Abstract
Social environments experienced at different developmental stages profoundly shape adult behavioural and neural phenotypes, and may have important interactive effects. We asked if social experience before and after weaning influenced adult social cognition in male prairie voles. Animals were raised either with or without fathers and then either housed singly or in sibling pairs. Males that were socially deprived before (fatherless) and after (singly housed) weaning did not demonstrate social recognition or dissociate spatial from social information. We also examined oxytocin and vasopressin receptors (OTR and V1aR) in areas of the forebrain associated with social behaviour and memory. Pre- and post-wean experience differentially altered receptor expression in several structures. Of note, OTR in the lateral septum—an area in which oxytocin inhibits social recognition—was greatest in animals that did not clearly demonstrate social recognition. The combination of absentee fathers on V1aR in the retrosplenial cortex and single housing on OTR in the septohippocampal nucleus produced a unique phenotype previously found to be associated with poor reproductive success in nature. We demonstrate that interactive effects of early life experiences throughout development have tremendous influence over brain–behaviour phenotype and can buffer potentially negative outcomes due to social deprivation.
Keywords: early life social environments, social recognition, Microtus ochrogaster, pair bond, monogamy, cognitive ecology
1. Introduction
The quality and composition of the postnatal social environment can profoundly affect brain organization, and this can have cascading effects that alter developmental trajectories into adulthood [1]. Indeed, most species face highly dynamic and variable early life social experiences, and the extent to which social environments can shape both the brain and behaviour has remained an area of potent interest. Despite the clear importance that early life experiences have on shaping phenotypes, most studies have focused on single developmental periods and have overlooked a crucial element: social experiences at different developmental periods might interact. Indeed ignoring that development is unidirectional in time and builds on previous experience also ignores that the adult phenotype is the outcome of complex phenotypic sculpting by many early life experiences. Examining the interactions of varied environments at distinct developmental stages is necessary if we are to meet the goal of providing a more thorough understanding of how adult behavioural and neural phenotypes emerge.
Pre-weaning stages of development in rodents are incredibly influential. Most work on the influences of early life social environments has focused on the impact of pre-weaning mother–infant interactions [2,3], largely because most mammals only engage in uni-parental care [4]. It is, however, also particularly important to understand how father–infant interactions influence offspring development in bi-parental species. Relatively few non-human studies have investigated the effects of paternal care and the importance of fathers at the nest [5]. Such studies have identified several socio-behavioural deficits associated with paternal absence. For example, the absence of fathers in the natal nest of bi-parental rodents adversely influences maturation, partner-preference formation, alloparental behaviour, aggression and social recognition in adulthood [6–12]. However, in order to fully understand the influence of early life experiences on development, we must consider the impact of social environments beyond the natal nest. Adolescence and early adulthood are also periods of life known to assert a profound influence over developmental trajectories. For example, comparisons of rodents living in isolated versus group housing conditions post-weaning suggest that isolated animals develop high anxiety-like phenotypes and stress-induced behavioural and neuroendocrine changes [13–15], indicating that the social environment continues to impact development.
Post-weaning social environments could potentially impact development in a manner that is distinct from pre-weaning social environments. Furthermore, because these important developmental stages are separated in time, they may interact to shape neural phenotype and social behaviour in complex ways. For example, environmental enrichment in later life modifies differences in neurodevelopment and anxiety-like behaviour that emerge from having high or low licking and grooming mothers [16]. Post-wean environmental enrichment also reverses the influence of impoverished mother–pup interactions on hippocampal physiology and spatial memory [17,18]. These studies underscore the need to consider how brain and behaviour can be affected by interactions between social environments in early and late development. Doing so will enable a deeper understanding of social development in ways that focusing on social experiences at single developmental periods does not capture.
Prairie voles (Microtus ochrogaster) provide an excellent opportunity to investigate how early social experiences at different life stages interact to shape complex brain–behaviour phenotypes. Prairie voles are most known for their tendencies to form long-term pairbonds [19,20] and their socially monogamous mating system is closely associated with bi-parental care. While most breeding units in nature consist of a heterosexual pair (referred to as ‘residents’) and their offspring, some reproductively active animals are single owing to a member of the pair defecting (‘divorce’), predation or what appears to be an active choice to forgo pairbonding [21–23]. Furthermore, ‘wandering’ individuals can be male or female [21–24]. These differences in mating tactics result in natural variation in the postnatal social environment; both parents will raise some offspring, and just mothers will raise others. Moreover, as pups mature into sub-adults, some continue to reside at the nest, while others leave the nest to join the reproductive population. Thus, sometime after weaning, some sub-adult animals will live in social groups, while others will live singly.
The neurobiology that modulates prairie vole pairbonds is also well understood. Much of this work has focused on the nonapeptides oxytocin (OT) and vasopressin (VP). For example, region-specific manipulations of OT, VP or their receptors (OTR and V1aR) in the lateral septum (LS, [25]), ventral pallidum (VPall, [26]), nucleus accumbens (NAcc, [27,28]) and prefrontal cortex (PFC, [29]) can enhance or eliminate the propensity to bond, which has led to their inclusion in a ‘pairbonding neural circuit’ [20]. Not surprisingly, most of these structures are deeply associated with the ‘social behaviour network’ [30,31], a set of nuclei that are frequently implicated in modulating social behaviours and in which nonapeptides assert a tremendous influence. What has been less appreciated recently, but has deep historical roots, is the influence of OT and VP in memory [32,33]. Although much of the original work was on avoidance and appetitive learning, more recently their roles in social recognition (in the LS) and spatial memory (in the septohippocampal nucleus (SHi), hippocampus (Hi) and the retrosplenial cortex (RSC)) have been topics of interest [34–37]. In addition to their aforementioned influences on social behaviour and memory, nonapeptide systems are open to the influences of early life social experience in numerous species, including prairie voles [6,38–40].
