Abstract
Differential allocation occurs when individuals adjust their reproductive investment based on their partner's traits. However, it remains unknown whether animals differentially allocate based on their partner's past experiences with predation risk. If animals can detect a potential mate's experience with predators, this might inform them about the stress level of their potential mate, the likelihood of parental effects in offspring and/or the dangers present in the environment. Using threespined stickleback (Gasterosteus aculeatus), we examined whether a female's previous experience with being chased by a model predator while yolking eggs affects male mating effort and offspring care. Males displayed fewer conspicuous courtship behaviours towards females that had experienced predation risk in the past compared with unexposed females. This differential allocation extended to how males cared for the resulting offspring of these matings: fathers provided less parental care to offspring of females that had experienced predation risk in the past. Our results show for the first time, to our knowledge, that variation among females in their predator encounters can contribute to behavioural variation among males in courtship and parental care, even when males themselves do not encounter a predator. These results, together with previous findings, suggest that maternal predator exposure can influence offspring development both directly and indirectly, through how it affects father care.
Keywords: differential allocation, maternal effects, parental care, predator stress, threespined stickleback, transgenerational plasticity
1. Introduction
In many species, individuals adjust their reproductive investment based on characteristics of their potential mate (differential allocation hypothesis [1,2]). For example, after mating with a preferred or high-quality male, females often produce larger or more numerous offspring (positive differential allocation [1–3]). In species that provide parental care after fertilization, differential allocation might extend to how both parents invest in parental care. For example, parents often provision offspring of unattractive mates less than offspring of attractive mates [3]. Individuals might also use the previous experiences of a potential mate, particularly those tied to receptivity and fecundity, to inform their reproductive decisions. For example, males can often detect a female's prior mating experience and early resource environment through changes in female behaviour and/or chemical cues, and adjust their reproductive investment accordingly [4–6].
Whether a potential mate's previous experiences with other ecological factors, such as predation risk, also affect an individual's reproductive investment decisions are unknown. Repeated encounters with predators and predator cues within an individual's lifetime can affect their morphology, growth, circulating stress hormones and behaviour [7,8]. If condition-dependent traits used in mating interactions are sensitive to stressful encounters with predators (as they are to other environmental factors), then these traits could reveal the stress level of a potential mate. Thus, information about a potential mate's experience with predators (and their stress level) might inform partner reproductive decisions in a similar way to information about a potential mate's early rearing environment, or previous mating experience. Additionally, if predator encounters have distinct effects on particular traits or behaviours, then these traits could signal the dangers present in the environment. For example, the cautiousness of an individual during courtship might communicate their perception of predation risk to others.
When a mother's experiences influence her offspring, it might be advantageous for males to differentially allocate their reproductive investment in response to the potential for such maternal effects on offspring. Maternal stress during pregnancy or while yolking eggs can have a number of consequences for offspring due to the physiological link between mothers and offspring [9–11]. For example, in threespined stickleback (Gasterosteus aculeatus), maternal exposure to high predation risk while yolking eggs has negative consequences for offspring (reared without post-fertilization care): offspring show learning deficits [12], as well as less anti-predator behaviour and lower survival when they themselves encounter a predator [13]. Altogether, differential allocation into mating effort and later offspring care by fathers could be in response to cues of their partner's experiences and/or in response to how maternal experiences might affect offspring traits [1,14,15].
Determining whether individuals adjust their reproductive investment based on the experiences of their mate can be challenging in species where both parents are able to adjust their investment. For example, females might adjust their hormonal investment into eggs depending on their experiences and these maternal effects might then affect offspring behaviour and parental care [16]. Similarly, there is substantial evidence in biparental species that when one parent is removed or handicapped after mating, the other parent will increase their offspring care to partially compensate [17]. In these cases, it is difficult to distinguish between fathers adjusting their offspring care based on the prior experiences of their partner or based on the current parental care provided by their partner. It is easier to disentangle maternal from paternal effects in species where allocation decisions into offspring are disassociated in time, such as in many fish species where males are the sole providers of care. In threespined sticklebacks, males and females interact only during courtship. Females cannot adjust their investment into eggs based on the traits of a recently encountered male because eggs are already fully mature prior to courtship. Males cannot adjust their offspring care in response to any female care because females do not provide care. Any differential allocation decisions by males must be based on either the brief courtship interaction with the female and/or in response to offspring cues during development.
