Abstract
Context
High blood pressure is an important risk factor for cardiovascular disease (CVD) and stroke, the leading cause of death in the U.S. and a substantial national burden through lost productivity and medical care. A recent Community Guide systematic review found strong evidence of effectiveness of team-based care in improving blood pressure control. The objective of the present review was to determine from the economic literature whether team-based care for blood pressure control is cost-beneficial and/or cost-effective.
Evidence acquisition
Electronic databases of papers published January 1980 – May 2012 were searched to find economic evaluations of team-based care interventions to improve blood pressure outcomes, yielding 31 studies for inclusion.
Evidence synthesis
In analyses conducted in 2012, intervention cost, healthcare cost averted, benefit-to-cost ratios, and cost-effectiveness were abstracted from the studies. The quality of estimates for intervention and healthcare cost from each study were assessed using three elements: intervention focus on blood pressure control; incremental estimates in the intervention group relative to a control group; and inclusion of major cost-driving elements in estimates. Intervention cost per unit reduction in systolic blood pressure was converted to lifetime intervention cost per quality-adjusted life-year (QALY) saved using algorithms from published trials.
Conclusion
Team-based care to improve blood pressure control is cost-effective based on evidence that 26 of 28 estimates of $/QALY gained from 10 studies were below a conservative threshold of $50,000. This finding is salient to recent health care reforms in the U.S. and coordinated patient-centered care through formation of Accountable Care Organizations (ACOs).
Context
High blood pressure presents a substantial economic burden in the U.S., fueled by increased medical expenditures, reduced worksite productivity from associated absences, and premature death. Recent U.S. studies estimate annual costs at $47.5 billion in direct medical expenses and $3.5 billion in lost productivity.1 High blood pressure is an important risk factor for cardiovascular disease (CVD) and stroke: the 2014 statistical update from the American Heart Association estimated that CVD and stroke cost $193.4 billion in medical care and about $122 billion in lost productivity from premature death in 2010.2
The objective of this review was to determine whether team-based care (TBC) for blood pressure (BP) control is cost-beneficial and/or cost-effective. Briefly, in TBC, a nurse, pharmacist, and/or other healthcare personnel work together with a provider and patient to manage the patient’s care. A recent Community Guide systematic review3 found strong evidence that TBC improves BP control. This paper provides estimates of intervention cost, healthcare cost averted, productivity gains, and health effects associated with TBC interventions to improve BP control. These estimates are crucial for understanding the economic merits of TBC.
Evidence Acquisition
General methods for Community Guide systematic economic reviews are available at www.thecommunityguide.org/about/economics.html. Methods specific to the present review are detailed below.
A systematic review team (the team) was constituted, including subject matter experts on CVD from various agencies, organizations, and academic institutions together with qualifıed systematic reviewers from The Community Guide branch at CDC. The team worked under the oversight of the Community Preventive Services Task Force.
Conceptual Approach
Team-based care to improve BP control is a health systems-level, organizational intervention that incorporates a multidisciplinary team to improve the quality of hypertension care for patients. The team comprises the patient, the patient’s primary care provider, and other professionals who support and share the responsibilities of hypertension care including medication management, patient follow-up, and adherence and self-management. The complete definition is at www.thecommunityguide.org/cvd/RRteambasedcare.html. TBC is usually implemented in private or public healthcare settings, and is likely to be financed by healthcare organizations or covered by insurers. Thus, evaluation studies may take a healthcare system perspective that only considers costs and benefits of TBC related to the healthcare delivery system. Because the healthcare system perspective and the broader societal perspective are each useful ways to assess economic effects of TBC, both perspectives were considered in this review.
Appropriate study design and measurement are important in identifying the true economic effect of an intervention, an important element of which is the use of a control group. Therefore, studies that included a control group and those in which the control group received usual care or treatment are identified.
Intervention cost is the monetized value of labor and non-labor resources needed to implement and maintain TBC to improve BP control; it reflects the incremental cost of TBC beyond the cost of usual care. The components of intervention cost are the cost of provider time, patient time, and rent and utilities.
The impact of TBC on healthcare cost is the difference in cost of healthcare products and services used by the intervention group and by the control group or the pre to post change where there is no control group. The components of healthcare cost are outpatient visits, medications, hospital inpatient stays, emergency room visits, and patient-time.
Effective TBC interventions to control BP lead to reduced systolic and diastolic blood pressure (SBP and DBP, respectively) and increase the number of patients achieving BP control. The reduction in BP, in turn, reduces morbidity and mortality and increases the quantity and quality of years lived, measured as quality-adjusted life-years (QALYs).