Here, we evaluate the interactive influences of pre-weaning (presence or absence of fathers) and post-weaning (group- versus single-housed) social environments on OT and VP receptor expression across brain regions of the pairbonding neural circuit, social behaviour network and socio-spatial memory structures in male prairie voles. Because social and spatial memory arguably form the foundation of social cognition (knowing who and where conspecifics are in space), we focus on performance in a modified social discrimination test that places social discrimination into a spatial context to ask how early life experiences from different stages of development affect socio-spatial memory in adults.
2. Material and methods
(a). Animals
All subjects came from the first litter of breeding pairs (n = 64) created specifically for this experiment. The breeders originated from our colony of voles, which were originally trapped in Champaign County, IL, USA. The animals were housed in standard polycarbonate rodent cages (29 × 18 × 13 cm) and kept on a 14 L : 10 D cycle, with lights on at 06.00 h. They were provided Rodent Chow 5000 (Harlan Teklad, Madison, WI, USA) and water ad libitum. After pairing, mating behaviour was monitored closely and used to estimate the expected parturition date.
(b). Early life manipulations
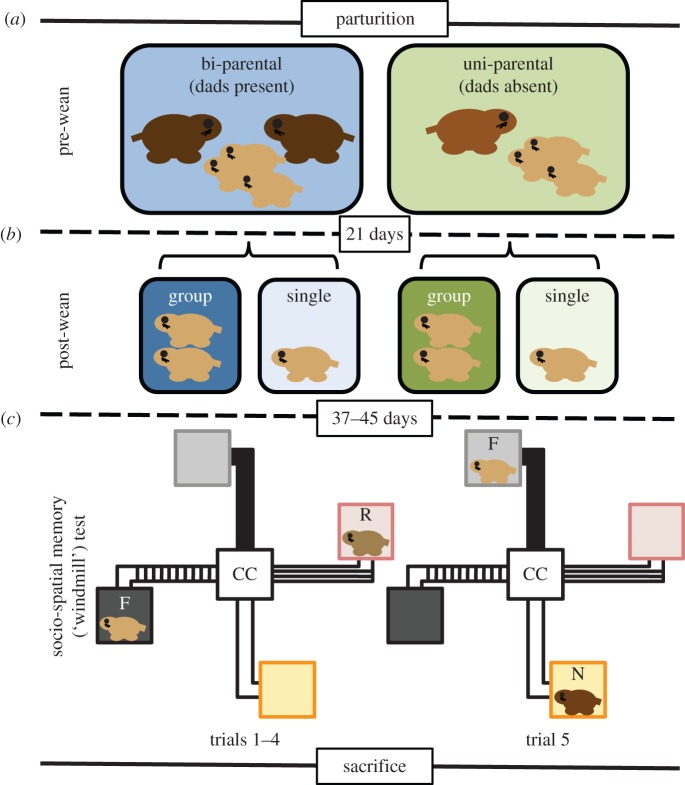
We created four rearing conditions (a 2 × 2 design) that contrasted pre- and post-wean social experience (figure 1a,b). Just before females were expected to give birth, we closely checked breeding pairs daily for newborn offspring. We recorded the number of offspring when a new litter was discovered. When pups were born, the fathers from half of the breeding pairs were removed from the cage (‘dad absent’); the other half of the fathers was left undisturbed (‘dad present’). The conditions were maintained until the pups were 21 days old, at which point the pups were sexed and weaned. At weaning, half of the pups from each group (dads absent or present) were assigned into post-wean groups. Animals serving in group-housed treatments were housed with a same-sex littermate; animals serving in single-housed treatments were housed alone. Post-wean housing conditions were maintained for the duration of the study. Behavioural testing began just after animals reached sub-adult age (approx. 35 days). Our behavioural experiments focused on males. Taken together we created four groups: dad present/group-housed (n = 15), dad present/single-housed (n = 14), dad absent/group-housed (n = 18) or dad absent/single-housed (n = 17).
Figure 1.
Experimental design and timeline of early life social manipulations and the socio-spatial memory test. (a) Between parturition and weaning (21 days) subjects were reared bi-parentally (blue) or uni-parentally (green). (b) Subjects were then housed with a same-sex sibling (group; dark shade) or alone (single; light shade). (c) Between 37 and 45 days, all subjects were tested in a socio-spatial memory test. The experimental set-up for the socio-spatial memory test trials 1–4 is depicted on the left; the set-up for the final test trial is depicted on the right. Hypothetical placement of the familiar stimulus animal used throughout the five trials (F), the familiar stimulus animal that was used in trials 1–4 and removed in trial 5 (R) and the novel stimulus animal presented only in trial 5 (N) is presented to illustrate the experimental procedure. Colour and shades of the stimulus chambers correspond to figures below (grey: location of F in trials 1–4 (dark) and trial 5 (light); red: location of R in trials 1–4; orange: location of N in trial 5). CC, center chamber, where the subject was placed at the beginning of each trial. Brains were extracted and processed after serving in the behavioural test.
(c). Socio-spatial memory test
Between 37 and 46 days old, subject males were tested in a socio-spatial memory test (figure 1c). This test functions as a modified social discrimination test [41] that incorporates social recognition in a spatial context. Traditional social discrimination tests present a subject with two unfamiliar conspecifics over a series of presentations to give the subject the opportunity to become familiar with the two stimulus animals (a familiarization phase). Social investigation is expected to decline as a subject becomes more familiar with the stimulus conspecifics. To test recognition, one of the two familiar stimulus animals is replaced by a third novel stimulus animal, and the time the subject spends investigating the novel animal compared with the familiar animal provides a measure of social discrimination. A subject is expected to spend more time investigating the novel animal, presumably because it is gathering information about the new identity.
In our test, two stimulus subjects were placed in the same respective arms of a four-arm apparatus (figure 1c, left; see below) during the familiarization phase. At the time of the test, one familiar stimulus animal was moved to a previously unoccupied arm, while the other was removed and an unfamiliar stimulus animal was placed in the other previously unoccupied arm (figure 1c, right). Thus, our test was designed to assess social recognition, but in a context where the spatial associations between identity and location were broken to contrast the reliance on spatial and social information. In any given trial, all stimulus and subject animals were unrelated.