In this study, we examined whether male threespined stickleback adjusted their reproductive decisions, during both courtship and paternal care, in response to a female's previous experience with predators. We might predict that males should be sensitive to female experiences with predators as spawning decisions represent a significant reproductive investment for males owing to the extended costly father-only offspring care [4,5]. Thus, detecting the predation risk experienced by a potential mate might allow males to adjust their courtship and subsequent offspring care in order to increase their own survival in such a predator-filled environment [18] and/or to increase the survival of their offspring by preparing those offspring for living in such a risky environment (anticipatory parental effects [19]).
2. Material and methods
(a). Collection, maintenance and female predator exposure
Sticklebacks were collected from Navarro River, CA, USA, before the mating season (as juveniles: November 2011; as adults: April 2012; the electronic supplemental material) and transported to the University of Illinois. Sticklebacks from this population typically live for 1 year and sticklebacks in general have multiple breeding attempts during the mating season, adjusting their reproductive investment as the mating season progresses [18]. This population has piscivorous predators such as the prickly sculpin (Cottus asper), which, at large size, are a threat to adult stickleback ([20]; the electronic supplementary material). Fish were maintained at 20.6°C on a summer photoperiod schedule (16 L : 8D) and fed ad libitum daily.
We randomly assigned females to one of eight predator-exposed (experimental) or one of eight unexposed (control) treatment tanks (10 females per 37.8 l tank), similar to treatments in [13,21]. Each tank had gravel, four artificial plants and opaque plastic on all sides. Within each tank, females were individually marked with a combination of spine clips. We chased experimental females in predator-exposed tanks for 45 s once a day with a 10 cm realistic rubber model of a prickly sculpin attached to a stiff metal rod. During the daily predator exposure, we moved the model along the bottom and made repeated lunges at each female, similar to the predator's behaviour. Experimental females were chased at a random time each day so predator exposure was unpredictable. Control females in the unexposed treatment were undisturbed. Females experienced their treatments for 32 ± 2 days before being tested on average but total treatment time was variable depending on when females became gravid (range 3–77 days). Importantly, there was no significant association between female treatment time and courtship behaviour (electronic supplementary material, table S1).
Males from the same population were housed singly (but within sight of one another [22]; the electronic supplementary material) in 9.5 l tanks with gravel, one artificial plant and a nest-box with sand and filamentous algae for nest building.
(b). Courtship
Gravid females were paired randomly with males with completed nests. Females were weighed and added to male tanks in the evening (20.00–22.00 h) at which time we measured courtship behaviour. During courtship, male threespined sticklebacks perform a conspicuous zigzag dance. Males also attempt to lead females to their nest, poking at the nest opening and fanning it with their pectoral fins, as they would in paternal care regardless of whether the nest has eggs. There is mutual mate choice and females show a variety of preference behaviours. We recorded male behaviour directed at the female (number of zigzags) and directed at the nest entrance (number of pokes and fanning bouts) as well as total female preference behaviours (number of head-ups, follows and nest inspections) for 5 min after the male noticed the female. The next morning (08.00–10.00 h), females were weighed, inspected for spawning and returned to their treatment tank.
We recorded courtship data for 109 different males. Of these 109 trials, 67 trials resulted in a spawn. While males spawned only once (67 unique males), some females were re-used as they became gravid (48 unique females). Trials without spawns could be owing to females having immature eggs, or female or male choice. Importantly, however, the likelihood of spawning was not significantly affected by female treatment (the electronic supplementary material). To standardize female reproductive state, only the 67 trials with spawns were included: 37 males with a control female and 30 males with an experimental female. Thus, our conclusions regarding differential allocation only pertain to situations where both males and females are able and willing to invest in reproduction.
We examined whether female treatment affected two aspects of male courtship (1 = female-directed zigzags, and 2 = sum of nest-directed pokes + fanning bouts) using separate mixed models (see the electronic supplementary material for additional details of statistical analyses). As female behaviour probably influences male behaviour, we included the total female preference behaviour (sum of all head-ups + follows + nest inspections) during the 5 min assay as a single covariate. The random effect of female identity was tested with log-likelihood tests. Behavioural data were natural log-transformed after adding 1 to data. Female mass and clutch size were not affected by female treatment (electronic supplementary material, table S2). Females of both treatments encountered males of similar sizes and throat coloration (electronic supplementary material, table S2).
