Abstract
Current high losses of honeybees seriously threaten crop pollination. Whereas parasite exposure is acknowledged as an important cause of these losses, the role of insecticides is controversial. Parasites and neonicotinoid insecticides reduce homing success of foragers (e.g. by reduced orientation), but it is unknown whether they negatively affect flight capacity. We investigated how exposing colonies to the parasitic mite Varroa destructor and the neonicotinoid insecticide imidacloprid affect flight capacity of foragers. Flight distance, time and speed of foragers were measured in flight mills to assess the relative and interactive effects of high V. destructor load and a field-realistic, chronic sub-lethal dose of imidacloprid. Foragers from colonies exposed to high levels of V. destructor flew shorter distances, with a larger effect when also exposed to imidacloprid. Bee body mass partly explained our results as bees were heavier when exposed to these stressors, possibly due to an earlier onset of foraging. Our findings contribute to understanding of interacting stressors that can explain colony losses. Reduced flight capacity decreases the food-collecting ability of honeybees and may hamper the use of precocious foraging as a coping mechanism during colony (nutritional) stress. Ineffective coping mechanisms may lead to destructive cascading effects and subsequent colony collapse.
Keywords: Apis mellifera, flight performance, body mass, wing dimensions, pollination
1. Introduction
Current high losses of honeybees seriously threaten pollination of crops [1–3]. Several factors may cause these losses, such as parasite and disease loads, and exposure to neonicotinoid insecticides, although the relative contribution of these factors is still unknown [3–6]. Exposure to the parasitic mite Varroa destructor, and related diseases, is generally seen as one of the important stressors in honeybee colonies. The role of insecticides in causing high colony losses remains highly debated [7–9]. Recent studies show it is unlikely that field-realistic, sub-lethal doses of neonicotinoid insecticides are the sole cause of colony declines [10,11]. The question remains to what extent insecticide exposure negatively influences foraging behaviour in combination with other stressors such as V. destructor.
Both parasitic infestation and neonicotinoid insecticides reduce homing success of forager bees [12–16]. For neonicotinoids, it was suggested that reduced homing is due to impaired orientation abilities [13], while for parasites it was interpreted as adaptive behaviour of parasitized bees to remove pathogens from the colony [12,16]. However, it is unknown whether insecticides or V. destructor affect the flight capacity (distance, time and speed) of forager bees. Flight in honeybees is energetically costly and flight metabolic rates are therefore high [17]. Moreover, flight is vital for foraging success as foraging for nectar and pollen sometimes involves several hours of flying a day and carrying heavy loads [14,18]. Negative effects on foraging performance may reduce pollen availability in the colony (as suggested by Wolf et al. [16]), which reduces the number of reared bees [19], weakens newly hatched bees by reduced body mass and protein content [20,21], and induces precocious foraging [22,23]. A reduced food-collecting ability due to multiple stressors may (partly) prevent the coping mechanism of precocious foraging, while the purpose of precocious foraging is to replenish the colony resources. Ultimately, this may trigger a positive feedback that could lead to colony collapse [24].
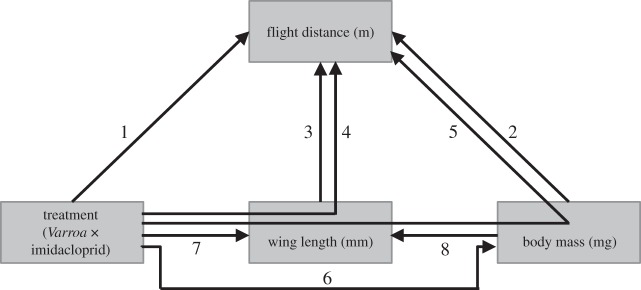
In this paper, we test both the relative and interaction effects of a neonicotinoid insecticide and high V. destructor loads on flight capacity. We used a chronic, sub-lethal dose of the neonicotinoid insecticide imidacloprid to simulate field-realistic insecticide colony exposure. Flight capacity of forager honeybees was assessed in flight mills. We test the hypothesis that bees from colonies exposed to imidacloprid or V. destructor will have a lower flight capacity and that this effect will be stronger when honeybees are exposed to both stressors. We analyse the direct and indirect effects of the treatments on flight capacity, including the possible effects of the treatments on morphological parameters, namely forewing length and body mass (figure 1).
Figure 1.
The direct and indirect relationships that were tested explaining flight distance of forager honeybees tethered on a flight mill. The arrow numbers refer to the models in table 1. For example, model 4 includes the effects of the treatments (Varroa destructor, imidacloprid and the interaction) and wing length on flight distance.
2. Material and methods
(a). Honeybee colonies and treatments
To study the effects of imidacloprid and V. destructor on flight capacity, honeybees were used from a large field experiment (started June of 2013) that hosted 40 colonies in a 2 × 2 experimental set-up at an apiary of Wageningen University and Research Centre in Wageningen, The Netherlands (51°59′32.1612″ N and 5°39′47.0772″ E). Honeybee colonies were kept in one-storey wooden hives with 10 frames. Colonies of the different treatment groups were randomly placed in rows around 1.5 m apart to avoid drifting of foragers. For this experiment, only pollen foragers were selected (see Flight experiment). We monitored the survival of the colonies after winter. In April 2014, we scored whether the colonies survived or not. Colonies were scored dead when no living honeybees were inside the hive.
