Abstract
Several properties of food webs—the networks of feeding links between species—are known to vary systematically with the species richness of the underlying community. Under the ‘latitude–niche breadth hypothesis’, which predicts that species in the tropics will tend to evolve narrower niches, one might expect that these scaling relationships could also be affected by latitude. To test this hypothesis, we analysed the scaling relationships between species richness and average generality, vulnerability and links per species across a set of 196 empirical food webs. In estuarine, marine and terrestrial food webs there was no effect of latitude on any scaling relationship, suggesting constant niche breadth in these habitats. In freshwater communities, on the other hand, there were strong effects of latitude on scaling relationships, supporting the latitude–niche breadth hypothesis. These contrasting findings indicate that it may be more important to account for habitat than latitude when exploring gradients in food-web structure.
Keywords: food webs, scaling, trophic level, generality, vulnerability, link density
1. Introduction
Food webs—networks of feeding links between species—have been used for several decades to summarize the structure of ecological communities [1–3], and to understand how that structure relates to environmental variables such as habitat type [4,5], primary productivity [6–8] and climate [9,10]. The latter variables in turn have strong gradients over latitude, with productivity and temperature both being higher in the tropics, while climate is more variable at high latitudes [11]. These variables affect both the resources available and species' metabolisms [12–15], and have been proposed as determinants of the strength of interspecific interactions [16–18]. By modulating interactions between species, latitudinal gradients may also shape food-web structure. Indeed, these latitudinal environmental gradients have been put forward as potential drivers for the latitudinal gradient in species richness, one of the most general and robust patterns in ecology [16,19,20].
One proposed link between species richness and latitude is the ‘latitude–niche breadth hypothesis' [21]. This hypothesis predicts that decreased seasonality in the tropics should lead to more stable populations, which in turn should evolve smaller niches [21]. These narrow niches should therefore allow more species to coexist in the tropics than at higher latitudes. Alternatively, the higher productivity of the tropics [22] may result in a broader niche space [23], which could also sustain greater biodiversity even if niche sizes are globally similar. Although the assumptions of the latitude–niche breadth hypothesis are only equivocally supported [21], it remains a compelling potential mechanism for the latitudinal gradient in species richness [24–26].
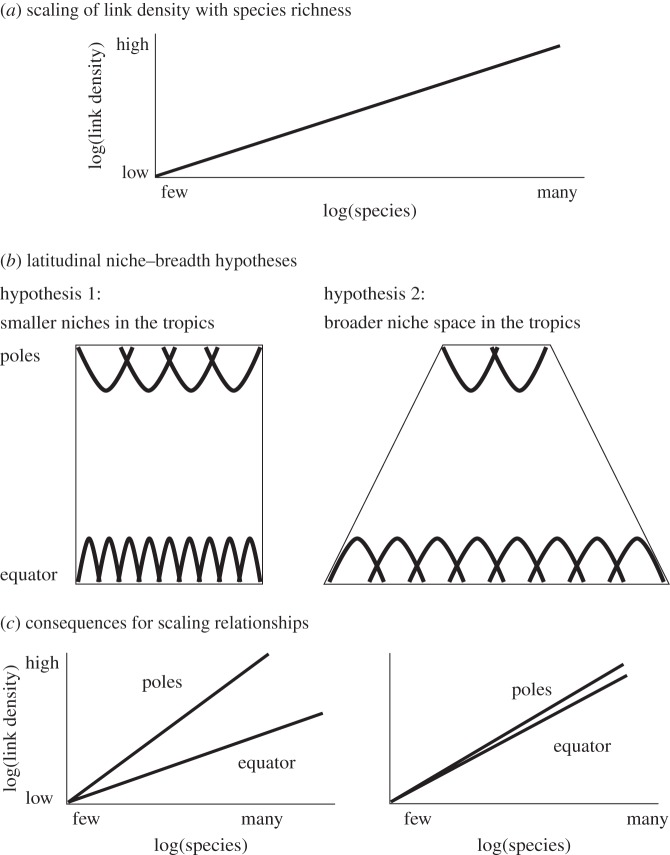
If the latitude–niche breadth hypothesis is correct, then there should also be direct relationships between latitude and the degree of specialization (i.e. the breadth of the Eltonian niche [27,28]) of species within food webs. Specifically, narrower niches in the tropics would equate to greater specialization (narrower niches), while constant niche sizes but greater productivity would translate to constant specialization and niche width across latitude (figure 1). Attempts to unravel these effects, however, are complicated by known relationships between species richness and many other network properties [29]. For example, narrower niches imply fewer links per species (i.e. greater specialization) in the tropics [30,31] (but see [18]). However, average numbers of links per species tend to increase in larger food webs [29,32]. This means that a latitudinal effect on specialization may be obscured by a latitudinal gradient in species richness.