(d). Apparatus
The acrylic apparatus resembled the arms of a windmill (figure 1c) and consisted of a central chamber (22.86 × 22.86 cm) that opened into four distinctly marked hallways (45.7 × 7.6 cm). Each hallway turned a corner at the distal end (7.6 × 7.6 cm), at which point a wire mesh barrier separated the hallway from an end chamber (22.86 × 22.86 cm) in which stimulus animals could be presented. Each arm was uniquely marked (black, white, horizontal stripes or vertical stripes) to enable local cue discrimination. The end chambers were placed around a corner so that subject animals were unable to see if they contained a stimulus animal without walking to the end of each hallway.
(e). Procedure
To begin a trial, subjects were placed in the central chamber for 30 min. During this time, opaque acrylic squares blocked access to the hallways. Two stimulus animals were placed on opposite ends of the apparatus from each other in their own end chamber. Their placement with respect to the arm markings was random. The acrylic squares were removed, and the subject was allowed to explore the apparatus freely for 5 min. Next, the subject was returned to the central chamber and the acrylic squares were replaced to block access to the hallways for 15 min. This procedure was repeated five times. Before the fifth presentation period began, one stimulus animal was rotated to the previously unoccupied arm to the right to serve as a familiar stimulus animal (F). The other stimulus animal was removed (R) and replaced with a third novel stimulus animal (N) that was placed in the remaining previously unoccupied presentation chamber (figure 1c).
We used EthoVisionXT (Noldus Information Technology, Leesburg, VA, USA) to measure the time spent in the main chamber and in each of the four arms for each trial. We subdivided each arm into the hallway and a ‘social interaction zone’ (SIZ; 15.2 × 7.6 cm). Each SIZ was approximately 1.5 body lengths. In trial 5, the difference between time spent in the SIZs of the arms containing stimulus animals indicated if subjects discriminated between familiar and unfamiliar conspecifics. The time spent in the SIZs of the vacant chambers (where stimulus animals were previously housed during trials 1–4) indicated a subject's ability to dissociate previously paired social and spatial information. In other words, it assessed the reliance on spatial cues and social cues.
(f). Oxytocin and vasopressin 1a receptors autoradiography
After behavioural trials were complete, subjects were sacrificed and we immediately extracted brains, froze them on powdered dry ice and stored them at −80°C. Later, we coronally cryosectioned brains at 20 µm and mounted sections at 100 µm intervals on Superfrost Plus slides (Fisher Scientific Co., Pittsburgh, PA, USA). Each of four sets was then stored at −80°C until they were used to visualize receptor density using autoradiography as previously described [42]. We used 125I-labelled radioligands to visualize OTR (ornithine vasotocin analogue ([125I]-OVTA); NEX254, PerkinElmer; Waltham, MA, USA) and V1aR (vasopressin (Linear), V-1A antagonist (Phenylacetly1, O-Me-d-Tyr2, [125I-Arg6]-); NEX310, PerkinElmer). We exposed radiolabelled tissue to film (GE Healthcare, Little Chalfont, UK) for 4 days. The relative density of ligand binding was assessed by inferring that receptor density relates to the optical density of exposed film and, in this way, optical density measurements serve as a proxy for receptor density. We used 125I-labelled radiographic standards (American Radiolabeled Chemicals, St Louis, MO, USA) to allow for conversion of optical density to receptor density. We digitized films on a Microtek ArtixScan M1 (Microtek, Santa Fe Springs, CA, USA) and measured optical densities using Image-J (NIH, Bethesda, MD, USA). We calculated receptor density by first converting optical density to disintegrations per minute (dpm), adjusted for tissue equivalence (TE; for 1 mg in the rat brain), by using a log function to fit curves generated by radiographic standards. We measured optical density across brain regions within the pairbonding circuit, social behaviour network and memory circuits (OTR:PFC, NAcc, septo-hippocampal nucleus (SHi), LS,Hi, central amygdala (CeA), basolateral amygdala (BLA); V1aR:VPall, LS, medial bed nucleus of the stria terminalis (BSTm), anterior hypothalamus (AH), RSC), CeA medial amygdala (MeA) and ventromedial hypothalamus (VMH). The optic density for each brain region was averaged over three measurements (once in a series of three brain sections, bilaterally). We also measured optical density of portions of film adjacent to the brain slices analysed. The average values for each structure were converted to dpm mg−1 TE, and adjusted for film effects by subtracting film optical densities from binding measurements. Some tissue sections were damaged during processing. As a result, sample sizes slightly varied by structure and receptor type (see the electronic supplementary material, table S1).
3. Results
(a). Early life social environments, social discrimination and socio-spatial memory
To begin, we first asked if early life social experience affected social discrimination. Social discrimination scores were calculated by subtracting the time spent in the SIZ of the familiar stimulus animal from the time spent in the SIZ of the novel stimulus animal in trial 5. To assess the degree of social discrimination, we compared the mean social discrimination scores to zero using one-sample t-tests (figure 2a). For all statistical tests we used α = 0.05. Social discrimination scores for dad present/single-housed and dad absent/group-housed males were significantly greater than zero (t17 = 3.367, p = 0.004; t13 = 3.129, p = 0.008, respectively), demonstrating social discrimination. Dad present/group-housed males did not significantly differ from zero, but they did show a non-significant tendency towards social discrimination (t14 = 1.64, p = 0.12; figure 2a). Males in the dad absent/single-housed group clearly did not differ from zero (t16 = 0.4729, p = 0.64), indicating potential deficits in social discrimination.
Figure 2.
