Abstract
Classic findings have demonstrated an important role for sex steroids as regulators of aggression, but this relationship is lacking within some environmental contexts. In mammals and birds, the adrenal androgen dehydroepiandrosterone (DHEA), a non-gonadal precursor of biologically active steroids, has been linked to aggression. Although females, like males, use aggression when competing for limited resources, the mechanisms underlying female aggression remain understudied. Here, we propose a previously undescribed endocrine mechanism regulating female aggression via direct action of the pineal hormone melatonin on adrenal androgens. We examined this in a solitary hamster species, Phodopus sungorus, in which both sexes are highly territorial across the seasons, and display increased aggression concomitant with decreased serum levels of sex steroids in short ‘winter-like' days. Short- but not long-day females had increased adrenal DHEA responsiveness co-occurring with morphological changes in the adrenal gland. Further, serum DHEA and total adrenal DHEA content were elevated in short days. Lastly, melatonin increased DHEA and aggression and stimulated DHEA release from cultured adrenals. Collectively, these findings demonstrate that DHEA is a key peripheral regulator of aggression and that melatonin coordinates a ‘seasonal switch’ from gonadal to adrenal regulation of aggression by direct action on the adrenal glands.
Keywords: conflict, stress physiology, HPA axis, zona reticularis, competitive phenotypes, seasonality
1. Background
Among the vast array of social behaviours, one of the most important is aggression. Aggression is a complex behaviour displayed by virtually all organisms and serves a wide range of adaptive functions in both sexes, including acquisition and maintenance of limited resources such as food or mates [1,2]. Appropriate displays of aggression require integration of both environmental and physiological factors. At a mechanistic level, steroid hormones mediate the expression of aggression across environmental contexts. Numerous studies have demonstrated a positive relationship between gonadal steroids, particularly testosterone (T), and aggression in males and females of many vertebrates, including birds, lizards and mammals (e.g. [3–6]). Traditionally, these studies have focused on males in reproductive condition, often in laboratory animals such as rats and mice (reviewed in [7]). When both males and females of seasonally breeding species are examined, noteworthy alternatives to the well-established connection between gonadal steroids and breeding aggression emerge [7,8]. It is becoming increasingly clear that steroid hormones in addition to T may play important roles in the regulation of aggression in both males and females [9–16].
Consistent with the general hypothesis of a lack of a positive relationship between gonadal steroids and aggression, we have previously shown that male and female Siberian hamsters (Phodopus sungorus) housed in short ‘winter-like’ days undergo gonadal regression and decreases in gonadal steroids, but marked increases in aggression compared with animals in long ‘summer-like’ days [17,18]. Replacement of gonadal steroids (i.e. 17β-oestradiol; E2) in short-day females (to mimic long-day levels) does not reduce aggression [18]. This lack of a relationship between gonadal steroids and aggression in short-day hamsters suggests additional neuroendocrine mechanisms regulating aggression independent of the gonads; these mechanisms, however, are less well understood in females.
Here we propose a previously undescribed endocrine mechanism regulating female aggression; changes in melatonin act directly on the adrenal glands to coordinate a ‘seasonal switch’ from gonadal to adrenal regulation of aggression. It is known that day length (photoperiod) is the primary cue used by mammals to coordinate seasonal changes in morphology, physiology and behaviour [19]. Further, photoperiodic information is transduced by the pineal gland into a melatonin signal, with peak concentrations of the hormone occurring during the night and basal levels occurring during the day [19]. Maintenance of animals in short days (i.e. long nights) results in a prolonged duration of melatonin secretion; the pattern of melatonin serves as the biochemical signal of day length. Short-day patterns of melatonin induce a suite of traits including gonadal regression and decreases in sex steroids. Further, melatonin receptors are distributed throughout the brain and periphery, including at the level of both the gonads and adrenal glands [19]. Melatonin links the environment with physiology and behaviour by coordinating seasonal changes in a variety of vertebrate species, including frogs, lizards, mammals and snakes ([20–23]; reviewed in [7]). Therefore, the endocrine mechanism described here may also regulate seasonal aggression in other species.
Many species, including birds, lizards and mammals, display decreases in aggression concurrent with decreases in reproduction during the non-breeding season [4,5]. By contrast, in some species aggression is unchanged [3,6,13] or even increased [12,17,18] during the non-breeding season. For example, aggression persists during the non-breeding season in song sparrows, Melospiza melodia morphna [16], and is further elevated in Siberian hamsters, P. sungorus [17,18]. Species like song sparrows and Siberian hamsters, unlike other species where reproduction and aggression often co-occur, are ideal for addressing non-gonadal mechanisms of aggression because they display high levels of aggression that occur independently of reproduction, allowing the relationship between gonadal steroids and aggression to be uncoupled (see also [10,11]).
Siberian hamsters occur at low population densities (i.e. one to six individuals per km2), are solitary and polygynous, displaying little paternal care [24]. Importantly, unlike most rodents where females limit aggression to pregnancy and lactation during the breeding season, female Siberian hamsters display substantial levels of aggression across both the breeding and non-breeding seasons. At an ultimate level of analysis, non-breeding aggression might confer an evolutionary advantage when food availability is relatively low and competition for limited resources is high; food restriction increases aggression in male and female hamsters housed in an intermediate photoperiod mimicking the transition between breeding and non-breeding seasons (A. M. Bailey, N. M. Rendon and G. E. Demas 2015, unpublished results). Photoperiod-induced changes in aggression provide an ecologically relevant model of female aggression, which also has relevance for our understanding of adrenal regulation of aggression broadly.
At a proximate level of analysis, both male and female hamsters secrete significant amounts of adrenal dehydroepiandrosterone (DHEA; for similar reports in sparrows, spotted antbirds and squirrels, see [9,13,16]). By contrast, rats and mice lack the specific enzyme 17α-hydroxylase necessary to synthesize and secrete adrenal androgens ([25], reviewed in [7,26]), making them an inappropriate model for the study of adrenal-derived androgens. DHEA and aggression appear positively correlated in humans [27,28], and individuals who are deficient in cortisol (CORT) synthesis (i.e. congenital adrenal hyperplasia) show elevated DHEA concomitant with increased aggression [29,30]. Evidence from male hamsters suggests that changes in adrenocortical steroids regulate, at least in part, short-day aggression. Increased aggression in male hamsters treated with short-day-like melatonin is blocked by bilateral adrenalectomies (which eliminate both adrenal steroids and catecholamines), but not demedullations (which eliminate catecholamines, leaving adrenal steroids intact) [31]. Short-day aggression is also independent of both CORT levels and glucocorticoid receptor expression in male hamsters [32]. These data suggest that the adrenal gland, likely via regulation of adrenal androgens, serves as a key peripheral target tissue regulating aggression in a previously undescribed endocrine manner.