We exposed honeybees to imidacloprid by providing sugar water in glass jars (330 ml) with feeding holes in the lid, upside-down on top of the inner cover (crown board). Half of the colonies were exposed to imidacloprid (I+) during the 13-week period between 20 June and 20 September 2013: weekly feeding of 660 ml (2 × 330 ml) of sugar water (1 : 1 w/w sucrose and water) with on average 5.98 ± 0.22 ng ml−1 a.i. imidacloprid, which is a worst-case field-realistic concentration (see the electronic supplementary material, appendix S1 for the justification of the imidacloprid treatment). The other half of the colonies were fed clean sugar water (I−) to ensure similar feeding conditions. All colonies continuously had additional access to sugar dough and were able to forage near the apiary (organic research farm).
With respect to the factor V. destructor, half of the colonies were treated against V. destructor infestation (V−) with formic acid during the last week of June and during the first week of August [25]. At the end of August, two strips of Apistan were hung inside the colonies for six weeks. The other half of the colonies were not treated with any acaricides against V. destructor (V+). In October, the colonies were sampled for V. destructor mite levels. Per colony 20 g of bees were sampled from the outer frame of the brood nest. These bees were weighed and rinsed with soap, and extracted mites were counted [26].
(b). Flight experiment
The flight experiment was carried out from the end of August until the first week of October 2013. Not all colonies from the field experiment were used for our flight experiment: six or seven colonies were selected from each treatment group, with an average of 10.3 ± 1.4 (range 1–21) bees per colony. Only pollen-carrying foragers without V. destructor mites on their body were selected. These bees had obviously flown before and were easily detectable when entering the hive. As foragers deposit the pollen themselves [27], they could not be mistaken for an in-hive bee.
Bees were kept in small plastic cages with air holes and available sugar water (1 : 1 w/w sucrose and water) for a maximum of 3 days before using them in the flight experiment. Each honeybee was tethered on a flight mill (see the electronic supplementary material, movie in appendix S2) by first gluing a needle with plastic tube to the thorax, without hindering the bee's (wing) movement. We used standard contact glue (Pattex) and bees were not anaesthetized. Four flight mills were used for the experiment, each with a 24.0 cm diameter and associated revolution of 75.4 cm. To leverage the weight of the bee, bees were weighed together with needle attached before flying, after which a counterweight with a maximum deviation of 2.0 mg was attached to the other side of the flight mill. The flight mill was stationed in a climate-regulated room of an average temperature of 25°C, as temperature could affect flight capacity of honeybees [28]. The lights in the room were standard daylight fluorescent tube lamps (2 × 36 W/33): flights were performed between 10.00 and 17.00. A windscreen with drawings on the side surrounded the flight mill (see the electronic supplementary material, appendix S2 for an impression). The windscreen minimized wind circulation and protected bees from gusts from neighbouring flight mills, whereas the drawings created visual cues, which can be important for the bee's orientation [29]. The flight mill was linked to a computer where the lap times and numbers were registered. We also recorded the flight mill number and the colony number.
Flight protocols were derived from [18]. Before testing, each bee was stimulated to fly in the flight mill by pulling away a small paper ball the bee was holding with its legs until the bee was observed flying at a stable speed and with continuous wing beats (a ‘continuous flight’ that produced a more monotonous and higher sound than short and interrupted flights). Bees that did not show a continuous flight within 20 min of flight stimulation were discarded and recorded as ‘unsuccessful’. To standardize our measurements, bees that showed a continuous flight were stimulated first to deplete their readily available energy reserves (an ‘emptying flight’). Once the bee actively stopped flying in the flight mill, it was stimulated again by pulling away the small paper ball the bee was given to hold on to with its legs until the bee only showed weak movements (we assumed the bee was ‘empty’). After the emptying flight, the bee was fed a glucose solution with a pipette, followed by a resting period (to ingest sugar) of approximately 5 min. During this whole procedure, the bee remained attached to the flight mill and held the paper ball. Then the computer measurement was started and the bee was stimulated to fly again. This method was used to ensure bees were tested with comparable energy reserves in their stomachs following standardized feeding conditions. The same feeding regimes were used by Brodschneider et al. [18], performing measuring flights after feeding bees a 10 µl 1 M and a 10 µl 2 M glucose solution, as honeybees may show a different energy efficiency when feeding on more concentrated glucose solutions. We therefore tested the interaction between the added glucose solution and the treatments. Most bees were fed 10 µl 1 M glucose solution before the first measurement flight, followed by a 10 µl 2 M feeding before their second flight. Some bees were unwilling to fly, and some bees flew only one flight, either the 1 M or the 2 M flight. Therefore, some bees were first fed with a 10 µl 2 M glucose solution, so their first measurement flight was a 2 M flight to prevent a small sample size of the 2 M treatment if several bees only performed the 1 M flight. Finally, the tested bee was stored in an −80°C freezer for later morphological measurements.
In total, we had 54 bee flights, of which 22 bees flew both the 1 M and the 2 M measurement flight. The percentage of successful flights varied between 10 and 60% per day (note that our experiment took place at the end of the flying season; earlier in the foraging season, the success rate will probably be higher). We did not find differences in the number of successful flights between the treatments (neither for the first flight: χ2-test, χ2 = 2.07, d.f. = 3, p = 0.56, nor for the second flight: χ2-test: χ2 = 4.03, d.f. = 3, p = 0.26). To calculate the expected values for the χ2-tests, we used the overall proportion of successful flights assuming no difference between the treatments.
(c). Forewing length and body mass
To be able to measure the wing length (mm), bees were pinned down and photos of the bees were taken with spread wings. Using a reference of a known length next to each bee, we calculated the wing length of the forewing, using ImageJ (adapted from [30]). The fresh masses of each bee were measured to the nearest 0.1 mg.