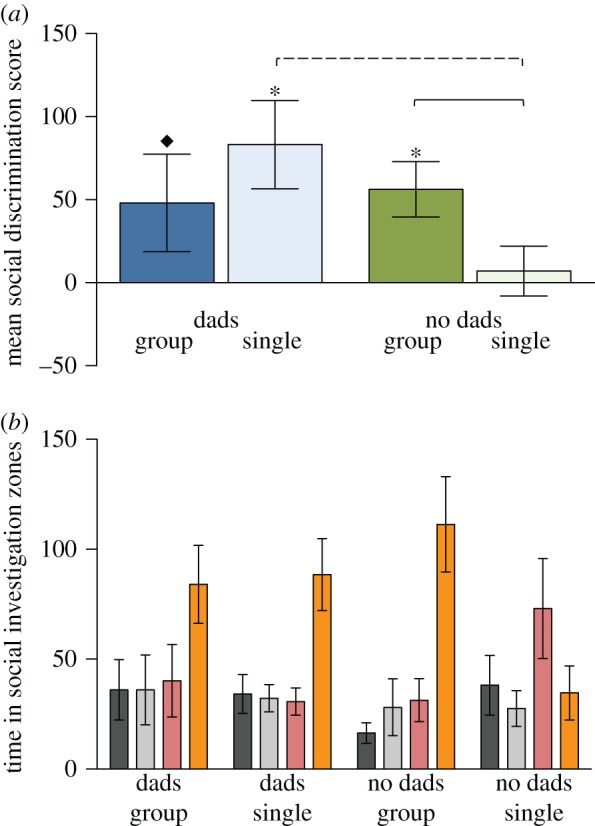
(a) Mean (±s.e.) male social discrimination scores were calculated by taking the average of the differences in time subjects were located in the social interaction zone (SIZ) of the novel and familiar stimulus animals in trial 5 for each treatment group. Males that were reared with their fathers present are coloured blue; males raised without fathers are coloured green. Dark shades indicate that males were group housed post-wean; light shades indicate that males were housed alone. Asterisks indicate the treatment group was significantly greater than zero; the diamond indicates a non-significant trend. Solid (p ≤ 0.05) and dashed (p ≤ 0.01) lines indicate significant post hoc differences between groups. (b) Mean (±s.e.) time (in seconds) males spent in the SIZ of each stimulus chamber in trial 5 for males from each rearing treatment. Colours for bars correspond to figure 1c: (grey location of the familiar stimulus animal (F) in trials 1–4 (dark) and trial 5 (light); red: location of the familiar stimulus animal that was removed for trial 5 (R); orange: location of the novel stimulus animal in trial 5 (N)).
When the social discrimination scores across the four treatment groups were analysed with a two-way ANOVA, there was no significant main effect of pre-weaning (F1,60 = 2.41, p = 0.13) or post-weaning (F1,60 = 0.10, p = 0.75), but the data did show a significant interaction between the two treatments (F1,60 = 3.71, p = 0.05; figure 2a), where dad absent/single-housed males had significantly lower social discrimination scores than males raised with dads and housed alone (post hoc Student's t-test; t29 = 2.609, p = 0.01) or without dads and group housed (t33 = 2.183, p = 0.04). However, males raised without dads and housed alone did not significantly differ from males raised with dads and housed in groups (t30 = 1.288, p = 0.21). Taken together, the significant interaction supports the hypothesis that the combination of pre-and post-wean socially depleted environments disrupt or delay social discrimination.
We compared the amount of time that subjects from each group spent in the SIZs for each arm to determine if they used social cues (i.e. location in trial 5) or spatial cues (i.e. location in previous trials) to guide investigation behaviour. We expected that subjects would spend the majority of their time in the SIZ nearest the novel stimulus animals, as this would presumably be the most salient socio-spatial cue available. Our results showed a main effect for subjects, who spent the majority of their time in the SIZ near the novel stimulus animals (F3,180 = 8.80, p < 0.0001; figure 2b). Although we found no main effect for time spent in the four SIZs across treatment group (F3,180 = 0.43, p = 0.73), we did find a significant interaction across treatments and SIZs (F9,180 = 2.17, p = 0.03; figure 2b). The interaction effect shows that the subjects experiencing the depleted pre- and post-wean social environments showed a significantly different pattern of investigation from all other subjects. Specifically, dad absent/single-housed subjects spent the majority of their time in the SIZ where the replaced stimulus animal was located during trials 1–4, whereas all other subjects spent the majority of their time in the SIZ near the novel stimulus animal (figure 2b).
To confirm that this result was not a function of social avoidance, we compared the time dad absent/single-housed subjects spent in the empty arms and the arms containing a conspecific for each presentation trial (trials 1–5). In trial 5 (after stimulus animals had been rotated in space) dad absent/single-housed males spent more time in the arms housing no conspecifics (F3,60 = 3.44, p = 0.02; electronic supplementary material, figure S1). By contrast, and like all other groups of males, during trials 1–4, these males spent more time in arms containing conspecifics (all Fs3,60 ≤ 1.48, ps ≥ 0.23; electronic supplementary material, figure S1). These data demonstrate that dad absent/singly-housed animals did not avoid social contact in trial 5, but rather that they were using a different search pattern to explore the apparatus after the social and spatial associations had been experimentally disrupted.
