Abstract
Evolution of body size is likely to involve trade-offs between body size, growth rate and longevity. Within species, larger body size is associated with faster growth and ageing, and reduced longevity, but the cellular processes driving these relationships are poorly understood. One mechanism that might play a key role in determining optimal body size is the relationship between body size and telomere dynamics. However, we know little about how telomere length is affected when selection for larger size is imposed in natural populations. We report here on the relationship between structural body size and telomere length in wild house sparrows at the beginning and end of a selection regime for larger parent size that was imposed for 4 years in an isolated population of house sparrows. A negative relationship between fledgling size and telomere length was present at the start of the selection; this was extended when fledgling size increased under the selection regime, demonstrating a persistent covariance between structural size and telomere length. Changes in telomere dynamics, either as a correlated trait or a consequence of larger size, could reduce potential longevity and the consequent trade-offs could thereby play an important role in the evolution of optimal body size.
Keywords: body size, telomere length, life-history trade-off, longevity, Passer domesticus, selection experiment
1. Introduction
Understanding the mechanisms that shape life-history strategies is at the heart of evolutionary ecology. Much of our current theoretical and empirical research is based around the premise that the balance of costs and benefits of different life-history components results in natural selection producing the optimal compromise [1,2]. Hence, we predict that trade-offs will occur among growth, self-maintenance and reproduction. Differences in the optimal resolution of these trade-offs through individual differences in resource acquisition and allocation [3] underlie the individual variation that we see in growth rate, body size, reproductive rate and longevity at the population level.
It is well recognized that in practice such trade-offs are difficult to demonstrate [1,3–6]. Considerable effort has been devoted to investigating how variation in reproductive investment influences other life-history traits [7–9]. The relationships among growth, body size and longevity have received much less attention [10], but are coming more to the fore as the importance of understanding intra- and interspecific variation in the rate of ageing is recognized as an important question in modern biology [6,11]. A paradox that has puzzled evolutionary ecologists and biogerontologists is the opposing patterns that frequently are observed in the relationship between body size and longevity among and within species. Across species, there is a strong positive relationship between body size and maximal lifespan: big species tend to live longer than small ones. Within species however, the opposite relationship has often been found: larger individuals have shorter lives than conspecifics of smaller size, and this has been demonstrated in the wild and in captivity (e.g. [10,12–17]). The within-species variation in lifespan is also exemplified in species subjected to artificial selection to produce variation in body size such as the domestic dog (Canis lupus familiaris). Great variation in body size is present in dogs; larger dog breeds have shorter lifespans, and this has recently been shown to occur because they age faster [12]. Similarly, when laboratory species such as mice and rats are selected or genetically engineered for larger or smaller size, lifespan is again reduced or increased [16,18,19].
We do not currently understand how the long-term costs associated with larger size are incurred. There are a number of potential, non-exclusive routes whereby this could occur. A candidate process is reduced telomere length owing to more or faster cell division and/or telomere attrition rate [5,6,10,20]. Given that telomere length is linked to organismal senescence [21–23], and that average telomere length in tissues at the end of growth is predictive of lifespan [24,25], this may point to a mechanistic process that could explain the lifespan penalty associated with larger size. This could be the case in dogs, where lifespan is negatively correlated with body size among breeds and telomere length in larger dog breeds is reported to be shorter than in the smaller breeds. However, the extreme variation in size created in this species (100 fold) could have created aberrant patterns that are not evident under natural conditions [26]. A key question then is whether telomere length covaries with intraspecific variation in body size in natural conditions, in the absence of such extreme size variation.
We used samples that had been collected during an experiment conducted in a population of wild house sparrows (Passer domesticus) in northern Norway, in which a selection pressure for larger body size was experimentally imposed on the breeding population over a 4-year period, which clearly increased the size of both adults and their offspring (see details in §2). We examined the extent to which telomere length and body size were related in fledglings, and, importantly, whether any relationship was maintained when fledgling body size was increased under the selection regime.
2. Material and methods
(a). Study site
The study was conducted in the house sparrow population on the island Leka situated ca 3 km from the mainland in the Nord-Trøndelag county in northern Norway (65°06′ N, 11°38′ E, see map in Hagen et al. [27]) and was initiated in 2002. Leka covers 57 km2 and is dominated by cultivated areas (mainly silage production for dairy cattle), marsh, deciduous forests, mountains and heath areas.