Figure 1.
(a) We show the known scaling relationship between link density (links per species) and species richness. This scaling relationship is a power law and therefore linear in a log–log plot. (b) We show two versions of the latitudinal–niche breadth hypothesis that have been proposed to explain this gradient. Hypothesis 1 posits that greater environmental stability in the tropics will allow species to evolve narrower niches (indicated by parabolas) than those at the poles. Hypothesis 2 suggests that species will have constant niche sizes over latitude but that greater primary productivity in the tropics creates a larger niche space such that each species still occupies a smaller proportion of the total niche space. These two hypotheses have different implications for the scaling of food-web properties such as the number of feeding links per species with species richness. (c) If hypothesis 1 is true, then the exponent of the scaling relationship between link density and species richness should be larger towards the poles, where each additional species in the food web will have a larger niche (i.e. more feeding links). If hypothesis 2 is true, then the exponent of this distribution should not vary significantly over latitude.
If this is the case, it may still be possible to uncover effects of latitude on specialization by examining the shape of the scaling relationship between specialization and species richness over changing latitude. By testing whether latitude affects the scaling of each property with species richness, we test for the effects of latitude on specialization predicted by the latitude–niche breadth hypothesis (figure 1). If the scaling of specialization with species richness is weaker in the tropics (i.e. if species gain fewer links, predators or prey as the size of the network increases), then this will indicate narrower niches at the tropics. If, however, the scaling of specialization with species richness does not vary over latitude, this will indicate that niches are similarly sized worldwide but that there is a broader niche space in the tropics. Additionally, as food webs describing different ecosystem types may differ in their topology [5,33], we also explored the differences in scaling relationships across ecosystem types. Here, we use three measures of specialization: mean links per species, mean generality (number of prey) and mean vulnerability (number of predators).
2. Material and methods
(a). Dataset
We compiled a list of 196 empirical food webs from multiple sources (see the electronic supplementary material, appendix S1 for web origins and selection criteria). We recorded study site latitude from the original source where possible or, where study sites were described but exact coordinates were not given, obtained estimated coordinates using Google Earth [34]. If a range of latitudes (e.g. 42°–49° N) was provided, we used the midpoint of this range. We grouped food webs by ecosystem type (stream, n = 71; lake, n = 47; marine, n = 28; estuarine, n = 18; terrestrial, n = 31) according to their designation in previous aggregations of food webs (i.e. [35–37]).
As the food webs in this dataset are derived from a variety of sources and were compiled over many decades, it is likely that they vary in their resolution and in the amount of sampling effort invested in their assembly. Many analyses of food-web structure attempt to reduce this variation by using food webs composed of ‘trophic species'—aggregations of species with identical sets of predators and prey—rather than species per se [8,33,37,38]. As our study is concerned directly with the number of species at a particular latitude, however, we did not ignore species with identical sets of interactions. We therefore analysed both original (i.e. without aggregating any species) and trophic species (i.e. after aggregating species with identical predators and prey) versions of the dataset, in each case using the number of species and feeding links in each web to calculate the mean link density (number of links per species), mean generality (number of prey per species) and mean vulnerability (number of predators per species) of the web. The version of the dataset used did not qualitatively change the results, suggesting that the scaling relationships between species richness and specialization across ecosystem type and latitude are very similar whether or not species with identical sets of predators and prey are included. For simplicity, we present here only the results for the original webs.
(b). Gradients over latitude
To put our dataset in the context of other research on latitudinal gradients in species richness, we first examined simple linear relationships between latitude and each of species richness, links per species, generality and vulnerability. We fitted models of the form
| 2.1 |
where Si is the species richness of web i, Li its absolute latitude (degrees north or south regardless of direction), Ei is a categorical variable indicating the ecosystem type of network i (comprising terms for stream, marine, lake and terrestrial networks with estuarine networks corresponding to Ei = 0) and 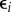 is a residual error term. We next calculated the AIC of the maximal model as well as the AICs of a suite of candidate simplified models identified using the R [39] function dredge from package MuMIn [40]. Simplified models were obtained by systematically removing all possible combinations of terms from the full model. The best-fitting model was then determined to be the model with the fewest terms where ΔAIC < 2, as this model is the least likely to suffer from over-fitting.
is a residual error term. We next calculated the AIC of the maximal model as well as the AICs of a suite of candidate simplified models identified using the R [39] function dredge from package MuMIn [40]. Simplified models were obtained by systematically removing all possible combinations of terms from the full model. The best-fitting model was then determined to be the model with the fewest terms where ΔAIC < 2, as this model is the least likely to suffer from over-fitting.