Evidence in non-mammalian systems suggests that non-gonadal steroid precursors can be converted to biologically active androgens or oestrogens, and that these precursors are critical regulators of aggression [7,8]. In song sparrows that exhibit year-round territorial aggression, DHEA serves as a critical regulator of aggression when sex steroids are low [16,33]. These non-gonadal mechanisms of aggression are likely shared across vertebrates. Mammals, as with songbirds, may also regulate aggression through adrenal DHEA metabolism when circulating sex steroids are relatively unavailable [7]. In this study, we examined regulation of aggression in highly territorial female Siberian hamsters by examining the effects of photoperiod and melatonin on peripheral target tissues, specifically adrenal gland morphology and DHEA responsiveness, and gonadal and adrenal release of DHEA. We hypothesized that short-day females would be more aggressive, mount a more robust DHEA response, and display discrete, layer-specific increases in adrenocortical tissue (specifically in the zona reticularis, the predominant site of DHEA release) compared with long-day females. We further hypothesized that melatonin serves as the endocrine switch mediating adrenal regulation of aggression, and that in vivo and in vitro melatonin would increase adrenal DHEA release, and stimulate increased aggression in vivo, mimicking patterns observed in short days.
2. Material and methods
(a). Animal housing
Subjects were adult (more than 60 days of age) female Siberian hamsters (P. sungorus) from a colony maintained at Indiana University. Hamsters were bred and housed under long days (16 L : 8 D h), group-housed at weaning and given ad libitum access to water and laboratory chow (Lab Diet 5001, PMI Nutrition). Ambient temperature was 20 ± 2°C, and relative humidity was 55 ± 5%.
(b). Photoperiodic manipulations
Hamsters (n = 129) were individually housed for a one-week acclimation under long days; a subset of hamsters were transferred to short days (8 L : 16 D), while the remaining hamsters were kept in long days. Long- (n = 45) and short-day (n = 84) animals were in these photoperiods for 10 weeks.
(c). Reproductive phenotypes
Following the 10 week photoperiodic treatment, reproductive phenotypes were determined based on a priori criteria for this species [18]. Long-day females displayed oestrous cyclicity, had functional reproductive tissue masses, no change in body mass and brown pelage. By contrast, short-day females were anoestrus or acyclic, had regressed reproductive tissues, lost more than 10% of their body mass and had white pelage. A subset of short-day animals (n = 18) failed to respond to the photoperiodic treatment; these non-responders [34] were excluded from the study owing to insufficient numbers for statistical analysis.
(d). Adrenal responsiveness and behavioural testing
After photoperiodic treatment, hamsters underwent same-sex territorial aggression trials, followed by adrenocorticotropic hormone (ACTH) challenges. The pituitary hormone ACTH is the tropic hormone regulating DHEA and CORT secretion from the adrenal cortex [35], but is not associated with the release of mineralocorticoids [35] or adrenomedullary catecholamines [36]. Therefore, using an ACTH challenge allows for direct assessment of adrenal DHEA and CORT output, without affecting changes in other adrenal hormones.
First, aggression was measured in all animals (long days, n = 45; short days, n = 66), using a 5 min female–female resident–intruder paradigm described previously ([18], electronic supplementary material). Aggression (i.e. latency to first attack (seconds), number and duration of attacks, number and duration of chases) and non-aggressive behaviour (i.e. number and duration of facial and anogenital investigations) were quantified for experimental animals. Aggression data were reduced to a composite ‘aggression score’, and social investigations were reduced to a composite ‘investigation score’ using principal component analyses (PCA), with the extracted components explaining 76.9%, and 69.2% of the total variance, respectively (electronic supplementary material, table S1). Three days following testing (to decrease the probability that behaviour would affect the ACTH challenge), animals were randomly assigned to either an ACTH injection (long days, n = 27; short days, n = 31) or a saline control injection (long days, n = 18; short days n = 35) group to assess the capacity of the adrenal glands to secrete steroids. All animals had pre-challenge samples taken at Time 0 (T0) between 4 and 6 h into the light phase. Animals were allowed 15 min to recover, and then received an intra-muscular injection of either 4 IU kg−1 synthetic ACTH (Cortrosyn, Henry Schein Animal Health, Melville, NY, USA) or a saline control. Subsequent blood samples were collected at T30 (n = 40), T60 (n = 35) or T120 (n = 36) post-injection; two blood samples per individual. The ACTH challenge protocol, including dosage and time points, was chosen based on a protocol in squirrels [9]; these time points reflected maximal response (T30) and a return to baseline DHEA (T120), respectively [9].
(e). Adrenal morphology and total steroid content
Adrenal glands were histologically processed using a haematoxylin and eosin (H&E) stain via previously described methods [37]. The area encompassed by each cortical layer (i.e. zona reticularis, zona glomerulosa and zona fasciculata), as well as the adrenal medulla, were quantified across photoperiods (long days, n = 9; short-days, n = 9). Only animals that received control injections were selected to avoid any potential confounding effects of previous ACTH administration on adrenal morphology (see electronic supplementary material). Owing to the unexpected findings related to adrenal morphology (see §3), we housed an additional group of hamsters in long (n = 6) or short (n = 6) days to determine total adrenal DHEA and CORT content via a modification of previously described methods ([38], electronic supplementary material).
(f). In vivo melatonin administration
Melatonin profiles were manipulated in an additional group of long-day female hamsters (n = 24) via timed injections of melatonin using previously described methods [21,39]. Half of the females (n = 12) received daily subcutaneous injections of melatonin (15 µg day−1 (M5250; Sigma Chemical, Saint Louis, MO, USA) dissolved in a 1 : 10 ethanol: saline solution) for 10 days [21], whereas the other half of the females (n = 12) received injections of the control (i.e. ethanol : saline) solution. Injections were administered 2 h before lights out to extend the long-day pattern of endogenous melatonin secretion mimicking that seen in short days [39]. Because melatonin treatment was administered on a relatively short-term basis (i.e. 10 days), it is not sufficient to trigger gonadal regression and, unlike prolonged maintenance in short days, leaves gonadal steroids unaffected [21]. In addition, this protocol allows us to examine the influence of melatonin on adrenal steroids and behaviour during a critical transitional period between long and short days. Following melatonin treatment, reproductive phenotypes were determined, and aggression and serum DHEA were quantified. Aggression data were reduced to a composite ‘aggression score’ (as above) using a PCA, yielding one component explaining 86.1% of the total variance (electronic supplementary material, table S2).