(c). Paternal care
Paternal care data were collected for a subset of spawnings (n = 16): eight males reared offspring from control mothers and eight males reared offspring from previously predator-exposed mothers. Female traits (mass, clutch size) and male traits (size, throat colour) did not differ between treatments for the subset and courtship behaviours showed a similar pattern to that of the larger sample size above (the electronic supplementary material).
We observed paternal care for 5 min on four separate days (at 10.00–12.00 h), with two observations occurring before embryos had hatched (days 3 and 4 after fertilization), and two observations occurring after hatching when offspring were fry (days 7 and 8 after fertilization). We recorded and summed: (i) the number of nest pokes and fanning bouts directed at the nest entrance, and (ii) the number of nest visits (i.e. father hovering within a body length of nest) and offspring retrievals (i.e. father retrieves offspring in his mouth and releases at nest). We kept these paternal care behaviours separate as males often hover above the nest-box and circle the area (i.e. guarding or retrieving fry) during a nest visit, but do not necessarily perform nest pokes or fans. Nests were observed behind a blind several feet away. Observers were blind to maternal treatment.
We used repeated measures analyses to examine the effect of maternal treatment on paternal care behaviour with father identity as the repeating subject through time (days 3,4,7,8 post-fertilization). The random effect of father identity was tested with log-likelihood tests. Nest pokes and fanning bouts were natural log-transformed after adding 1 to data, and nest visits and retrievals were untransformed.
In all analyses, non-significant interactions were removed when it improved model fit based on Akaike information criterion values. Degrees of freedom were estimated using the Satterthwaite approximation. Residuals were examined visually to assess model assumptions. Analyses were conducted using SAS v. 9.3. Additional methods, results, and trait means are in the electronic supplementary material.
3. Results
(a). Courtship
Males showed fewer zigzags (conspicuous courtship behaviour) towards females that had experienced predation risk in the past compared with control females (table 1 and figure 1). In contrast with female-directed courtship, nest-directed courtship was not significantly affected by female treatment (table 1 and figure 1).
Table 1.
Effect of female predator exposure and total preference behaviour (as covariate) on male (a) female-directed courtship and (b) nest-directed courtship. n = 67 males.
| effect | d.f. | F-value | p-value |
|---|---|---|---|
| (a) number of female-directed zigzags | |||
| female predator exposure | 1, 64 | 9.18 | 0.0035 |
| female preference behaviour | 1, 64 | 1.75 | 0.1905 |
| random effect of female identity | χ2 difference = 0 | 1.0 | |
| (b) number of nest-directed pokes and fanning bouts | |||
| female predator exposure | 1, 40 | 2.88 | 0.0975 |
| female preference behaviour | 1, 61.4 | 17.15 | 0.0001 |
| random effect of female identity | χ2 difference = 3.2 | 0.0736 | |
Figure 1.
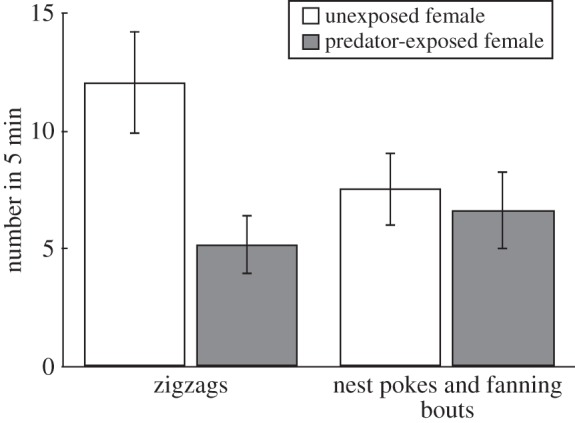
Effect of female predator exposure on male courtship behaviour directed at the female (i.e. zigzags) and directed at the nest (i.e. nest pokes and fanning bouts). Means ± s.e. (n = 67 males).