(c). Scaling relationships with species richness
The scaling relationship between link density (Z) and species richness (S) has been shown to be a power law [29] of the form
| 2.2 |
which is often re-expressed in logarithmic form
| 2.3 |
As the two forms imply a statistical fit of the data to different error distributions, neither of which has strong a priori justification in our dataset, we followed the recommendations in [41] to compare the two model formulations explicitly (see the electronic supplementary material, appendix S2 for details). The logarithmic form (equation (2.3)) provided the better fit to the data, as did the logarithmic forms of similar models for the scaling of generality and vulnerability. We therefore used and present logarithmic models throughout the rest of the analyses.
(d). Effect of latitude on scaling
After determining the appropriate form of the scaling relationship, we then assessed the impact of latitude on the scaling relationships between species richness and link density, generality and vulnerability. In the context of the scaling relationships above, note that this implies that we wished to determine the effect of latitude on the scaling exponent β. We included a categorical variable for ecosystem type (stream, lake, terrestrial, marine or estuary), as well as interactions between food-web type and latitude.
We therefore began by considering models of the form
| 2.4 |
where Si is the species richness of web i, Li its absolute latitude (degrees north or south regardless of direction), Ei is a categorical variable indicating the ecosystem type of network i (comprising terms for stream, marine, lake and terrestrial networks with estuarine networks corresponding to Ei = 0) and 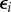 is a residual error term. The logarithmic formulation of this model is
is a residual error term. The logarithmic formulation of this model is
| 2.5 |
We then simplified versions of equation (2.5) for link density, generality and vulnerability following the procedure described above. As a supplemental check to ensure that variation in sampling effort across food webs was not responsible for the trends we observed, we then repeated our analyses using jackknifed datasets in which we (i) sequentially removed each food web in the dataset and (ii) sequentially removed sets of food webs that shared a common author. The first jackknife essentially controls for the influence of any single outlier, whereas the second controls for the influence of particular research groups, some of which contributed large numbers of food webs (up to 27) to the dataset. Parameter estimates for the simplified models varied very little across either jackknife test (see the electronic supplementary material, appendix S3 for details), indicating that the trends we observed were not due to either strong outliers or to substantial differences in sampling effort across research groups.
3. Results
Link density (mean number of feeding links per species), generality (mean number of prey per species) and vulnerability (mean number of predators per species) were strongly and positively correlated (R2 = 0.891 for link density and generality, R2 > 0.999 for link density and vulnerability, and R2 = 0.890 for generality and vulnerability). Contrary to the expected latitudinal gradient, the best-fitting version of equation (2.1) did not include a significant effect of latitude on species richness for any ecosystem type. Further, there were no significant relationships between link density, generality and vulnerability with latitude for any ecosystem type.