(g). In vitro melatonin administration
To test the hypothesis that melatonin modulates peripheral DHEA release, we used an in vitro assay that measured the DHEA response to exogenous melatonin in adrenals and ovaries across photoperiods. We individually housed adult female hamsters (n = 96), in long and short days (as above). Both long- (n = 44) and short-day (n = 52) animals underwent aggression trials (long days, n = 44; short days, n = 52, electronic supplementary material). Aggression was quantified (as above), yielding one PCA component explaining 82.1% of the total variance (electronic supplementary material, table S3).
Three days following behavioural testing (as above), adrenal glands and ovaries of long- and short-day animals were dissected and weighed within 2 h before lights off, when tissues are sensitive to exogenous melatonin [39]. For adrenal glands, one-quarter of the samples in culture were treated with ACTH (0.4 IU ACTH; long days, n = 11; short days, n = 12) to stimulate DHEA release, a quarter of the samples received melatonin (450 µM melatonin; long days, n = 11; short days, n = 13) to determine whether direct action of melatonin stimulated DHEA release, a quarter of the samples received both ACTH and melatonin (0.4 IU ACTH + 450 µM melatonin; long days, n = 11; short days, n = 12) to determine whether melatonin enhances the DHEA response, and the remaining samples received a control solution (Krebs-Ringer Solution with 0.01 M glucose and 1% albumin; long days, n = 11; short days, n = 11) in place of both ACTH and melatonin. Subsets of ovaries from both photoperiods were treated with either melatonin (450 µM melatonin; long days, n = 9; short days, n = 9) or a control solution (Krebs-Ringer Solution with 0.01 M glucose and 1% albumin; long days, n = 9; short days, n = 7). DHEA was measured from the liquid media of cultured glands. The in vitro assay protocol, including dosage and time points, was chosen based on slight modifications of a previous protocol ([40]; electronic supplementary material).
(h). Blood and tissue collection and quantification of steroid hormones
Serum DHEA and CORT, DHEA and CORT content from adrenal gland homogenates, and DHEA in media were quantified using commercially available enzyme immunoassays (electronic supplementary material). Tissues were collected at the end of each experiment at necropsy.
(i). Statistical analyses
Statistical analyses were performed in JMP v. 11.0.0 (SAS Institute, Inc., Cary, NC, USA), and statistical significance was reported if p < 0.05. If parametric statistical tests were used, data were transformed to attain normality and homogeneity of variances. Two-tailed t-tests were used to compare physiological and behavioural changes that occurred between long- and short-day animals or to compare melatonin-induced changes in long-day melatonin- and control-treated females. A Mann–Whitney U test was used to compare aggression scores and investigation scores across photoperiods. Repeated-measures ANOVAs were used to compare pre- and post-challenge levels of hormones with pair-wise comparisons conducted using two-tailed t-tests. Spearman's rank correlations were run on pre- and post-levels of DHEA and CORT at T30 in females that received ACTH. P-values for these correlations were Bonferroni corrected to account for multiple comparisons. A two-way ANOVA was used to examine the photoperiodic effects on area of adrenal layer, and to examine photoperiodic effects on in vitro DHEA release. Tukey's highly-significant difference (HSD) post-hoc analyses were used to examine pair-wise comparisons.
3. Results
(a). Reproductive phenotypes and behaviour
Following 10 weeks of photoperiodic treatment, short-day females had regressed reproductive tissues (p < 0.01), decreased body mass (more than 10%; p < 0.01), higher circulating DHEA (t109 = 2.78; p < 0.01) and lower circulating CORT (t67 = −2.69; p < 0.01), compared with long-day females (electronic supplementary material, table S4). Short-day females displayed more aggression (aggression score: U = 1169.5, z = −8.11; p < 0.01; attacks: t90 = 11.73; p < 0.01; figure 1a,b), and less investigation (investigation score: U = 2916.5, z = 2.38; p = 0.02; anogenital investigations: t94 = −2.23; p = 0.03; electronic supplementary material, figure S1a,b) than long-day females (see the electronic supplementary material for additional behavioural measures).
Figure 1.
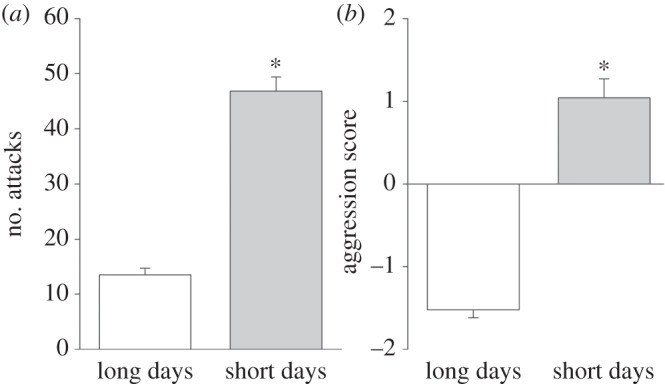
Seasonal change in aggressive behaviours. (a) Number of attacks and (b) aggression score. Long days (n = 45), short days (n = 66). Bar heights represent means ± s.e.m. *p < 0.05.
