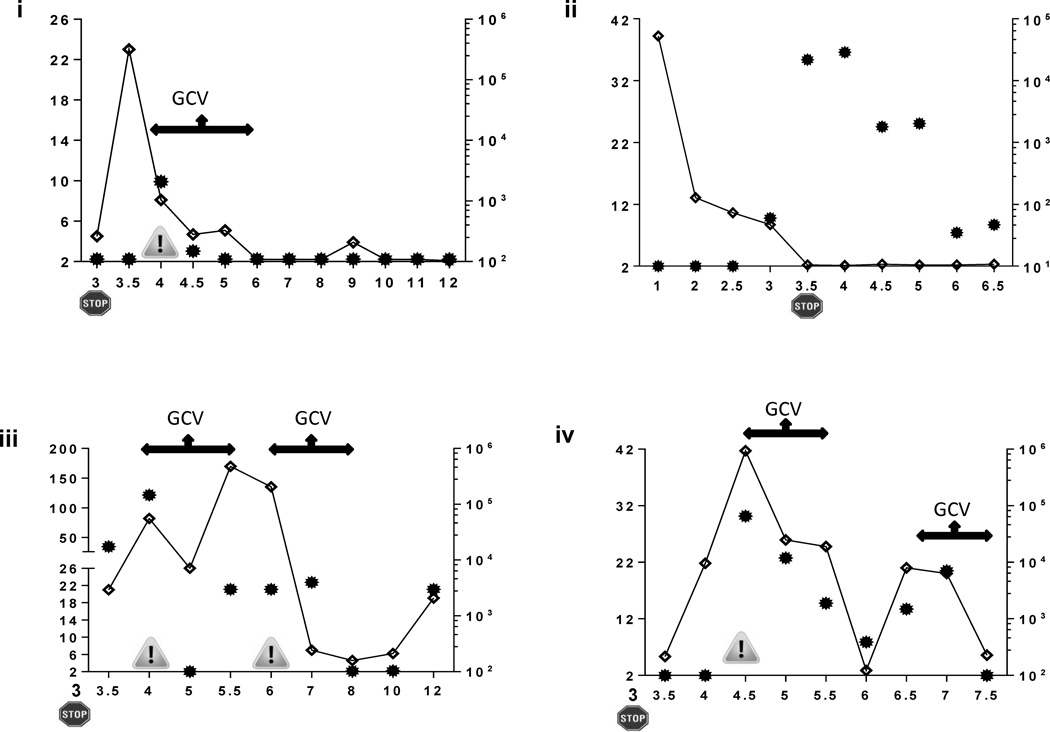
While IL-10 levels generally declined and/or remained low during prophylaxis administration[22], they varied widely after VALGAN discontinuation. Figure 2 provides longitudinal profiles of plasma IL-10 levels together with viremia levels and timing of GCV therapy for representative patients. In UPN 15, IL-10 increased after the end of prophylaxis, just before CMV-dz was diagnosed. After a course of GCV therapy, CMV viremia was controlled, symptoms resolved, and IL-10 became undetectable for three months (Figure 2 i). However, for UPN 3* and UPN 23 in whom antivirals were discontinued when IL-10 levels were still elevated, viremia or symptomatic relapse occurred; re-treatment was necessary, though resistance was not observed (Figure 2 iii, iv). In contrast, UPN 2 (Figure 2 ii, max CMV DNaemia 301830 copies/ml at 5 months post-SOT) and 25 (data not shown, max CMV DNAemia 4030 copies copies/ml at 5.5 months post-SOT) who had undetectable IL-10 at the end of prophylaxis, controlled asymptomatic CMV viremia after prophylaxis discontinuation without the need for antiviral treatment.
Figure 2.
Representative longitudinal profiles of plasma IL-10 (continuous lines) and viremia levels (filled dots) in CMV-dz and asymptomatic D+R− patients. In the panels, the STOP sign indicates the post-SOT month (x axes) of VALGAN prophylaxis discontinuation. The danger sign shows the time of diagnosis of CMV-dz; the arrow lines, the time length of GCV antiviral therapy. In UPN 15 (i), 3* (iii) and 23 (iv), high levels of IL-10 (y axes) preceded the onset of CMV-dz. In UPN 8 (ii), levels of IL-10 became undetectable at prophylaxis discontinuation; sustained CMV viraemia (z axes) remained asymptomatic and was eventually controlled.