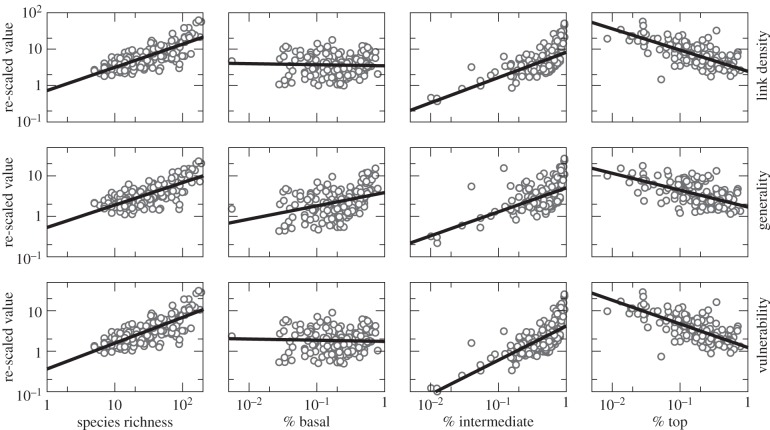
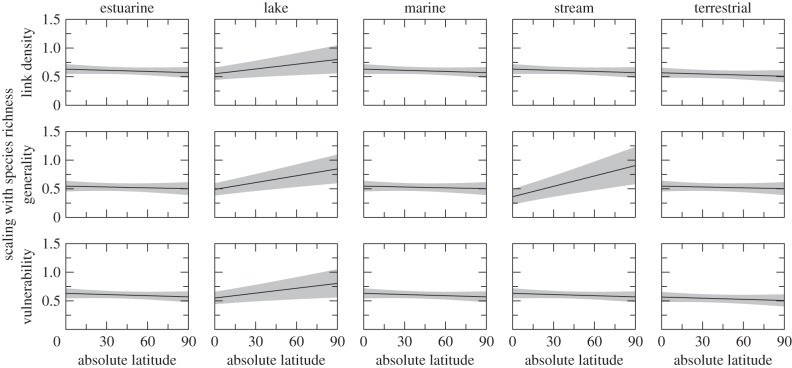
Each measure of specialization increased with increasing species richness (β0 = 0.637, p < 0.001; β0 = 0.553, p < 0.001; and β0 = 0.637, p < 0.001, respectively; figure 2). For estuarine, marine and terrestrial food webs, the strength of this scaling did not vary with latitude (βLatitude = −0.001, p = 0.365 for link density; βLatitude = −0.001, p = 0.535 for generality; βLatitude = −0.001, p = 0.366 for vulnerability; figure 3). In lake food webs, however, the scaling of each property was stronger towards the poles (βLatitude Lake = 0.004, p = 0.019; βLatitude Lake = 0.005, p = 0.004; and βLatitude Lake = 0.004, p = 0.018, respectively). In stream food webs, generality increased more rapidly towards the poles (βLatitude Stream = 0.007, p = 0.001) while link density and vulnerability did not vary with latitude (i.e. the interaction term βLatitude Stream was not retained in the best-fitting models).
Figure 2.
Scaling relationships for re-scaled link density, generality and vulnerability relative to the species richness of a food web. Link density, generality and vulnerability were each re-scaled to remove the effects of latitude and ecosystem type. As these relationships take the form of power laws, we did this by dividing the food-web property (e.g. link density) by species richness raised to an exponent including the effects of latitude and, where applicable, ecosystem type and the interaction between ecosystem type and latitude. Note that in all cases estuarine food webs were treated as the baseline ecosystem type, but that at most two ecosystem types had interactions between ecosystem type and latitude retained in the best-fitting model (see Results for specifics). For each relationship, we show the re-scaled values (white circles) as well as the overall scaling relationship using estuarine ecosystems as a baseline (black line; n = 196 food webs). For a figure with the uncorrected values, see the electronic supplementary material, figure S7 and appendix S4.
Figure 3.
Changes to the scaling of link density, generality and vulnerability with species richness across ecosystem types and over latitude. We show the estimated scaling exponent for species richness (black line) with its 95% CI (in grey), based on n = 196 empirical food webs. Latitude is given in degrees from the equator regardless of direction.
4. Discussion
The tendency of food-web structure to exhibit scaling relationships with species richness has been well established [29,33]. As species richness in particular is also known to vary systematically over latitude [16,19,20,42], intuitively one might suspect that any relationship between food-web properties such as generality might be due to the latitudinal gradient in species richness. In this dataset, however, we found no evidence to support latitudinal gradients in species richness, links per species, generality or vulnerability.
The lack of a latitudinal gradient in species richness in this dataset contrasts strongly with other studies [16,19,20,42]. As numbers of species and links included in a food web vary strongly with sampling effort as well as with the underlying diversity of the study area, it is possible that the lack of latitudinal trends here is a result of researchers tending to expend similar amounts of sampling effort across studies. This could result in food webs describing species-rich tropical communities omitting more species and links than studies of species-poor arctic communities if research groups spend similar person-hours assembling webs and can observe similar numbers of species and links per person-hour. In addition, it is worth noting that gradients in species richness are generally measured for a single taxonomic group at a time [16,19,20,42]. It is possible that these taxa are not well represented in our food webs and that the dominant taxa that are represented do not have an underlying latitudinal gradient in richness. In either case, the lack of a strong association between observed species richness and latitude in any ecosystem type in our dataset means that any effect of latitude on other scaling relationships is not being driven by the scaling of specialization with species richness in our food webs. This is fortunate because the lack of confounding effects of latitude allows us to more clearly assess effects of latitude on scaling with species richness.