There was a strong association at the individual level between female preference behaviour and male nest-directed behaviour, regardless of treatment (table 1). The total number of female preference behaviours during the 5 min encounter was positively correlated with the number of male nest pokes and fanning bouts (Spearman's r = 0.49, p < 0.0001, n = 67) but not with the number of zigzags (Spearman's r = 0.08, p = 0.5190, n = 67). Note that we cannot determine whether female preference behaviour incited males to increase nest-directed courtship or whether elevated levels of male nest-directed courtship induced females to show preference behaviour. While the directionality behind this pattern is unclear, it is likely that feedback and negotiations between males and females are important in mutual mate choice. Importantly, we did not detect an effect of treatment on female preference behaviour (the electronic supplementary material, F1,65 = 1.23, p = 0.2713, n = 67).
(b). Paternal care
Males behaved differently towards offspring of females that had experienced predation risk in the past compared with offspring of control females, both pre- and post-hatching. Fathers performed fewer nest pokes and engaged in fewer nest fanning bouts when their offspring were from experimental mothers compared with control mothers, particularly during the pre-hatch embryo stage (table 2a and figure 2a). Similarly, fathers visited their nest less often to guard and retrieve offspring when their offspring were from experimental mothers compared with control mothers, particularly post-hatching when offspring were mobile (table 2b and figure 2b).
Table 2.
Effect of maternal predator exposure on paternal care towards pre-hatch embryos (days 3–4 post-fertilization) and post-hatch fry (days 7–8 post-fertilization). n = 16 fathers.
| effect | d.f. | F-value | p-value |
|---|---|---|---|
| (a) number of nest pokes and fanning bouts | |||
| maternal predator exposure | 1, 19.9 | 5.72 | 0.0268 |
| day (3,4,7,8) | 3, 39.5 | 10.26 | <0.0001 |
| treatment × day | 3, 39.5 | 0.15 | 0.9300 |
| random effect of father identity | χ2 difference = 1.5 | 0.2207 | |
| (b) number of nest visits and offspring retrievals | |||
| maternal predator exposure | 1, 19.9 | 5.31 | 0.0321 |
| day (3,4,7,8) | 3, 40.3 | 4.64 | 0.0071 |
| treatment × day | 3, 40.3 | 0.87 | 0.4646 |
| random effect of father identity | χ2 difference = 3.0 | 0.0833 | |
Figure 2.
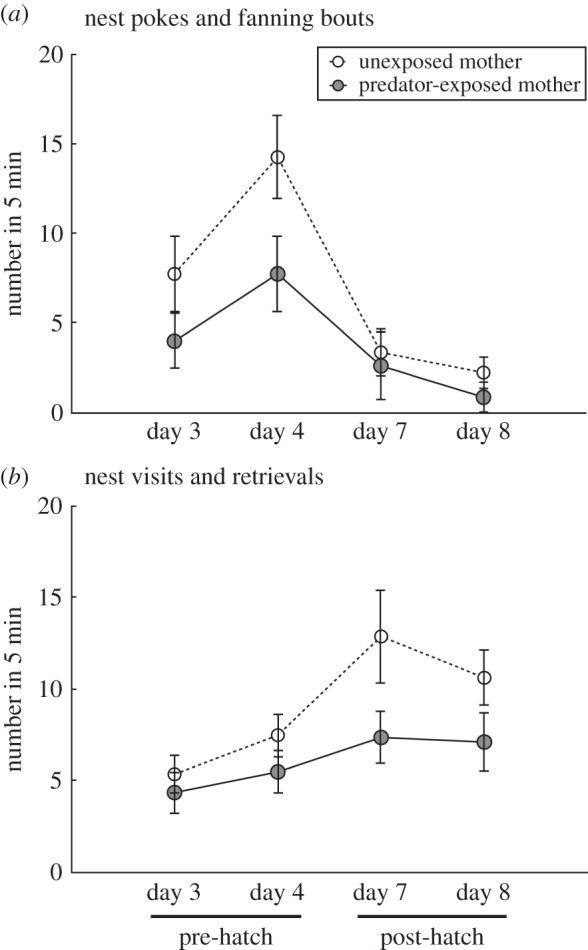
Maternal predator exposure and offspring age affect father (a) nest pokes and fanning bouts and (b) nest-guarding and offspring retrieval. X-axis indicates days post-fertilization (days 3–4: pre-hatch embryos; days 7–8: hatched fry). Means ± s.e. (n = 16 fathers).
