Abstract
Mass bleaching events are predicted to occur annually later this century. Nevertheless, it remains unknown whether corals will be able to recover between annual bleaching events. Using a combined tank and field experiment, we simulated annual bleaching by exposing three Caribbean coral species (Porites divaricata, Porites astreoides and Orbicella faveolata) to elevated temperatures for 2.5 weeks in 2 consecutive years. The impact of annual bleaching stress on chlorophyll a, energy reserves, calcification, and tissue C and N isotopes was assessed immediately after the second bleaching and after both short- and long-term recovery on the reef (1.5 and 11 months, respectively). While P. divaricata and O. faveolata were able to recover from repeat bleaching within 1 year, P. astreoides experienced cumulative damage that prevented full recovery within this time frame, suggesting that repeat bleaching had diminished its recovery capacity. Specifically, P. astreoides was not able to recover protein and carbohydrate concentrations. As energy reserves promote bleaching resistance, failure to recover from annual bleaching within 1 year will likely result in the future demise of heat-sensitive coral species.
Keywords: Caribbean, annual bleaching, long-term recovery, energy reserves, C and N isotopes, calcification
1. Introduction
Climate change is one of the main threats to coral reefs today [1], and mass bleaching events due to periods of elevated ocean surface temperatures have increased in frequency over the past decades [2,3]. Furthermore, annual severe bleaching is expected to occur worldwide later this century, putting more than 90% of reefs at risk of long-term degradation [4]. In the Caribbean, this is projected to occur as early as 2040 [5]. Yet, the effects of annually recurring bleaching on coral physiology, recovery capacity and resilience remain largely unstudied.
There is mounting evidence that thermal history has strong impacts on coral bleaching susceptibility [6–8]. However, the first study to experimentally address annual coral bleaching was Grottoli et al. [9], which showed that bleaching stress occurring in 2 consecutive years can dramatically alter the thermal tolerance of coral, and that while some species were able to acclimate rapidly to repeat bleaching events, others became more susceptible. Although this study was a major step forward, it did not address the impacts of annual bleaching on long-term recovery (i.e. more than 1.5 months). It is currently unknown if corals can recover between annual bleaching events, or if their recovery capacity is overwhelmed by such frequent bleaching stress.
During bleaching, corals lose a significant portion of their algal endosymbionts (Symbiodinium spp.) and/or photosynthetic pigments, giving the colony a pale (hence bleached) appearance [10,11]. As healthy corals typically meet most of their daily energy requirements via photosynthesis by their endosymbiotic algae (e.g. [12]), bleaching results in severe reductions in algal photosynthesis and translocation of photosynthate to the coral host accompanied by dramatic changes in physiology. Thus, bleached corals typically decrease calcification (e.g. [13,14]) and catabolize energy reserves (e.g. [15–17]), and some species may increase heterotrophy [18]. Generally, the size of energy reserves (i.e. lipids, protein, carbohydrates), endosymbiont type, as well as heterotrophic capacities are critical in promoting bleaching resilience and recovery [9,18–21].
In addition to physiological measurements, coral tissue isotopes can be useful indicators of bleaching recovery. Specifically, the carbon isotopic composition of the animal host (δ13Ch) and endosymbiont (δ13Ce) track changes in the allocation of auto- and heterotrophically derived C to the tissues, whereas their nitrogen isotopic composition (δ15Nh and δ15Ne) records sources and rate of nitrogen incorporation into coral tissues during bleaching and recovery [14,22–24]. Detailed physiological measurements coupled with isotopic analyses are therefore powerful tools to study the short- and long-term recovery from annual coral bleaching.
This study is the first to assess short- and long-term recovery from annual coral bleaching in three Caribbean corals using both physiological and biogeochemical methods. We conducted a 2-year-long study to determine (i) if the coral holobiont can recover from annually recurring bleaching within 1 year, and (ii) which traits are associated with short- and long-term recovery.
2. Material and methods
(a). Repeat bleaching experiment
A detailed description of this experiment can be found in Schoepf et al. [25], and a flow diagram of the experimental design is shown in the electronic supplementary material, figure S1. Briefly, coral fragments of Porites divaricata, Porites astreoides and Orbicella faveolata were collected in July 2009 near Puerto Morelos, Mexico (20°50′ N, 86°52′ W region; see [9] for details). Peak sea surface temperatures (SST) at this location typically occur in August/September with the maximum monthly mean (MMM) SST being 29.0°C; the bleaching threshold SST is therefore 30.0°C (MMM + 1°C, NOAA Coral Reef Watch Virtual Station Puerto Morelos, Mexico). Corals were then buoyantly weighed, and allowed to recover in shaded outdoor flow-through seawater tanks for 5 days. In mid-July, temperature in half of the tanks was gradually raised to 31.5 ± 0.20°C (single bleaching treatment) over 7 days and then maintained at the elevated temperature, while the remaining tanks received ambient reef water (controls; 30.6 ± 0.24°C). After 15 days, all corals were placed on the reef in a random arrangement for 1 full year at ambient reef temperatures (electronic supplementary material, figures S1 and S2). During the time on the reef, corals were considered naturally fed but were not artificially fed during the tank portion of the study.
In late July/early August 2010, the experiment was repeated (repeat bleaching treatment) and the treatment corals from the previous year were exposed to elevated temperatures again (31.6 ± 0.24°C) while the control fragments from 2009 were maintained at ambient temperature (30.4 ± 0.23°C) (electronic supplementary material, figures S1 and S2). After 17 days, coral fragments were visually assessed for their health status [17] (see definition of each health status category in the electronic supplementary material, figure S3), buoyantly weighed and then one third of all fragments were frozen for physiological and isotopic analyses (i.e. 0 months on the reef). The remaining fragments were placed back on the reef in a random arrangement at ambient reef temperatures (electronic supplementary material, figures S1 and S2). To assess short- and long-term recovery from repeat bleaching, half of the remaining fragments were collected after 1.5 and 11 months on the reef, respectively. Fragment health status was visually assessed, they were buoyantly weighed and then frozen for physiological and isotopic analyses (see below).