(b). Artificial selection experiment
As part of a large study on body size variation, each year the whole adult house sparrow population at Leka was captured by mist netting in February and held temporarily in a barn for up to 12 days, which was the time it took to capture all the individuals on the island. The house sparrows were given ad libitum water and food, which included concentrate feeds for cattle, sunflower seeds and bread. The temperature in the barn was kept at 10–12°C, which is the normal temperature inside cowsheds in this area during winter. All the sparrows were measured for morphological traits as well as blood sampled before they were released into the barn. We used tarsus length to indicate structural body size in this study as tarsus size has been shown to be a stable indicator of body size and is relatively resistant to local variations in environmental conditions during growth and in adulthood in house sparrows [28]. After practically all (ca. 95%) individuals at Leka had been captured, we separately estimated the mean and the standard deviation of tarsus length of males and females. Based on this information, we determined the cut-off tarsus size where all individuals with shorter tarsus lengths were removed and released into distant populations of house sparrows away from Leka [29]. This artificial selection procedure was conducted annually from 2002 until 2005 (i.e. four selection events). Thus, each winter we removed ca 50–60% of the individuals with the smallest tarsus lengths. As a consequence of this annual artificial selection protocol at Leka, which allowed only the larger individuals to breed, tarsus length of males increased during the 4 years of selection. This is indicated in a generalized linear mixed model (brood identity as random effect, fitted with the lme4 package [30] R Core Team [31]) where the average size among fledglings that were later recaptured as juveniles or adults showed that their full-grown tarsus lengths increased from 2002 (19.47 mm, s.e. = 0.21) to 2005 (20.36 mm, s.e. = 0.37), in which year 2002 is represented by the intercept (βIntercept = 19.473, s.e. = 0.211, t = 92.3, β2003 = 0.633, s.e. = 0.308, t = 2.06, β2004 = 1.227, s.e. = 0.459, t = 2.67, β2005 = 0.887, s.e. = 0.425, t = 2.09, n = 28).
In this study, we examined telomere lengths from red blood cell samples collected from known age fledglings from whom body size measures (tarsus length) were also taken. This was done for a sample of fledglings produced in the first year of selection (2002) and a sample produced in the last year of selection (2005). We could only use samples from which DNA of sufficient quality and quantity could be extracted, and for which tarsus length data had been collected. The sex of the birds was not known at the time of sample collection. We confined our analysis to males, for which we had the larger sample presumably for stochastic reasons, since the pattern of growth in body size differs between the sexes in house sparrows [32], and the sample size for females was too small to enable investigation of the differences between the sexes.
(c). Sampling procedures
In the study region, the house sparrows lay one to three clutches during the breeding season, early May to mid-August [33]. During this period, we searched for nests and recorded all that were accessible. Typically, the nests were located under the ceiling in barns and cowsheds at dairy farms. Each nest was visited about once a week and the number of eggs, the number of nestlings and their age were recorded. Fledglings were measured for tarsus length between 9 and 15 days of age, when we also collected a small sample of blood (25 µl) used here for genetic sexing and analyses of telomere lengths (for more details on fieldwork procedures, see [34,35]). The age range at which the 44 fledglings in this study were sampled was small, covering a 6-day period.
(d). Molecular methods
Until DNA extraction, the blood was preserved in 96% ethanol at room temperature. The blood was lysed in 60 µl Lairds buffer [36], with 90 µg proteinase K (Sigma Aldrich), and incubated at 50°C for 3 h. The genomic DNA was extracted from the lysate using the ReliaPrep Large Volume HT gDNA Isolation System (Promega), automated on a Biomek NXp robot (Beckman Coulter) following the manufacturer's recommendations, the only exception being elution of DNA in 25 mM Tris HCl (pH 8). The DNA concentrations were measured using a FLUROostar Omega scanner (BMG Labtech). Samples with DNA concentrations above 60 ng µl−1 were normalized to a concentration of 50 ng µl−1 with 25 mM Tris HCl (pH 8). DNA samples were stored at −20°C. For further details, see [27].
The sex of fledglings was determined by molecular genetic methods using DNA extracted from the blood samples. A copy of the CHD-gene is located on both the avian Z and W chromosomes. We amplified the CHD-gene by polymerase chain reaction (PCR) using P2 and P8 primers [37]. Because the two copies vary in size in house sparrows, CHD-Z is 370 bp and CHD-W is 388 bp, they can be separated by electrophoresis. PCR amplification was carried out in 10 µl reactions containing 5 µl 2× QIAGEN Multiplex PCR Master Mix (QIAGEN), 3 µl extracted DNA (ca 20 ng µl−1) and 0.125 µM of each CHD primer. The CHD primers were included in a 2 µl multiplex primer mix that also contained the seven microsatellite loci Ase18 and Pdo10 [38], Pdoμ1 and Pdoμ3 [39], Pdoµ5 [40], Pdo33 and Pdo40 [41]. We used a touchdown PCR profile: 94°C for 15 min; 12 cycles of 94°C for 30 s, 62°C for 1 min 30 s, and 72°C for 1 min, where the annealing temperature was dropped 1°C each cycle; 19 cycles of 94°C for 30 s, 50°C for 1 min 30 s, and 72°C for 1 min; 60°C for 5 min. The PCR product was kept at 4°C until 1 µl was taken out and mixed with 0.5 µl GeneScan 600 LIZ size standard (Applied Biosystems) and 10 µl HiDi Formamide (Applied Biosystems) prior to capillary electrophoresis on an ABI 3130xl Genetic Analyzer (Applied Biosystems). The P8 primer was fluorescently labelled using NED (Applied Biosystems), enabling us to score Z- and W-copies of the CHD-gene in the GeneMapper v. 4.0 software (Applied Biosystems). In birds, males are the homogametic sex and were thus identified by being homozygous for the CHD-gene (i.e. carrying two short copies).