(b). Early life social environments and nonapeptide receptor densities
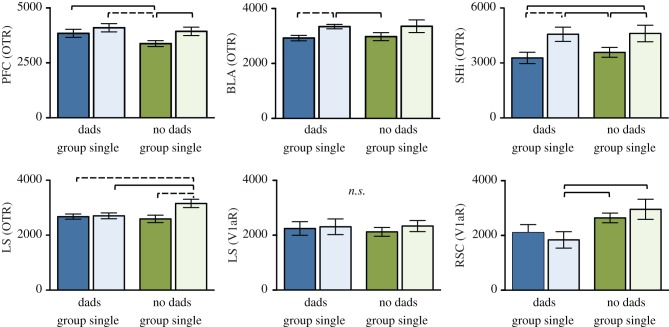
Early life social experience did not affect OTR or V1aR expression in many areas of the pairbonding neural circuit, the social behaviour network or the socio-spatial memory structures that we examined (electronic supplementary material, table S1). However, pre- and post-wean social experience did differentially affect nonapeptide receptor expression in some structures in three interesting ways. First, we found a main effect of pre-wean experience on V1aR density in the RSC (ANOVA: F1,56 = 8.335, p = 0.006; figure 3) and the MeA (F1,56 = 3.936, p = 0.052; electronic supplementary material, table S1), with pups without fathers expressing greater V1aR than pups reared with both parents. The effect in the MeA was relatively subtle (electronic supplementary material, figure S2). Second, we found that post-wean social experience influenced OTR density in the PFC (F1,55 = 5.430, p = 0.02), BLA (F1,54 = 6.763, p = 0.01) and SHi (F1,55 = 10.40, p = 0.002). In all three cases, socially isolated males had higher OTR expression than group-housed males (figure 3). Finally, like the PFC, BLA and SHi, the LS showed a main effect for more OTR among the socially isolated males (F1,56 = 5.575, p = 0.02). However, OTR in the LS also showed an interaction between pre- and post-wean social environments (F1,56 = 4.596, p = 0.04; figure 3), in which dad absent/single-housed males expressed greater OTR density than males from all other groups. In other words, the same males that demonstrated deficits in social discrimination also expressed more OTR in the LS. By contrast, we found no main effect for pre-wean experience on LS OTR (F1,56 = 2.177, p = 0.15) and no significant effects of early life social experience on V1aR in the LS (figure 3; electronic supplementary material, table S1).
Figure 3.
Mean (±s.e.) oxytocin receptor (OTR) and vasopressin 1a receptor (V1aR) density (dpm mg−1 TE) in the prefrontal cortex (PFC), basolateral amygdala (BLA), septo-hipocampal nucleus (SHi), lateral septum (LS) and retrosplenial cortex (RSC). Colours and shades for treatment groups correspond with figure 2a. Post hoc differences are indicated with solid (p ≤ 0.05) and dashed (p ≤ 0.01) lines.
4. Discussion
Our results show that early- and late-stages of postnatal development in voles interact to impact social discrimination, socio-spatial memory and the nonapeptide mechanisms that are closely linked with these behaviours. We found that reduced social exposure (i.e. males raised without fathers and later housed alone) interfered with male prairie vole social discrimination and how they approach socio-spatial challenges. We also found that reduced social exposure at different periods of early life can differentially affect nonapeptide expression patterns. While V1aR in the RSC and MeA are altered by the presence or absence of fathers, several oxytocinergic structures (PFC, BLA, LS and SHi) are influenced by post-wean social conditions. Strikingly, OTR, but not V1aR expression in the LS—a structure in which both OT and VP are known to influence social recognition—differed among the same groups of animals that showed deficits in social recognition. Taken together, these results demonstrate that social environments experienced during pre-weaning and post-weaning influence neural development in a region-specific manner, and can interactively shape neural and behavioural phenotype.
(a). Paternal influences on social cognition and development
Early life social environments can profoundly influence the phenotypic development of an individual. Pioneering work by classic and recent studies has shown that disruption of mother–infant interactions has consequences for the physiology and social behaviour of adult offspring [43–46]. Despite the significant advances that studies such as these have made, the majority of research performed has focused on maternal care and has overlooked the contribution of fathers on offspring development. In species with bi-parental care, both parents invest heavily in rearing offspring. Bi-parental care is closely related to adopting a monogamous mating system, something found in only 5% of mammalian species [4]. Because the occurrence of mammalian paternal care is relatively rare, it is particularly difficult to assess the importance of paternal behaviour among mammals, with most work having been conducted in humans. The potential impact the presence of caring fathers has on developing offspring is of significant importance as, among humans, for example, it may determine a child's appropriate emotional and cognitive development [47], health [48], and likelihood to engage in violent activity and crime [49]. On the other hand, a positive paternal influence on developing children increases cognitive competence (e.g. [50]) and adult psychological adjustment (e.g. [51]). Non-human work on paternal care is beginning to gain appreciation and it is clear that the role of the father in bi-parental species is significant [5]. For instance, paternal absence in prairie voles retards development, and can interfere with pairbonding [6,11,12]. Our results add to this story indicating that the lack of fathers in the postnatal environment also interferes with socio-cognitive behaviours.
(b). Protective influences of post-wean environments on social cognition
Our results demonstrated that male prairie voles which were raised without fathers showed no evidence for social discrimination, but only if they later lived in social isolation. These results are unique in that they highlight a particularly compelling role for social housing in later life, which may act as a social buffer to protect animals from demonstrating socio-cognitive deficits that might result from reduced paternal care. Although the combination of environmental and social enrichment is known to reverse the influence of impoverished mother–pup interactions [16–18], these studies were unable to directly attribute their effects to either social or physical enrichment. To our knowledge our study is the first demonstration of a cognitive deficit that is potentially both caused and rescued by social context alone.
Furthermore, the roles of OT and VP acting in the LS on social recognition are well established [32,34,52,53]. Septal VP facilitates social recognition, while endogenous levels of OT inhibit social recognition. Relatively elevated levels of OTR in the LS should therefore increase animals' sensitivity to the inhibitory effects of OT on social recognition. The fact that males raised without fathers and later housed alone demonstrated both deficits in social discrimination and greater OTR density suggests that continued social deprivation throughout early life affects septal OTR phenotype, which in turn affects social recognition. A similar pattern was not evident in septal V1aR, which was surprising.
(c). Did males with the greatest social experience also show recognition deficits?
Based on our data, it was unclear if males raised with fathers and then group housed (arguably males with the most social exposure) showed evidence for social discrimination or not. Although this is a peculiar result, we note that this group did show a non-significant trend towards showing social discrimination, and group performance was more similar to the groups that clearly showed recognition than the one group that clearly did not (figure 2a). This result might better represent an underpowered sample rather than a lack of evidence for social recognition. We have repeatedly demonstrated that animals raised with fathers and then group housed show social recognition [54]. Moreover, we showed testing animals in contexts that lack pre-existing social cues (i.e. clean testing apparatus) produces high variance in social recognition. Similarly, dad present/group-housed males showed the greatest variation of all four treatment groups in the current study, a result primarily driven by three animals. It is possible that these animals were particularly affected by the lack of social cues in the testing apparatus. Removal of any one of these three animals from the analyses would have produced a significant result. Taken together, we feel that this result is most probably a sampling error and not evidence for lack of recognition.