(b). Adrenal responsiveness
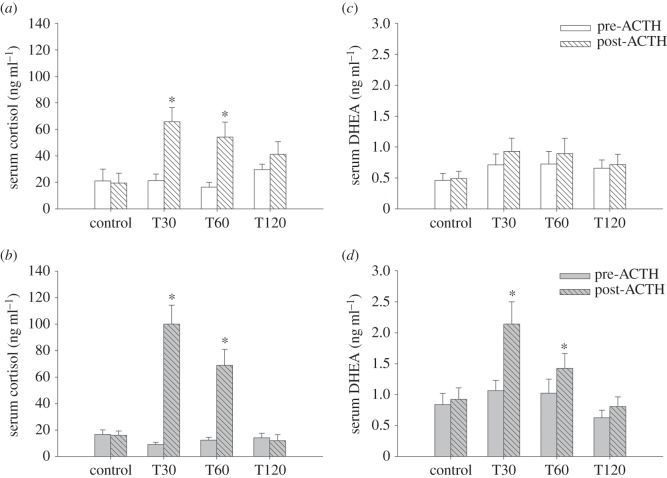
Both long- and short-day females elevated CORT in response to an ACTH challenge (treatment effect: long days, F1,39 = 22.66, p < 0.01; short days: F5,60 = 40.05, p < 0.01), and the CORT response differed across sampling times (treatment × time effect: long days: F2,39 = 3.46; p = 0.04; short days: F1,60 = 7.99, p < 0.01; figure 2a,b). CORT was maximally elevated at T30 (long days: t9 = 4.85, p < 0.01; short days: t11 = 3.86, p < 0.01), remained elevated at T60 (long days: t7 = 3.48, p = 0.01; short days: t10 = 2.69, p = 0.02) and returned to baseline by T120 (long days: t8 = 1.62, p = 0.14; short days: t7 = 2.98, p = 0.21; figure 2a,b). Short-, but not long-day females exhibited elevated DHEA in response to an ACTH challenge (long days, F1,39 = 1.70, p = 0.20; treatment effect: short days, F1,60 = 17.44, p < 0.01), and the DHEA response differed across sampling times (treatment × time effect: long days, F2,39 = 1.53, p = 0.23; treatment × time effect: short days, F1,60 = 7.99, p < 0.01; figure 2c,d). In short-day animals, DHEA was maximally elevated at T30 (t11 = 3.86, p < 0.01), remained elevated at T60 (t10 = 2.69, p = 0.02) and returned to baseline by T120 (t7 = 2.98, p = 0.21; figure 2d). By contrast, DHEA levels in long-day females were not altered in response to an ACTH challenge (T30: t9 = 1.12, p = 0.29; T60: t7 = 1.22, p = 0.26; T120: t8 = 0.33, p = 0.75; figure 2c). CORT and DHEA levels of animals that received saline controls, regardless of photoperiod, were not affected (p < 0.01; electronic supplementary material).
Figure 2.
ACTH challenge time course for CORT and DHEA across photoperiods. CORT levels for (a) long-day females, (b) short-day females; DHEA levels for (c) long-day females and (d) short-day females receiving either an ACTH or control treatment. White bars, long days; grey bars, short days. Bar heights represent means ± s.e.m. *p < 0.05.
(c). Associations between endocrine responses and aggression
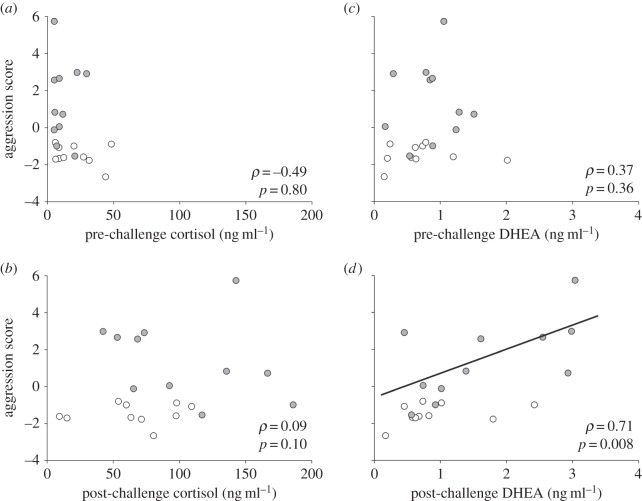
Aggression was not associated with pre-challenge CORT (p = 0.80), post-challenge CORT (p > 0.10) or pre-challenge DHEA (p = 0.36) (figure 3a–c). Aggression and post-challenge DHEA, by contrast, were positively associated (p < 0.01). Short-day females (p = 0.04), but not long-day females (p = 0.24), drive the positive association between aggression and post-challenge DHEA (figure 3d).
Figure 3.
Relationships between seasonal aggression and adrenal steroids. Aggression score and (a) pre-challenge CORT, (b) post-challenge CORT, (c) pre-challenge DHEA and (d) post-challenge DHEA. White circles, long days; grey circles, short days. Regression line generated from Spearman's rank correlation within short days; p < 0.05.
(d). Adrenal morphology
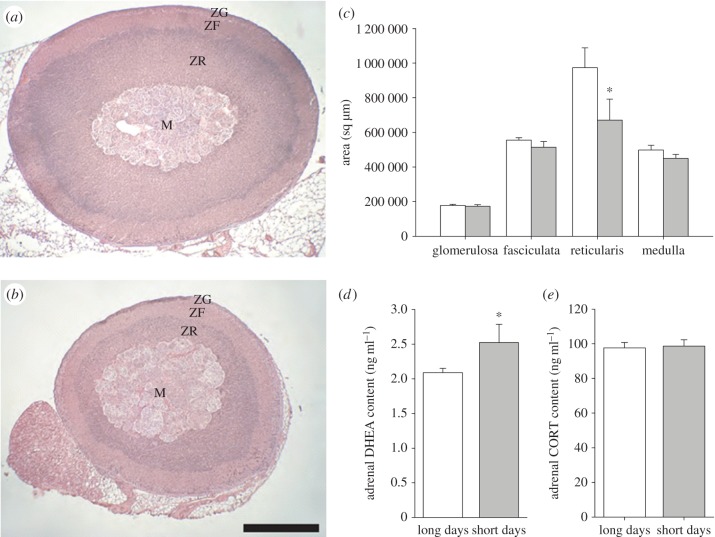
Photoperiod did not affect adrenal mass (t109 = 0.17; p = 0.87; electronic supplementary material, table S4); however, total cross-sectional areas through the adrenal epicentre of long-day females were significantly larger (31%) than those of short-day females (t15 = 3.32; p < 0.01; figure 4a,b). There was a photoperiodic effect on adrenal cortical zones and medulla (F1,45 = 11.02, p < 0.01), and a photoperiod by cortical layer interaction (F3,45 = 6.76, p < 0.01). Areas of long-day zonae reticulara were 72% larger than those of short-day animals (F1,15 = 8.96, p < 0.01); areas of other adrenal components did not differ across photoperiods (F1,30 = 2.70, p = 0.1214; figure 4c).
Figure 4.