Scaling of links per species, generality and vulnerability with species richness varied strongly across ecosystem types. In estuarine, marine and terrestrial food webs, scaling of each property varied little with latitude. This is consistent with the idea that species' niche breadths do not vary systematically with temperature and productivity but that the niche space might be larger in species-rich communities [23]. Rather than niche space depending on temperature and productivity, it may be that species diversity itself affects the biotic niche space available to species (although climate may affect speciation rates and therefore the diversity in a region [43]). For example, as the plant diversity of a community increases both the variety of food available to herbivores and the structural variety of the habitat will also increase.
Unlike other ecosystem types, the scaling of generality in lake and stream food webs was stronger (i.e. generality increased more steeply with increasing species richness) in higher-latitude food webs. In lake food webs, this trend was echoed in the scaling relationships between species richness and vulnerability, and links per species. This means that species in tropical freshwater communities gain fewer additional feeding links per additional species in the web and that species in tropical lakes also gain fewer predators, and fewer links in general, per additional species than species in high-latitude lakes. These trends are consistent with the hypothesis that greater stability in the tropics leads to narrower niches [21] and a higher proportion of specialists.
That freshwater food webs supported the hypothesis of narrower niches in the tropics—while other ecosystem types did not—is noteworthy given that these ecosystems (especially streams) are known for being highly variable and that seasonal variability is one of the proposed drivers of the latitude–niche breadth hypothesis [21]. Both streams and lakes can experience severe changes in water temperature and volume (e.g. floods, drying, freezing) that remove food or other resources (notably oxygen during freezing events) [44,45]. These events are often linked to seasonal events such as snowmelts or summer drought [44]. Further, both temperate streams and lakes tend to experience seasonal strong pulses of allochthonous inputs (e.g. fallen leaves, terrestrial invertebrates [46–48]). These trends combined mean that, relative to estuarine and marine communities, freshwater food webs may experience high turnover in both community composition and productivity [49–51]. Notable exceptions from the above trends are New Zealand stream communities (representing 31 of the 71 stream food webs in our dataset), which experience unpredictable flooding and drying throughout the year and do not receive seasonally pulsed subsidies [44,52]. However, as this subset of webs is very tightly grouped in latitude (44.64–46.41° S, within an overall range of 23.00–69.02° for stream communities), it is unlikely that they have greatly influenced our results (see also the electronic supplementary material, appendix S3). Moreover, just as in highly variable communities where said variation is more seasonal, New Zealand communities are dominated by ecological generalists [44,52], implying that they appear to fit the general pattern of streams worldwide.
Importantly, while terrestrial communities are also strongly seasonal at high latitudes and can receive significant allochthonous inputs [46], terrestrial consumers tend to be morphologically specialized for feeding on particular prey [53]. This means that primarily gape-limited aquatic consumers tend to be more generalist across all types of aquatic habitats than terrestrial consumers [5,53]. This trend also held in our data (μAquatic = 5.47, μTerrestrial = 3.82; p = 0.007 for μAquatic > μTerrestrial). The key to this argument is therefore whether freshwater ecosystems experience more severe seasonal variation than marine and estuarine ecosystems. Although we are not aware of any study explicitly comparing seasonal variation in freshwater and saltwater ecosystems at similar latitudes, we believe that freshwater ecosystems are indeed likely to experience more severe changes because of their small size. While oceans and estuaries certainly vary in terms of water temperature and nutrients over the course of a year [51], these changes are likely to be slower and milder than in freshwater because marine and estuarine communities are buffered by being open to the ocean rather than isolated amid a terrestrial matrix. Supporting this hypothesis, net primary productivity is much more variable over the course of a year in non-marine communities [11]. Thus suggests that niche breadths may also be more variable over the course of the year.
5. Conclusion
Overall, our results were inconsistent with the latitude–niche breadth hypothesis in estuarine, marine and terrestrial communities, but consistent with the hypothesis of greater specialization in the tropics in stream and lake food webs. This suggests that different mechanisms may structure food webs in different habitat types and that freshwater food webs in particular may be strongly affected by seasonal variation. Freshwater food webs also appear to have different predator–prey biomass ratios than other ecosystem types [36]. Although it is not clear whether these ratios are related to seasonality, this could be a promising avenue for future research. In addition, different relationships between latitude and niche breadth in different habitat types go some way towards explaining the equivocal support for the opposing hypotheses of narrower niches in the tropics [21] and broader niche space in the tropics [23]. Our study indicates that both have merit, but would appear to apply to different systems.
