Abstract
Metabolic programming occurs when variations in nutrition during a specific developmental window result in long-term metabolic effects. It has been studied almost exclusively in humans and other mammals but never in an ecological context. Here, we report metabolic programming and its functional consequences in a marine fish, red drum. We demonstrate that maternal provisioning of eggs with an essential fatty acid, docosahexaenoic acid (DHA), varies with DHA content of the maternal diet. When offspring are reared on a DHA-replete diet, whole-body DHA content of offspring depends upon the amount of DHA that was in the egg. We further demonstrate that whole-body DHA content is correlated with traits related to offspring fitness (escape responses, routine swimming, growth, and survival). DHA content of red drum eggs produced in nature is in the range where the effects of metabolic programming are most pronounced. Our findings indicate that during a brief developmental window, DHA plays a role in establishing the metabolic capacity for its own uptake or storage, with protracted and possibly permanent effects on ecologically important survival skills of individuals and important implications for dynamics of populations and food webs.
Keywords: transgenerational effects, nutrition, development, performance, docosahexaenoic acid
1. Introduction
Most animals must obtain certain nutrients, including some fatty acids, amino acids, vitamins, and minerals, from their diet because they are unable to synthesize an adequate supply to meet their physiological needs. These are known as essential nutrients, and embryos and neonates use them to fuel development and growth [1–4]. Mothers transfer ingested essential nutrients to offspring through the yolk, placenta, or lactation. Some animals, those referred to as capital breeders [5], accumulate and store nutrients for reproduction over a long period before releasing those nutrients to eggs or embryos. This tactic should produce relatively consistent nutrition for offspring because it draws upon somatic reserves. Income breeders, however, supply their offspring with nutrients from recently ingested food, which creates the potential for variations in availability of essential nutrients to the mother to affect embryonic nutrition, with consequences for offspring development, growth, and survival.
Recent research has shown that changes in the essential fatty acid (EFA) composition of the diet of the marine fish red drum (Sciaenops ocellatus) and gilthead seabream (Sparus aurata) quickly produce changes in the EFA content of their eggs [6,7], identifying these species as income breeders. Further, the amount of the EFA docosahexaenoic acid (DHA, 22 : 6ω3) in red drum eggs is correlated with escape response performance and survival of three-week-old larvae, even when the larval diet is enriched with DHA [8,9]. These findings suggest that deficiencies in maternal provisioning of eggs with DHA induced by variations in maternal diet produce extended and possibly irreversible effects on larvae. The proximate cause of this transgenerational effect of nutrition on offspring fitness is not clear, nor is its likelihood in nature. Here, we report results of experiments that provide evidence for metabolic programming in which the maternal dietary intake of an essential nutrient alters the ability of offspring to absorb or retain that nutrient from their own diet which, in turn, has protracted effects on offspring performance, growth, and survival. We also show that DHA concentrations in red drum eggs in nature are in the range where effects of metabolic programming are most pronounced.
2. Material and methods
We conducted three experiments to measure the effect of maternal provisioning of DHA in eggs on larval fatty acid composition and its consequences for offspring fitness. By manipulating the diet of adult red drum, we created batches of eggs that varied widely in DHA content. Individual batches were divided, and larvae were reared on diets containing different amounts of DHA. Experiments were terminated when larvae reached 9–10 mm total length at which time larvae were assayed for fatty acid composition, ecological performance, growth, and survival.
(a). Adult diets and egg composition
Different levels of DHA in eggs were achieved by adjusting proportions of shrimp (Litopenaeus setiferus or Farfantepenaeus aztecus), squid (Loligo opalescens), Spanish sardine (Sardinella aurita), Atlantic mackerel (Scomber scombrus), beef liver, and a fatty acid supplement (Algamac ARA, Aqua-fauna Bio-Marine) in the adult diet. The fatty acid supplement was intended to alter the ratio of arachidonic acid (ARA, the dominant ω6 EFA) to DHA in eggs. Fish were induced to spawn using photothermal manipulation. Eggs were sampled from 25 spawns (6, 8, and 11 spawns for Experiments 1, 2, and 3, respectively) produced by eight tanks of broodstock, each containing one to three females. Additional details about the fish and methods were published previously [9].
(b). Experiments and larval diets
The DHA content of larval diets were 0.6 and 8.6 mg g−1 dry weight (DW) for Experiment 1 and 3.9, 8.6, and 22.3 mg g−1 for Experiment 2. After analysing results from these experiments, Experiment 3 (15.0 and 23.1 mg DHA g−1 DW) was conducted to fill the gap in the diets used in Experiments 1 and 2.