An high proportion of the CMV-dz patients (6 out of 13, Table 1) received treatment for acute rejection[24], which has been shown to influence Treg reconstitution and can lead to increased IL-10 production[10]. A subset of interest consists of the 13 patients who received an SOT transplant as a consequence of hepatitis B (HBV) of C (HCV) cirrhosis (Table 1), in which high levels of IL-10 have been commonly reported[22,27]. Among those 13 recipients, 4 recipients maintained elevated levels of IL-10 and progressed to CMV-dz (Table 1); while for the remaining 9 asymptomatic recipients, levels of IL-10 were undetectable by the time of prophylaxis discontinuation.
Post-prophylaxis levels of IL-10 in CMV-dz vs. asymptomatic patients
The majority of asymptomatic patients had undetectable levels of IL-10 after discontinuation of antiviral prophylaxis (Figure 1 and 2). In contrast, high IL-10 levels were most often measured in D+R− patients who subsequently progressed to CMV-dz [22], although CMV-dz was not always preceded by high or increasing IL-10 levels (Figure 1). The apparent relevance of driving IL-10 to undetectable levels, illustrated in Figure 2, suggests a focus on detectable IL-10, rather than on quantitative levels. In 11 of 13 CMV-dz patients (Table 1, shaded rows), detectable levels of IL-10 in the immediate post-prophylaxis interval preceded CMV-dz (Figure 1); while in 2 CMV-dz patients levels of IL-10 were undetectable before CMV-dz diagnosis (UPN 26 and UPN 34, Figure 1). Thus, monitoring IL-10 plasma levels soon after prophylaxis suspension has a potentially useful level of 85% sensitivity (CI 55% - 98%), and appears reasonably robust to the timing of the measurement. Even the CMV-dz events that occurred more than four months post-prophylaxis discontinuation were preceded by IL-10 levels that were detectable at the end of prophylaxis, and through most of the ensuing time period (Figure 1). Of the 27 patients who remained asymptomatic during the 12 month post-SOT, 6 had detectable IL-10 at the first post-prophylaxis measurement, which results in an estimated specificity of 78% (CI 54% - 89%).