Consistent with other stickleback studies [23], paternal care changed over time (table 2 and figure 2), with the number of nest pokes and fanning bouts being highest pre-hatching (days 3–4 post-fertilization) and nest-guarding and offspring retrieval being highest post-hatching (days 7–8 post-fertilization).
4. Discussion
Our results show that whether females have encountered predators in the past affects both male mating effort and paternal care. During courtship, males showed fewer zigzags to experimental females compared with control females. This behavioural difference extended to how males cared for the offspring produced from these matings. Fathers provided less paternal care, both pre- and post-hatching, to offspring of experimental mothers, suggesting that males differentially allocate offspring care based on their mate's previous experience with predation risk. Whether this adjustment then affects future breeding attempts and reflects an adaptive trade-off between current and future reproduction is unclear. However, our results demonstrate for the first time, to our knowledge, that variation among females within a population in their encounters with predators can contribute to courtship variation among males and can affect the care those offspring will receive from fathers, even when males themselves do not encounter a predator.
Why might fathers invest less effort into courting previously predator-exposed females and caring for the resulting offspring? A possible explanation is that repeatedly encountering predators renders females unattractive and males reduce their reproductive investment accordingly [1,2]. A number of studies have found that both males and females reduce their courtship, parental care, and in the case of females, their prenatal investment into eggs when mated with an unattractive mate [2,3]. However, we did not find differences between females in their preference behaviour, mass or clutch size (the electronic supplementary material). It is also possible that males adjust their effort based on the potential quality of offspring produced by females. Maternal stress can have important consequences for offspring [9–11] and maternal predator exposure in threespined stickleback negatively affects a variety of offspring behaviours including survival with a predator [13]. Thus males might value the lower quality offspring produced by stressed females less than offspring produced by unstressed females and as a result, males reduce their mating effort and paternal care [1,2,5].
An alternative, but not mutually exclusive, perspective is that males use cues from potential mates to assess the predation risk in the environment, and adjust their willingness to take risks, in both courtship and offspring care, to this inferred level of predation risk. Whether experiencing a recent predator encounter or an extended period of high predation risk (as in our study) have similar effects on these cues is unknown. There is substantial evidence, including in threespined stickleback [22], that when predation risk is high, males decrease conspicuous courtship displays that could attract predators with benefits for both signallers and receivers [6,7,24]. There is also substantial evidence that parents decrease offspring care when predation risk is high [25]. For example, threespined stickleback fathers reduce their paternal care when presented with a predator model [26]. Interestingly, the way males in this study reduced their paternal care in response to female past experience with predation risk is strikingly similar to the way males reduce paternal care in response to their own (direct) experience with predation risk [26]. Examining whether males reduce their reproductive investment when females have been exposed to stressors other than predators (e.g. high density or food unpredictability) that are not indicative of any immediate threat to males (or receiving females) would allow us to assess whether males are using female cues to assess predation risk, or female stress more generally.
The cues that males use to inform their courtship and care decisions are currently unknown. We did not detect any differences in female behaviour or size between female treatments (the electronic supplementary material), making it unlikely that males are using these female traits as cues. We also did not detect any differences between female treatments in the size or throat colour of the spawning males (the electronic supplementary material). Thus, it is unlikely that the pattern we see is a result of predator-exposed females being less choosy and accepting lower quality males (who might provide less courtship or parental care), as has been found in studies examining female choosiness under current predation risk [6,27]. We also found that female preference behaviour was strongly associated with nest-directed courtship, not zigzags (also [22]), and nest-directed courtship was unaffected by female treatment. Thus, males are probably assessing female experience based on other female traits, such as stress hormones excreted into the water, for example. Once fathers are parenting, it is unclear whether they continue to use cues detected during their brief courtship to inform parental care decisions or whether they might also use cues from the developing offspring. For example, embryos might excrete stress hormones during development that are indicative of their mother's experiences. After hatching, fry from different mothers might behave differently, perhaps inducing differential retrieval and guarding behaviour in their fathers (similar to bird begging differences [14–16]). Determining whether fathers adjust their care to mother or offspring cues would be interesting to pursue by experimentally cross-fostering clutches from predator-exposed and unexposed mothers, as well as measuring offspring hormonal excretion and behaviour throughout development.