Each tank of larvae was reared from a sample of 5 ml (approx. 5 000 eggs) (Experiment 1) or 3 ml (3 000 eggs) (Experiments 2 and 3). An additional sample (2 ml) was rinsed twice in deionized water and frozen (−80°C) for analysis of fatty acids. All larvae were fed rotifers (Brachionus plicatilis) enriched for 45 min with Algamac 3050 (Aqua-fauna Bio-Marine) once daily at a concentration of 10 000 l−1 for the first 10 days posthatching (dph). At 10–11 dph, larvae were also fed non-enriched 1-day-old Artemia nauplii at a concentration of 250 l−1 once daily.
Beginning 12 dph and through the end of each experiment (19–30 dph), larvae were fed one of the experimental diets only (table 1). Different levels of DHA in the experimental diets were achieved by changing the amount of enrichment product per Artemia nauplius. One diet used a different enrichment product in order to alter the proportional composition of EFAs (table 1). While the objective of using different larval diets was to vary DHA levels, the method resulted in coordinated variation in other EFAs. Principal component analysis showed that the other ω3 EFAs (18-, 20-, and 22-carbon polyunsaturated) varied in parallel with DHA and that the ω6 EFAs varied independently of them. Fatty acid composition of each diet and their interrelationships are provided in the electronic supplemental material, table S1.
Table 1.
Diets fed to red drum larvae and levels of DHA (mean ± s.e.) in those diets. Artemia were enriched overnight for all diets. DW: dry weight.
| experiment | enrichment |
DHA (mg g−1 DW) | ||
|---|---|---|---|---|
| product | amount (g) | no. Artemia | ||
| 1 | Algamac 3050 | 0.025 | 300 000 | 0.6 ± 0.1 |
| 1 | Algamac 3050 | 0.050 | 150 000 | 8.6 ± 1.4 |
| 2 | Algamac ARA | 0.200 | 100 000 | 3.9 ± 0.4 |
| 2 | Algamac 3050 | 0.050 | 150 000 | 8.6 ± 1.4 |
| 2 | Algamac 3050 | 0.100 | 100 000 | 22.3 ± 1.7 |
| 3 | Algamac 3050 | 0.052 | 100 000 | 15.0 ± 0.8 |
| 3 | Algamac 3050 | 0.076 | 100 000 | 23.1 ± 1.1 |
(c). Larval performance, growth, and survival
To assess the impact of DHA (maternally provisioned and in the larval diet) on offspring fitness, we measured performance traits that are critical to survival in nature—escape performance, routine swimming, growth, and survival. For Experiments 1 and 2, when larvae in each tank reached an average of 9–10 mm total length (19–30 dph), the performance of 24 individuals was measured in two assays: escape response to a looming visual stimulus and routine swimming. For each tank, mean values were computed for the following escape response variables: responsiveness, response latency, response distance, response duration, and response speed; and routine swimming variables: routine swimming speed and turning rate (defined by previous research [8,9]). Larvae used in performance trials were frozen (−80°C) for subsequent analysis of fatty acid composition. The performance of larvae from Experiment 3 was not measured, but larval fatty acid composition was analysed using 20, 9 to 10 mm individuals from each tank. Mean growth rate was measured using all larvae in each rearing tank (Experiments 1 and 2) or a subsample of 20 larvae (Experiment 3) and was corrected for the density of fish in each tank (residual of the regression of growth against number surviving). Survival was calculated from the total number of larvae surviving in each tank. Additional details about these methods were published previously [9].
(d). Field collection of eggs
Red drum eggs were collected during the annual spawning aggregation (early September to early November) from the University of Texas Marine Science Institute pier, located on the Aransas Pass Ship Channel (27°50.3′ N, 97°3.1′ W). Collections were made on multiple dates each year (four dates in 2009, 10 in 2010 and four in 2011) using a 500 µm mesh plankton net, and red drum eggs were separated from the few other species based on their shape, diameter, yolk and oil globule characteristics, and lack of pigmentation. Eggs were rinsed twice in deionized water and frozen (−80°C) for fatty acid analysis.