(d). Early life social experience influences how environmental cues are used
Differences resulting from social deprivation spanning postnatal and sub-adult periods of development extended beyond social discrimination. Indeed these animals also demonstrated distinct ways of using environmental information. Whereas most animals appeared to focus on social cues to guide behaviour in the socio-spatial memory test, animals experiencing reduced social exposure appeared to rely more heavily on spatial cues. It is hard to interpret why these animals appeared to preferentially visit the arm where a familiar animal that can no longer be located used to be, however, it is clear it was not a social aversion (electronic supplementary material, figure S1). Furthermore, all animals were able to locate novel and familiar conspecifics in the experiment during the test period; animals in all groups spent some proportion of time in every arm (SIZ) of the maze. One interpretation of these results is that animals experiencing reduced social exposure are less able to resolve broken associations between spatial location and social identity. This notion is consistent with the performance of these animals, in which they spent similar, relatively low, amounts of time investigating arms containing stimulus animals (either familiar or unfamiliar) in the test trial, but returned throughout this period to the arm that formerly contained an animal that was nowhere to be found. Another possibility is that the social search strategies of male prairie voles are influenced by postnatal social experiences, producing differential motivation to either engage with novel animals or account for all conspecifics (particularly ones that have gone missing). If this is true, then the relatively poor performance in social discrimination that these animals showed might be explained by an altered social search strategy rather than actual deficiency in social discrimination. However, we think this is unlikely considering that the deficits in social recognition could be accounted for by a well-supported proximate explanation: the tight link between septal OT/OTR action and its role in disrupting social discrimination combined with the powerful observation that the same group of animals which showed recognition deficits also showed elevated OTR expression (see section above). Nevertheless, deficient social recognition could very well contribute to an altered search strategy. A third possibility is that postnatal social experiences alter the weighting that male prairie voles give to spatial or social cues. In other words, perhaps animals experiencing reduced social exposure are more attentive to spatial cues over social cues, thereby demonstrating an over-reliance on spatial cues. Further studies are clearly necessary to explain the nature of this difference in cognitive behaviour.
(e). Nonapeptide receptor sensitivity to developmental social environments
Results from our autoradiographic analyses showed several intriguing outcomes. First, we found that nonapeptide receptor expression is relatively robust throughout postnatal development. The presence or absence of fathers only affected receptor expression in a handful of structures. The expression patterns of V1aR in the RSC and MeA were higher in males raised without fathers. On the other hand, social isolation after weaning was associated with greater expression of OTR in the PFC (an area of particular importance for pairbonding), the BLA (a central part of the social behaviour network involved in emotional processing and valuation), the LS (an area already noted for its importance in pairbonding and social recognition, and that is part of the social behaviour network) and the SHi (an area that relays between the Hi and LS and is known to be important in socio-spatial memory). This general influence of neonatal and juvenile social experience in V1aR and OTR, respectively, suggests that nonapeptide receptor phenotype is sensitive to the social environment across several inter-related neural circuits and systems.
(f). Can early life social experience shape reproductive success among mating tactics?
Prairie voles are socially monogamous, and have received much notoriety for their usefulness in understanding the mammalian neurobiology that underlies social attachment. An occasionally overlooked aspect of their natural history is that while a majority of males and females form bonds and establish socially monogamous breeding units (‘residents'), an important minority of prairie voles remains single and traverses large undefended home ranges (‘wanderers') [21–23,55]. Although the V1aR and OTR profiles of residents and wanderers do not differ among structures involved in the ‘pairbonding neural circuit’, structures important for socio-spatial memory robustly predict whether residents or wanderers sire offspring [35,36]. Specifically, these studies revealed an interaction between mating tactic (resident/wanderer) and reproductive success (sired offspring or did not), with reproductively unsuccessful wanderers having significantly more nonapeptide receptors than successful wanderers in key socio-spatial memory neural structures, in particular, RSC and SHi [35,36]. These and other results suggest socio-spatial memory is important in shaping mating tactics [24,35–37,54].
In this study, differences in RSC V1aR were attributed to the presence or absence of fathers and differences in SHi OTR were shaped by the post-wean social environment. Interestingly, the combination of these two effects produced a particularly striking outcome for the individuals experiencing reduced social exposure throughout development. These males had both high V1aR in the RSC and high OTR in the SHi, which recapitulates a major component of the unique phenotype exhibited by the wanderers that did not sire offspring in the field [35,36]. Although many other mechanisms are sure to be involved, this result raises the provocative possibility that reduced social interaction during both pre- and post-wean development might contribute to a neural phenotype that disadvantages wanderers under natural conditions. This idea is clearly speculative and merits further testing.
5. Conclusion
We have shown that socio-cognitive development and neural phenotype are susceptible to the influences of early life social environments. Some studies have demonstrated that pre-weaning environments have the potential to shape adult pro-social behaviour, thereby facilitating the establishment of relationships (e.g. attachment and affiliation; [6]). Meanwhile, the post-weaning environments may influence stress reactivity and other aspects of behaviour that may modulate anti-social behaviours [13,14]. Such experiences have been suggested as means for informing and preparing individuals to survive when environments that they are likely to experience as adults are dynamic, variable or relatively unpredictable [16]. The relative survival value that socio-spatial cognition may have extends the potential importance of early life social experiences on relevant neural and behavioural mechanisms. We have long appreciated that quality and quantity of parental care has long-term implications for adult behaviour (e.g. [45,46]). Our results indicate that subsequent social experiences can serve to protect and even potentially rescue aspects of social cognition in individuals that experience social adversity early in their lives. In addition to promoting survival and social relationships, the combination of childhood and adolescent social experiences may predispose individuals to successfully navigate the reproductive challenges that await them in adulthood.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Aubrey Kelly and two anonymous reviewers for their helpful comments on the manuscript.