The concentration and quality of DNA samples were assessed using a Nanodrop-8000 Spectrophotometer. Relative telomere lengths were measured using the qPCR method. While this gives a relative rather than an absolute measure, it is widely used in telomere studies and very suitable for the very small blood samples we had [42]. This method also includes any interstitial repeats of the telomere sequence, the level of which is not known for this species. We therefore further examined a small sample of fledglings for which we had sufficient DNA, using both the standard Southern blot TRF method and in-gel TRF method [42]; telomere length was in the 15–20 kb range, and no substantial levels of interstitial repeats were observed when the gels were examined. Telomere measurements were made using the qPCR method as described by Criscuolo et al. [43] with the following modifications. We verified that Gapdh was a suitable control gene in this species by carrying out a melt curve analysis and checking that the dissociation curve was a single peak. As the melting temperature of the product is sequence dependent, a single peak indicates amplification of a single product. The amplification products, from a random selection of samples, were also run on a gel to confirm that the amplification was of a single product. DNA samples (10 ng) were assayed using the Absolute blue qPCR SYBR green Low Rox master mix (Thermo scientific) with telomere primers (Tel1b and Tel2b) at a final concentration of 500 nM and Gapdh primers at a final concentration of 200 nM. Forward Gapdh primer was 5′-GAG GTG CTG CTC AGA ACA TTA T-3′ and reverse Gapdh primer was 5′-ACG GAA AGC CAT TCC AGT AAG-3′. The telomere thermal profile was 15 min at 95°C, followed by 27 cycles of 15 s at 95°C, 30 s at 58°C, 30 s at 72°C. The Gapdh thermal profile was 15 min at 95°C, followed by 40 cycles of 15 s at 95°C, 15 s at 60°C. Both assays were followed by melt curve analysis of (58–95°C 1oc/5 s ramp).The reference sample was serially diluted (from 40 to 2.5 ng well−1) to produce a standard curve for each plate. This was used to calculate plate efficiencies, all of which fell well within the acceptable range of 100 ± 10% (mean efficiency for our telomere assay 98.6% and for our Gapdh assay 94.6%) and only samples that fell within the bounds of the standard curve were included. As described in Criscuolo et al. [43], relative telomere measurements were calculated using the ΔΔCt method. This provides a ratio of the abundance of the telomeric sequence to the abundance of the reference single copy gene (henceforth, T/S ratio). Since the qPCR efficiencies were tightly controlled, these data were highly correlated with the values obtained when the calculations were done using the Pfaffl method, which corrects for differences in plate efficiencies (rPearson = 0.998).
(e). Statistical analyses
We used linear mixed models assuming Gaussian residual distribution, where all models included the identity of the nest as random factor in order to account for any non-independence of fledglings from the same brood (R v. 3.0.2. [31], package lme4, function lmer [30]). The dataset comprised 24 and 20 male fledglings in 2002 and 2005, respectively, distributed among 21 nests, with structural body size being represented by tarsus length. The significance of the explanatory variables was evaluated by likelihood-ratio tests, which compare the difference in deviance between two models after excluding a term with the χ2-distribution. All comparisons were 1 d.f. in this study. We tested whether the average fledgling tarsus length changed between 2002 and 2005 and accounted for any effect of the slight variation in fledgling age at sampling by retaining this variable in all models. Likewise we tested whether the average telomere length differed between 2002 and 2005, respectively. We also tested whether a linear relationship between telomere length (response variable) and tarsus length was present when the samples from 2002 and 2005 were pooled and whether a similar linear relationship existed within each year by testing the significance of the interaction term (year : tarsus length). Also here we included the fledgling age at sampling in the model. Age was not significantly correlated with tarsus length in the small age range covered by this dataset (rPearson = 0.05, p > 0.1; n = 44). Assumptions of all statistical models were evaluated visually by diagnostic plots.
The growth of the fledglings was not monitored during the selection experiment, since they were measured only once for logistic reasons and to minimize disturbance. However, to give an insight into how tarsus measured as a fledgling related to adult size, we used data for fledglings that were recaptured later as juveniles and/or adults and from which the full-grown tarsus length was then obtained. The sample size for this was small (10 individuals from the 2002 cohort and five from the 2005 cohort, distributed over 10 nests). We examined the relationship between tarsus size (standardized to the age of 10 days to account for variation in the age of measurement of fledglings), and tarsus size when fully grown by using a generalized linear mixed model (package lme4, function lmer, accounting for nest identity as random factor). To obtain the standardized size at the age of 10 days, we modelled the relationship between age and tarsus length (as response variable) by a quadratic model based on a large sample (n = 10 517) from our main study area in northern Norway [33]. From this model we extracted the predicted tarsus length at the age of 10 days for each individual.
3. Results
(a). Body size variation
During the period over which selection for larger structural size in the breeding population was imposed, the average tarsus length in the fledglings increased significantly (by 4.3%) in the 2005 cohort as compared to the 2002 cohort (βtarsus 2005 = 0.823, s.e. = 0.243; χ2 = 7.887, p = 0.005; n = 44; figure 1a). This is because (as can be seen in figure 3) the upper range of tarsus lengths in fledglings was extended as expected. Our capacity to examine whether the increase in fledgling tarsus length in 2005 also resulted in an increase in full-grown tarsus length compared to in 2002 was limited, since we only had data for the 15 birds measured both as fledglings and as full-grown adults. Nonetheless, figure 2 shows that tarsus length standardized to 10 days of age was predictive of adult tarsus size (β = 0.593, s.e. = 0.076; χ2 = 25.244, p < 0.001; n = 15; figure 2). As can be seen in this figure, the larger tarsus sizes in fledglings produced as a consequence of the selection regime gave rise to larger adults.