Seawater temperatures during the tank and field portion of the study are shown in the electronic supplementary material, figure S2. On average, treatment corals were exposed to estimated heat stress levels of 3–4 degree heating weeks (DHW), which is similar to those recorded in the Caribbean in 1998 but much lower than during the severe regional bleaching event in 2005, where up to 16 DHW were recorded in some locations [26].
(b). Physiological and isotopic analyses
Chlorophyll a was determined according to Jeffrey & Humphrey [27] and standardized to surface area [28]. Chlorophyll a values at 0 and 1.5 months on the reef following repeat bleaching are from Schoepf et al. [29]. Total soluble lipids, animal soluble protein and animal soluble carbohydrate were determined on ground, frozen coral fragments using established methods [17,30] and then converted to Joules per gram ash-free dry weight [31] (see the electronic supplementary material for more information). Net calcification was determined using the buoyant weight technique [32] and standardized to surface area. Calcification rates at 0 and 1.5 months on the reef following repeat bleaching are from Grottoli et al. [9].
Tissue C and N isotopic analyses were performed on separated animal host and endosymbiont fractions using established methods [14,33]. The difference between δ13Ch and δ13Ce (i.e. δ13Ch−e) was calculated to determine the relative contribution of photoautotrophic versus heterotrophic carbon to the coral [14,23]. Repeated measurements of commercial standards (USGS-24, IAEA-N2) had a standard deviation of ±0.04‰ for δ13C (n = 55) and ±0.11‰ for δ15N (n = 51).
(c). Statistical analyses
Multivariate one-way analysis of similarity (ANOSIM) was used to test whether the overall bleaching and recovery response differed significantly between the three species. As there was a significant species effect (see Results), two-way ANOSIMs were conducted individually for each species to test for treatment (control, treatment) and time (0, 1.5 and 11 months on the reef) effects. Furthermore, SIMPER analyses were conducted to determine which variables contributed most to the observed physiological changes due to treatment and time effects. Non-metric multidimensional scaling (NMDS) was used to graphically represent relationships between response variables, species and treatments in multidimensional space. In order to prevent bias from including all five isotopic variables, only δ13Ch−e and δ15Ne were included in the multivariate analyses along with chlorophyll a, lipid, protein, carbohydrate and calcification.
Univariate three-way analysis of variance (ANOVA) was then used to test the effects of temperature, time and genotype for each species individually for all physiological and isotopic variables. Post hoc slice tests (e.g. tests of simple effects [34]) determined if the control and treatment averages differed significantly within each time interval and species. If a variable was significantly different between treatment and control averages at 0 and/or 1.5 months of recovery but no longer differed at 1.5 and/or 11 months on the reef, it was deemed to be fully recovered from repeat bleaching. Conversely, if one or more variables still had significantly lower treatment averages than the control after 11 months on the reef, this was deemed indicative of impaired long-term recovery capacity. More detailed information is provided in the electronic supplementary material.
3. Results
(a). Multivariate analyses
Over the entire 11 months following repeat bleaching, all three species significantly differed from each other (one-way ANOSIM R = 0.39, p = 0.001). Porites divaricata was most distinct from the other two species (P. d. versus O. f.: R = 0.70; P. d. versus P. a.: R = 0.39; P. a. versus O. f.: R = 0.19; all p < 0.05), consistent with the NMDS results (electronic supplementary material, figure S3 and table S2).
For each species, significant treatment and time effects were also observed (table 1). Only P. astreoides showed clear separation of the treatment and control groups (R = 0.567; table 1; electronic supplementary material, figure S3), which was largely driven by lower chlorophyll a concentrations, higher δ15Ne and lower calcification rates in the treatment corals (electronic supplementary material, table S1 and figure S3). In contrast, P. divaricata showed the greatest change in responses over time (R = 0.504; table 1), largely due to differences in chlorophyll a concentration (0 versus 2 months), δ15Ne and carbohydrate concentration (0 versus 11 months), as well as lipid concentration (2 versus 11 months and 2 versus 11 months) (electronic supplementary material, table S1). In O. faveolata, significant overlap among treatment and time groups was observed (R < 0.5; table 1; electronic supplementary material, figure S3); thus the response of each variable is best assessed individually for each time point (see below).
Table 1.
Results from two-way ANOSIMs testing the effects of temperature and time on the reef on the overall bleaching and recovery response of each of the three study species following repeat bleaching. Significant p-values (p ≤ 0.05) are highlighted in bold.