Together with our previous studies showing maternal effects on stickleback offspring [12,13,21], these results suggest that maternal and paternal effects on offspring are not independent of one another [14,15]. Thus, previous predator experiences of mothers can influence offspring traits and survival both directly [12,13,21] and indirectly through how these experiences affect paternal care (this study). Paternal care in this species affects offspring behavioural [26,28,29] and morphological [26] development, brain gene expression [28] and is linked to offspring survival with predators [28]. Whether paternal care can compensate for the negative effects of maternal predator exposure on offspring, or complements these maternal effects, remains unknown. This interplay between individual experiences, behavioural flexibility and environmental conditions could alter the benefits of mate choice and affect the outcome of sexual selection.
An intriguing possibility is that it is the combination of maternal and paternal effects arising from predator exposure that ‘programs’ offspring for a high-risk environment (anticipatory parental effects [19]). For example, offspring might be ‘programmed’ for high predation risk by mothers via their pre-fertilization provisioning into eggs [21] and by fathers via adjustments to offspring care [26]. This would, in part, explain why maternal predator exposure results in maladaptive offspring behaviour when offspring are reared without care (as orphans [13]) and experience a ‘mismatch’ between maternal and paternal cues of high predation risk. This study emphasizes the importance of interactions between parental effects by demonstrating that the experiences of one parent can influence offspring phenotype directly through changes to offspring development, as well as indirectly through changes to the behaviour of the other parent, and thus, their parental effects.
Supplementary Material
Acknowledgements
We sincerely thank D. Roche, M. Schrader, the Behavioural Ecology group at Cambridge, the Bell Laboratory, H. Klug and two referees.
Ethics
This study was approved by the Animal Care and Use Committee at University of Illinois (no. 12118).
Data accessibility
All data are deposited in Dryad http://dx.doi.org/10.5061/dryad.rp3d0.
Authors' contributions
K.E.M. and A.M.B. designed the study, K.E.M., S.F. and S.L. coordinated the study and collected data, K.E.M analysed data and wrote a first draft. All authors contributed to revisions and gave final approval.
Competing interests
We have no competing interests.
Funding
NSF IOS 1121980 to A.M.B. and K.E.M, and NIH PHS 1 R01 GM082937 to A.M.B. and M. Band. S.F. was supported by NSF REU funding. S.L. was supported by the Research Experience Program at the College for Veterinary Medicine at the University of Illinois.
References
- 1.Sheldon BC. 2000. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15, 397–402. ( 10.1016/S0169-5347(00)01953-4) [DOI] [PubMed] [Google Scholar]
- 2.Ratikainen II, Kokko H. 2010. Differential allocation and compensation: who deserves the silver spoon? Behav. Ecol. 21, 195–200. ( 10.1093/beheco/arp168) [DOI] [Google Scholar]
- 3.Horvathova T, Nakagawa S, Uller T. 2012. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. B 279, 163–170. ( 10.1098/rspb.2011.0663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonduriansky R. 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. Camb. Philos. Soc. 76, 305–339. ( 10.1017/S1464793101005693) [DOI] [PubMed] [Google Scholar]
- 5.Edward DA, Chapman T. 2011. The evolution and significance of male mate choice. Trends Ecol. Evol. 26, 647–654. ( 10.1016/j.tree.2011.07.012) [DOI] [PubMed] [Google Scholar]
- 6.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. Camb. Philos. Soc. 72, 283–327. ( 10.1017/s0006323196005014) [DOI] [PubMed] [Google Scholar]
- 7.Lima SL. 1998. Stress and decision-making under the risk of predation: recent developments from behavioral, reproductive and ecological perspectives. Adv. Study Behav. 27, 215–290. ( 10.1016/S0065-3454(08)60366-6) [DOI] [Google Scholar]
- 8.Clinchy M, Sheriff MJ, Zanette LY. 2013. Predator-induced stress and the ecology of fear. Funct. Ecol. 27, 56–65. ( 10.1111/1365-2435.12007) [DOI] [Google Scholar]