(e). Fatty acid and statistical analyses
Concentrations of 27 fatty acids in adult and larval diets, eggs, and larvae were measured (expressed as mg fatty acid g−1 DW) using established methods [10]. For each sample, a measured amount of tricosanoic acid (23 : 0) was added before the sample was homogenized as an internal standard for quantification of mg g−1 dry mass of fatty acids. Total lipids were cold extracted by homogenizing a sample of approximately 50 mg (DW) in a chloroform–methanol solution (2 : 1 v/v). Fatty acid methyl esters were prepared by saponification in potassium hydroxide, followed by methylation in a 14% boron trifluoride in methanol solution. Fatty acid concentrations were determined by gas chromatography (Shimadzu GC-2014 Scientific Instruments; www.ssi.shimadzu.com) set with a Phenomenex ZB-WAX plus capillary column (30 m long, 0.53 mm ID, 1.0 µm thick, www.phenomenex.com). Individual fatty acids were identified by comparison to commercial standards (Supelco, Inc.; www.sigmaaldrich.com).
Analysis of covariance (ANCOVA) was used to test the effect of maternal provisioning (DHA content of eggs) on uptake and retention of DHA by larvae (measured as DHA content of larvae) under different levels of DHA in the larval diet. Pearson correlation coefficients (r) were used to assess the strength of relationships between larval DHA content and the measures of performance, growth, and survival (Experiments 1 and 2). Routine swimming speed and survival were transformed using a logarithmic and arcsine transformation, respectively, in order to meet the assumption of normally distributed errors.
3. Results
The DHA content of eggs varied roughly twofold in each experiment (22.0–45.4 mg g−1 DW in Experiment 1, 30.1–59.2 mg g−1 DW in Experiment 2, and 27.8–51.2 mg g−1 DW in Experiment 3). The DHA content of whole larvae (9–10 mm total length) was significantly affected by the DHA content of both the eggs and the larval diet in each of the three experiments (ANCOVA, p < 0.001; figure 1). A response surface computed from the combined data from the three experiments (adjusted R2 = 0.77, p < 0.001) showed that when the DHA content of the larval diet was low, the DHA content of larvae was low and relatively insensitive to differences in DHA content of the eggs (figure 2). Under these conditions, dietary availability of DHA probably limits DHA uptake and storage by larvae. When the DHA content of the larval diet was higher, however, the DHA content of larvae was 1.5 to 2.5 times greater when eggs contained higher concentrations of DHA compared with eggs that contained lower levels of DHA (figure 2).
Figure 1.
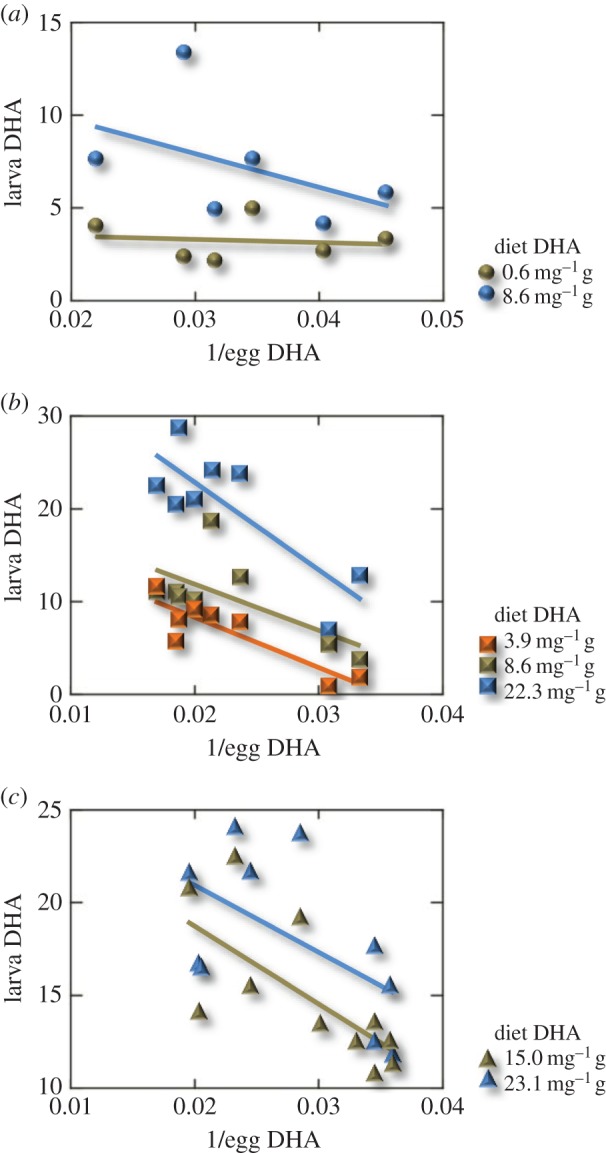
DHA content of larval tissue in response to differences in DHA content of eggs and larval diet. (a) Experiment 1, N = 6 batches of eggs, each divided into two treatments. (b) Experiment 2, N = 8 batches of eggs, each divided into three treatments. (c) Experiment 3, N = 11 batches of eggs, each divided into two treatments. The inverse of egg DHA is plotted to linearize the relationships for statistical analysis. All DHA values are expressed in mg g−1 DW.