(d). Statistical analysis
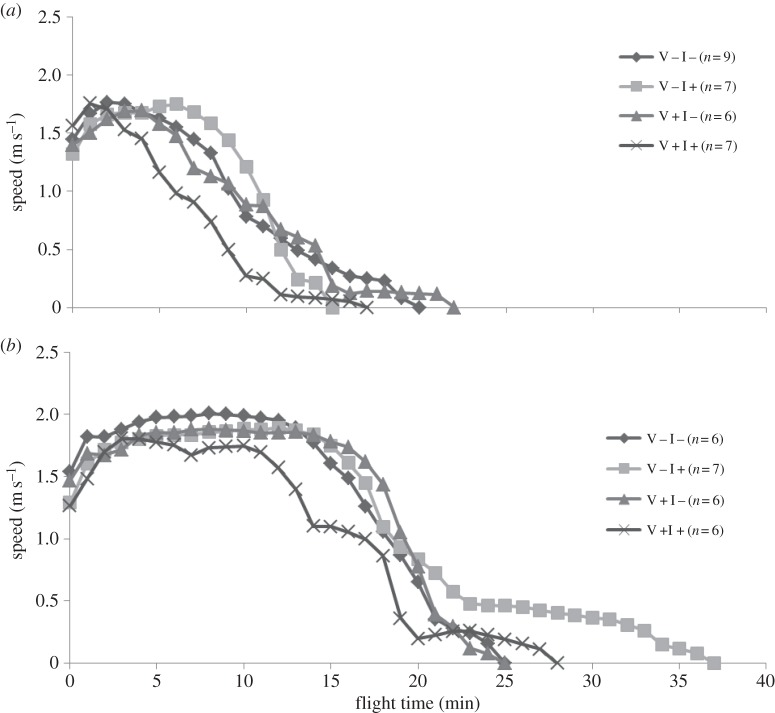
Lap times were used to calculate the tethered flight variables: distance covered, flight time, and average and maximum speed. Total distance (m) was calculated by the number of laps multiplied by the distance covered per lap. Flight time (s) was calculated by the sum of the total time per lap. Average speed was calculated as the total distance (m) divided by the total time flown (s). Maximum speed was defined by the maximum speed recorded during the total time flown (figure 3). The flight data were analysed in linear mixed models (LMMs; figure 1; electronic supplementary material, figure S1 in appendix S3) with the 1 or 2 M flight as a repeated fixed factor (‘1–2 M’) and colony as random factor. We included colony in the model, even when shown to be redundant. We also tested the interactions between ‘1 and 2 M’ and V. destructor and imidacloprid in our models, and tested for the effect of flight mill number as a random factor. These interactions and flight mill number never showed any effect and were therefore not included any further. The best-fitting model was selected by choosing the covariance matrix with the lowest AICc (AIC corrected for small datasets) index. Additionally, either the restricted maximum likelihood (REML) or maximum likelihood (ML) was used, depending on the lowest AICc score.
Figure 3.
Mean flight speed per minute of honeybees in a flight mill that are exposed to one of the four treatments (V− refers to colonies that were treated against V. destructor, whereas the colonies V+ were not treated, I− refers to colonies with no exposure to a field-realistic, chronic sub-lethal dose of imidacloprid, whereas colonies I+ were exposed) after being fed (a) a 10 µl 1 M glucose solution or (b) a 10 µl 2 M glucose solution. Mean speed was calculated for every flying minute from all individual bees per treatments (indicated in the legend), including those bees that have stopped flying due to depletion of their reserves. Honeybees that stopped flying at a certain time were included for illustration purposes and were considered to have a speed of zero.
The treatments imidacloprid, V. destructor, and the interaction between V. destructor and imidacloprid were fixed factors in the LMM (models 1 and 4–7 in figure 1). We entered wing length (models 3–5) and body mass (models 2 and 8) as covariates. Body mass and wing length were the same for one individual that flew both the 1 and 2 M flight, therefore the models 6–8 were run with 32 unique individuals and the factor 1–2 M was excluded from the model. As body mass differed between the treatments, it could not be included in the same model as treatment. We therefore first corrected flight distance for body mass (the residuals of model 2) and then determined the effect of treatment and wing length on the residuals of flight distance that were independent of body mass (model 5). For flight distance, an additional analysis was performed, where we tested the mean distance in relation to colony survival in April the next year (dead/alive). The remaining part of the model was similar to model 1. For each LMM, Sidak post-hoc tests were conducted for the pairwise comparison between groups. Flight distance, time and maximum speed were log-transformed to meet the assumptions of normality.
We used Pearson correlation tests to determine the relationships between the morphological data (models 9–13 in the electronic supplementary material, figure S2 in appendix S3). A two-way ANOVA was used to determine the effect of the treatments on the number of V. destructor mites per gram of bees (log-transformed (+0.01)), followed by a Sidak post-hoc test. The data met the assumptions of normality required for the different tests.