Figure 1.
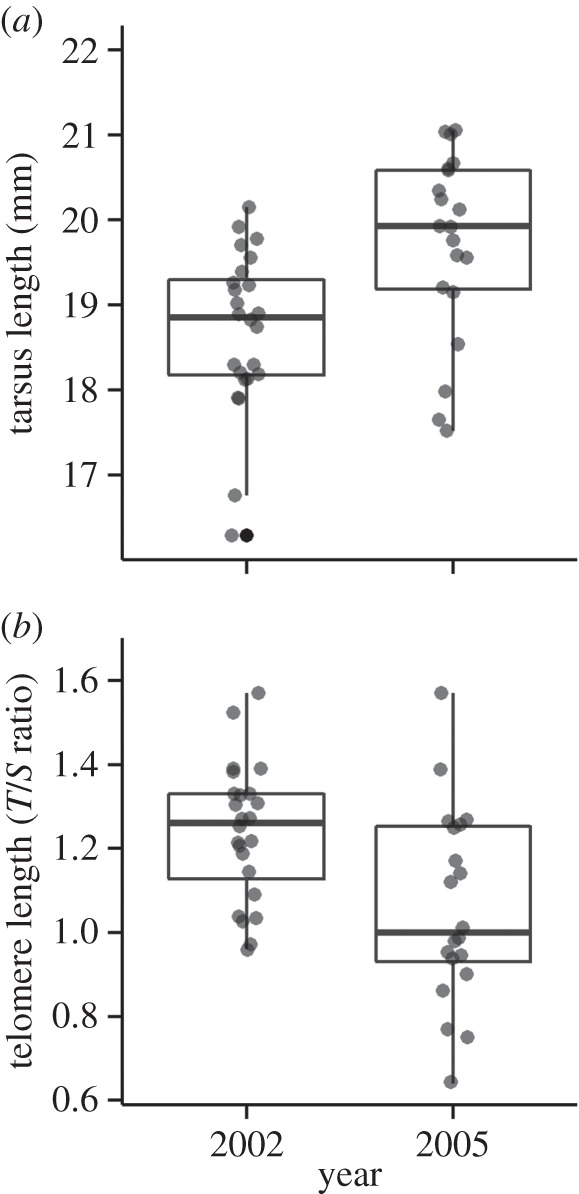
An artificial selection experiment for increased tarsus size was conducted among breeding adults in a house sparrows population at the island Leka in northern Norway during 2002–2005. (a) The tarsus length for fledglings increased significantly from 2002 to 2005 (n = 44). (b) During the same period, the telomere lengths of the fledglings decreased significantly (n = 44). In (a), the fledgling tarsus length was standardized to the age of 10 days for illustration (but not in the statistical analyses, see §2). The boxplots represents the 25–75% quartiles, where the horizontal lines represent the median of the data and the vertical lines correspond to the 95% CI. Individual values are given as grey dots. See §3 for further details.
Figure 3.
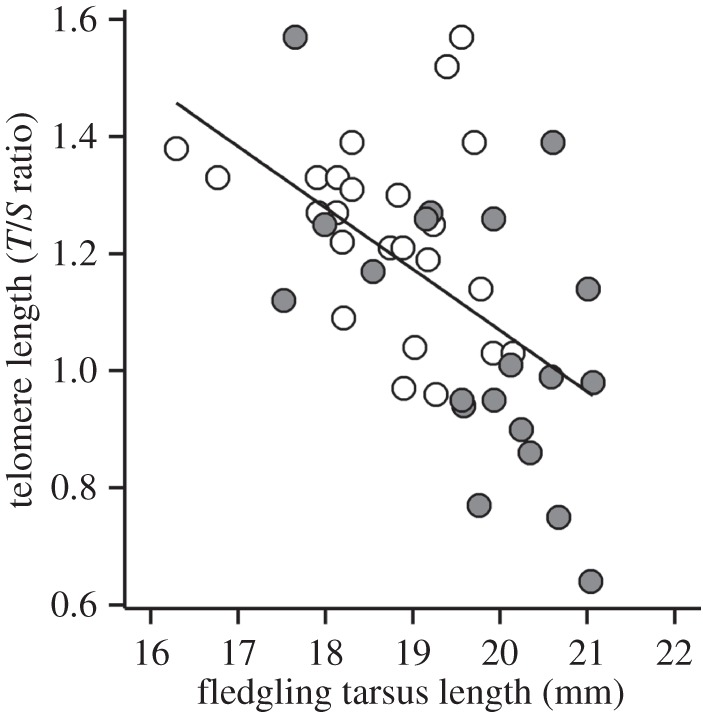
The relationship between tarsus length and telomere length of fledglings. In this figure, the fledgling tarsus length was standardized to the age of 10 days for illustration (but not in the statistical analyses, see §2). Data from the first and the last years of the selection experiment (2002 and 2005, respectively) are indicated by white and dark circles, respectively (n = 44).
Figure 2.