Figure 2.
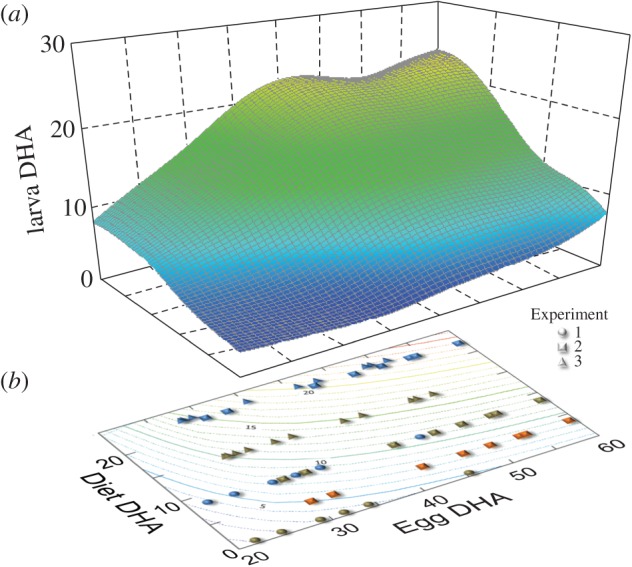
Response of DHA content of larval tissue to DHA content of eggs and larval diet. Perspective plot (a) is fitted with a distance-weighted least-squares smoothing function. Contour plot (b) shows location of data points (N = 58) and contours of an approximation of the smoothed response surface (larval DHA = 8.14–154.51/egg DHA + 1.11 × diet DHA – 16.84 × diet DHA/egg DHA). All DHA values are expressed as mg g−1 DW.
Five larval traits—escape response duration and speed, routine swimming speed, growth, and survival—were significantly correlated with the amount of DHA present in larvae. Three of these traits were also significantly correlated with the DHA content of eggs, and two traits were significantly correlated with the DHA content of the larval diet, although the correlations with the larval diet were much weaker (table 2).
Table 2.
Correlation coefficients (r) and statistical significance (p) for relationships between means of larval performance traits and mean DHA concentration in eggs, larval diet, and larvae. Italicized values signify a statistically significant correlation (p < 0.05).
| performance trait | DHA content (mg g−1 DW) |
|||||
|---|---|---|---|---|---|---|
| egg | p | diet | p | larva | p | |
| escape response | ||||||
| responsiveness | −0.15 | 0.44 | 0.07 | 0.70 | 0.05 | 0.79 |
| response latency | −0.28 | 0.13 | 0.00 | 0.98 | −0.22 | 0.25 |
| response distance | −0.10 | 0.61 | −0.04 | 0.82 | 0.12 | 0.53 |
| response duration | 0.01 | 0.97 | 0.24 | 0.20 | 0.43 | 0.02 |
| response speed | −0.23 | 0.22 | −0.33 | 0.07 | −0.46 | 0.01 |
| routine swimming | ||||||
| routine speed (log-transformation) | −0.43 | 0.02 | −0.32 | 0.09 | −0.40 | 0.03 |
| turning rate | −0.12 | 0.51 | −0.22 | 0.23 | −0.19 | 0.33 |
| growth and survival | ||||||
| growth rate | −0.59 | <0.01 | −0.39 | 0.02 | −0.57 | <0.01 |
| survival (arcsine transformation) | 0.44 | <0.01 | 0.35 | 0.03 | 0.51 | <0.01 |
Wild red drum eggs contained an average of 26.8 ± 2.8, 20.6 ± 4.2, and 25.9 ± 2.6 mg DHA g−1 DW in 2009, 2010 and, 2011, respectively. These values are at the lower end of eggs used in our experiments (figure 2).