Histological and steroid content analyses of adrenal glands. Representative sections (i.e. representative of their respective group mean) through the adrenal epicentres of a (a) long-day and (b) short-day female stained with H&E. Zona glomerulosa (ZG), zona fasciculata (ZF), zona reticularis (ZR) and medulla (M) shown. Scale bar = 500 µm; (c) Cross-sectional areas of adrenal cortical zones and medulla from long-day (n = 9) and short-day (n = 9) animals. Adrenal (d) DHEA and (e) CORT content from long-day (n = 6) and short-day (n = 6) animals. Bar heights represent means ± s.e.m. *p < 0.05. (Online version in colour.)
(e). Adrenal steroid content
Photoperiod did not alter absolute (F1,10 = 0.19; p = 0.68) or relative (F1,10 = 2.13; p = 0.18) adrenal mass. Adrenal DHEA content, however, was greater in short- compared with long-day females (F1,6 = 6.13; p = 0.04; figure 4d); there was no difference in adrenal CORT content in either photoperiod (F1,10 = 0.04; p = 0.85; figure 4e).
(f). In vivo melatonin administration
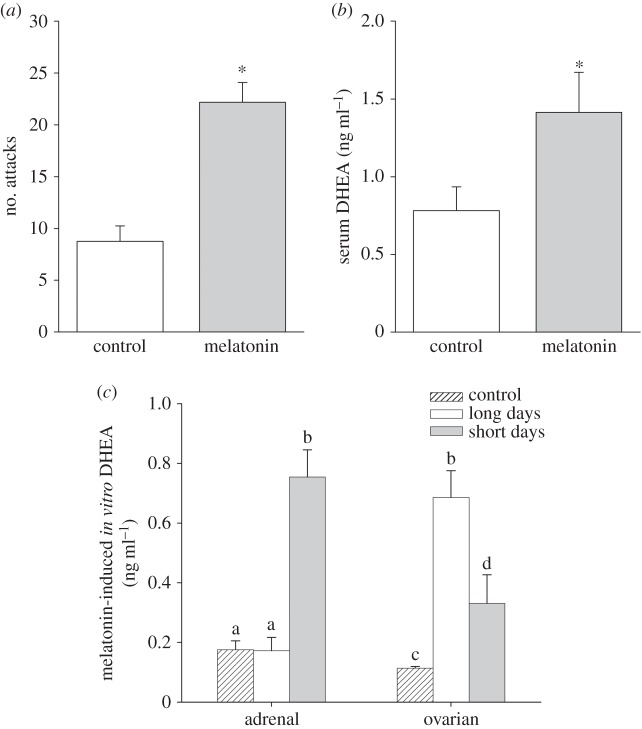
Body mass did not differ among groups (initial: t19 = 0.58; p = 0.57; post-treatment: t19 = −1.10; p = 0.92), and short-term melatonin treatment did not affect reproductive physiology (p > 0.05; electronic supplementary material). Melatonin-treated females had elevated aggression (attacks: t21 = −5.47; p < 0.01; figure 5a; aggression score: t20 = −5.10; p < 0.01; electronic supplementary material, figure S2), and elevated circulating levels of DHEA (i.e. short-day like) (t21 = −2.33, p < 0.01; figure 5b) compared with control females. See the electronic supplementary material for additional aggression measures.
Figure 5.
Melatonin-induced changes in aggression and DHEA. In vivo-induced (a) number of attacks, (b) serum DHEA concentrations; white bars, control-treated females (n = 12); grey bars, melatonin-treated females (n = 12). (c) In vitro DHEA response to melatonin (bars with no patterning, ovarian: long days, n = 9, short days, n = 9; adrenal: long days, n = 11, short days, n = 13) or controls (bars with stippling, ovarian: long days, n = 9, short days, n = 7; adrenal: long days, n = 11, short days, n = 11). Bar heights represent means ± s.e.m. Means with different letters are statistically significantly different (Tukey's HSD, p < 0.05).
(g). In vitro melatonin administration
Short-day females had regressed reproductive physiology (p > 0.05; see the electronic supplementary material, table S5) and elevated aggression (attacks: t53 = 11.40; p < 0.01; aggression score: t55 = 9.89; p < 0.01) when compared with long-day females (see the electronic supplementary material for additional aggression measures). Photoperiod and in vitro treatments increased DHEA release in cultured adrenal glands (photo × treatment: F3,84 = 4.14; p = 0.009) and ovarian tissue (photo × treatment: F1,30 = 11.20; p = 0.002; figure 5c). ACTH increased DHEA release from adrenal glands in both photoperiods when compared with control groups (treatment: F3,84 = 18.36; p < 0.0001). Melatonin alone increased DHEA release from adrenal glands in short-day, but not long-day females; the melatonin-induced DHEA release from adrenal glands was elevated when compared with the ACTH-induced DHEA release from adrenal glands (photo × treatment: F3,84 = 4.14; p = 0.009; figure 5c). For short-day females, the melatonin-induced increase in DHEA release was statistically indistinguishable from melatonin given in combination with ACTH. Melatonin alone also increased gonadal DHEA release from both photoperiods when compared to control groups (treatment: F1,30 = 74.93; p < 0.0001; figure 5c). In sum, short-day females displayed increased adrenal DHEA release, whereas long-day females displayed increased gonadal DHEA release.
4. Discussion
Here, we provide empirical support for the hypothesis of a seasonal switch from gonadal to adrenal regulation of aggression in female hamsters. We demonstrate for the first time that short-day females display elevated aggression concomitant with a DHEA-specific increase in adrenal responsiveness. Increased DHEA following an ACTH challenge was short-day specific; DHEA, but not CORT response predicted aggression. Commensurate with changes in physiology and behaviour, we showed a layer-specific morphological change in the adrenal gland, with substantially smaller zonae reticulara in short- compared with long-day females, but no differences in other adrenal layers. Further, adrenal DHEA but not CORT content was elevated in short-day females, consistent with photoperiodic changes in DHEA output. Lastly, we isolated the effects of photoperiod versus melatonin using in vivo and in vitro approaches. In vivo melatonin increased aggression as well as circulating DHEA independent of changes of photoperiod and reproductive physiology. Short-day adrenals treated with in vitro melatonin had increased DHEA but decreased gonadal DHEA release; long-day gonads displayed the opposite effect. These findings support the hypothesis of a seasonal switch from gonadal to adrenal regulation of steroid hormones and implicate a previously undescribed non-gonadal mechanism regulating seasonal aggression. Further, these findings demonstrate a key role of melatonin in regulating both peripheral androgens and aggression in females; this is the first finding of a direct action of melatonin on adrenal DHEA release. This peripheral mechanism provides an alternative to the previously described actions of gonadal steroids acting on the brain to regulate behaviour [17,18], and described actions of DHEA regulating aggression [7,16,33,41]. From a comparative perspective, this seasonal mechanism may also regulate aggression in other vertebrate species, including frogs, lizards, mammals and snakes, where melatonin also facilitates seasonal changes ([20–23]; reviewed in [7]).