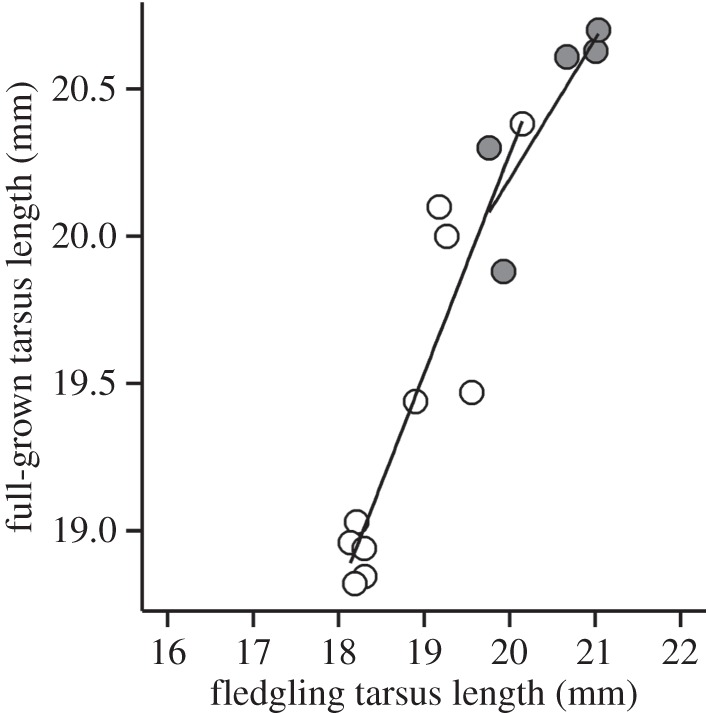
The tarsus length of house sparrows at fledgling stage was positively predictive for the tarsus length measured when individuals were recaptured later at full-grown size (n = 15) at the island Leka in northern Norway. Individuals from 2002 and 2005 are indicated with white and dark circles, respectively. In this analysis, the fledgling tarsus length was standardized to the age of 10 days to allow for small differences in the age at measurement. See §3 for further details.
(b). Size and telomere length
The increase in fledgling tarsus length was accompanied by a significant reduction in average telomere lengths from 2002 to 2005 (βtelomere length 2005 = −0.163, s.e. = 0.070; χ2 = 4.640, p = 0.031, n = 44, figure 1b). We examined whether there was a significant negative relationship between size and telomere length, whether this was evident in 2002 at the start of the selection regime, and whether the relationship changed when structural body size had increased significantly at the end of the selection regime. Figure 3 shows these relationships. An overall negative relationship between tarsus length and telomere length was present for fledglings when the samples from 2002 and 2005 were pooled (βtarsus length = −0.105, s.e. = 0.030; χ2 = 8.710, p = 0.003; n = 44), as seen in figure 3. Fledglings with larger structural size had shorter telomeres. The slopes of the negative relationships between telomere length and tarsus length did not differ significantly between 2002 and 2005, as the interaction effect (year : tarsus length) was not significant (p > 0.1). Thus, structural size and telomere length were negatively related, and the extension of the upper range of tarsus length in fledglings extended, but did not change, this relationship. All the models above controlled for the actual age of the fledglings at measurement, which had no significant effect (p > 0.05) in all models.
4. Discussion
In an isolated population of house sparrows in northern Norway, we have shown a negative relationship in fledglings between structural size (as indicated by tarsus length) and telomere length. This relationship continued when fledgling size was increased by an experimental protocol that increased the structural body size of the breeding population, demonstrating the persistent phenotypic link between the two traits. To our knowledge, this is the first study that demonstrates that large size is accompanied by a reduction in telomere length in a wild vertebrate species. Because the study was carried out in the wild, unmeasured, correlated factors could influence the results, and the sample size was relatively small. However, that the negative relationship between structural size and telomere length was maintained when the size was extended at the upper end by the selection regime suggests that the negative covariation between the two traits is not coincidental.
The reduced telomere length could be a consequence of associations between structural size and gene expression [44,45], or a downstream phenotypic consequence of larger size, for example by influencing oxidative damage incurred during growth [46]. Alternatively, or additionally, it could involve gametic, embryonic or post-natal changes in cell division rates to enable a larger size to be achieved [47,48]. The size of fledglings was predictive of adult size, and the larger fledglings produced under the selection regime were larger both as chicks and as adults (figure 2). It is also possible that the reduction in telomere length occurred as a consequence not simply of larger size, but also as a result of faster growth needed to reach this adult size within the defined time frame likely to occur in seasonally breeding species. Thus, growth and body size could both be involved. With respect to the effect of extending the upper range of body size, it would obviously have been useful to have had replicates of the selection experiment [49], but this was not possible owing to the logistical effort required to undertake this kind of work in the field. Furthermore, the negative relationship is unlikely to have arisen as a consequence of processes such as genetic drift (see electronic supplementary material, S1, for a further evaluation of the potential role of genetic drift), since the same relationship was present in the first year, and before the selection experiment was really underway.