4. Discussion
Our results demonstrate metabolic programming in a marine fish and suggest physiological mechanisms and ecological consequences. Specifically, (i) DHA content of the adult diet alters offspring performance of survival skills through differential provisioning of embryos with DHA; (ii) lower amounts of DHA in eggs result in reduced whole-body DHA content in offspring after weeks of exogenous feeding on a DHA-replete diet; and (iii) several measures of offspring performance are strongly linked to whole-body DHA content. Metabolic programming is known almost exclusively from mammals and it is believed to be a cause of important medical disorders in humans [11–13]. Numerous epidemiological and experimental studies indicate that foetal nutrition alters development, and various molecular, cellular, metabolic, neuroendocrine, physiological, and epigenetic pathways have been proposed for the resulting permanent effects on metabolism [14,15]. Our findings provide a clear demonstration of metabolic programming that connects maternal nutrition to measures of offspring fitness in a fish and indications of the general mechanism involved. Specifically, DHA appears to play a role during embryonic (pre-feeding) stages in establishing the metabolic capacity for its own accumulation in tissues, and this effect appears to be irreversible. Similar findings have been obtained for domesticated mammals, where DHA levels in the hypothalamus of young rats [16] and the plasma and red blood cells of lambs [17] and rats [18] were affected by levels of EFAs in the maternal, perinatal diet despite the young animals receiving a DHA-replete post-weaning diet. In contrast, differences in the DHA content of the maternal diet of Senegalese sole (Solea senegalensis) generated small differences in the DHA content of eggs and early larvae which became negligible by 7 dph [19].
(a). Physiological mechanisms of metabolic programming
The total amount of DHA in a 9 to 10 mm fish fed higher levels of DHA was 11–20 times the amount of DHA in an egg, which means that at least 90–95% of the DHA in the larval body was exogenous. Variation in DHA content of young fish could arise from differences in the (i) amount of food ingested (appetite); (ii) efficiency of DHA digestion and absorption by the gut; (iii) rate of metabolic oxidation or storage of DHA (energy homeostasis); (iv) rate of de novo synthesis of DHA; or (v) some combination of these. Under certain conditions, undernutrition or overnutrition of rats in utero and while suckling results in hyperphagia later in life (references in [14]). Metabolic programming of appetite might explain our results if deficiency of DHA to the embryo inhibits absorption of DHA by larvae. Then, an increased appetite might be a compensatory mechanism which would increase food intake and result in faster growth without a concomitant increase in whole-body DHA. This is consistent with the opposing relationships we observed for growth and whole-body DHA content with respect to egg DHA content (table 2). These opposing relationships for growth and DHA content are, however, also consistent with the possibility that offspring from eggs containing low amounts of DHA are programmed to metabolize more of their dietary DHA, thereby providing more energy for growth while leaving less DHA for deposition in tissues. Results from experiments on rats support this; undernourished male foetuses were programmed towards higher levels of fatty acids in muscle tissue later in life [20].
With regard to DHA synthesis, mammals use the Δ6 desaturase enzyme to convert α-linolenic acid (ALA) to DHA, and experiments on rats indicate that a maternal diet low in ALA can reduce the ability of pups to synthesize DHA when fed a diet high in ALA [16]. The rate of de novo synthesis is unlikely to be the primary mechanism that explains our results because marine fishes generally lack Δ6 desaturase [21] or their capacity to synthesize DHA is insufficient to account for the more than twofold difference in whole-body DHA associated with variation in egg DHA content. Further, studies of gene expression in European sea bass (Dicentrarchus labrax) and Senegalese sole suggest that early EFA deficiencies in the diet (maternal or larval) might increase the capacity of early larvae to synthesize DHA [19,22]. This kind of compensatory response is the opposite of what we observed. Regardless of the mechanism, the developmental window during which metabolic programming is established in red drum appears to be during the first five days after fertilization, prior to differentiation of the organs involved in exogenous feeding and digestion. This points to an epigenetic mechanism for metabolic programming that influences the function of tissues that differentiate at a later time.
Oviparous fishes may be a useful alternative to mammalian models for investigating some mechanisms of metabolic programming, especially those aspects related to lipid metabolism. The nutritional environment of a developing fish (from fertilization until yolk absorption) is fixed prior to fertilization. It is not subject to the fluctuations in maternally derived nutrients, hormones, and metabolites that developing mammals experience through the placenta and lactation. The embryonic nutritional environment of eggs can be altered through the maternal diet (as demonstrated in the current study), injections into the yolk, or through the larval diet to access different stages of embryonic development. Further, since much of organogenesis, especially that of the digestive system, occurs while the fishes are feeding exogenously, different kinds of experimental manipulations are possible to explore the physiological mechanisms of metabolic programming.