Supplementary Material
Acknowledgements
We thank members of the Romanuk, Stouffer and Tylianakis laboratories for their comments on the manuscript. We also thank Angus McIntosh for valuable discussion on stream food webs, and Matthias Schleuning, Robby Stoks and one anonymous reviewer for their insightful questions.
Data accessibility
Food webs used in this study were retrieved from the University of Canberra's GlobalWeb database [35] (www.globalwebdb.com) and from two papers [36,37]. Original sources for the food webs are given in the electronic supplementary material, appendix S1.
Authors' contributions
A.R.C., D.B.S. and T.N.R. designed the study. A.R.C. collected published data, performed the analyses and wrote the first draft. D.B.S. and T.N.R. substantially revised the article. All authors approved the final version.
Competing interests
We have no competing interests.
Funding
This research was supported by an NSERC USRA undergraduate scholarship and NSERC PGS-D graduate scholarship (to A.R.C.), a Marsden Fund Fast-Start grant (UOC-1101) and a Rutherford Discovery Fellowship, both administered by the Royal Society of New Zealand (to D.B.S.), and an NSERC Discovery grant (to T.N.R.).
References
- 1.Paine RT. 1966. Food web complexity and species diversity. Am. Nat. 100, 65–75. ( 10.1086/282400) [DOI] [Google Scholar]
- 2.Williams RJ, Martinez ND. 2000. Simple rules yield complex food webs. Nature 404, 180–183. ( 10.1038/35004572) [DOI] [PubMed] [Google Scholar]
- 3.Petchey OL, Beckerman AP, Riede JO, Warren PH. 2008. Size, foraging, and food web structure. Proc. Natl Acad. Sci. USA 105, 4191–4196. ( 10.1073/pnas.0710672105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briand F. 1983. Environmental control of food web structure. Ecology 64, 253–263. ( 10.2307/1937073) [DOI] [Google Scholar]
- 5.Shurin JB, Gruner DS, Hillebrand H. 2006. All wet or dried up? Real differences between aquatic and terrestrial food webs. Proc. R. Soc. B 273, 1–9. ( 10.1098/rspb.2005.3377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend CR, Thompson RM, Mcintosh AR, Kilroy C, Edwards E, Scarsbrook MR. 1998. Disturbance, resource supply, and food-web architecture in streams. Ecol. Lett. 1, 200–209. ( 10.1046/j.1461-0248.1998.00039.x) [DOI] [Google Scholar]
- 7.Thompson RM, Townsend CR. 2005. Energy availability, spatial heterogeneity and ecosystem size predict food-web structure in streams. Oikos 108, 137–148. ( 10.1111/j.0030-1299.2005.11600.x) [DOI] [Google Scholar]
- 8.Vermaat JE, Dunne JA, Gilbert AJ. 2009. Major dimensions in food-web structure properties. Ecology 90, 278–282. ( 10.1890/07-0978.1) [DOI] [PubMed] [Google Scholar]
- 9.Petchey OL, Brose U, Rall BC. 2010. Predicting the effects of temperature on food web connectance. Phil. Trans. R. Soc. B 365, 2081–2091. ( 10.1098/rstb.2010.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baiser B, Gotelli NJ, Buckley HL, Miller TE, Ellison AM. 2012. Geographic variation in network structure of a nearctic aquatic food web. Glob. Ecol. Biogeogr. 21, 579–591. ( 10.1111/j.1466-8238.2011.00705.x) [DOI] [Google Scholar]
- 11.Field CB. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. ( 10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 12.White CR, Blackburn TM, Martin GR, Butler PJ. 2007. Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc. R. Soc. B 274, 287–293. ( 10.1098/rspb.2006.3727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor MI, Piehler MF, Leech DM, Anton A, Bruno JF. 2009. Warming and resource availability shift food web structure and metabolism. PLoS Biol. 7, 3–8. ( 10.1371/journal.pbio.1000178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hechinger RF, Lafferty KD, Dobson AP, Brown JH, Kuris AM. 2011. A common scaling rule for abundance, energetics, and production of parasitic and free-living species. Science 333, 445–448. ( 10.1126/science.1204337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White CR, Grémillet D, Green JA, Martin GR, Butler PJ. 2011. Metabolic rate throughout the annual cycle reveals the demands of an Arctic existence in Great Cormorants. Ecology 92, 475–486. ( 10.1890/09-1951.1) [DOI] [PubMed] [Google Scholar]
- 16.Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Sys. 40, 245–269. ( 10.1146/annurev.ecolsys.39.110707.173430) [DOI] [Google Scholar]