| global R-statistic | p-value | possible no. permutations | actual no. permutations | no. permuted statistics ≥ global R | |
|---|---|---|---|---|---|
| Porites divaricata | |||||
| temperature | 0.173 | 0.020 | 20 490 624 | 999 | 19 |
| time | 0.504 | 0.001 | large number | 999 | 0 |
| Porites astreoides | |||||
| temperature | 0.567 | 0.001 | large number | 999 | 0 |
| time | 0.387 | 0.001 | large number | 999 | 0 |
| Orbicella faveolata | |||||
| temperature | 0.108 | 0.041 | large number | 999 | 40 |
| time | 0.395 | 0.001 | large number | 999 | 0 |
(b). Physiology following repeat bleaching
(i). Porites divaricata
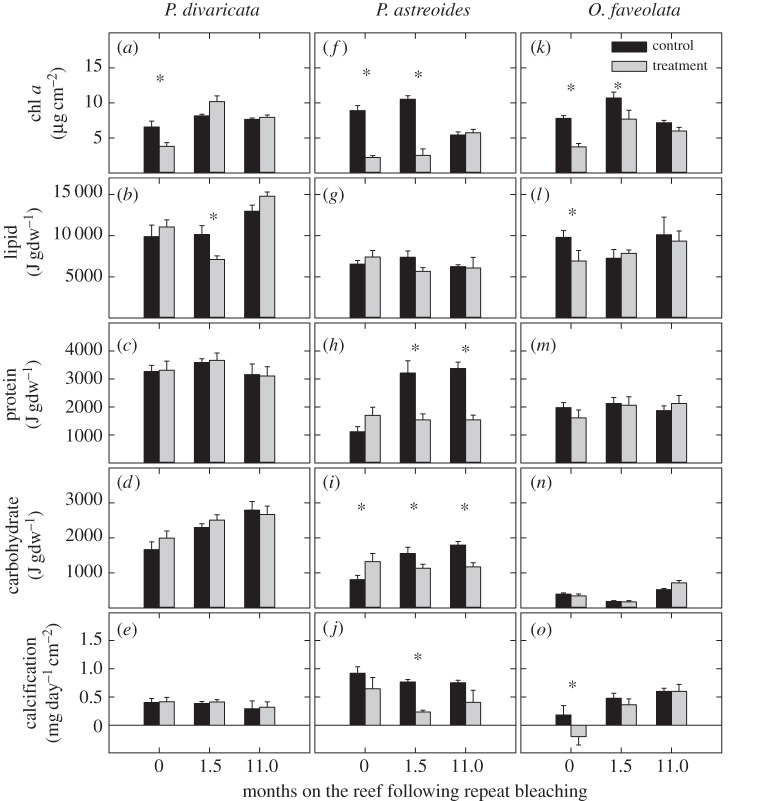
Chlorophyll a concentrations of treatment corals were 42% lower immediately after repeat bleaching, but had fully recovered after 1.5 months on the reef (figure 1a; electronic supplementary material, table S3) [29]. Lipid concentrations of treatment corals did not differ from controls immediately after repeat bleaching, but were 30% lower at 1.5 months on the reef and had fully recovered after 11 months (figure 1b; electronic supplementary material, table S3). Protein and carbohydrate concentrations were the same for both treatment and controls in the year following repeat bleaching, though carbohydrate concentrations increased steadily (figure 1c,d; electronic supplementary material, table S3). Calcification rates were the same for both treatment and control corals throughout the 11 months following repeat bleaching (figure 1e; electronic supplementary material, table S3) [9].
Figure 1.
Average chlorophyll a (chl a), lipid, protein, carbohydrate and calcification rates of (a–e) Porites divaricata, (f–j) Porites astreoides and (k–o) Orbicella faveolata after 0, 1.5 and 11 months on the reef following repeat bleaching. Averages are shown ±1 s.e. Asterisks indicate significant differences between treatment and control corals within a time point and species. Sample size per average ranges from 5–9. Statistical results shown in the electronic supplementary material, table S2. Chl a and calcification values at both 0 and 1.5 months on the reef are reproduced from Schoepf et al. [29] and Grottoli et al. [9], respectively.
(ii). Porites astreoides
Chlorophyll a concentrations of treatment corals were 75% and 76% lower than in controls after both 0 and 1.5 months on the reef, respectively [29], but were fully recovered after 11 months (figure 1f; electronic supplementary material, table S3). Lipid concentrations were the same for both treatment and control corals throughout the 11 months following repeat bleaching (figure 1g; electronic supplementary material, table S3). In contrast, protein concentrations of treatment corals were 52% and 54% lower after 1.5 and 11 months on the reef, respectively (figure 1h). Carbohydrate concentrations of treatment corals were initially 64% higher than in controls, but then were 27% and 35% lower than in controls after 1.5 and 11 months on the reef, respectively (figure 1i; electronic supplementary material, table S3). Thus, neither protein nor carbohydrate concentrations were fully recovered by the end of the study. Calcification rates of treatment corals were the same as in controls immediately following bleaching, but were 69% lower after 1.5 months on the reef (figure 1j; electronic supplementary material, table S3) [9]. Although calcification rates were still 46% lower in the treatment corals after 11 months on the reef, this was not statistically significant due to high variability between fragments (p < 0.43) (figure 1j; electronic supplementary material, table S3).
(iii). Orbicella faveolata
Chlorophyll a concentrations were 52% and 28% lower in treatment corals compared with controls after 0 and 1.5 months on the reef, respectively [29], but had fully recovered after 11 months (figure 1k; electronic supplementary material, table S3). Chlorophyll a also showed seasonal variability with higher concentrations after 1.5 months than at the other two sampling points. Lipid concentrations were 29% lower in treatment than control corals immediately after repeat bleaching, but were fully recovered after 1.5 months on the reef (figure 1l; electronic supplementary material, table S3). Protein and carbohydrate concentrations did not differ between treatment and control corals at any point following repeat bleaching (figure 1m,n; electronic supplementary material, table S3), though carbohydrate concentrations varied over time. In contrast, calcification rates of treatment corals were significantly compromised (−212%) and net skeletal dissolution was observed immediately after repeat bleaching (figure 1o; electronic supplementary material, table S3) [9]. However, calcification rates had fully recovered 1.5 months later [9] and remained no different from the controls after 11 months (figure 1o).
Results from the visual assessment of health status at each time point are given in the electronic supplementary material, Additional results and figure S4. Lipid, protein and carbohydrate values in gram per gram ash-free dry weight are shown for each species in the electronic supplementary material, figure S5 to facilitate comparison with other studies.