- 9.Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ. 2014. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J. Neuroendocrinol. 26, 707–723. ( 10.1111/jne.12175) [DOI] [PubMed] [Google Scholar]
- 10.Weinstock M. 2008. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32, 1073–1086. ( 10.1016/j.neubiorev.2008.03.002) [DOI] [PubMed] [Google Scholar]
- 11.Schoech SJ, Rensel MA, Heiss RS. 2011. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr. Zool. 57, 514–530. [Google Scholar]
- 12.Roche DP, McGhee KE, Bell AM. 2012. Maternal predator-exposure has lifelong consequences for offspring learning in threespined sticklebacks. Biol. Lett. 8, 932–935. ( 10.1098/rsbl.2012.0685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGhee KE, Pintor LM, Suhr EL, Bell AM. 2012. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in threespined stickleback. Funct. Ecol. 26, 932–940. ( 10.1111/j.1365-2435.2012.02008.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crean AJ, Bonduriansky R. 2014. What is a paternal effect? Trends Ecol. Evol. 29, 554–559. ( 10.1016/j.tree.2014.07.009) [DOI] [PubMed] [Google Scholar]
- 15.Braun K, Champagne FA. 2014. Paternal influences on offspring development: behavioural and epigenetic pathways. J. Neuroendocrinol. 26, 697–706. ( 10.1111/jne.12174) [DOI] [PubMed] [Google Scholar]
- 16.Smiseth PT, Scott MP, Andrews C. 2011. Hormonal regulation of offspring begging and mediation of parent–offspring conflict. Anim. Behav. 81, 507–517. ( 10.1016/j.anbehav.2010.11.029) [DOI] [Google Scholar]
- 17.Harrison F, Barta Z, Cuthill I, Székely T. 2009. How is sexual conflict over parental care resolved? A meta-analysis. J. Evol. Biol. 22, 1800–1812. ( 10.1111/j.1420-9101.2009.01792.x) [DOI] [PubMed] [Google Scholar]
- 18.Candolin U. 1998. Reproduction under predation risk and the trade-off between current and future reproduction in the threespine stickleback. Proc. R. Soc. Lond. B 265, 1171–1175. ( 10.1098/rspb.1998.0415) [DOI] [Google Scholar]
- 19.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 20.Foster S, Ploch S. 1990. Determinants of variation in antipredator behavior of territorial male threespine stickleback in the wild. Ethology 84, 281–294. ( 10.1111/j.1439-0310.1990.tb00803.x) [DOI] [Google Scholar]
- 21.Giesing ER, Suski CD, Warner RE, Bell AM. 2011. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proc. R. Soc. B 278, 1753–1759. ( 10.1098/rspb.2010.1819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Candolin U. 1997. Predation risk affects courtship and attractiveness of competing threespine stickleback males. Behav. Ecol. Sociobiol. 41, 81–87. ( 10.1007/s002650050367) [DOI] [Google Scholar]
- 23.Stein LR, Bell AM. 2012. Consistent individual differences in fathering in threespined stickleback, Gasterosteus aculeatus. Curr. Zool. 58, 45–52. ( 10.1007/s00265-014-1835-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes NK, Kelley JL, Banks PB. 2012. Dangerous liaisons: the predation risks of receiving social signals. Ecol. Lett. 15, 1326–1339. ( 10.1111/j.1461-0248.2012.01856.x) [DOI] [PubMed] [Google Scholar]
- 25.Ghalambor CK, Peluc SI, Martin TE. 2013. Plasticity of parental care under the risk of predation: how much should parents reduce care? Biol. Lett. 9, 20130154 ( 10.1098/rsbl.2013.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein LR, Bell AM. 2014. Paternal programming in sticklebacks. Anim. Behav. 95, 165–171. ( 10.1016/j.anbehav.2014.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak GM, Boughman JW. 2015. Predator experience overrides learned aversion to heterospecifics in stickleback species pairs. Proc. R. Soc. B 282, 20143066 ( 10.1098/rspb.2014.3066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGhee KE, Bell AM. 2014. Paternal care in a fish: epigenetics and fitness enhancing effects on offspring anxiety. Proc. R. Soc. B 281, 20141146 ( 10.1098/rspb.2014.1146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tulley JJ, Huntingford FA. 1987. Paternal care and the development of adaptive variation in anti-predator responses in sticklebacks. Anim. Behav. 35, 1570–1572. ( 10.1016/S0003-3472(87)80034-9) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are deposited in Dryad http://dx.doi.org/10.5061/dryad.rp3d0.