3. Results
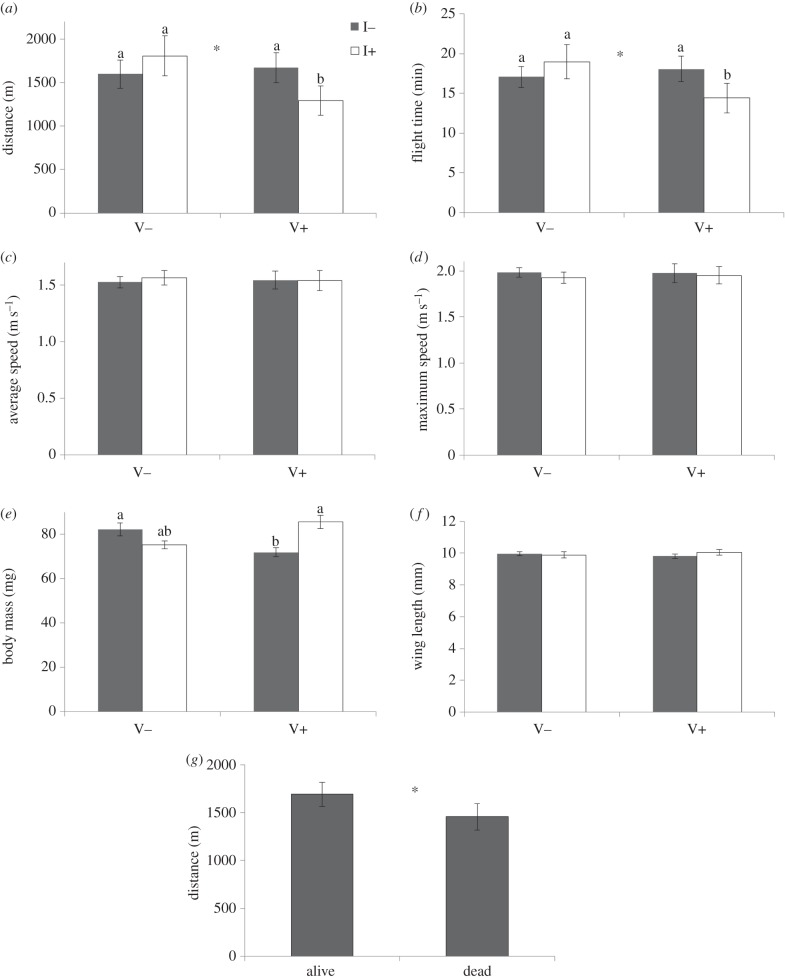
Our results show that pollen foragers from colonies exposed to both a field-realistic, chronic sub-lethal dose of imidacloprid and high levels of V. destructor fly shorter distance and time, and that these effects are indeed more negative than when exposed to a single stressor (figures 2 and 3 and table 1; electronic supplementary material, table S1 in appendix 3). Additionally, the differences in flight distance and time for bees exposed to the single factor V. destructor were larger than the differences due to exposure to the single factor imidacloprid. The effect size for flight distance was larger than for flight time. We did not find an effect of these stressors on the average or maximum flight speed (electronic supplementary material, table S1 in appendix S3). Moreover, imidacloprid and V. destructor affected body mass of the bees (model 6 in figure 1 and table 1), with bees exposed to only V. destructor having the lowest body mass (figure 2e). Differences in body mass were mostly related to abdomen mass (electronic supplementary material, figure S2 and table S2 in appendix S3).
Figure 2.
(a) Mean flight distance, (b) mean flight time, (c) average flight speed and (d) maximum flight speed of honeybees tethered on a flight mill that were exposed to different levels of Varroa destructor infestation (V− refers to colonies that were treated against V. destructor, whereas the colonies V+ were not treated) and to different concentrations of a field-realistic, chronic sub-lethal dose of the neonicotinoid insecticide imidacloprid (I− refers to colonies with no exposure, whereas colonies I+ were exposed). Error bars indicate the standard error of the mean. The letters give the significant differences between groups based on linear mixed models (see statistics in table 1 and electronic supplementary material, table S1 in appendix S3). The asterisks in panels (a,b) indicate significant differences for the main effect V. destructor. (e–g) Mean (e) body mass and (f) wing length for the treatments (respectively, model 6 and model 7 in table 1), and (g) mean flight distance for the colonies that survived April of the next year and the colonies that did not survive. The asterisk indicates a significant difference (see text for statistics of this test).
Table 1.
Results of the linear mixed models for the effects of the stressors Varroa destructor and a field-realistic, chronic sub-lethal dose of the neonicotinoid insecticide imidacloprid including their interaction on the flight distance, body mass and wing length of forager honeybees tethered on a flight mill. The model numbers refer to the different arrows in figure 1. For model 5, we took the residuals from model 2 as the dependent variable. For each factor in the model, the F- and p-values are given. Some models include a significant covariate (BM = body mass, WL = wing length) from which we report the parameter estimate and the standard error of the estimate. For the random variable colony, the Wald statistic and p-value are given. For each model, we give the applied method of estimation (ML = maximum likelihood, REML = restricted maximum likelihood), whether we log-transformed the dependent variable (Log), the sample size (n), the value of the AICc and the repeated covariance type (DIAG = diagonal). Dashes (—) indicate that this variable was not tested in the model; asterisks (*) indicate that although this variable was redundant (the test statistic and confidence interval could not be computed) we kept the variable in the model.