(c). Tissue isotopes following repeat bleaching
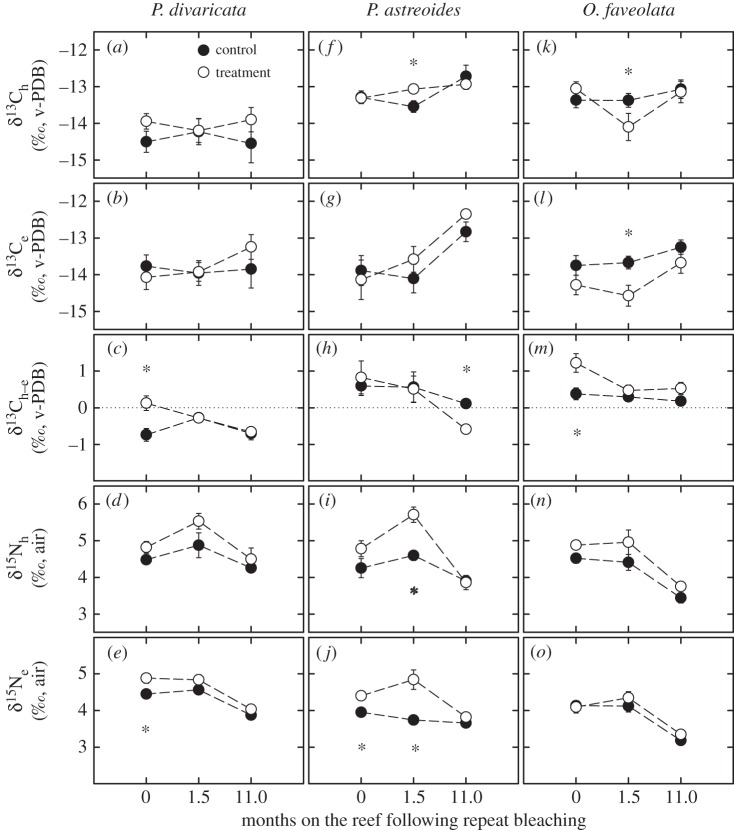
Results for tissue C and N isotopes for all three species (figure 2; electronic supplementary material, table S4) are detailed in the electronic supplementary material, Additional results.
Figure 2.
Average stable carbon isotopes of the animal host (δ13Ch) and endosymbiont (δ13Ce), δ13Ch−e and the stable nitrogen isotopes of the animal host (δ15Nh) and endosymbiont (δ15Ne) of (a–e) Porites divaricata, (f–j) Porites astreoides and (k–o) Orbicella faveolata after 0, 1.5 and 11 months on the reef following repeat bleaching. For δ13Ch−e, heterotrophic C is preferentially incorporated into tissues when the difference is less than 0, whereas photosynthetic C is preferentially incorporated when the difference is greater than or equal to 0. Averages are shown ±1 s.e. Asterisks indicate significant differences between treatment and control corals within a time point and species. Sample size per average ranges from 3 to 9. Statistical results shown in the electronic supplementary material, table S3.
4. Discussion
(a). Evidence for impaired long-term recovery capacity after repeat bleaching in Porites astreoides
Mounding P. astreoides has recently increased in abundance throughout the Caribbean, possibly in part due to its history of high thermal tolerance [35] and the ability to survive disturbance better than other species [36]. This is consistent with previous published findings that P. astreoides was only modestly impacted by single bleaching stress and had fully recovered from single bleaching within 1.5 months [9]. In stark contrast, P. astreoides was much more severely affected by repeat bleaching [9] (table 2) and did not fully recover all variables within 11 months (figures 1h–j, 2h; electronic supplementary material, figure S4b). This is further corroborated by the multivariate analyses showing that the overall physiology of treatment P. astreoides strongly differed from the controls for the 11 months following repeat bleaching (table 1; electronic supplementary material, figure S3). As annual bleaching significantly increased the bleaching susceptibility of this species [9], it is therefore not surprising that the resulting cumulative damage overwhelmed its capacity to recover within 1 year.
Table 2.
Months after single and repeat bleaching when response variables no longer differed significantly between treatment and control corals of Porites divaricata (P. d.), Porites astreoides (P. a.) and Orbicella faveolata (O. f.). The capacity to recover from annual bleaching was defined as impaired if one or more variables were not fully recovered within 11 months after repeat bleaching. The symbol ‘–’ denotes no statistically significant difference between treatment and control corals at any time and ‘>11’ indicates that bleached corals had not recovered by 11 months.
| variable | single bleaching |
repeat bleaching |
||||
|---|---|---|---|---|---|---|
| P. d. | P. a. | O. f. | P. d. | P. a. | O. f. | |
| symbiont density (cells cm−2)a | 1.5 | 1.5 | 1.5 | — | 11 | 1.5 |
| chlorophyll a (μg cm−2)b | 1.5 | 11 | 11 | |||
| calcification (mg day−1 cm−2)c | 1.5 | 1.5 | 11 | — | 11f | 1.5 |
| total energy reserves (J gdw−1)d | 11 | — | — | — | >11 | 1.5 |
| δ13Ch−e (‰)e | 1.5 | >11 | 1.5 | |||
| δ15Ne (‰)e | 1.5 | 11 | — | |||
| capacity to recover from annual bleaching | not impaired | impaired | not impaired | |||
Overall, treatment P. astreoides was characterized by a combination of lower chlorophyll a, protein and carbohydrate concentrations, lower calcification rates and higher δ15Ne values (electronic supplementary material, table S1 and figure S3). Critically, both protein and carbohydrate concentrations were still 54% and 35% lower than in the controls after 11 months on the reef (figure 1h,i), respectively, and had thus not fully recovered within the study period. In contrast, Grottoli et al. [9] showed that P. astreoides did not catabolize total energy reserves in response to single bleaching stress. Similarly, calcification rates declined by 69% after repeat bleaching and showed a trend of still being lower (−46%) than the controls after 11 months on the reef (figure 1j). Further, the relative contribution of heterotrophic versus photoautotrophic C (δ13Ch−e) indicated that treatment P. astreoides was incorporating a disproportionate amount of heterotrophic C into its tissues even after 11 months on the reef (figure 2h), suggesting that they needed additional energy to promote the ongoing recovery process. This is consistent with findings from single bleaching studies that showed that heterotrophic C can play a vital role in the long-term recovery of tissue and energy reserves from bleaching [39,40].