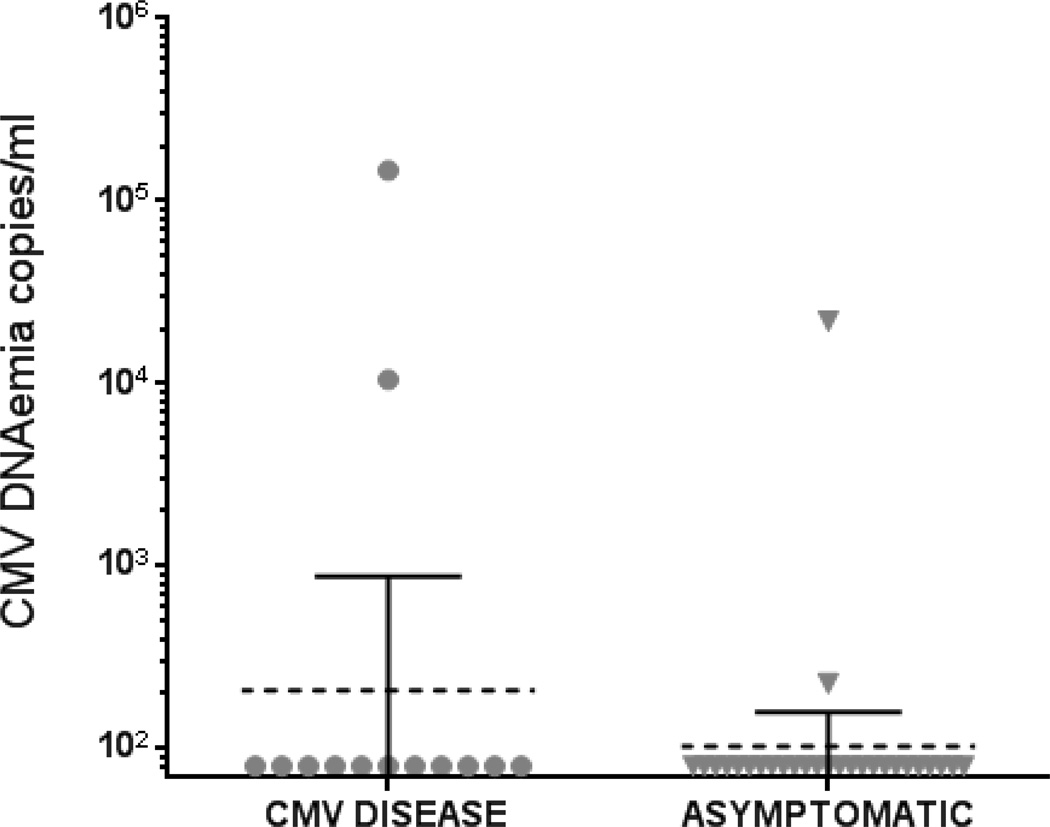
Post-prophylaxis viremia was detected in 5 out of 27 CMV asymptomatic outpatients at multiple time points, and sometimes at high levels (Figure 2 ii). In all these cases CMV viremia remained clinically asymptomatic, and was spontaneously controlled without any additional antiviral therapy. In contrast to the promising prognostic performance of detectable IL-10, CMV viremia measurements (assay threshold DNAemia ≥ 100 copies/ml, see Material and Methods), evaluated in parallel, showed that only 2 of the 13 CMV-dz cases reached detectable CMV DNAemia[5,15,26], within 1 month after prophylaxis discontinuation (Figure 3) prior to diagnosis of CMV-dz. Thus, in agreement with previous reports, CMV DNAemia was of modest value in predicting CMV-dz in D+R− SOT recipients[15,23].
Figure 3.
Levels of CMV DNAemia as copies/ml measured within 1 month of antiviral prophylaxis discontinuation. The measurements obtained in the SOT recipient population are plotted for CMV-dz (N=13) and asymptomatic patients (N=27), as specified on the x axes of the scatter plots. The dotted bars represent the geometric mean and the filled bars, 95% confidence interval.
Further analysis of IL-10 levels
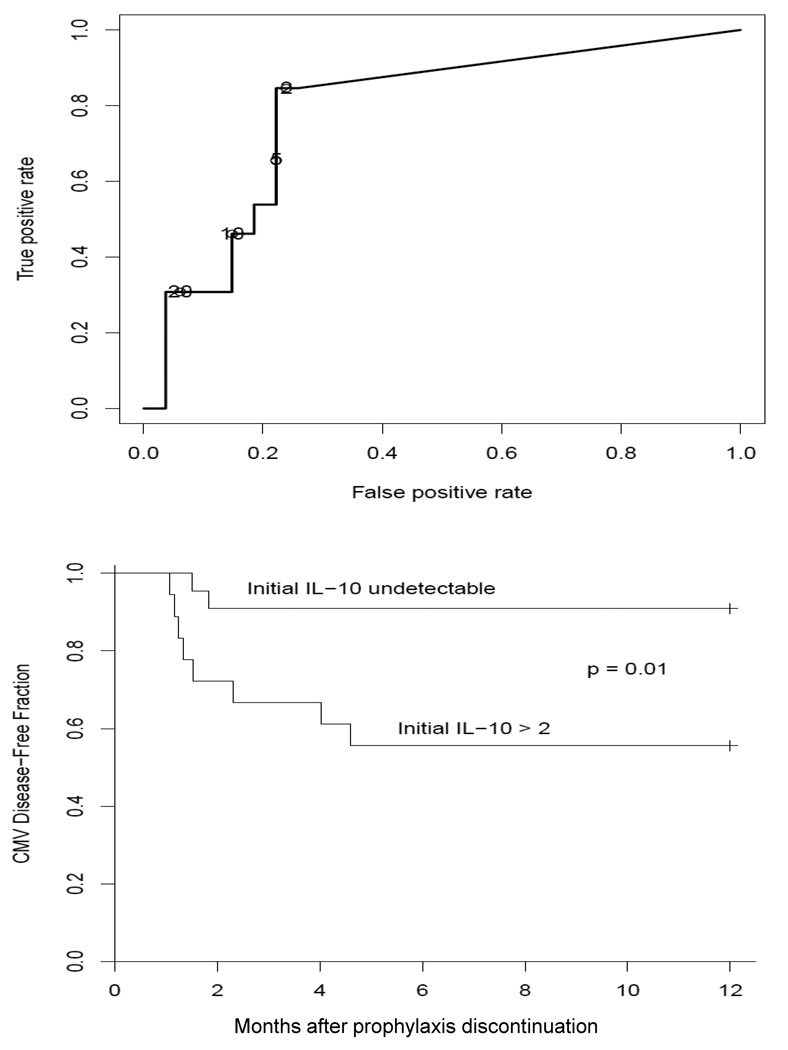
The upper panel of Figure 4 shows a ROC curve, which was constructed as a more formal evaluation of the decision to focus on detectable IL-10 (≥ 2 pg/ml, assay sensitivity see Materials and Methods), as opposed to a higher threshold. The lowest detectable level clearly provides the closest approach to the desirable upper left corner of the curve, where both specificity and sensitivity are highest.