Ethics
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Oklahoma State University.
Data accessibility
All data are available online on Dryad: http://dx.doi.org/10.5061/dryad.43pv1.
Authors' contributions
G.S.P. performed the brain analyses, analysed data and wrote the manuscript. L.F. and A.R. developed the experimental design, performed the developmental and behavioural procedures, and contributed to data analysis and writing. A.G.O. conceptualized the experiment, developed the experimental design, analysed data and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to A.G.O. (HD065604 and HD079573), the Niblack Research Fellowship at Oklahoma State University to L.F and research support to A.G.O from the Cornell University College of Arts and Sciences.
References
- 1.Kundakovic M, Champagne FA. 2015. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology 40, 141–153. ( 10.1038/npp.2014.140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champagne FA. 2013. Early environments, glucocorticoid receptors, and behavioral epigenetics. Behav. Neurosci. 127, 628–636. ( 10.1037/a0034186) [DOI] [PubMed] [Google Scholar]
- 3.Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. 2011. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology 36, 843–853. ( 10.1016/j.psyneuen.2010.11.007) [DOI] [PubMed] [Google Scholar]
- 4.Kleiman DG. 1977. Monogamy in mammals. Q. Rev. Biol. 52, 39–69. ( 10.1086/409721) [DOI] [PubMed] [Google Scholar]
- 5.Braun K, Champagne FA. 2014. Paternal influences on offspring development: behavioural and epigenetic pathways. J. Neuroendocrinol. 26, 697–706. ( 10.1111/jne.12174) [DOI] [PubMed] [Google Scholar]
- 6.Ahern TA, Young LJ. 2009. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster). Front. Behav. Neurosci. 3, 1–19. ( 10.3389/neuro.08.017.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bester-Meredith JK, Marler CA. 2007. Social experience during development and female offspring aggression in Peromyscus mice. Ethology 113, 889–900. ( 10.1111/j.1439-0310.2007.01393.x) [DOI] [Google Scholar]
- 8.Cao Y, Wu R, Tai F, Zhang X, Yu P, An X, Qiao X, Hao P. 2014. Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen receptor α mRNA expression in the MeA and NAcc, and serum oxytocin in mandarin voles. Horm. Behav. 65, 57–65. ( 10.1016/j.yhbeh.2013.11.005) [DOI] [PubMed] [Google Scholar]
- 9.Frazier CRM, Trainor BC, Cravens CJ, Whitney TK, Marler CA. 2006. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm. Behav. 50, 699–707. ( 10.1016/j.yhbeh.2006.06.035) [DOI] [PubMed] [Google Scholar]
- 10.Schradin C, Pillay N. 2005. The influence of the father on offspring development in the striped mouse. Behav. Ecol. 16, 450–455. ( 10.1093/beheco/ari015) [DOI] [Google Scholar]
- 11.Wang Z, Novak MA. 1994. Alloparental care and the influence of father presence on juvenile prairie voles, Microtus ochrogaster. Anim. Behav. 47, 281–288. ( 10.1006/anbe.1994.1040) [DOI] [Google Scholar]
- 12.Wang ZX, Novak MA. 1992. Influence of the social environment on parental behavior and pup development of meadow voles (Microtus pennsylvanicus) and prairie voles (Microtus ochrogaster). J. Comp. Psychol. 106, 163–171. ( 10.1037/0735-7036.106.2.163) [DOI] [Google Scholar]
- 13.Lukkes JL, Watt MJ, Lowry CA, Forster GL. 2009. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front. Behav. Neurosci. 3, 18 ( 10.3389/neuro.08.018.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. 2007. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32, 966–980. ( 10.1016/j.psyneuen.2007.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. 2009. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci. Lett. 454, 67–71. ( 10.1016/j.neulet.2009.02.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champagne FA, Meaney MJ. 2007. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav. Neurosci. 121, 1353–1363. ( 10.1037/0735-7044.121.6.1353) [DOI] [PubMed] [Google Scholar]
- 17.Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. 2003. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience 118, 571–576. ( 10.1016/S0306-4522(02)00918-1) [DOI] [PubMed] [Google Scholar]
- 18.Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ. 2004. Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur. J. Neurosci. 20, 1355–1362. ( 10.1111/j.1460-9568.2004.03599.x) [DOI] [PubMed] [Google Scholar]
- 19.Carter CS. 1998. Neuroendocrine perspectives on social attachment and love. Pyschoneuroendocrinology 23, 779–818. ( 10.1016/S0306-4530(98)00055-9) [DOI] [PubMed] [Google Scholar]
- 20.Young LJ, Wang ZX. 2004. The neurobiology of pair bonding. Nat. Neurosci. 7, 1048–1054. ( 10.1038/nn1327) [DOI] [PubMed] [Google Scholar]
- 21.Getz LL, McGuire B, Pizzuto T, Hofmann J, Frase B. 1993. Social organization of the prairie vole (Microtus ochrogaster). J. Mammal. 74, 44–58. ( 10.2307/1381904) [DOI] [Google Scholar]
- 22.McGuire B, Getz LL, Bemis WE, Oli MK. 2013. Social dynamics and dispersal in free-living prairie voles (Microtus ochrogaster). J. Mammal. 94, 40–49. ( 10.1644/11-MAMM-A-387.1) [DOI] [Google Scholar]
- 23.Ophir AG, Phelps SM, Sorin AB, Wolff JO. 2008. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim. Behav. 75, 1143–1154. ( 10.1016/j.anbehav.2007.09.022) [DOI] [Google Scholar]
- 24.Zheng D-J, Larsson B, Phelps SM, Ophir AG. 2013. Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behav. Brain Res. 246, 139–147. ( 10.1016/j.bbr.2013.02.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Curtis JT, Wang ZX. 2001. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav. Neurosci. 115, 910–919. ( 10.1037/0735-7044.115.4.910) [DOI] [PubMed] [Google Scholar]