Grottoli et al. [9] suggested that the increased bleaching susceptibility of P. astreoides was due to a combination of a low flexibility to associate with different genetic types of Symbiodinium and low overall total energy reserve concentrations compared with the other species studied. Here, we show that the low overall energy reserves were due to lower concentrations of lipid reserves compared with the other two species (figure 1b,g,l) and also the failure to recover protein and carbohydrates during the 11 months of recovery on the reef. As lipids have the highest energetic value when compared with protein and carbohydrates [31], their low concentrations in P. astreoides have a disproportionate effect on total energy reserves. We hypothesize that the low baseline levels of lipids in this species force it to catabolize protein and carbohydrates when bleached. The low lipid levels could be related to the reproductive cycle of P. astreoides, which is a brooder with a long reproductive season lasting from January through September [41]. Consequently, lipid levels are expected to be lowest towards the end of this period, which coincides with peak SST at our study site. Such unfortunate timing likely influences resistance to heat stress [19] and may contribute to the increased bleaching susceptibility and impaired recovery capacity of this species in response to annual bleaching.
These findings highlight that following repeat bleaching, recovery of the animal can take much longer than the recovery of the endosymbiont. Both chlorophyll a concentrations and endosymbiont densities of treatment P. astreoides were fully recovered 11 months after repeat bleaching (this study; [37]), which is also reflected in the N isotopic data (figure 2d,e). δ15Nh and δ15Ne typically increase in bleached and/or recovering corals as the remaining endosymbionts are released from nitrogen limitation and take up more dissolved inorganic nitrogen to promote growth and chlorophyll a recovery [15], thus leading to less discrimination against 15N during uptake [14]. This trend was generally observed in treatment corals of P. astreoides as well as the other two coral species (figure 2; electronic supplementary material, figure S3 and table S1), although it was not always statistically significant, and is thus consistent with other studies showing δ15N enrichment in singly bleached corals [14,24,42].
Overall, this suggests that even when translocation of photosynthetic carbon to the animal has been restored, recovery of energy reserves and possibly also calcification rates often requires additional time and energy or even depends on additional factors. This is consistent with other, single bleaching studies from the Caribbean and the Pacific [14,15,17,43]. Therefore, in a future with annual bleaching stress, failure to fully recover energy reserves and calcification within a year could negatively affect coral reproduction (e.g. [44]), compromise resistance to further stress events (e.g. [19]) and diminish a coral's capacity to compete for space.
(b). Evidence for sustained long-term recovery capacity after repeat bleaching in Porites divaricata and Orbicella faveolata
In contrast to P. astreoides, branching P. divaricata and mounding O. faveolata were both able to fully recover from repeat bleaching stress within 11 months (figures 1 and 2), consistent with their response to single bleaching [9] (table 2). This is corroborated by the multivariate analyses showing that repeat bleaching affected the overall physiology of these two species much less than that of P. astreoides (table 1; electronic supplementary material, figure S3). Porites divaricata was particularly distinguished due to its high resistance to repeat bleaching and consequent rapid recovery (electronic supplementary material, figure S3). Nevertheless, the underlying mechanism of their recoveries was quite different and highlights the species-specific recovery from annual bleaching stress (electronic supplementary material, figure S3).
P. divaricata was barely affected by repeat bleaching, only showing declines in chlorophyll a immediately after repeat bleaching and declines in lipid at 1.5 months on the reef (figure 1a,b). Further, both chlorophyll a and lipid were fully recovered after 1.5 and 11 months on the reef, respectively (figure 1a,b). A substantial shift in Symbiodinium dominance from C47 to A4 coupled with high total energy reserve concentrations appeared to be the underlying mechanism for this rapid acclimation and recovery [9]. Interestingly, all three energy reserve pools were higher in this species compared with P. astreoides or O. faveolata (figure 1b–d; electronic supplementary material, figure S3), possibly because peak reproductive output in this brooding species occurs in spring [45], thus leaving time to rebuild lipid reserves before water temperatures reach their maximum. Overall, it is likely that the remarkable acclimation and recovery capacity of this species will make P. divaricata significantly more competitive than many other species in a future of annual bleaching events.
Orbicella faveolata also showed a remarkable capacity to recover from repeat bleaching, especially since Grottoli et al. [9] showed that this species was much more severely affected by repeat bleaching compared with single bleaching stress. Even so, O. faveolata recovered most variables within the first 1.5 months and the remainder after 11 months on the reef (figures 1k–o and 2k–o). For example, calcification stopped completely immediately after repeat bleaching resulting in net dissolution of skeletal material but had fully recovered only 1.5 months later (figure 1o). Similarly, lipids declined by 29% immediately after repeat bleaching, but were fully recovered 1.5 months later (figure 1l). High lipid levels at the time of the repeat bleaching (late July/early August) may have contributed to the remarkable recovery capacity and were probably linked to the reproductive cycle of this species, which typically spawns in August/September [41] and thus when water temperatures reach their maximum.
Only chlorophyll a levels took up to 11 months on the reef to fully recover (figure 1k). This was also evident in the δ13Ce values, which were systematically lower than in the controls for large parts of the study (figure 2l; electronic supplementary material, table S4). Decreases in δ13Ce are typically indicative of a reduction in the photosynthetic rate and/or the incorporation of photosynthetically derived C into the endosymbiont cells [14,23]. Although all measured variables of treatment O. faveolata were fully recovered by the end of the study, these systematically lower values could indicate that their endosymbionts (incl. a higher prevalence of Symbiodinium trenchii [46]) had associated physiological trade-offs compared with the original A and B types [9,47,48]. Thus, although O. faveolata overall appears to be able to recover from repeat bleaching within 1 year, additional studies are needed to fully test this over several years of annual bleaching.