A key finding in our study is that short-day females displayed high aggression along with significant DHEA-specific increases in adrenal responsiveness. By using an ACTH challenge, we examined the roles of DHEA and CORT in regulating aggression. We report significant increases in CORT in both long- and short-day females. DHEA, by contrast, was significantly elevated, but only in short-day females; long-day hamsters showed no appreciable increase in this hormone. The robust response of ACTH-induced CORT secretion across photoperiods shows that females were sensitive to ACTH at the dose used. Further, short-day females have increased adrenal responsiveness that is specific to DHEA, whereas there is a lack of a DHEA response in long-day animals. Unlike baseline levels, post-challenge levels of DHEA, but not CORT, were positively associated with short-day levels of aggression, suggesting that this short-day specific increase in adrenal responsiveness accounts for the observed increased aggression.
Another key finding of this study is the marked photoperiodic changes in adrenocortical zonation, specifically decreased zona reticularis, in short- compared with long-day females. There were no photoperiodic changes in either the zona glomerulosa (the site of adrenal mineralocorticoid synthesis) or zona fasciculata (glucocorticoid synthesis) within the adrenal cortex, or the adrenal medulla (catecholamine synthesis), indicating that the changes in adrenal morphology are specific to the zona reticularis, the predominant androgen-secreting layer of the adrenal (homologous in humans and non-human primates). Although we predicted increased zona reticularis area as a potential mechanism to explain increased DHEA output in short days, we found the opposite. Despite this seemingly counterintuitive finding, we showed that total adrenal DHEA content was increased in short compared with long days, suggesting that total area may not be an accurate measure of steroid metabolism and secretion (see also [42]). Decreased zonae reticulara, but increased total adrenal DHEA content, strongly suggests that cells in the short-day reticularis may be more efficient at synthesizing DHEA, likely reflecting changes in DHEA output. Future studies will address the molecular mechanisms of the adrenal gland, and characterize adrenal cell types (i.e. steroidogenic versus non-steroidogenic) to establish the precise role of these cells in steroid metabolism (reviewed in [35]; but see [43]).
We tested the role of melatonin independent of photoperiod-induced changes in reproductive physiology on aggression, and our data strongly support the idea that melatonin drives seasonal changes in DHEA synthesis and aggression. Melatonin administered to long-day hamsters (i.e. to mimic short days) increased aggression, consistent with previous findings in male hamsters [21]. Exogenous melatonin also increased circulating DHEA, suggesting a direct action of melatonin on adrenal androgens in female hamsters that has not been previously reported in males. In further support of this idea, female hamsters given daily melatonin injections to mimic short-day patterns of the hormone (i.e. ‘timed’) showed increased aggression, increased circulating levels of DHEA, and low levels of E2 compared with either control hamsters or hamsters injected with a melatonin profile that does not mimic short days (i.e. ‘mis-timed’; N. M. Rendon and G. E. Demas 2013, unpublished results). In male hamsters where DHEA was measured before and after aggressive interactions either during the day (i.e. low melatonin) or at night (i.e. high melatonin), aggression was significantly higher at night. Further, DHEA levels decreased, but T levels increased following aggression, but only in hamsters tested during the night [15]. Aggressive encounters occurring in the presence of melatonin (e.g. during night time or in the ‘timed’ melatonin group) likely cause rapid increases in converting enzymes (e.g. 3β-hydroxysteroid dehydrogenase (HSD), 17β-HSD, aromatase) similar to what has been shown in songbirds [33,41], effectively converting prohormones to biologically active androgens, or oestrogens [7]. These data suggest that circulating melatonin likely triggers changes in aggression via metabolism of circulating DHEA to androgens and oestrogens that, in turn, act on target tissues (e.g. brain) to promote aggressive behaviour [7]. In accordance with these findings, exogenous melatonin administered to human clinical populations is associated with increased aggression [44,45].
Consistent with in vivo studies, we show that in vitro melatonin acts directly on the adrenal glands to stimulate DHEA release from cultured adrenal glands. Based on previous work in males, we predicted that melatonin would increase DHEA output from adrenal glands combined with ACTH, suggesting a permissive effect of melatonin on DHEA secretion [8]. The finding that melatonin acts directly on the adrenal glands, independent of ACTH, to increase DHEA secretion was unexpected, but demonstrates for the first time that melatonin acts directly on the adrenal gland in female hamsters in a way not previously reported in males. These results suggest that melatonin, like ACTH, serves as a key tropic hormone regulating both adrenal and gonadal androgens in a season-specific manner in females. Collectively, these data strongly suggest that melatonin acts directly on the adrenal gland to regulate a seasonal switch between gonadal and adrenal DHEA production, and thus serves as an important mechanism mediating the actions of DHEA on aggression. Taken together, DHEA is a key player in a previously undescribed, peripheral mechanism underlying seasonal changes in aggression in hamsters and likely other vertebrate species.
Supplementary Material
Acknowledgements
We thank Dr. L. Michael Romero for the suggestion to examine adrenal histology, Dr. Bruce S. McEwen for feedback on an earlier draft, the Common Themes in Reproductive Diversity faculty and trainees for insightful conversations regarding this project. We also thank L. K. Achury, A. C. Amez, J. Bazan Villicaña, E. A. St. John, P. G. Flores, N. K. Khan, K. J. O'Malley, E. R. Weigel and L. R. Wright for assistance.
Ethics
All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Bloomington Institutional Animal Care and Use Committee at Indiana University.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.tk4b5.
Authors' contributions
N.M.R. and G.E.D. designed the experiments. N.M.R. performed photoperiodic and pharmacological treatments, staged behavioural interactions, collected vaginal cytology samples, performed necropsies, collected blood samples, determined reproductive phenotypes, analysed video files and ran hormone assays. L.M.R. performed procedures for adrenal gland histological analysis. Data were analysed by N.M.R. and D.R.S. All authors interpreted results and wrote the manuscript.
Competing interests
The authors declare they have no competing interests.