- 17.Lang B, Rall BC, Brose U. 2012. Warming effects on consumption and intraspecific interference competition depend on predator metabolism. J. Anim. Ecol. 81, 516–523. ( 10.1111/j.1365-2656.2011.01931.x) [DOI] [PubMed] [Google Scholar]
- 18.Schleuning M, et al. 2012. Specialization of mutualistic interaction networks decreases toward tropical latitudes. Curr. Biol. 22, 1925–1931. ( 10.1016/j.cub.2012.08.015) [DOI] [PubMed] [Google Scholar]
- 19.Kaufman DM. 1995. Diversity of New World mammals: universality of the latitudinal gradients of species and bauplans. J. Mammal. 76, 322–334. ( 10.2307/1382344) [DOI] [Google Scholar]
- 20.Macpherson E. 2002. Large-scale species-richness gradients in the Atlantic Ocean. Proc. R. Soc. Lond. B 269, 1715–1720. ( 10.1098/rspb.2002.2091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vázquez DP, Stevens RD. 2004. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 164, E1–E19. ( 10.1086/421445) [DOI] [PubMed] [Google Scholar]
- 22.Brown JH. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 23.Davies KF, Harrison S, Safford HD, Viers JH. 2007. Productivity alters the scale dependence of the diversity–invasibility relationship. Ecology 88, 1940–1947. ( 10.1890/06-1907.1) [DOI] [PubMed] [Google Scholar]
- 24.Lappalainen J, Soininen J. 2006. Latitudinal gradients in niche breadth and position—regional patterns in freshwater fish. Naturwissenschaften 93, 246–250. ( 10.1007/s00114-006-0093-2) [DOI] [PubMed] [Google Scholar]
- 25.Krasnov BR, Shenbrot GI, Khokhlova IS, Mouillot D, Poulin R. 2008. Latitudinal gradients in niche breadth: empirical evidence from haematophagous ectoparasites. J. Biogeogr. 35, 592–601. ( 10.1111/j.1365-2699.2007.01800.x) [DOI] [Google Scholar]
- 26.Slove J, Janz N. 2010. Phylogenetic analysis of the latitude-niche breadth hypothesis in the butterfly subfamily Nymphalinae. Ecol. Entom. 35, 768–774. ( 10.1111/j.1365-2311.2010.01238.x) [DOI] [Google Scholar]
- 27.Elton C. 1927. Animal ecology. New York, NY: Macmillan Co. [Google Scholar]
- 28.Leibold MA, Chase JM, Shurin JB, Downing AL. 1997. Species turnover and the regulation of trophic structure. Annu. Rev. Ecol. Syst. 28, 467–494. ( 10.1146/annurev.ecolsys.28.1.467) [DOI] [Google Scholar]
- 29.Riede JO, Rall BC, Banasek-Richter C, Navarrete SA, Wieters EA, Emmerson MC, Jacob U, Brose U. 2010. Scaling of food-web properties with diversity and complexity across ecosystems. In Advances in ecological research, vol. 42 (ed Woodward G.), pp. 139–170. Burlington, VT: Elsevier. [Google Scholar]
- 30.Marra PP, Remsen JV. 1997. Insights into the maintenance of high species diversity in the neotropics: habitat selection and foraging behavior in understory birds of tropical and temperate forests. Ornithol. Monogr. 48, 445–483. ( 10.2307/40157547) [DOI] [Google Scholar]
- 31.Dyer LA, et al. 2007. Host specificity of Lepidoptera in tropical and temperate forests. Nature 448, 696–699. ( 10.1038/nature05884) [DOI] [PubMed] [Google Scholar]
- 32.Dunne JA. 2006. The network structure of food webs. In Ecological networks: linking structure to dynamics in food webs (eds Pascual M, Dunne JA), pp. 27–86. New York, NY: Oxford University Press. [Google Scholar]
- 33.Dunne JA, Williams RJ, Martinez ND. 2004. Network structure and robustness of marine food webs. Mar. Ecol. Prog. Ser. 273, 291–302. ( 10.3354/meps273291) [DOI] [Google Scholar]
- 34.Google Inc. 2015. Google Earth (version 7.1.2.2041). See http://earth.google.com. [Google Scholar]
- 35.Caffrey L, Thompson R. 2015. GlobalWeb: an online collection of food webs. University of Canberra; See http://www.globalwebdb.com. [Google Scholar]
- 36.Riede JO, Brose U, Ebenman B, Jacob U, Thompson R, Townsend CR, Jonsson T. 2011. Stepping in Elton's footprints: a general scaling model for body masses and trophic levels across ecosystems. Ecol. Lett. 14, 169–178. ( 10.1111/j.1461-0248.2010.01568.x) [DOI] [PubMed] [Google Scholar]
- 37.Dunne JA, et al. 2013. Parasites affect food web structure primarily through increased diversity and complexity. PLoS Biol. 11, e1001579 ( 10.1371/journal.pbio.1001579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez ND. 1991. Artifacts or attributes? Effects of resolution on the Little Rock Lake food web. Ecol. Monogr. 61, 367–392. ( 10.2307/2937047) [DOI] [Google Scholar]
- 39.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 40.Barton K. 2014. MuMIn: multi-model inference. R package v. 1.10.5 See https://cran.r-project.org/web/packages/MuMIn/index.html.