Directional selection on body size is commonly reported in the literature [50] and it is well established within quantitative genetics that directional selection on one focal trait will often alter other genetically correlated traits indirectly, depending on the strength and the sign of the genetic correlations [44,51]. Since telomere length at the end of the growth period is related to lifespan [24], it seems likely that the negative fitness consequences of reduced longevity that accompany larger size could constrain evolution of larger body size. A prerequisite for such an evolutionary mechanism is that body size and telomere length are heritable traits [51]. Body size has been shown to be heritable in house sparrows [52,53], heritability of body size is well documented in avian literature [54,55]. Significant heritability of telomere lengths has so far been demonstrated non-human species such as sand lizard (Lacerta agilis) [56], collared flycatcher (Ficedula albicollis), kakapo (Strigops habroptilus) [57,58], king penguins (Aptenodytes patagonicus) [59] and the great reed warbler (Acrocephalus arundinaceus) [60]. Heritability of telomere length has also been found in humans [61–63]. However, the genetic correlations between telomere length and other phenotypic traits [51] have yet to be investigated in detail. This study reveals evidence of a mechanism that could underpin a trade-off between body size and longevity within species. Since lifespan is a key component of lifetime reproductive success in iteroparous breeders like birds (see e.g. [34]), the link between structural size and telomere length could have important fitness consequences.
Acknowledgements
We are grateful to the fieldworkers who collected the data, in particular to Ole Roar Davidsen. We are also grateful for the help by laboratory technician Randi Røsbak in Trondheim, and to Neil Metcalfe for useful comments.
Ethics
The project was conducted in accordance with permits from the Norwegian Environmental Agency (permit numbers: 2001/6427-ARTS/VI/ARE and 2004/1671 ARTS-VI-ID) and from Bird Ringing Centre at Museum Stavanger, Norway. The project was conducted in accordance with Norwegian legal policy for animal welfare approved by the Norwegian Animal Research Authority (permit numbers: S-2603-01 and S-2004-8032-1). These permits cover all research activity involving animals described in this study. No animals were killed or sacrificed in this study.
Data accessibility
The dataset supporting this article have been uploaded as electronic supplementary material, S2.
Authors' contributions
B.-E.S., T.H.R. and H.J. designed the selection experiment. T.H.R., H.J. and B.R. collected data. H.J. and I.J.H. were responsible for the extraction of DNA in Trondheim, whereas W.B. and R.G. were responsible for the analyses of telomere lengths in Glasgow. T.H.R. conducted the statistical analyses with significant contributions from H.P., T.K. and H.H. H.P. also made the figures. T.H.R. and P.M. wrote the manuscript. P.M. and T.H.R. designed the telomere investigation. B.R., T.K., I.J.H., H.J., H.P., H.H., W.B., R.G. and B.-E.S. contributed during the process with discussions and manuscript revisions.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the European Research Council (ERC-2010-AdG 268562) and the Research Council of Norway (221956 and 2232571F50). The laboratory work in Glasgow was supported by ERC Advanced grant no. 268926.
References
- 1.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Roff DA. 2002. Life history evolution. Sunderland, MA: Sinauer. [Google Scholar]
- 3.van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources—their influence on variation in life-history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 4.Reznick DN. 1997. Life history evolution in guppies (Poecila reticulata): guppies as a model for studying the evolutionary biology of aging. Exp. Gerontol. 32, 245–258. ( 10.1016/s0531-5565(96)00129-5) [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/s0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe NB, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940. ( 10.1016/S0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 7.Martin TE. 1995. Avian life-history evolution in relation to nest sites, nest predation and food. Ecol. Monogr. 65, 101–127. ( 10.2307/2937160) [DOI] [Google Scholar]
- 8.Reznick D, Nunney L, Tessier A. 2000. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425. ( 10.1016/s0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- 9.Nilsson J-Å, Svensson E. 1996. The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc. R. Soc. Lond. B 263, 711–714. ( 10.1098/rspb.1996.0106) [DOI] [Google Scholar]
- 10.Blanckenhorn WU. 2000. The evolution of body size: what keeps organisms small? Q. Rev. Biol. 75, 385–407. ( 10.1086/393620) [DOI] [PubMed] [Google Scholar]
- 11.Austad SN. 2010. Methusaleh's zoo: how nature provides us with clues for extending human health span. J. Comp. Pathol. 141, S10–S21. ( 10.1016/j.jcpa.2009.10.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraus C, Pavard S, Promislow DEL. 2013. The size–life span trade-off decomposed: why large dogs die young. Am. Nat. 181, 492–505. ( 10.1086/669665) [DOI] [PubMed] [Google Scholar]