Funding
This work was supported by National Science Foundation Grant IOB-0543798 (to G.E.D.), National Science Foundation Doctoral Dissertation Improvement Grant IOS-1406063 (to N.M.R. and G.E.D), National Institutes of Health Training Grant T32HD049336; Common Themes in Reproductive Diversity (to N.M.R.), National Science Foundation Graduate Research Fellowship (to N.M.R.) and Indiana University.
References
- 1.Clutton-Brock T. 2009. Sexual selection in females. Anim. Behav. 77, 3–11. ( 10.1016/j.anbehav.2008.08.026) [DOI] [Google Scholar]
- 2.Stockley P, Bro-Jørgensen J. 2011. Female competition and its evolutionary consequences in mammals. Biol. Rev. 86, 341–366. ( 10.1111/j.1469-185X.2010.00149.x) [DOI] [PubMed] [Google Scholar]
- 3.Gill SA, Alfson ED, Hau M. 2007. Context matters: female aggression and testosterone in a year-round territorial neotropical songbird (Thryothorus leucotis). Proc. R. Soc. B 274, 2187–2194. ( 10.1098/rspb.2007.0457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lincoln G, Guinness F, Short R. 1972. The way in which testosterone controls the social and sexual behavior of the red deer stag (Cervus elaphus). Horm. Behav. 3, 375–396. ( 10.1016/0018-506X(72)90027-X) [DOI] [Google Scholar]
- 5.Moore MC. 1988. Testosterone control of territorial behavior: tonic-release implants fully restore seasonal and short-term aggressive responses in free-living castrated lizards. Gen. Comp. Endocrinol. 70, 450–459. ( 10.1016/0016-6480(88)90121-9) [DOI] [PubMed] [Google Scholar]
- 6.Wingfield JC, Ball GF, Dufty AM, Hegner RE, Ramenofsky M. 1987. Testosterone and aggression in birds. Amer. Sci. 75, 602–608. [Google Scholar]
- 7.Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE. 2015. DHEA effects on brain and behavior: insights from comparative studies of aggression. J. Steroid Biochem. Mol. Biol. 145, 261–272. ( 10.1016/j.jsbmb.2014.05.011) [DOI] [PubMed] [Google Scholar]
- 8.Demas GE, Soma KK, Albers HE. 2011. DHEA and aggression. In DHEA in human health and aging (ed. Watson RR.), pp. 415–431. London, UK: Taylor & Francis Group LLC. [Google Scholar]
- 9.Boonstra R, Lane JE, Boutin S, Bradley A, Desantis L, Newman AEM, Soma KK. 2008. Plasma DHEA levels in wild, territorial red squirrels: seasonal variation and effect of ACTH. Gen. Comp. Endocrinol. 158, 61–67. ( 10.1016/j.ygcen.2008.05.004) [DOI] [PubMed] [Google Scholar]
- 10.Crews D. 1984. Gamete production, sex hormone secretion, and mating behavior uncoupled. Horm. Behav. 18, 22–28. ( 10.1016/0018-506X(84)90047-3) [DOI] [PubMed] [Google Scholar]
- 11.Crews D, Moore MC. 1986. Evolution of mechanisms controlling mating behavior. Science 231, 121–125. ( 10.1126/science.3941893) [DOI] [PubMed] [Google Scholar]
- 12.Gutzler SJ, Karom M, Erwin WD, Albers HE. 2009. Photoperiodic regulation of adrenal hormone secretion and aggression in female Syrian hamsters. Horm. Behav. 56, 481–489. ( 10.1016/j.yhbeh.2009.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hau M, Stoddard ST, Soma KK. 2004. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm. Behav. 45, 40–49. ( 10.1016/j.yhbeh.2003.08.002) [DOI] [PubMed] [Google Scholar]
- 14.Moore MC, Whittier JM, Crews D. 1985. Sex steroid hormones during the ovarian cycle of an all-female, parthenogenetic lizard and their correlation with pseudosexual behavior. Gen. Comp. Endocrinol. 60, 144–153. ( 10.1016/0016-6480(85)90308-9) [DOI] [PubMed] [Google Scholar]
- 15.Scotti M-AL, Schmidt KL, Newman AEM, Bonu T, Soma KK, Demas GE. 2009. Aggressive encounters differentially affect serum dehydroepiandrosterone and testosterone concentrations in male Siberian hamsters (Phodopus sungorus). Horm. Behav. 56, 376–381. ( 10.1016/j.yhbeh.2009.07.004) [DOI] [PubMed] [Google Scholar]
- 16.Soma KK, Wingfield JC. 2001. Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen. Comp. Endocrinol. 123, 144–155. ( 10.1006/gcen.2001.7657) [DOI] [PubMed] [Google Scholar]
- 17.Jasnow AM, Huhman KL, Bartness TJ, Demas GE. 2000. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus). Horm. Behav. 38, 102–110. ( 10.1006/hbeh.2000.1604) [DOI] [PubMed] [Google Scholar]
- 18.Scotti M-AL, Place NJ, Demas GE. 2007. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus). Horm. Behav. 52, 183–190. ( 10.1016/j.yhbeh.2007.03.029) [DOI] [PubMed] [Google Scholar]
- 19.Hardeland R. 2009. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors 35, 183–192. ( 10.1002/biof.23) [DOI] [PubMed] [Google Scholar]
- 20.Howard CM, Lutterschmidt DI. 2015. The effects of melatonin on brain arginine vasotocin: relationship to sex and seasonal differences in MT1 in green treefrogs (Hyla cinerea). J. Neuroendocrinol. 27, 670–679. ( 10.1111/jne.12292) [DOI] [PubMed] [Google Scholar]
- 21.Jasnow AM, Huhman KL, Bartness TJ, Demas GE. 2002. Short days and exogenous melatonin increase aggression of male Syrian hamsters (Mesocricetus auratus). Horm. Behav. 42, 13–20. ( 10.1006/hbeh.2002.1797) [DOI] [PubMed] [Google Scholar]
- 22.Lutterschmidt DI, Mason RT. 2009. Endocrine mechanisms mediating temperature-induced reproductive behavior in red-sided garter snakes (Thamnophis sirtalis parietalis). J. Exp. Biol. 212, 3108–3118. ( 10.1242/jeb.033100) [DOI] [PubMed] [Google Scholar]
- 23.Underwood H. 1981. Effects of pinealectomy and melatonin on the photoperiodic gonadal response of the male lizard Anolis carolinensis. J. Exp. Zool. 217, 417–422. ( 10.1002/jez.1402170313) [DOI] [Google Scholar]