The expected economic benefit of TBC is the sum of savings from averted healthcare cost and the increased productivity of patients at their worksites owing to reduced morbidity and mortality. Cost-benefit analysis compares economic benefit to intervention cost, where both benefit and cost are monetized and expressed in dollar terms; an intervention is cost-beneficial when economic benefit exceeds intervention cost.
The ratio of intervention cost to QALY gained produces cost-utility, a type of cost-effectiveness assessment: an intervention is cost-effective when cost per QALY gained is less than a conservative threshold of $50,000.4 Because the threshold is based on net cost (intervention cost plus healthcare cost) per QALY gained, an additional set of estimates of net cost per QALY gained is also computed. This review defines other cost-effectiveness measures based on additional health outcomes: intervention cost per unit reduction in BP ($/mmHg) and intervention cost per additional person achieving BP control. Interventions targeting BP control can be readily compared to each other based on results expressed as cost per unit reduction in BP or additional person achieving BP control. Results expressed as cost per QALY gained facilitate comparison of interventions to control BP with other health interventions.
For studies that reported an intermediate health outcome, such as reduction in SBP, along with the cost of intervention, reductions in SBP were converted to QALY gained using existing algorithms to allow estimation of cost per QALY gained. A comprehensive registry of cost-effectiveness studies on a wide variety of diseases and treatments was searched to identify studies that translated SBP to QALY (research.tufts-nemc.org/cear4/).5 Two search terms were used—BP/hypertension and QALY—so that the yield would be broadly inclusive. Review of titles and abstracts from 44 papers and further review of full text of 15 studies identified two studies6,7 that converted a reduction in SBP to QALY gained. Both studies were for populations with diabetes. The present review adopted the two conversion algorithms used in the studies.
This first reference study6 assumed that a 5.7 mmHg reduction in SBP sustained over a lifetime would result in a gain of 0.53 QALYs, where QALY/mmHg=0.093. The translation in this study conducted in the United Kingdom was based primarily on results from the UK Prospective Diabetes Study (UKPDS: www.dtu.ox.ac.uk/ukpds/), which followed a group of adults (mean age: 56 years) with diabetes over a period of 8 years. Risk of myocardial infarction and stroke were incorporated based on both UKPDS8 and on the Framingham Heart Study9–11 with weights for QALY drawn from the CDC Diabetes Cost-Effectiveness Study Group.12 The second reference study7 demonstrated that each unit reduction in mmHg of SBP is associated with approximately 0.009 QALYs gained during each annual cycle of a simulation model. This study modeled the experience of a cohort of people with diabetes aged 20–74 years (mean age ~52 years) over 20 years. Risks of myocardial infarction, stroke, peripheral artery disease, end stage renal disease, and severe visual loss were drawn from the UKPDS13–15 and the Framingham Heart Study,9 with QALY based on patient reported quality-of-life.16 The QALY gained per unit reduction in SBP in the two studies is not far apart, considering that the second study was focused on a slightly younger cohort and based on patient-reported quality of life.
Cost and economic benefit estimates from included studies were standardized to a per person per year basis when possible. All monetary values were then converted to 2010 U.S. dollars; the Consumer Price Index from the Bureau of Labor Statistics (www.bls.gov/cpi/tables.htm) was used to adjust for inflation and Purchasing Power Parity indices from the World Bank (data.worldbank.org/indicator/PA.NUS.PRVT.PP) to convert from foreign currencies. Considerable variability may remain, owing to various factors including composition of the team providing the care, allocation of activities among team members, and incomplete accounting for costs and benefits associated with the intervention. The major elements that drive intervention costs and benefits were identified a priori based on knowledge and information gained from peer-reviewed literature and subject matter experts. Finally, what variability remained was acknowledged by presenting medians of individual estimates with interquartile intervals.
Team-based care interventions that go beyond BP control, with additional objectives such as treatment of hyperlipidemia and hyperglycemia, are likely to cost more to implement than interventions focused on BP control, and also likely to avert greater healthcare cost when they are effective. Separate estimates are provided in this paper from the full set of studies and from the studies of TBC interventions that focused on BP control.