Figure 4.
In the upper panel, the ROC curve indicates sensitivity and specificity in predicting CMV-dz at different threshold of IL-10 levels. In the lower panel, a Kaplan-Meier curve separates the patients into two groups based on the presence/absence of measurable IL-10 levels at the first post-prophylaxis measurement (within 1 month of antiviral prophylaxis discontinuation), and compares estimates of the proportion of patients who remain CMV-dz free.
As an alternative aspect of the predictive value of IL-10, Kaplan-Meier curves were estimated separately for D+R− SOT patients with detectable versus undetectable post-prophylaxis IL-10 (Figure 4, lower panel). These curves are somewhat biased toward a high overall hazard, as three subjects (UPN 3*, 8* and 15*) were included retrospectively to increase the number of CMV-dz patients, but the comparison is fair with regard to relative hazard, as IL-10 values were not involved in patient selection. This comparison is striking, and statistically significant. Subjects who have detectable IL-10 levels at the time of prophylaxis discontinuation are at enhanced risk of CMV-dz, while approximately 90% of patients with undetectable IL-10 levels will remain CMV asymptomatic 1 year after SOT.
Assuming a one in three rate of CMV-dz among D+R− SOT patients (~30%[25]), and our estimates of 85% sensitivity and 78% specificity, the predictive value of detectable IL-10 is approximately 62%. This finding indicates that detectable IL-10 at prophylaxis discontinuation nearly doubles the risk of CMV-dz. This is of course based on preliminary estimates, using the same data that suggest formalizing the risk factor as detectable IL-10 in the immediate post-prophylaxis measurement. For this reason we suggest that further evaluation of post-prophylaxis IL-10 be undertaken to confirm its prognostic value. If the prognostic value is reasonably well-confirmed, establishing the ability of IL-10 guided extension of prophylactic therapy to reduce CMV-dz rates would be feasible in a clinical trial of modest size.
Discussion
Late-onset CMV-dz is strongly and independently associated with an increased risk of morbidity, allograft failure and death[25]. After a standard 3-month course of prophylaxis, approximately 1/3 of high-risk D+R− SOT patients still develop CMV-dz. Prolonging prophylaxis has shown efficacy and safety in a randomized controlled trial, however it exposes a large number of transplant patients to the cost, toxicity, and threat of resistance of prolonged antiviral therapy[14]. To date, the optimal duration of antiviral prophylaxis remains debated[2,13,18]. Thus, new approaches to prevent CMV-dz are an important priority and of high relevance for improving SOT outcomes in high-risk D+R− patients [19].
Our observational study suggests that detectable IL-10 may have adequate sensitivity and specificity to enable IL-10 guided strategies to decrease the incidence of CMV-dz in high-risk D+R− SOT patients. Differently from other proposed immunological markers involving T cell functions and complex flow cytometry analyses[11,21,22], measurements of plasma IL-10 have the clinical advantage of requiring only limited amount of blood specimens, are easy to perform and standardize, since a commercially available kit can be used (see Material and Methods).