| Model no. |
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| independent variables | statistics | flight distance (m) | flight distance (m) | flight distance (m) | flight distance (m) | residuals of model 2 | body mass (mg) | wing length (mm) |
| 1–2 M | F | 113.27 | 107.45 | 112.18 | 113.86 | — | — | — |
| p | <0.001 | <0.001 | <0.001 | <0.001 | — | — | — | |
| Varroa | F | 6.61 | — | — | 7.37 | 5.41 | 0.04 | 0.04 |
| p | 0.01 | — | — | 0.01 | 0.03 | 0.84 | 0.84 | |
| imidacloprid | F | 2.29 | — | — | 2.87 | 0.53 | 0.53 | 0.28 |
| p | 0.14 | — | — | 0.10 | 0.47 | 0.48 | 0.60 | |
| Varroa × imidacloprid | F | 5.47 | — | — | 9.43 | 1.23 | 9.41 | 0.13 |
| p | 0.02 | — | — | <0.001 | 0.27 | 0.01 | 0.72 | |
| body mass (mg) | F | — | 7.40 | — | — | — | — | — |
| p | — | 0.02 | — | — | — | — | — | |
| wing length (mm) | F | — | — | 3.97 | 7.17 | 7.38 | — | — |
| p | — | — | 0.05 | 0.01 | 0.01 | — | — | |
| colony | Wald Z | * | 0.17 | 1.33 | * | — | 0.52 | * |
| p | * | 0.86 | 0.18 | * | — | 0.60 | * | |
| covariate estimate | — | −0.010 | 0.012 | 0.014 | 0.013 | — | — | |
| s.e. | — | 0.004 | 0.006 | 0.005 | 0.005 | — | — | |
| estimation method | ML | ML | ML | ML | ML | REML | REML | |
| transformation | Log | Log | Log | Log | — | — | — | |
| n | 54 | 54 | 54 | 54 | 54 | 32 | 32 | |
| AICc | 18.55 | 17.87 | 18.67 | 15.14 | 9.86 | 205.87 | 197.11 | |
| repeated covariance type | DIAG | DIAG | DIAG | DIAG | DIAG | — | — | |
For flight distance, we found that higher body mass was related to shorter distances (model 2 in figure 1 and table 1), while longer wings increased flight distance (model 3). Differences in flight distance could be better explained by the V. destructor and imidacloprid treatments in combination with wing length (model 4) than without wing length (model 1), even though wing length did not differ between the treatments (model 7; figure 2f) and there was no relationship between wing length and body mass (model 8; electronic supplementary material, table S2 in appendix S3). We found that bees had a longer flight distance when given the dose of 10 µl 2 M compared to the dose of 10 µl 1 M.
Given these different relationships, we then addressed the question whether the stressors directly affected flight distance (models 1–4 in figure 1 and table 1) or also indirectly due to their effect on body mass (models 5 and 6). When correcting flight distance for the indirect effect of body mass of the flying bees (model 5), we still found a direct effect of V. destructor, independent of body mass, where the bees from colonies with higher V. destructor levels and shorter wing lengths flew shorter distances. The effect of imidacloprid disappeared, suggesting that the effect of imidacloprid was mostly indirect via body mass.
Equal patterns were observed for flight time, but not for flight speed (electronic supplementary material, figure S1 and table S1 in appendix S3). Apparently, speed was not affected by the stressors, which was confirmed by flight distances that were highly correlated with flight times (Pearson correlation: r = 0.92, p < 0.001, n = 54), while less strongly correlated with flight speeds (Pearson correlation between distance and average speed: r = 0.46, p < 0.001, n = 54; Pearson correlation between distance and maximum speed: r = 0.38, p < 0.001, n = 54).
Finally, the mean flight distance flown by bees from colonies that were still alive in April the next year, was longer than from those colonies that were dead by that time (figure 2g, LMM: ML; repeated covariance type diagonal; distance log-transformed; n = 54; AIC = 15.84; 1–2 M: F = 117.1, p < 0.01; survival: F = 5.8, p = 0.03; colony: Wald Z = 0.66, p = 0.51).
The colonies treated with acaricides to reduce mite levels indeed showed much lower V. destructor mite levels compared to colonies not treated with acaricides (electronic supplementary material, figure S3 and table S3 in appendix S3). However, for the group of bees that did fly in the flight mill, there was also an interaction between V. destructor and imidacloprid, where colonies with low V. destructor levels had even fewer mites when also exposed to imidacloprid (V−I+). Compared to the large (×8) difference between the V− and V+ group, however, this small (×2) difference between the V−I+ and V−I− group was negligible (note that the electronic supplementary material, figure S3 shows log-transformed values (+0.01)).
4. Discussion
We show for the first time that flight distances and flight times reduce for forager bees that were raised in colonies exposed to both high levels of V. destructor mites and a field-realistic, chronic sub-lethal dose of imidacloprid. This effect was more pronounced for the stressor V. destructor. The interaction between the two stressors suggests the possible effect of one stressor on the vulnerability of bees for other stressors: bees weakened by one stressor, here high loads of V. destructor, may be vulnerable to the negative effects of an insecticide. The distance covered is a vital proxy for the flight capacity of honeybees as it integrates speed with endurance [27]. Furthermore, flight distance is an indicator of the foraging range of insects and their pollination potential [31,32] (e.g. honeybees live until they have flown around 800 km [27]).
Our data showed that forager bees exposed to both stressors fly on average only three-quarters of the distance that non-exposed or single-exposed bees could fly (figure 2a: 1.3 km for bees exposed to both stressors, indicated with ‘b’, versus on average 1.7 km for non-exposed or single-exposed bees, indicated with ‘a’). Reduced flight distance per flight due to these stressors means that either bees cannot meet this 800 km of foraging potential (i.e. they can only fly 612 km on the same amount of fuel) or they need to fuel up more often between shorter flights, resulting in these 800 km being more energetically costly (i.e. less efficient). Most probably, the foraging range of bees from stressed colonies will be reduced.
The negative effects of V. destructor may be the result of infestation during pupation, as it reduces the body mass of newly emerged bees [21]. Flight muscles are mainly formed during the pupal life stage before emergence [33]. Drones that flew in a wind tunnel also showed reduced flight time when infested with V. destructor during pupation [34], but to date this effect is unknown for forager bees that perform a crucial task for colony survival. A homing experiment comparing bees of infested colonies showed that homing success was reduced and return time prolonged for bees that carried V. destructor mites [12]. It was hypothesized that this prolonged returning time was an adaptive response to get rid of the parasite.