(b). Ecological implications of metabolic programming
Levels of DHA found in wild red drum eggs were in the range where they have the most pronounced influence on the ability of larvae to accumulate DHA from their diet and the concomittant effects on larval performance, growth, and survival. DHA is also important for visual and nervous system function [23], schooling ability [24], and escape responses [25] of young fishes. Therefore, metabolic programming could have important consequences in nature because the functions affected by DHA influence predation, starvation, and the duration of the period of high mortality during early life.
Details of the reproductive and feeding habits of adult red drum illustrate how metabolic programming can be important to population dynamics of income breeders. In the western Gulf of Mexico adult red drum reside offshore for much of the year and migrate to coastal inlets for spawning in late summer. As a result of this migration, their diet shifts from approximately 55% fish and 38% crustaceans in winter and spring (offshore) to 28% fish and 65% crustaceans in late summer (inshore), principally replacing DHA-rich menhaden (Brevoortia patronus) with white shrimp [26,27] at the time when oocytes are being supplied with yolk. Thus despite containing somewhat less DHA, the shrimp (together with blue crab, Callinectes sapidus, which does not vary seasonally in the diet) represent an important source of DHA for red drum eggs. Commercial harvest or environmental conditions that reduce the abundances of shrimp and crabs in coastal areas could reduce the availability of DHA to adult red drum and the DHA content of their eggs, resulting in larvae that are less able to accumulate DHA from their own diet and altering their growth, survival, and performance of skills that are critical in nature.
On a broader scale, there is growing recognition that EFAs also play an important role in food webs and ecosystem structure and function. EFAs that animals require for their own well-being are synthesized almost entirely by primary producers, principally phytoplankton and macroalgae in marine ecosystems, and they reach animals through a variety of food web linkages [28]. Changes in environmental conditions, such as upwelling, eutrophication, and ocean acidification can alter communities of primary producers, resulting in changes in the availability of EFAs to higher trophic levels which can alter the fatty acid composition of animals [29,30], the timing of reproduction [31], and the structure of communities on the scale of regime shifts [32]. The transgenerational link that metabolic programming establishes between ingestion of EFAs by adults and offspring fitness represents one specific pathway through which variations in EFA synthesis by primary producers might transmit through food webs to alter animal populations and communities.
Acknowledgements
We thank Cynthia Faulk for conducting Experiment 3, measuring fatty acid profiles of eggs, larvae, and diets, and providing constructive comments on the manuscript. We thank Jeffrey Kaiser and Rene Lopez for care of broodstock and Alfredo Ojanguren for making field collections of eggs. The manuscript benefitted from our discussions with Ken Webb and Cynthia Faulk.
Ethics
Experiments were conducted under an Animal Use Protocol approved by The University of Texas at Austin.
Data accessibility
Data deposited in Dryad (http://dx.doi.org/10.5061/dryad.74cj2).
Authors' contributions
Both authors participated in conceiving the study, designing and coordinating the experiments, analysing the data and preparing the manuscript. L.A.F. secured funding for the research.
Competing interests
We have no competing interests.
Funding
This research was supported by funding from Texas Sea Grant and the Guy Harvey Ocean Foundation.