The rationale of such tailored and individualized intervention is based on accumulating evidence implicating IL-10 involvement in inducing profound immune dysregulation during CMV infection [20,22,28,30,33]. However, the exact role of IL-10 with its multiple immunoregulatory effects during CMV infection remains unclear. Produced by a wide variety of cells, IL-10 is a pleiotropic immunomodulatory cytokine that suppresses T-cell responses, primarily via effects mediated on APCs, and it is considered the master regulator of immunity to infection[3,33]. During chronic viral infection, this mechanism is often targeted and exploited by the virus to orchestrate an environment that limits the capacity of the host immune response to clear infection[6,28,33]. The expression of a functional IL-10 ortholog (viral IL-10) by CMV indicates its importance in the suppression of CMV-specific immunity, and as a significant stimulator of viral replication, dissemination, establishment of latency and/or persistent infection[8,34]. Intriguingly, viral IL-10 appears to provide a peculiar immune escape strategy, having the capacity to increase human IL-10 production, and directly inhibits expression of MHC II on the cell surface and memory CD4 T-cell recognition[33].
These virus-encoded functions may overwhelmingly perturb nascent CMV immune responses in immunosuppressed D+R− SOT patients, and could be involved in the clinical events leading to CMV-dz. In this context, limiting viral replication by administration of antivirals may reduce the CMV-driven and IL-10 based inhibitory milieu, favoring the expansion of functional antiviral T-cells. Previous studies both in HIV and CMV transplant patients have shown that IL-10 levels were significantly higher at viraemic time points compared to time points at which viraemia was undetectable[22,28,31,36]. Our group found that while IL-10 levels dramatically declined during prophylaxis administration[22], high levels of IL-10 contributed to diminish cell proliferation in both CMV-specific CD8 and CD4 T-cell compartments[20]. Additionally, primary CMV-specific responses were detectable early post-SOT during VALGAN prophylaxis, but exclusively when levels of plasma IL-10 were ≤20 pg/ml[22]. Prophylaxis may therefore reduce inhibitory IL-10 levels, and promote primary CMV immunity in D+R− SOT patients[20,22]. Thus, prolonging or resuming VALGAN specifically in patients showing dysregulated levels of IL-10 at suspension of antiviral prophylaxis may ultimately reduce the incidence of CMV-dz in high-risk SOT recipients.
High levels of IL-10 are commonly detected in several pathological contexts, including chronic liver disease, caused by HBV or HCV infection [9,17,22,27,35], and levels of IL-10 may remain sustained in these patients following SOT. Due to the limited size of our study, larger SOT cohorts should be analyzed to assess a possible mechanistic association between pre-transplant viral history and post-SOT levels of IL-10.
Finally anti-suppressant therapy for acute rejection is known to influence Treg reconstitution with consequent increase of IL-10[10]. Thus, SOT patients undergoing anti-rejection treatments may become more susceptible to develop CMV-dz, as our limited data suggest (Table 1) and as previously reported[16,19,29].
In summary, though putative mechanisms underlying IL-10 dysregulation in SOT patients may significantly differ, our data suggest that measurable plasma IL-10 within 1 month after prophylaxis discontinuation may be an acceptably sensitive (85%) and specific (78%) indicator of subsequent CMV-dz.
Findings from our current analysis are encouraging, however only a prospective clinical trial can establish safety and efficacy of an individualized immune-guided prevention strategy, in which duration of antiviral prophylaxis and/or treatment will be defined by the patient’s plasma IL-10 levels. This innovative strategy will lay the ground work for changing clinical practice of high-risk D+R− patients, from the current “one size fits all” approach to an individualized strategy to prevent CMV-dz and improving SOT outcomes.
Acknowledgments
We gratefully acknowledge Aparna Krishnan for her technical expertise, and the assistance in volunteer recruiting, scheduling, and specimen shipment by UWMC General Clinical Research Center personnel including Sarah Johnson, Kristin Salmi, and Cherry Thuntarug. We thank volunteers and PCPs for kindly participating to the study. The administrative assistance of Peter Kwon and Donna Packer is gratefully acknowledged.
Funding.
These studies were partially supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID; R21 AI084019); from the National Cancer Institute (NCI; R01-CA77544 and P01-CA30206, Project III); from NCI (CA33572), and by The Edwin and Bea Wolfe Charitable Foundation.