Concerning the effect of imidacloprid, several homing studies showed that return time was prolonged and homing success was reduced after exposure to a sub-lethal dose of neonicotinoid insecticides [13–15]. So far, homing failure due to imidacloprid has mainly been related to impaired orientation ability and learning performance of honeybees [14,15,35,36]. Our results provide an alternative explanation for reduced homing success besides this impaired orientation ability and learning performance, namely that neonicotinoid insecticides reduce flight capacity, in particular when bees are simultaneously exposed to another stressor. We tested the effect of imidacloprid on the flight capacity of foragers raised in colonies that were fed a field-realistic dose (6 ng ml−1; see the electronic supplementary material, appendix S1 for the justification of the imidacloprid treatment), which is lower than used in most of the homing studies. Also, we assessed chronic oral toxicity on the colony level, in contrast to measuring the direct acute toxicity on the individual level. Acute toxicity may induce more stress to honeybees, as reduced mobility and a phase of motionlessness was observed after exposure to a high LD50 dose of imidacloprid [7,8,14,37]. Chronic sub-lethal oral exposure on the colony level may show less acute toxicity, but may reduce performance and thereby reduce colony survival.
Our field-realistic exposure was lower than the no-observed effect concentration (NOEC) found for foraging activity (waggle dance), colony development, changes in brood or pollen/nectar supplies of 20 ng ml−1 after chronic oral field exposure [7,38–40]. We nonetheless found a negative effect on flight performance. Our study therefore suggests that high levels of V. destructor can decrease the NOEC for imidacloprid (cf. [41]).
The selected bees looked healthy upon visual inspection: they neither had V. destructor mites attached nor deformed wings. As we did not mark bees at emergence, we do not know whether the selected bees were infested with mites during pupation. Also, we do not know whether the selected bees had imidacloprid in their body. The selected bees were caught in the act of returning with pollen from a foraging flight (i.e. they were healthy enough to make it to the foraging stage). Our approach however mimics reality as the collected bees were raised in colonies that were exposed to low or high levels of V. destructor mites and to the absence or the presence of imidacloprid. This is in contrast to the often used approaches where individual bees were treated with a single stressor [12–15]. These studies severely neglect resilience mechanisms of the social honeybee colonies to counteract the negative effects of these stressors, such as precocious foraging [24]. These results were found despite relatively low sample sizes. We cannot reject the idea that additional effects would be detected using larger sample sizes.
Our results suggest that the effect of imidacloprid on flight capacity is mainly indirectly via body mass, whereas V. destructor affects flight capacity both indirectly via body mass as well as directly as a result of exposure to the stressor. We found that bees exposed to the single stressor V. destructor had lower body mass than the control bees exposed to neither stressor. Varroa destructor is known to reduce body mass of bees [21]. Unexpectedly, bees exposed to both V. destructor and imidacloprid had higher body mass, which may be explained by an earlier onset of foraging when bees are exposed to both stressors. Honeybees reduce around 40% in body mass before becoming a forager to reduce the energetic costs of flying [42]. There is evidence that precocious foragers are indeed heavier and less efficient flyers than older foragers [43]. This precocious foraging due to parasite infestation was previously observed in bees exposed to Nosema ceranae infestation [44].
Fewer mites in colonies with low V. destructor levels when also exposed to imidacloprid (V−I+) could explain the slightly higher (n.s.) flight capacity of bees from the group exposed to imidacloprid (V−I+) than of bees from the control group (V−I−). Perhaps applying imidacloprid increased the effectiveness of the active compound tau-fluvalinate in Apistan to kill V. destructor mites. Mixing in-hive pesticides and fungicides previously increased toxicity for bees [45,46], but was not previously investigated for pests or diseases.
(a). Effects on the colony
Together with a reduced homing success, reduction of the flight distance and time of forager bees as found in our study can lead to lower pollen and nectar loads that are brought to the hive. Pollen loads were previously reduced in foragers from colonies with high V. destructor infestation [23]. Hence, we expect that the negative effect on the flight distance and time reduces the forage capacity of the colony, resulting in nutritional stress on the colony [21]. As we only selected honeybees after a pollen-collecting flight, we may underestimate the effects of V. destructor and imidacloprid on the foraging capacity of the colony: V. destructor-infested bees may not even reach the foraging stage due to early death or incapacitating V. destructor-related diseases such as deformed wing virus [25]. Reduced flight capacity decreases the food-collecting ability of honeybees and may hamper the use of precocious foraging as a coping mechanism during colony (nutritional) stress. Ultimately, ineffective coping mechanisms may lead to cascading effects and subsequent colony collapse [24].
Reduced nectar availability—which equals low fuel availability—restricts future foraging flights, but it also limits winter food storage and thereby thermoregulation. Reduction of flight distance and time may also directly cause a reduction of the colony's capacity to regulate the temperature during winter, because the same muscles that bees use for flight are also used for thermoregulation [27].
Although colony survival is not solely driven by flight performance, our data showed that bees from colonies that were still alive in April the next year had a larger flight capacity; they flew per flight on average 235 m further, for either 1 M or 2 M, compared with bees from colonies that were dead by that time. We cannot distinguish whether this difference is caused by differences in flight distance, or also due to other effects caused by our experimental treatment. However, our findings support the suggested theory described above and relate reduced flight distance to colony loss during winter.