(c). Importance of energy reserves and heterotrophy for long-term recovery from annual bleaching
Resilience to and recovery from coral bleaching may be promoted by heterotrophic plasticity, shifts in endosymbiont type and the size of energy reserves [9,18–21]. This study confirms that high levels of energy reserves play an important role in long-term recovery from annual bleaching stress as the species with the highest energy reserves, P. divaricata, was only minimally affected by repeat bleaching and was able to recover rapidly (table 2). However, it also highlights that location-specific, seasonal dynamics of different reproductive strategies and cycles, lipid levels and SST interact and significantly influence how species recover from bleaching. Additionally, the specific energy reserve pool catabolized during recovery from repeat bleaching appears to be highly species-specific with P. astreoides exclusively catabolizing protein and carbohydrates (figure 1g–i) and P. divaricata and O. faveolata catabolizing only lipid (figure 1b,l). This demonstrates that coral lipids are not necessarily the most important source of energy reserves as is often assumed, which has important implications for coral energy budgets.
In contrast to the size of energy reserves, heterotrophic plasticity did not play an important role in promoting short-term recovery from annual bleaching in these corals. None of the three species increased zooplankton feeding rates immediately after repeat bleaching, and zooplankton feeding of treatment corals contributed less than 10% to daily animal respiration [9]. Although corals may have accessed other sources of heterotrophic carbon such as bacteria and dissolved and particulate organic matter (e.g. [24,49,50]), this was probably not a significant contribution to the fixed carbon pool given the positive δ13Ch−e values for all treatment corals at 0 months on the reef (figure 2c,h,m) indicating that autotrophic C was preferentially incorporated into tissues.
However, heterotrophy appears to play an important role in the long-term recovery from annual bleaching as observed in δ13Ch−e of P. astreoides 11 months after repeat bleaching (figure 2h). Increased heterotrophy for up to 11 months post-bleaching has also been observed in singly bleached corals [39,40], and it has been debated whether this is a sign of prolonged stress and impaired recovery or indicates acclimation and increased resistance to future bleaching [39]. The current study is the first to show that this mechanism likely indicates prolonged stress, at least in repeat bleached P. astreoides given its incomplete recovery 11 months after repeat bleaching.
(d). Implications for the future of coral reefs
Overall, this study highlights that annually recurring bleaching events will disproportionately affect the long-term recovery of different coral species (table 2), which will likely change future coral community composition, diversity and reef functioning. It is encouraging that two of the species studied here were able to fully recover within a year, and acclimation combined with the capacity to recover between annual bleaching events will likely result in significantly fewer severe bleaching events and coral mortality by 2100 (e.g. [51]). However, it needs to be cautioned that the temperature treatments in this study simulated short bleaching events relative to naturally occurring events and that one of the species studied here nevertheless experienced cumulative damage and impaired long-term recovery capacity. The resilience of corals to future annual bleaching could also depend on potential interactive effects of heat and pCO2 stress although recent evidence suggests that ocean acidification does not affect bleaching susceptibility [52]. Importantly, models should integrate a range of trajectories to account for species-specific responses to annual bleaching (e.g. unaltered versus impaired recovery capacity) to provide more realistic predictions of future coral mortality, reef degradation and occurrence of mass bleaching events.
Supplementary Material
Acknowledgements
We thank R. Iglesias-Prieto, A. Banaszak, S. Enriquez, R. Smith and the staff of the Instituto de Ciencias del Mar y Limnologia, Universidad Nacional Autonoma de Mexico, in Puerto Morelos for their generous time and logisitical support. We also thank T. Huey, D. Borg, E. Zebrowski, J. Scheuermann and M. McBride for help in the field and laboratory.
Ethics
All work undertaken in this study complied with the current laws of Mexico and the United States of America for collecting and importing/exporting coral specimens.
Data accessibility
The datasets supporting this manuscript will be deposited at www.bco-dmo.org/project/516103.
Authors' contributions
A.G.G. and M.E.W. designed and coordinated the study; all authors participated in the fieldwork; V.S. carried out all laboratory analyses except for chlorophyll a; V.S. and A.G.G. analysed the data; V.S. drafted the manuscript; M.D.A. analysed chlorophyll a concentrations; all authors contributed to revising the manuscript and gave final approval for publication.
Competing interests
The authors declare that no conflict of interest exists.
Funding
This work was funded by the National Science Foundation (OCE#0825490 to A.G.G. and OCE#0825413 to M.E.W.).