- 26.Lim MM, Young LJ. 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45. ( 10.1016/j.neuroscience.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 27.Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. 2003. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 23, 3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. 2009. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 29, 1312–1328. ( 10.1523/JNEUROSCI.5039-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young LJ, Lim MM, Gingrich B, Insel TR. 2001. Cellular mechanisms of social attachment. Horm. Behav. 40, 133–138. ( 10.1006/hbeh.2001.1691) [DOI] [PubMed] [Google Scholar]
- 30.Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22. ( 10.1016/j.yhbeh.2005.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman SW. 1999. The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann. NY Acad. Sci. 877, 242–257. ( 10.1111/j.1749-6632.1999.tb09271.x) [DOI] [PubMed] [Google Scholar]
- 32.Bohus B, Kovacs GL, Dewied D. 1978. Oxytocin, vasopressin and memory: opposite effects on consolidation and retrieval processes. Brain Res. 157, 414–417. ( 10.1016/0006-8993(78)90052-5) [DOI] [PubMed] [Google Scholar]
- 33.McEwen B. 2004. The roles of vasopressin and oxytocin in memory processing, 640 San Diego, CA: Elsevier Academic Press. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson JN, Young LJ, Insel TR. 2002. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 23, 200–224. ( 10.1006/frne.2002.0229) [DOI] [PubMed] [Google Scholar]
- 35.Ophir AG, Gessel A, Zheng D-J, Phelps SM. 2012. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm. Behav. 61, 445–453. ( 10.1016/j.yhbeh.2012.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ophir AG, Wolff JO, Phelps SM. 2008. Variation in neural V1aR predicts sexual fidelity and space use among prairie voles in semi-natural settings. Proc. Natl Acad. Sci. USA 105, 1249–1254. ( 10.1073/pnas.0709116105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ophir AG, Zheng D-J, Eans S, Phelps SM. 2009. Social investigation in a memory task relates to neural variation in oxytocin receptor but not vasopressin receptor 1a. Behav. Neurosci. 123, 979–991. ( 10.1037/a0016663) [DOI] [PubMed] [Google Scholar]
- 38.Bales KL, Perkeybile AM. 2012. Developmental experiences and the oxytocin receptor system. Hom. Behav. 61, 313–319. ( 10.1016/j.yhbeh.2011.12.013) [DOI] [PubMed] [Google Scholar]
- 39.Bales KL, Boone E, Epperson P, Hoffman G, Carter CS. 2011. Are behavioral effects of early experience mediated by oxytocin? Front. Psychiatry 2, 1–12. ( 10.3389/fpsyt.2011.00024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter CS. 2003. Developmental consequences of oxytocin. Physiol. Behav. 79, 383–397. ( 10.1016/S0031-9384(03)00151-3) [DOI] [PubMed] [Google Scholar]
- 41.Engelmann M, Wotjak CT, Landgraf R. 1995. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol. Behav. 58, 315–321. ( 10.1016/0031-9384(95)00053-L) [DOI] [PubMed] [Google Scholar]
- 42.Ophir AG, Sorochman G, Evans BL, Prounis GS. 2013. Stability and dynamics of forebrain vasopressin receptor and oxytocin receptor during pregnancy in prairie voles. J. Neuroendocrinol. 25, 719–728. ( 10.1111/jne.12049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bale TL, et al. 2010. Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319. ( 10.1016/j.biopsych.2010.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Champagne FA, Curley JP. 2009. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci. Biobehav. Rev. 33, 593–600. ( 10.1016/j.neubiorev.2007.10.009) [DOI] [PubMed] [Google Scholar]
- 45.Harlow HF. 1958. The nature of love. Am. Psychol. 13, 537–685. ( 10.1037/h0047884) [DOI] [PubMed] [Google Scholar]
- 46.Levine S. 1957. Infantile experience and resistance to physiological stress. Science 126, 405–406. ( 10.1126/science.126.3270.405) [DOI] [PubMed] [Google Scholar]
- 47.Phares V, Compas BE. 1992. The role of fathers in child and adolescent psycho-pathology: make room for daddy. Psychol. Bull. 111, 387–412. ( 10.1037/0033-2909.111.3.387) [DOI] [PubMed] [Google Scholar]
- 48.Flinn MV, England BG. 1997. Social economics of childhood glucocorticoid stress response and health. Am. J. Anthropol. 102, 33–53. () [DOI] [PubMed] [Google Scholar]
- 49.Ember C, Ember M. 1994. War, socialization, and interpersonal violence. J. Confl. Resolution 38, 620–646. ( 10.1177/0022002794038004002) [DOI] [Google Scholar]
- 50.Forehand R, Nousiainen S. 1993. Maternal and paternal parenting: critical dimensions in adolescent functioning. J. Fam. Psychol. 7, 213–221. ( 10.1037/0893-3200.7.2.213) [DOI] [Google Scholar]
- 51.Amato P. 1994. Father-child relations, mother-child relations, and offspring psychological well-being in adulthood. J. Marriage Fam. 56, 1031–1042. ( 10.2307/353611) [DOI] [Google Scholar]
- 52.Bielsky IF, Young LJ. 2004. Oxytocin, vasopressin, and social recognition in mammals. Peptides 25, 1565–1574. ( 10.1016/j.peptides.2004.05.019) [DOI] [PubMed] [Google Scholar]
- 53.Wacker DW, Ludwig M. 2012. Vasopressin, oxytocin, and social odor recognition. Horm. Behav. 61, 259–265. ( 10.1016/j.yhbeh.2011.08.014) [DOI] [PubMed] [Google Scholar]
- 54.Zheng D-J, Foley L, Rehman A, Ophir AG. 2013. Social recognition is context dependent in single male prairie voles. Anim. Behav. 86, 1085–1095. ( 10.1016/j.anbehav.2013.09.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGuire B, Getz LL. 2010. Alternative male reproductive tactics in a natural population of prairie voles Microtus ochrogaster. Acta Theriol. 55, 261–270. ( 10.4098/j.at.0001-7051.077.2009) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available online on Dryad: http://dx.doi.org/10.5061/dryad.43pv1.