5. Concluding remarks
We demonstrate for the first time that V. destructor and the interaction between V. destructor and imidacloprid reduces the flight capacity of honeybees, where the effect of V. destructor was larger than that of imidacloprid. The negative effect of neonicotinoid insecticides in combination with V. destructor on flight capacity could explain the observed decreased homing capacity of bees. Increasing our understanding of the relative and interactive effects of V. destructor and neonicotinoid insecticides on flight capacity and the accompanied food-collecting capacity is vital to explain colony collapse and reduced pollination services of honeybees.
Supplementary Material
Acknowledgements
We thank Robert Brodschneider for giving advice on the use of flight mills, Tjeerd Blacquière for helping with the determination of field-realistic, chronic sub-lethal dose of the neonicotinoid insecticide imidacloprid, Bram Cornelissen and Chula Hok-Ahin for feeding and taking care of the colonies, and Jolanda Tom and Maayke Broerse for helping to tether the bees to the flight mills. Dorset Group BV (Aalten, The Netherlands) produced the flight mills. Bayer Crop Science AG (Monheim am Rhein, Germany) kindly produced pure imidacloprid. Vita Europe Ltd (Basingstoke, UK) kindly provided Apistan.
Data accessibility
All raw data can be found at the Dryad repository ‘interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees’, doi:10.5061/dryad.4df64.
Authors' contributions
L.J.B., F.v.L. and C.v.D. designed the experiment and wrote the manuscript; L.J.B. did the measurements; L.J.B. and C.v.D. analysed the data.
Competing interests
We have no competing interests.
Funding
This project was funded by the Ministry of Economic Affairs (EZ) of The Netherlands as part of a multifactorial experiment to study colony losses of honeybees during winter (BO-20-003-003). C.v.D. was funded within this project.
References
- 1.Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313. ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aizen MA, Harder LD. 2009. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19, 915–918. ( 10.1016/j.cub.2009.03.071) [DOI] [PubMed] [Google Scholar]
- 3.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 4.VanEngelsdorp D, Meixner MD. 2010. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 103, S80–S95. ( 10.1016/j.jip.2009.06.011) [DOI] [PubMed] [Google Scholar]
- 5.Vanbergen AJ, the Insect Pollinators Initiative. 2013. Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ. 11, 251–259. ( 10.1890/120126) [DOI] [Google Scholar]
- 6.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 7.Blacquière T, Smagghe G, Van Gestel CAM, Mommaerts V. 2012. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotox 21, 973–992. ( 10.1007/s10646-012-0863-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfray HCJ, Blacquiere T, Field LM, Hails RS, Petrokofsky G, Potts SG, Raine NE, Vanbergen AJ, McLean AR. 2014. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B 281, 20140558 ( 10.1098/rspb.2014.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisa LW, et al. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 22, 68–102. ( 10.1007/s11356-014-3471-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dively GP, Embrey MS, Kamel A, Hawthorne DJ, Pettis JS. 2015. Assessment of chronic sublethal effects of imidacloprid on honey bee colony health. PLoS ONE 10, e0118748 ( 10.1371/journal.pone.0118748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rundlöf M, et al. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. ( 10.1038/nature14420) [DOI] [PubMed] [Google Scholar]
- 12.Kralj J, Fuchs S. 2006. Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie 37, 577–587. ( 10.1051/apido:2006040) [DOI] [Google Scholar]
- 13.Henry M, Béguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350. ( 10.1126/science.1215039) [DOI] [PubMed] [Google Scholar]
- 14.Schneider CW, Tautz J, Grünewald B, Fuchs S. 2012. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE 7, e30023 ( 10.1371/journal.pone.0030023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer J, Müller T, Spatz AK, Greggers U, Grünewald B, Menzel R. 2014. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS ONE 9, e91364 ( 10.1371/journal.pone.0091364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf S, McMahon DP, Lim KS, Pull CD, Clark SJ, Paxton RJ, Osborne JL. 2014. So near and yet so far: harmonic radar reveals reduced homing ability of Nosema infected honeybees. PLoS ONE 9, e103989 ( 10.1371/journal.pone.0103989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suarez RK, Lighton JR, Joos B, Roberts SP, Harrison JF. 1996. Energy metabolism, enzymatic flux capacities, and metabolic flux rates in flying honeybees. Proc. Natl Acad. Sci. USA 93, 12 616–12 620. ( 10.1073/pnas.93.22.12616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodschneider R, Riessberger-Gallé U, Crailsheim K. 2009. Flight performance of artificially reared honeybees (Apis mellifera). Apidologie 40, 441–449. ( 10.1051/apido/2009006) [DOI] [Google Scholar]
- 19.Brodschneider R, Crailsheim K. 2010. Nutrition and health in honey bees. Apidologie 41, 278–294. ( 10.1051/apido/2010012) [DOI] [Google Scholar]
- 20.Amdam GV, Hartfelder K, Norberg K, Hagen A, Omholt SW. 2004. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during overwintering? J. Econ. Entomol. 97, 741–747. ( 10.1093/jee/97.3.741) [DOI] [PubMed] [Google Scholar]