APL was partially supported by the National Institute of Allergy and Infectious Diseases (NIAID; R21 AI084019).
CLR was partially supported by the National Institute of Allergy and Infectious Diseases (NIAID; R21 AI084019) and from the National Cancer Institute (NCI; R01-CA77544 and P01-CA30206, Project III).
JL was partially supported by the National Cancer Institute (NCI; R01-CA77544 and P01-CA30206, Project III).
DJD was partially supported by the National Cancer Institute (NCI; R01-CA77544 and P01-CA30206, Project III).
Abbreviations
- CMV
cytomegalovirus
- CMV-dz
CMV disease
- COH
City of Hope Comprehensive Cancer Center
- D+R−
CMV naïve recipient of a solid organ from a CMV seropositive donor
- ESRD
End Stage Renal Disease
- GCV
Ganciclovir
- HBV
Hepatitis B
- HCV
Hepatitis C
- K
kidney recipient
- L
liver recipient
- m
month
- PD-1
programmed cell death 1
- SOT
solid organ transplantation
- Treg
regulatory T cell
- UPN
Unique Patient Number
- UWMC
University of Washington Medical Center
- VALGAN
valganciclovir
Footnotes
Disclosure.
The authors declare no conflicts of interest
APL, CLR, JL, DJD participated in research design and in the writing of the paper; APL, CLR, JL, DJD participated in the performance of the research and data analysis.
Contributor Information
Ajit P. Limaye, Email: ALimaye@medicine.washington.edu.
Corinna La Rosa, Email: clarosa@coh.org.
Jeff Longmate, Email: jlongmate@coh.org.
Don J. Diamond, Email: ddiamond@coh.org.
Reference List
- 1.Asberg A, Jardine AG, Bignamini AA, et al. Effects of the intensity of immunosuppressive therapy on outcome of treatment for CMV disease in organ transplant recipients. Am J Transplant. 2010;10:1881–1888. doi: 10.1111/j.1600-6143.2010.03114.x. [DOI] [PubMed] [Google Scholar]
- 2.Atabani SF, Smith C, Atkinson C, et al. Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy. Am J Transplant. 2012;12:2457–2464. doi: 10.1111/j.1600-6143.2012.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn SD and Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42:1142–1148. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boillat BN, Pascual M, Venetz JP, Nseir G, Meylan PR, Manuel O. Impact of a preemptive strategy after 3 months of valganciclovir cytomegalovirus prophylaxis in kidney transplant recipients. Transplantation. 2011;91:251–255. doi: 10.1097/TP.0b013e318200b9f0. [DOI] [PubMed] [Google Scholar]
- 6.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruminhent J and Razonable RR. Management of cytomegalovirus infection and disease in liver transplant recipients. World J Hepatol. 2014;6:370–383. doi: 10.4254/wjh.v6.i6.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang WL, Barry PA. Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc Natl Acad Sci U S A. 2010;107:22647–22652. doi: 10.1073/pnas.1013794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chau GY, Wu CW, Lui WY, et al. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552–558. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Serres SA, Sayegh MH, Najafian N. Immunosuppressive drugs and Tregs: a critical evaluation! Clin J Am Soc Nephrol. 2009;4:1661–1669. doi: 10.2215/CJN.03180509. [DOI] [PubMed] [Google Scholar]
- 11.Dirks J, Tas H, Schmidt T, et al. PD-1 analysis on CD28(−) CD27(−) CD4 T cells allows stimulation-independent assessment of CMV viremic episodes in transplant recipients. Am J Transplant. 2013;13:3132–3141. doi: 10.1111/ajt.12480. [DOI] [PubMed] [Google Scholar]
- 12.Eid AJ, Brown RA, Arthurs SK, et al. A prospective longitudinal analysis of cytomegalovirus (CMV)-specific CD4(+) and CD8(+) T cells in kidney allograft recipients at risk of CMV infection. Transpl Int. 2010;23:506–513. doi: 10.1111/j.1432-2277.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 13.Helantera I, Kyllonen L, Lautenschlager I, Salmela K, Koskinen P. Primary CMV infections are common in kidney transplant recipients after 6 months valganciclovir prophylaxis. Am J Transplant. 2010;10:2026–2032. doi: 10.1111/j.1600-6143.2010.03225.x. [DOI] [PubMed] [Google Scholar]
- 14.Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10:1228–1237. doi: 10.1111/j.1600-6143.2010.03074.x. [DOI] [PubMed] [Google Scholar]