References
- 1.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 2.Hoegh-Guldberg O. 1999. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwater Res. 50, 839–866. (doi:10.1071/MF99078) [Google Scholar]
- 3.Eakin CM, Lough JM, Heron SF. 2009. Climate variability and change: monitoring data and evidence for increased coral bleaching stress. In Coral bleaching: patterns, processes, causes and consequences (eds van Oppen MJH, Lough JM), pp. 41–67. Berlin, Germany: Springer. [Google Scholar]
- 4.Frieler K, Meinshausen M, Golly A, Mengel M, Lebek K, Donner SD, Hoegh-Guldberg O. 2013. Limiting global warming to 2°C is unlikely to save most coral reefs. Nat. Clim. Change 3, 165–170. (doi:10.1038/nclimate1674) [Google Scholar]
- 5.van Hooidonk R, Maynard JA, Liu Y, Lee S-K. 2015. Downscaled projections of Caribbean coral bleaching that can inform conservation planning. Glob. Change Biol. 21, 3389–3401. (doi:10.1111/gcb.12901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maynard JA, Anthony KRN, Marshall PA, Masiri I. 2008. Major bleaching events can lead to increased thermal tolerance in corals. Mar. Biol. 155, 173–182. (doi:10.1007/s00227-008-1015-y) [Google Scholar]
- 7.Guest JR, Baird AH, Maynard JF, Muttaqin E, Edwards AJ, Campbell SJ, Yewdall K, Affendi YA, Chou LM. 2012. Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 7, e33353 (doi:10.1371/journal.pone.0033353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middlebrook R, Hoegh-Guldberg O, Leggat W. 2008. The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J. Exp. Biol. 211, 1050–1056. (doi:10.1242/jeb.013284) [DOI] [PubMed] [Google Scholar]
- 9.Grottoli AG, Warner M, Levas SJ, Aschaffenburg M, Schoepf V, McGinley M, Baumann J, Matsui Y. 2014. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Change Biol. 20, 3823–3833. (doi:10.1111/gcb.12658) [DOI] [PubMed] [Google Scholar]
- 10.Hoegh-Guldberg O, Smith GJ. 1989. The effect of sudden changes in temperature, light, and salinity on the population density and export of zooxanthellae from the reef coral Stylophora pistillata Esper and Seriatopora hystrix Dana. J. Exp. Mar. Biol. Ecol. 129, 279–303. (doi:10.1016/0022-0981(89)90109-3) [Google Scholar]
- 11.Jokiel PL, Coles SL. 1990. Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 8, 155–162. (doi:10.1007/BF00265006) [Google Scholar]
- 12.Muscatine L, McCloskey LR, Marian RE. 1981. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 26, 601–611. (doi:10.4319/lo.1981.26.4.0601) [Google Scholar]
- 13.Leder JJ, Szmant AM, Swart P. 1991. The effect of prolonged ‘bleaching’ on skeletal banding and stable isotopic composition in Montastraea annularis. Coral Reefs 10, 19–27. (doi:10.1007/BF00301902) [Google Scholar]
- 14.Rodrigues LJ, Grottoli AG. 2006. Calcification rate and the stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Acta 70, 2781–2789. (doi:10.1016/j.gca.2006.02.014) [Google Scholar]
- 15.Fitt WK, Spero HJ, Halas J, White MW, Porter JW. 1993. Recovery of the coral Montastraea annularis in the Florida Keys after the 1987 Caribbean ‘bleaching event’. Coral Reefs 12, 57–64. (doi:10.1007/BF00302102) [Google Scholar]
- 16.Porter JW, Fitt WK, Spero HJ, Rogers AD, White MW. 1989. Bleaching in reef corals: physiological and stable isotopic responses. Proc. Natl Acad. Sci. USA 86, 9342–9346. (doi:10.1073/pnas.86.23.9342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues LJ, Grottoli AG. 2007. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol. Oceanogr. 52, 1874–1882. (doi:10.4319/lo.2007.52.5.1874) [Google Scholar]
- 18.Grottoli AG, Rodrigues LJ, Palardy JE. 2006. Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189. (doi:10.1038/nature04565) [DOI] [PubMed] [Google Scholar]
- 19.Anthony KRN, Hoogenboom MO, Maynard JF, Grottoli AG, Middlebrook R. 2009. Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Funct. Ecol. 23, 539–550. (doi:10.1111/j.1365-2435.2008.01531.x) [Google Scholar]
- 20.Ferrier-Pagès C, Rottier C, Beraud E, Levy O. 2010. Experimental assessment of the feeding effort of three scleractinian coral species during a thermal stress: effect on the rates of photosynthesis. J. Exp. Mar. Biol. Ecol. 390, 118–124. (doi:10.1016/j.jembe.2010.05.007) [Google Scholar]
- 21.Connolly SR, Lopez-Yglesias MA, Anthony KRN. 2012. Food availability promotes rapid recovery from thermal stress in a scleractinian coral. Coral Reefs 31, 951–960. (doi:10.1007/s00338-012-0925-9) [Google Scholar]
- 22.Reynaud S, Ferrier-Pages C, Sambrotto R, Juillet-Leclerc A, Jaubert J, Gattuso JP. 2002. Effect of feeding on the carbon and oxygen isotopic composition in the tissues and skeleton of the zooxanthellate coral Stylophora pistillata. Mar. Ecol. Prog. Ser. 238, 81–89. (doi:10.3354/meps238081) [Google Scholar]
- 23.Muscatine L, Porter JW, Kaplan IR. 1989. Resource partitioning by reef corals as determined from stable isotope composition. I. δ13C of zooxanthellae and animal tissue vs. depth. Mar. Biol. 100, 185–193. (doi:10.1007/BF00391957) [Google Scholar]
- 24.Levas SJ, Grottoli AG, Hughes AD, Osburn CL, Matsui Y. 2013. Physiological and biogeochemical traits of bleaching and recovery in the mounding species of coral Porites lobata: implications for resilience in mounding corals. PLoS ONE 8, e63267 (doi:10.1371/journal.pone.0063267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoepf V, Levas SJ, Rodrigues LJ, McBride MO, Aschaffenburg M, Matsui Y, Warner M, Hughes AD, Grottoli AG. 2014. Kinetic and metabolic isotope effects in coral skeletal carbon isotopes: a re-evaluation using experimental coral bleaching as a case study. Geochim. Cosmochim. Acta 146, 164–178. (doi:10.1016/j.gca.2014.09.033) [Google Scholar]