- 24.Wynne-Edwards KE. 2003. From dwarf hamster to daddy: the intersection of ecology, evolution, and physiology that produces paternal behavior. Adv. Study Behav. 32, 207–261. ( 10.1016/S0065-3454(03)01005-2) [DOI] [Google Scholar]
- 25.Van Weerden W, Bierings H, Van Steenbrugge G, De Jong F, Schröder F. 1992. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 50, 857–861. ( 10.1016/0024-3205(92)90204-3) [DOI] [PubMed] [Google Scholar]
- 26.Payne AH, Hales DB. 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25, 947–970. ( 10.1210/er.2003-0030) [DOI] [PubMed] [Google Scholar]
- 27.Gavrilova V, Ivanova S, Gusev S, Trofimova M, Bokhan N. 2012. Neurosteroids dehydroepiandrosterone and its sulfate in individuals with personality disorders convicted of serious violent crimes. Bull. Exp. Biol. Med. 154, 89–91. ( 10.1007/s10517-012-1882-6) [DOI] [PubMed] [Google Scholar]
- 28.Pajer K, Tabbah R, Gardner W, Rubin RT, Czambel RK, Wang Y. 2006. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology 31, 1245–1256. ( 10.1016/j.psyneuen.2006.09.005) [DOI] [PubMed] [Google Scholar]
- 29.Berenbaum SA, Resnick SM. 1997. Early androgen effects on aggression in children and adults with congenital adrenal hyperplasia. Psychoneuroendocrinology 22, 505–515. ( 10.1016/S0306-4530(97)00049-8) [DOI] [PubMed] [Google Scholar]
- 30.Mathews GA, Fane BA, Conway GS, Brook CG, Hines M. 2009. Personality and congenital adrenal hyperplasia: possible effects of prenatal androgen exposure. Horm. Behav. 55, 285–291. ( 10.1016/j.yhbeh.2008.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demas GE, Polacek KM, Durazzo A, Jasnow AM. 2004. Adrenal hormones mediate melatonin-induced increases in aggression in male Siberian hamsters (Phodopus sungorus). Horm. Behav. 46, 582–591. ( 10.1016/j.yhbeh.2004.07.001) [DOI] [PubMed] [Google Scholar]
- 32.Scotti M-AL, Rendon NM, Greives TJ, Romeo RD, Demas GE. 2015. Short-day aggression is independent of changes in cortisol or glucocorticoid receptors in male Siberian hamsters (Phodopus sungorus). J. Exp. Zool. A Ecol. Genet. Physiol. 323A, 331–342. ( 10.1002/jez.1922) [DOI] [PubMed] [Google Scholar]
- 33.Pradhan DS, Newman AEM, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. 2010. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm. Behav. 57, 381–389. ( 10.1016/j.yhbeh.2010.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlatt S, Niklowitz P, Hoffmann K, Nieschlag E. 1993. Influence of short photoperiods on reproductive organs and estrous cycles of normal and pinealectomized female Djungarian hamsters, Phodopus sungorus. Biol. Reprod. 49, 243–250. ( 10.1095/biolreprod49.2.243) [DOI] [PubMed] [Google Scholar]
- 35.Gallo-Payet N, Battista MC. 2014. Steroidogenesis—adrenal cell signal transduction. Compr. Physiol. 4, 889–964. ( 10.1002/cphy.c130050) [DOI] [PubMed] [Google Scholar]
- 36.Kirshner N. 2012. The adrenal medulla. In The structure and function of nervous tissue (ed. Bourne GH.), pp. 163–204. London, UK: Academic. [Google Scholar]
- 37.Di Curzio DL, Goldowitz D. 2011. The genetic basis of adrenal gland weight and structure in BXD recombinant inbred mice. Mamm. Genome 22, 209–234. ( 10.1007/s00335-011-9315-9) [DOI] [PubMed] [Google Scholar]
- 38.Maayan R, Touati-Werner D, Ram E, Galdor M, Weizman A. 2005. Is brain dehydroepiandrosterone synthesis modulated by free radicals in mice? Neurosci. Lett. 377, 130–135. ( 10.1016/j.neulet.2004.11.086) [DOI] [PubMed] [Google Scholar]
- 39.Stetson MH, Tay DE. 1983. Time course of sensitivity of golden hamsters to melatonin injections throughout the day. Biol. Reprod. 29, 432–438. ( 10.1095/biolreprod29.2.432) [DOI] [PubMed] [Google Scholar]
- 40.Haus E, Nicolau GY, Ghinea E, Dumitriu L, Petrescu E, Sackett-Lundeen L. 1996. Stimulation of the secretion of dehydroepiandrosterone by melatonin in mouse adrenals in vitro. Life Sci. 58, PL263–PL267. ( 10.1016/0024-3205(96)00079-3) [DOI] [PubMed] [Google Scholar]
- 41.Schlinger BA, Pradhan DS, Soma KK. 2008. 3β-HSD activates DHEA in the songbird brain. Neurochem. Int. 52, 611–620. ( 10.1016/j.neuint.2007.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benmouloud A, Amirat Z, Khammar F, Patchev AV, Exbrayat JM, Almeida OF. 2014. Androgen receptor-mediated regulation of adrenocortical activity in the sand rat, Psammomys obesus. J. Comp. Physiol. B 184, 1055–1063. ( 10.1007/s00360-014-0859-3) [DOI] [PubMed] [Google Scholar]
- 43.Freedman BD, Kempna PB, Carlone DL, Shah MS, Guagliardo NA, Barrett PQ, Gomez-Sanchez CE, Majzoub JA, Breault DT. 2013. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell 26, 666–673. ( 10.1016/j.devcel.2013.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill AP, Zuckerman KE, Hagen AD, Kriz DJ, Duvall SW, Van Santen J, Nigg J, Fair D, Fombonne E. 2014. Aggressive behavior problems in children with autism spectrum disorders: prevalence and correlates in a large clinical sample. Res. Autism Spectrum Disorders 8, 1121–1133. ( 10.1016/j.rasd.2014.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haffmans P, Sival RC, Lucius SA, Cats Q, van Gelder L. 2001. Bright light therapy and melatonin in motor restless behaviour in dementia: a placebo-controlled study. Int. J. Geriatric Psychiatry 16, 106–110. () [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.tk4b5.