References
- 1.Nettleton JA. 1993. Are n-3 fatty acids essential nutrients for fetal and infant development? J. Am. Diet. Assoc. 93, 58–64. ( 10.1016/0002-8223(93)92132-H) [DOI] [PubMed] [Google Scholar]
- 2.Izquierdo MS, Fernández-Palacios H, Tacon AGJ. 2001. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197, 25–42. ( 10.1016/S0044-8486(01)00581-6) [DOI] [Google Scholar]
- 3.Innis SM. 2005. Essential fatty acid transfer and fetal development. Placenta 26, S70–S75. ( 10.1016/j.placenta.2005.01.005) [DOI] [PubMed] [Google Scholar]
- 4.Uauy R, Dangour AD. 2006. Nutrition in brain development and aging: role of essential fatty acids. Nutr. Rev. 64, S24–S33. ( 10.1111/j.1753-4887.2006.tb00242.x) [DOI] [PubMed] [Google Scholar]
- 5.Jönsson KI. 1997. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66. ( 10.2307/3545800) [DOI] [Google Scholar]
- 6.Fuiman LA, Faulk CK. 2013. Batch spawning facilitates transfer of an essential nutrient from diet to eggs in a marine fish. Biol. Lett. 9, 20130593 ( 10.1098/rsbl.2013.0593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harel M, Tandler A, Kissil G, Applebaum S. 1994. The kinetics of nutrient incorporation into body tissues of gilthead seabream (Sparus aurata) females and the subsequent effects on egg composition and egg quality. Br. J. Nutr. 72, 45–58. ( 10.1079/BJN19940008) [DOI] [PubMed] [Google Scholar]
- 8.Fuiman LA, Ojanguren AF. 2011. Fatty acid content of eggs determines antipredator performance of fish larvae. J. Exp. Mar. Biol. Ecol. 407, 155–165. ( 10.1016/j.jembe.2011.06.004) [DOI] [Google Scholar]
- 9.Perez KO, Fuiman LA. 2015. Maternal diet and larval diet influence survival skills of larval red drum Sciaenops ocellatus. J. Fish Biol. 86, 1286–1304. ( 10.1111/jfb.12637) [DOI] [PubMed] [Google Scholar]
- 10.Faulk CK, Holt GJ. 2005. Advances in rearing cobia Rachycentron canadum larvae in recirculating aquaculture systems: live prey enrichment and greenwater culture. Aquaculture 249, 231–243. ( 10.1016/j.aquaculture.2005.03.033) [DOI] [Google Scholar]
- 11.Hanley B, et al. 2010. Metabolic imprinting, programming and epigenetics—a review of present priorities and future opportunities. Br. J. Nutr. 104(Suppl 1), S1–25. ( 10.1017/S0007114510003338) [DOI] [PubMed] [Google Scholar]
- 12.Innis SM. 2011. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern. Child. Nutr. 7(Suppl 2), 112–123. ( 10.1111/j.1740-8709.2011.00318.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming TP, Velazquez MA, Eckert JJ, Lucas ES, Watkins AJ. 2012. Nutrition of females during the peri-conceptional period and effects on foetal programming and health of offspring. Anim. Reprod. Sci. 130, 193–197. ( 10.1016/j.anireprosci.2012.01.015) [DOI] [PubMed] [Google Scholar]
- 14.McMillen IC, Robinson JS. 2005. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633. ( 10.1152/physrev.00053.2003) [DOI] [PubMed] [Google Scholar]
- 15.Sookoian S, Gianotti TF, Burgueño AL, Pirola CJ. 2013. Fetal metabolic programming and epigenetic modifications: a systems biology approach. Pediatr. Res. 73, 531–542. ( 10.1038/pr.2013.2) [DOI] [PubMed] [Google Scholar]
- 16.Li D, Weisinger HS, Weisinger RS, Mathai M, Armitage JA, Vingrys AJ, Sinclair AJ. 2006. Omega 6 to omega 3 fatty acid imbalance early in life leads to persistent reductions in DHA levels in glycerophospholipids in rat hypothalamus even after long-term omega 3 fatty acid repletion. Prostaglandins Leukot. Essent. Fatty Acids 74, 391–399. ( 10.1016/j.plefa.2006.03.010) [DOI] [PubMed] [Google Scholar]
- 17.Clayton EH, Lamb TA, Refshauge G, Kerr MJ, Bailes KL, Ponnampalam EN, Friend MA, Hopkins DL. 2014. Differential response to an algae supplement high in DHA mediated by maternal periconceptional diet: intergenerational effects of n-6 fatty acids. Lipids 49, 767–775. ( 10.1007/s11745-014-3926-3) [DOI] [PubMed] [Google Scholar]