- 26.Eakin CM, et al. 2010. Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS One 5, e13969 (doi:10.1371/journal.pone.0013969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffrey SW, Humphrey GF. 1975. New spectrophotometric equations for determining chlorophylls a, b, c1, and c2 in higher plants, algae, and natural phytoplankton. Biochem. Physiol. Pflanzen 167, 191–194. [Google Scholar]
- 28.Marsh JA. 1970. Primary productivity of reef-building calcareous red algae. Ecology 51, 255–263. (doi:10.2307/1933661) [Google Scholar]
- 29.Schoepf V, McCulloch MT, Warner ME, Levas SJ, Matsui Y, Aschaffenburg M, Grottoli AG. 2014. Short-term coral bleaching is not recorded by skeletal boron isotopes. PLoS One 9, e112011 (doi:10.1371/journal.pone.0112011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoepf V, et al. 2013. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE 8, e75049 (doi:75010.71371/journal.pone.0075049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnaiger E, Bitterlich G. 1984. Proximate biochemical composition and caloric content calculated from elemental CHN analysis: a stoichiometric concept. Oecologia 62, 289–298. (doi:10.1007/BF00384259) [DOI] [PubMed] [Google Scholar]
- 32.Jokiel PL, Maragos JE, Franzisket L. 1978. Coral growth: buoyant weight technique. In Coral reefs: research methods (eds Stoddart DR, Johannes RE), pp. 529–541. Paris, France: UNESCO. [Google Scholar]
- 33.Hughes AD, Grottoli AG, Pease TK, Matsui Y. 2010. Acquisition and assimilation of carbon in non-bleached and bleached corals. Mar. Ecol. Prog. Ser. 420, 91–101. (doi:10.3354/meps08866) [Google Scholar]
- 34.Winer BJ. 1971. Statistical principles in experimental design. New York, NY: McGraw-Hill. [Google Scholar]
- 35.Warner ME, LaJeunesse TC, Robison JD, Thur RM. 2006. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol. Oceanogr. 51, 1887–1897. (doi:10.4319/lo.2006.51.4.1887) [Google Scholar]
- 36.Green DH, Edmunds PJ, Carpenter RC. 2008. Increasing relative abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Mar. Ecol. Prog. Ser. 359, 1–10. (doi:10.3354/meps07454) [Google Scholar]
- 37.Aschaffenburg M. 2012. The physiological response of Symbiodinium spp. to thermal and light stress: a comparison of different phylotypes and implications for coral reef bleaching. PhD thesis, University of Delaware.
- 38.Levas SJ. 2012. Biogeochemistry and physiology of bleached and recovering Hawaiian and Caribbean corals. PhD thesis. The Ohio State University, Columbus, OH, USA.
- 39.Hughes AD, Grottoli AG. 2013. Heterotrophic compensation: a possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress? PLoS ONE 8, e81172 (doi:10.1371/journal.pone.0081172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumann J, Hughes AD, Matsui Y, Grottoli AG. 2014. Photoautotrophic and heterotrophic carbon in bleached and non-bleached coral lipid acquisition and storage. J. Exp. Mar. Biol. Ecol. 461, 469–478. (doi:10.1016/j.jembe.2014.09.017) [Google Scholar]
- 41.Szmant AM. 1986. Reproductive ecology of Caribbean reef corals. Coral Reefs 5, 43–54. (doi:10.1007/BF00302170) [Google Scholar]
- 42.Bessell-Browne P, Stat M, Thomson D, Clode PL. 2014. Coscinaraea marshae corals that have survived prolonged bleaching exhibit signs of increased heterotrophic feeding. Coral Reefs 33, 795–804. (doi:10.1007/s00338-014-1156-z) [Google Scholar]
- 43.Fitt WK, McFarland FK, Warner ME, Chilcoat GC. 2000. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 45, 677–685. (doi:10.4319/lo.2000.45.3.0677) [Google Scholar]
- 44.Ward S, Harrison PJ, Hoegh-Guldberg O. 2000. Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. In Proc. 9th Int. Coral Reef Symp., Bali, Indonesia, 23–27 October 2000, pp. 1123–1128.
- 45.McDermond J. 2014. Reproduction and population of Porites divaricata at Rodriguez Key: the Florida Keys, USA. Master thesis, Nova Southeastern University.
- 46.LaJeunesse TC, Wham DC, Pettay DT, Parkinson JE, Keshavmurthy S, Chen CA. 2014. Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) clade D are different species. Phycologia 53, 305–319. (doi:10.2216/13-186.1) [Google Scholar]
- 47.Pettay DT, Wham DC, Smith RT, Iglesias-Prieto R, LaJeunesse TC. 2015. Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc. Natl Acad. Sci. USA 112, 7513–7518. (doi:10.1073/pnas.1502283112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones A, Berkelmans R. 2010. Potential costs of acclimatization to a warmer climate: growth of a reef coral with heat tolerant versus sensitive symbiont types. PLoS ONE 5, e10437 (doi:10.1371/journal.pone.0010437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tremblay P, Naumann MS, Sikorski S, Grover R, Ferrier-Pagès C. 2012. Experimental assessment of organic carbon fluxes in the scleractinian coral Stylophora pistillata during a thermal and photo stress event. Mar. Ecol. Prog. Ser. 453, 63–77. (doi:10.3354/meps09640) [Google Scholar]
- 50.Anthony KRN. 2000. Enhanced particle-feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 19, 59–67. (doi:10.1007/s003380050227) [Google Scholar]
- 51.Logan CA, Dunne JP, Eakin CM, Donner SD. 2014. Incorporating adaptive responses into future projections of coral bleaching. Glob. Change Biol. 20, 125–139. (doi:10.1111/gcb.12390) [DOI] [PubMed] [Google Scholar]
- 52.Noonan SHC, Fabricius KE. 2015. Ocean acidification affects productivity but not the severity of thermal bleaching in some tropical corals. ICES J. Mar. Sci. (doi:10.1093/icesjms/fsv127) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this manuscript will be deposited at www.bco-dmo.org/project/516103.