- 13.Bartke A. 2012. Healthy aging: is smaller better? A mini-review. Gerontology 58, 337–343. ( 10.1159/000335166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austad SN. 2010. Animal size, metabolic rate, and survival, among and within species. In The comparative biology of aging (ed. Wolf NS.), pp. 27–41. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 15.Bernstein RM. 2010. The big and small of it: how body size evolves. Am. J. Phys. Anthropol. 143, 46–62. ( 10.1002/ajpa.21440) [DOI] [PubMed] [Google Scholar]
- 16.Miller RA, Harper JM, Galecki A, Burke DT. 2002. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell 1, 22–29. ( 10.1046/j.1474-9728.2002.00006.x) [DOI] [PubMed] [Google Scholar]
- 17.Samaras TT. 2009. Should we be concerned over increasing body height and weight? Exp. Gerontol. 44, 83–92. ( 10.1016/j.exger.2008.02.002) [DOI] [PubMed] [Google Scholar]
- 18.Miller RA, Chrisp C, Atchley W. 2000. Differential longevity in mouse stocks selected for early life growth trajectory. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 55, B455–B461. ( 10.1093/gerona/55.9.B455) [DOI] [PubMed] [Google Scholar]
- 19.Patronek GJ, Waters DJ, Glickman LT. 1997. Comparative longevity of pet dogs and humans: implications for gerontology research. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 52, B171–B178. ( 10.1093/gerona/52A.3.B171) [DOI] [PubMed] [Google Scholar]
- 20.Arendt JD. 1997. Adaptive intrinsic growth rates: an integration across taxa. Q. Rev. Biol. 72, 149–177. ( 10.1086/419764) [DOI] [Google Scholar]
- 21.Tarry-Adkins JL, Chen JH, Smith NS, Jones RH, Cherif H, Ozanne SE. 2009. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 23, 1521–1528. ( 10.1096/fj.08-122796) [DOI] [PubMed] [Google Scholar]
- 22.Monaghan P. 2010. Telomeres and life histories. The long and the short of it. In Year in evolutionary biology (eds Schlichting CD, Mousseau TA), pp. 130–142. Malden, MA: Wiley-Blackwell. [DOI] [PubMed] [Google Scholar]
- 23.Aubert G, Lansdorp PM. 2008. Telomeres and aging. Physiol. Rev. 88, 557–579. ( 10.1152/physrev.00026.2007) [DOI] [PubMed] [Google Scholar]
- 24.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aviv A, Susser E. 2013. Leukocyte telomere length and the father's age enigma: implications for population health and for life course. Int. J. Epidemiol. 42, 457–462. ( 10.1093/ije/dys236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fick LJ, Fick GH, Li ZC, Cao E, Bao B, Heffelfinger D, Parker HG, Ostrander EA, Riabowol K. 2012. Telomere length correlates with life span of dog breeds. Cell Rep. 2, 1530–1536. ( 10.1016/j.celrep.2012.11.021) [DOI] [PubMed] [Google Scholar]
- 27.Hagen IJ, Billing AM, Rønning B, Pedersen SA, Pärn H, Slate J, Jensen H. 2013. The easy road to genome-wide medium density SNP screening in a non-model species: development and application of a 10 K SNP-chip for the house sparrow (Passer domesticus). Mol. Ecol. Resour. 13, 429–439. ( 10.1111/1755-0998.12088) [DOI] [PubMed] [Google Scholar]
- 28.Killpack TL, Karasov WH. 2012. Growth and development of house sparrows (Passer domesticus) in response to chronic food restriction throughout the nestling period. J. Exp. Biol. 215, 1806–1815. ( 10.1242/jeb.066316) [DOI] [PubMed] [Google Scholar]
- 29.Skjelseth S, Ringsby TH, Tufto J, Jensen H, Sæther B-E. 2007. Dispersal of introduced house sparrows Passer domesticus: an experiment. Proc. R. Soc. B 274, 1763–1771. ( 10.1098/rspb.2007.0338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. (http://arxiv.org/abs/1406.5823) [Google Scholar]
- 31.R Core Team. 2013. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 32.Cleasby IR, Burke T, Schroeder J, Nakagawa S. 2011. Food supplements increase adult tarsus length, but not growth rate, in an island population of house sparrows (Passer domesticus). BMC Res. Notes 4, 431 ( 10.1186/1756-0500-4-431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ringsby TH, Sæther B-E, Tufto J, Jensen H, Solberg EJ. 2002. Asynchronous spatiotemporal demography of a house sparrow metapopulation in a correlated environment. Ecology 83, 561–569. ( 10.1890/0012-9658%282002%29083%5B0561%3AASDOAH%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 34.Jensen H, Sæther B-E, Ringsby TH, Tufto J, Griffith SC, Ellegren H. 2004. Lifetime reproductive success in relation to morphology in the house sparrow Passer domesticus. J. Anim. Ecol. 73, 599–611. ( 10.1111/j.0021-8790.2004.00837.x) [DOI] [Google Scholar]
- 35.Pärn H, Ringsby TH, Jensen H, Sæther B-E. 2012. Spatial heterogeneity in the effects of climate and density-dependence on dispersal in a house sparrow metapopulation. Proc. R. Soc. B 279, 144–152. ( 10.1098/rspb.2011.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl JA. 1989. Current Protocols in Molecular Biology New York, NY: John Wiley & Sons. [Google Scholar]
- 37.Griffiths R, Double MC, Orr K, Dawson RJG. 1998. A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075. ( 10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- 38.Griffith SC, Dawson DA, Jensen H, Ockendon N, Greig C, Neumann K, Burke T. 2007. Fourteen polymorphic microsatellite loci characterized in the house sparrow Passer domesticus (Passeridae, Aves). Mol. Ecol. Notes. 7, 333–336. ( 10.1111/j.1471-8286.2006.01598.x) [DOI] [Google Scholar]