- 41.Xiao X, White EP, Hooten MB, Durham SL. 2011. On the use of log-transformation vs. nonlinear regression for analyzing biological power laws. Ecology 92, 1887–1894. ( 10.1890/11-0538.1) [DOI] [PubMed] [Google Scholar]
- 42.Hillebrand H. 2000. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211. ( 10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 43.Currie DJ, et al. 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134. ( 10.1111/j.1461-0248.2004.00671.x) [DOI] [Google Scholar]
- 44.Winterbourn MJ. 1997. New Zealand mountain stream communities: stable yet disturbed? In Evolutionary ecology of freshwater animals (eds Streit B, Stadler T, Lively CM), pp. 31–53. Basel, Germany: Birkhauser. [Google Scholar]
- 45.Meding ME, Jackson LJ. 2001. Biological implications of empirical models of winter oxygen depletion. Can. J. Fish. Aquat. Sci. 58, 1727–1736. ( 10.1139/f01-109) [DOI] [Google Scholar]
- 46.Nakano S, Murakami M. 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl Acad. Sci. USA 98, 166–170. ( 10.1073/pnas.98.1.166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lennon JT. 2004. Experimental evidence that terrestrial carbon subsidies increase CO2 flux from lake ecosystems. Oecologia 138, 584–591. ( 10.1007/s00442-003-1459-1) [DOI] [PubMed] [Google Scholar]
- 48.Zeng QF, Kong FX, Zhang EL, Tan X, Wu XD. 2008. Seasonality of stable carbon and nitrogen isotopes within the pelagic food web of Taihu Lake. Ann. Limnol. Int. J. Lim. 44, 1–6. ( 10.1051/limn:2008019) [DOI] [Google Scholar]
- 49.Tilzer MM, Beese B. 1988. The seasonal productivity cycle of phytoplankton and controlling factors in Lake Constance. Schweiz. Z. Hydrol. 50, 1–39. ( 10.1007/BF02538370) [DOI] [Google Scholar]
- 50.Magalhães MF. 1993. Feeding of an Iberian stream cyprinid assemblage: seasonality of resource use in a highly variable environment. Oecologia 96, 253–260. ( 10.1007/BF00317739) [DOI] [PubMed] [Google Scholar]
- 51.Baird D, Ulanowicz R. 1989. The seasonal dynamics of the Chesapeake Bay ecosystem. Ecol. Monogr. 59, 329–364. ( 10.2307/1943071) [DOI] [Google Scholar]
- 52.Winterbourn MJ, Rounick JS, Cowie B. 1981. Are New Zealand stream ecosystems really different? New Zeal. J. Mar. Fresh. Res. 15, 321–328. ( 10.1080/00288330.1981.9515927) [DOI] [Google Scholar]
- 53.Liem KF. 1990. Aquatic versus terrestrial feeding modes: possible impacts on the trophic ecology of vertebrates. Am. Zool. 30, 209–221. ( 10.1093/icb/30.1.209) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Food webs used in this study were retrieved from the University of Canberra's GlobalWeb database [35] (www.globalwebdb.com) and from two papers [36,37]. Original sources for the food webs are given in the electronic supplementary material, appendix S1.