- 15.Humar A, Paya C, Pescovitz MD, et al. Clinical utility of cytomegalovirus viral load testing for predicting CMV disease in D+/R− solid organ transplant recipients. Am J Transplant. 2004;4:644–649. doi: 10.1111/j.1600-6143.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 16.Issa NC and Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48:772–786. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- 17.Kakumu S, Okumura A, Ishikawa T, et al. Serum levels of IL-10, IL-15 and soluble tumour necrosis factor-alpha (TNF-alpha) receptors in type C chronic liver disease. Clin Exp Immunol. 1997;109:458–463. doi: 10.1046/j.1365-2249.1997.4861382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotton CN. CMV: Prevention, Diagnosis and Therapy. Am J Transplant. 2013;13(Suppl 3):24–40. doi: 10.1111/ajt.12006. [DOI] [PubMed] [Google Scholar]
- 19.Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan A, Zhou W, Lacey SF, Limaye AP, Diamond DJ, La Rosa C. Programmed death-1 receptor and interleukin-10 in liver transplant recipients at high risk for late cytomegalovirus disease. Transpl Infect Dis. 2010;12:363–370. doi: 10.1111/j.1399-3062.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Rosa C, Krishnan A, Longmate J, et al. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197:25–33. doi: 10.1086/523652. [DOI] [PubMed] [Google Scholar]
- 22.La Rosa C, Limaye AP, Krishnan A, Blumstein G, Longmate J, Diamond DJ. Primary response against cytomegalovirus during antiviral prophylaxis with valganciclovir, in solid organ transplant recipients. Transpl Int. 2011;24:920–931. doi: 10.1111/j.1432-2277.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Rosa C, Limaye AP, Krishnan A, Longmate J, Diamond DJ. Longitudinal assessment of cytomegalovirus (CMV)-specific immune responses in liver transplant recipients at high risk for late CMV disease. J Infect Dis. 2007;195:633–644. doi: 10.1086/511307. [DOI] [PubMed] [Google Scholar]
- 24.Limaye AP, Bakthavatsalam R, Kim HW, et al. Late-onset cytomegalovirus disease in liver transplant recipients despite antiviral prophylaxis. Transplantation. 2004;78:1390–1396. doi: 10.1097/01.tp.0000145989.22373.03. [DOI] [PubMed] [Google Scholar]
- 25.Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 26.Lisboa LF, Preiksaitis JK, Humar A, Kumar D. Clinical utility of molecular surveillance for cytomegalovirus after antiviral prophylaxis in high-risk solid organ transplant recipients. Transplantation. 2011;92:1063–1068. doi: 10.1097/TP.0b013e31822fa4b7. [DOI] [PubMed] [Google Scholar]
- 27.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordoy I, Muller F, Nordal KP, et al. The role of the tumor necrosis factor system and interleukin-10 during cytomegalovirus infection in renal transplant recipients. J Infect Dis. 2000;181:51–57. doi: 10.1086/315184. [DOI] [PubMed] [Google Scholar]
- 29.Razonable RR. Management strategies for cytomegalovirus infection and disease in solid organ transplant recipients. Infect Dis Clin North Am. 2013;27:317–342. doi: 10.1016/j.idc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Sadeghi M, Daniel V, Naujokat C, et al. Dysregulated cytokine responses during cytomegalovirus infection in renal transplant recipients. Transplantation. 2008;86:275–285. doi: 10.1097/TP.0b013e31817b063d. [DOI] [PubMed] [Google Scholar]
- 31.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus- specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8:1486–1497. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 33.Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A. Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J Virol. 2009;83:9618–9629. doi: 10.1128/JVI.01098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer JV, Lockridge KM, Barry PA, et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol. 2002;76:1285–1292. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 36.Vingert B, Benati D, Lambotte O, et al. HIV Controllers Maintain a Population of Highly Efficient Th1 Effector Cells in Contrast to Patients Treated in the Long Term. J Virol. 2012;86:10661–10674. doi: 10.1128/JVI.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]