- 39.Neumann K, Wetton JH. 1996. Highly polymorphic microsatellites in the house sparrow Passer domesticus. Mol. Ecol. 5, 307–309. ( 10.1046/j.1365-294X.1996.00095.x) [DOI] [PubMed] [Google Scholar]
- 40.Griffith SC, Stewart IRK, Dawson DA, Owens IPF, Burke T. 1999. Contrasting levels of extra-pair paternity in mainland and island populations of the house sparrow (Passer domesticus): is there an ‘island effect’? Biol. J. Linn. Soc. 68, 303–316. ( 10.1111/j.1095-8312.1999.tb01171.x) [DOI] [Google Scholar]
- 41.Dawson DA, et al. 2012. Microsatellite resources for Passeridae species: a predicted microsatellite map of the house sparrow Passer domesticus. Mol. Ecol. Resour. 12, 501–523. ( 10.1111/j.1755-0998.2012.03115.x) [DOI] [PubMed] [Google Scholar]
- 42.Nussey DH, et al. 2014. Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol. Evol. 5, 299–310. ( 10.1111/2041-210x.12161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P. 2009. Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian. Biol. 40, 342–347. ( 10.1111/j.1600-048X.2008.04623.x) [DOI] [Google Scholar]
- 44.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 45.Roff DA, Fairbairn DJ. 2007. The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447. ( 10.1111/j.1420-9101.2006.01255.x) [DOI] [PubMed] [Google Scholar]
- 46.Richter T, von Zglinicki T. 2007. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 42, 1039–1042. ( 10.1016/j.exger.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 47.Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB. 1995. Telomere shortening is associated with cell division in vitro and in vivo. Exp. Cell Res. 220, 194–200. ( 10.1006/excr.1995.1306) [DOI] [PubMed] [Google Scholar]
- 48.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 20133151 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson N. 1997. Spurious associations in unreplicated selected lines. Behav. Genet. 27, 145–154. ( 10.1023/A:1025689425738) [DOI] [PubMed] [Google Scholar]
- 50.Kingsolver JG, Diamond SE. 2011. Phenotypic selection in natural populations: what limits directional selection? Am. Nat. 177, 346–357. ( 10.1086/658341) [DOI] [PubMed] [Google Scholar]
- 51.Lynch M, Walsch B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer. [Google Scholar]
- 52.Jensen H, Steinsland I, Ringsby T, Sæther B-E. 2008. Evolutionary dynamics of a sexual ornament in the house sparrow (Passer domesticus): the role of indirect selection within and between sexes. Evolution 62, 1275–1293. ( 10.1111/j.1558-5646.2008.00395.x) [DOI] [PubMed] [Google Scholar]
- 53.Jensen H, Sæther B-E, Ringsby TH, Tufto J, Griffith SC, Ellegren H. 2003. Sexual variation in heritability and genetic correlations of morphological traits in house sparrow (Passer domesticus). J. Evol. Biol. 16, 1296–1307. ( 10.1046/j.1420-9101.2003.00614.x) [DOI] [PubMed] [Google Scholar]
- 54.Merilä J, Sheldon BC. 2001. Avian quantitative genetics. Curr. Ornithol. 16, 179–181. ( 10.1007/978-1-4615-1211-0_4) [DOI] [Google Scholar]
- 55.Postma E. 2014. Four decades of estimating heritabilities in wild vertebrate popuations: improved methods, more data, better estimates? In Quantitative genetics in the wild (eds Charmantier A, Garant D, Kruuk L), pp. 16–39. Oxford, UK: Oxford University Press. [Google Scholar]
- 56.Olsson M, Pauliny A, Wapstra E, Uller T, Schwartz T, Blomqvist D. 2011. Sex differences in sand lizard telomere inheritance: paternal epigenetic effects increases telomere heritability and offspring survival. PLoS ONE 6, e17473 ( 10.1371/journal.pone.0017473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voillemot M, Hine K, Zahn S, Criscuolo F, Gustafsson L, Doligez B, Bize P. 2012. Effects of brood size manipulation and common origin on phenotype and telomere length in nestling collared flycatchers. BMC Ecol. 12, 17 ( 10.1186/1472-6785-12-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horn T, Robertson BC, Will M, Eason DK, Elliott GP, Gemmell NJ. 2011. Inheritance of telomere length in a bird. PLoS ONE 6, e17199 ( 10.1371/journal.pone.0017199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reichert S, Rojas ER, Zahn S, Robin JP, Criscuolo F, Massemin S. 2015. Maternal telomere length inheritance in the king penguin. Heredity 114, 10–16. ( 10.1038/hdy.2014.60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asghar M, Bensch S, Tarka M, Hansson B, Hasselquist D. 2014. Maternal and genetic factors determine early life telomere length. Proc. R. Soc. B 282, 20142263 ( 10.1098/rspb.2014.2263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graakjaer J, Der-Sarkissian H, Schmitz A, Bayer J, Thomas G, Kolvraa S, Londono-Vallejo JA. 2006. Allele-specific relative telomere lengths are inherited. Hum. Genet. 119, 344–350. ( 10.1007/s00439-006-0137-x) [DOI] [PubMed] [Google Scholar]
- 62.Broer L, et al. 2013. Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 21, 1163–1168. ( 10.1038/ejhg.2012.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Njajou OT, et al. 2007. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl Acad. Sci. USA 104, 12 135–12 139. ( 10.1073/pnas.0702703104) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting this article have been uploaded as electronic supplementary material, S2.