- 21.Van Dooremalen C, Stam E, Gerritsen L, Cornelissen B, Van der Steen J, Van Langevelde F, Blacquière T. 2013. Interactive effect of reduced pollen availability and Varroa destructor infestation limits growth and protein content of young honey bees. J. Insect Physiol. 59, 487–493. ( 10.1016/j.jinsphys.2013.02.006) [DOI] [PubMed] [Google Scholar]
- 22.Schulz DJ, Huang ZY, Robinson GE. 1998. Effects of colony food shortage on behavioural development in honey bees. Behav. Ecol. Sociobiol. 42, 295–303. ( 10.1007/s002650050442) [DOI] [Google Scholar]
- 23.Janmaat AF, Winston ML, Ydenberg RC. 2000. Condition-dependent response to changes in pollen stores by honey bee (Apis mellifera) colonies with different parasitic loads. Behav. Ecol. Sociobiol. 47, 171–179. ( 10.1007/s002650050008) [DOI] [Google Scholar]
- 24.Perry CJ, Søvik E, Myerscough MR, Barron AB. 2015. Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc. Natl Acad. Sci. USA 112, 3427–3432. ( 10.1073/pnas.1422089112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Dooremalen C, Gerritsen L, Cornelissen B, van der Steen JJM, van Langevelde F, Blacquière T. 2012. Winter survival of individual honey bees and honey bee colonies depends on level of Varroa destructor infestation. PLoS ONE 7, e36285 ( 10.1371/journal.pone.0036285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietemann V, et al. 2013. Standard methods for Varroa research. J. Apicul. Res. 52, 1–54. ( 10.3896/IBRA.1.52.1.13) [DOI] [Google Scholar]
- 27.Seeley TD. 1995. The wisdom of the hive: the social physiology of honey bee colonies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 28.Harrison JF, Nielsen DI, Page RE Jr. 1996. Malate dehydrogenase phenotype, temperature and colony effects on flight metabolic rate in the honey bee, Apis mellifera. Func. Ecol. 10, 81–88. ( 10.2307/2390265) [DOI] [Google Scholar]
- 29.Dittmar L, Egelhaaf M, Stürzl W, Boeddeker N. 2011. The behavioural relevance of landmark texture for honeybee homing. Front. Behav. Neurosci. 5, 20 ( 10.3389/fnbeh.2011.00020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaplane KS, Dag A, Danka RG, Freitas BM, Garibaldi LA, Goodwin RM, Hormaza JI. 2013. Standard methods for pollination research with Apis mellifera. J. Apicult. Res. 52, 1–28. ( 10.3896/IBRA.1.52.4.12) [DOI] [Google Scholar]
- 31.Schulke B, Waser NM. 2001. Long-distance pollinator flights and pollen dispersal between populations of Delphinium nuttallianum. Oecologia 127, 239–245. ( 10.1007/s004420000586) [DOI] [PubMed] [Google Scholar]
- 32.Osborne JL, Martin AP, Carreck NL, Swain JL, Knight ME, Goulson D, Hale RJ, Sanderson RA. 2008. Bumblebee flight distances in relation to the forage landscape. J. Anim. Ecol. 77, 406–415. ( 10.1111/j.1365-2656.2007.01333.x) [DOI] [PubMed] [Google Scholar]
- 33.Harrison JM. 1986. Caste-specific changes in honeybee flight capacity. Physiol. Zool. 59, 175–187. ( 10.1186/1471-2164-14-799) [DOI] [Google Scholar]
- 34.Duay P, De Jong D, Engels W. 2002. Decreased flight performance and sperm production in drones of the honeybee (Apis mellifera) slightly infested by Varroa destructor mites during pupal development. Genet. Mol. Res. 1, 227–232. [PubMed] [Google Scholar]
- 35.Decourtye A, Lacassie E, Pham-Delégue MH. 2003. Learning performances of honeybees (Apis mellifera L) are differentially affected by imidacloprid according to the season. Pest Manag. Sci. 59, 269–278. ( 10.1002/ps.631) [DOI] [PubMed] [Google Scholar]
- 36.Yang EC, Chuang YC, Chen YL, Chang LH. 2008. Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 101, 1743–1748. ( 10.1603/0022-0493-101.6.1743) [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto T. 2013. Reduction in homing flights in the honey bee Apis mellifera after a sublethal dose of neonicotinoid insecticides. B. Insectol. 66, 1–9. [Google Scholar]
- 38.Kirchner WH. 1999. Mad-bee disease? Sublethal effects of imidacloprid (‘Gaucho’) on the behaviour of honey-bees. Apidologie 30, 422 ( 10.1051/apido:19990405) [DOI] [Google Scholar]
- 39.Maus C, Curé G, Schmuck R. 2003. Safety of imidacloprid seed dressings to honey bees: a comprehensive overview and compilation of the current state of knowledge. B. Insectol. 56, 51–57. [Google Scholar]
- 40.Schmuck R, Stork A, Schramel O. 2001. Risk posed to honeybees (Apis mellifera L, Hymenoptera) by an imidacloprid seed dressing of sunflowers. Pest Manag. Sci. 57, 225–238. ( 10.1002/ps.270) [DOI] [PubMed] [Google Scholar]
- 41.Pettis JS, Vanengelsdorp D, Johnson J, Dively G. 2012. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99, 153–158. ( 10.1007/s00114-011-0881-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winston ML. 1987. The biology of the honey bee. Cambridge, MA: Harvard University Press. [Google Scholar]
- 43.Vance JT, Williams JB, Elekonich MM, Roberts SP. 2009. The effects of age and behavioral development on honey bee (Apis mellifera) flight performance. J. Exp. Biol. 212, 2604–2611. ( 10.1242/jeb.028100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goblirsch M, Huang ZY, Spivak M. 2013. Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 8, e58165 ( 10.1371/journal.pone.0058165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pilling ED, Jepson PC. 1993. Synergism between EBI fungicides and a pyrethroid insecticide in the honeybee (Apis mellifera). Pestic. Sci. 39, 293–297. ( 10.1002/ps.2780390407) [DOI] [Google Scholar]
- 46.Johnson RM, Pollock HS, Berenbaum MR. 2009. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 102, 474–479. ( 10.1603/029.102.0202) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data can be found at the Dryad repository ‘interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees’, doi:10.5061/dryad.4df64.