- 18.Muhlhausler BS, Miljkovic D, Fong L, Xian CJ, Duthoit E, Gibson RA. 2011. Maternal omega-3 supplementation increases fat mass in male and female rat offspring. Front. Genet. 2, 48 ( 10.3389/fgene.2011.00048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morais S, Mendes AC, Castanheira MF, Coutinho J, Bandarra N, Dias J, Conceição LEC, Pousão-Ferreira P. 2014. New formulated diets for Solea senegalensis broodstock: effects of parental nutrition on biosynthesis of long-chain polyunsaturated fatty acids and performance of early larval stages and juvenile fish. Aquaculture 432, 374–382. ( 10.1016/j.aquaculture.2014.04.033) [DOI] [Google Scholar]
- 20.Lane RH, Kelley DE, Ritov VH, Tsirka AE, Gruetzmacher EM. 2001. Altered expression and function of mitochondrial β-oxidation enzymes in juvenile intrauterine-growth-retarded fat skeletal muscle. Pediatr. Res. 50, 83–90. ( 10.1203/00006450-200107000-00016) [DOI] [PubMed] [Google Scholar]
- 21.Bell MV, Toucher DR. 2009. Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: general pathways and new directions. In Lipids in aquatic ecosystems (eds Arts MT, Brett MT, Kainz MJ), pp. 211–236. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 22.Vagner M, Zambonino Infante JL, Robin JH, Person-Le Ruyet J. 2007. Is it possible to influence European sea bass (Dicentrarchus labrax) juvenile metabolism by a nutritional conditioning during larval stage? Aquaculture 267, 165–174. ( 10.1016/j.aquaculture.2007.01.031) [DOI] [Google Scholar]
- 23.Bell MV, Batty RS, Dick JR, Fretwell K, Navarro JC, Sargent JR. 1995. Dietary deficiency of docosahexaenoic acid impairs vision at low light intensities in juvenile herring (Clupea harengus L.). Lipids 30, 443–449. ( 10.1007/BF02536303) [DOI] [PubMed] [Google Scholar]
- 24.Ishizaki Y, Masuda R, Uematsu K, Shimizu K, Arimoto M, Takeuchi T. 2001. The effect of dietary docosahexaenoic acid on schooling behaviour and brain development in larval yellowtail. J. Fish Biol. 58, 1691–1703. ( 10.1006/jfbi.2001.1579) [DOI] [Google Scholar]
- 25.Nakayama S, Masuda R, Takeuchi T, Tanaka M. 2003. Effects of highly unsaturated fatty acids on escape ability from moon jellyfish Aurelia aurita in red sea bream Pagrus major larvae. Fish. Sci. 69, 903–909. ( 10.1046/j.1444-2906.2003.00706.x) [DOI] [Google Scholar]
- 26.Boothby RN, Avault JW Jr. 1971. Food habits, length-weight relationship, and condition factor of the red drum (Scieanops ocellata) in southeastern Louisiana. Trans. Am. Fish. Soc. 100, 290–295. () [DOI] [Google Scholar]
- 27.Scharf FS, Schlicht KK. 2000. Feeding habits of red drum (Sciaenops ocellatus) in Galveston Bay, Texas: seasonal diet variation and predator–prey size relationships. Estuaries 23, 128–139. ( 10.2307/1353230) [DOI] [Google Scholar]
- 28.Fuiman LA, Connelly TL, Lowerre-Barbieri SK, McClelland JW. 2015. Egg boons: central components of marine fatty acid food webs. Ecology 96, 362–372. ( 10.1890/14-0571.1) [DOI] [PubMed] [Google Scholar]
- 29.Ahlgren G, Van Nieuwerburgh L, Wanstrand I, Pedersen M, Boberg M, Snoeijs P. 2005. Imbalance of fatty acids in the base of the Baltic Sea food web—a mesocosm study. Can. J. Fish. Aquat. Sci. 62, 2240–2253. ( 10.1139/F05-140) [DOI] [Google Scholar]
- 30.Litz M, Brodeur R, Emmett R, Heppell S, Rasmussen R, O'Higgins L, Morris M. 2010. Effects of variable oceanographic conditions on forage fish lipid content and fatty acid composition in the northern California Current. Mar. Ecol. Prog. Ser. 405, 71–85. ( 10.3354/meps08479) [DOI] [Google Scholar]
- 31.Røjbek M, Jacobsen C, Tomkiewicz J, Støttrup J. 2012. Linking lipid dynamics with the reproductive cycle in Baltic cod Gadus morhua. Mar. Ecol. Prog. Ser. 471, 215–234. ( 10.3354/meps10012) [DOI] [Google Scholar]
- 32.Litzow MA, Bailey KM, Prahl FG, Heintz R. 2006. Climate regime shifts and reorganization of fish communities: the essential fatty acid limitation hypothesis. Mar. Ecol. Prog. Ser. 315, 1–11. ( 10.3354/meps315001) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data deposited in Dryad (http://dx.doi.org/10.5061/dryad.74cj2).