Search Strategy and Search Yield
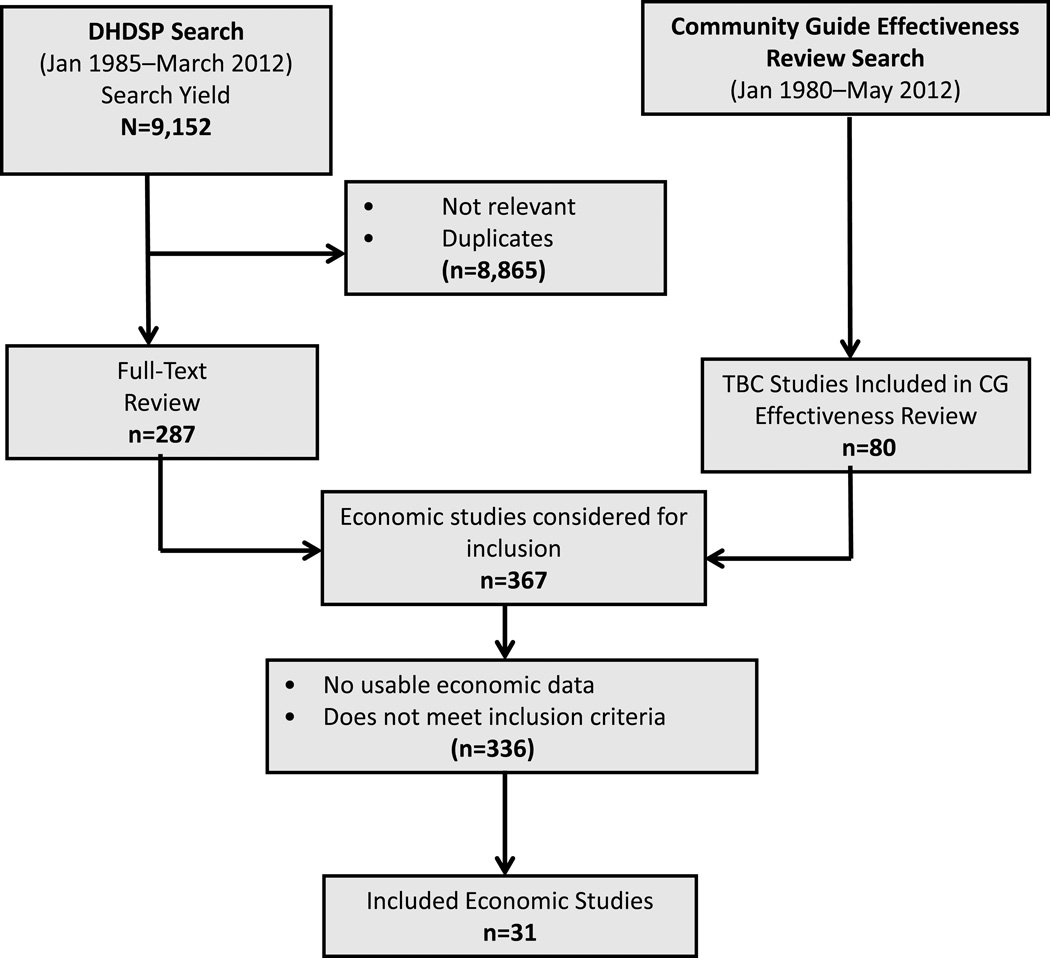
Included studies came from two separate searches. First, a broad search for economic studies of interventions that focused on BP control was conducted by CDC’s Division for Heart Disease and Stroke Prevention (DHDSP), from January 1985 to March 2012. Databases searched were OVID/Medline, EMBASE, PsycINFO, CINAHL, Sociological Abstracts, Web of Science, Cochrane, and EconLit. The search strategy used by DHDSP is available at www.thecommunityguide.org/cvd/supportingmaterials/SS-team-based-care-econ.html. Authors screened 9,152 titles from the DHDSP search for TBC intervention studies with economic outcomes, based on the conceptual approach for this review. In addition, any studies with economic information identified in the search for the review of TBC effectiveness3 were also included. That search strategy is available at www.thecommunityguide.org/cvd/supportingmaterials/SS-team-based-care.html. Screening from these two sources resulted in 31 included studies (Figure 1), where studies were included in the present review if they:
met the intervention definition;
were in English;
were implemented in a high-income economy;
reported the economic cost or economic benefit of the intervention;
had BP control as the primary intervention focus; and
did not include populations with secondary hypertension.
Figure 1.
Flow diagram: number of studies identified, reviewed in full text, reasons for exclusion, and total number of included studies. (CG, Community Guide; DHDSP, Division for Heart Disease and Stroke Prevention, CDC; TBC, team-based care)
Evidence Synthesis
Table 1 provides an overview of four characteristics of included studies: location, setting, presence of control group, and period of publication. Details of the included studies are available at www.thecommunityguide.org/cvd/supportingmaterials/SETecon-Team-Based-Care.pdf. Table 2 summarizes the type of economic analyses conducted in included studies.
Table 1.
Characteristics of Included Studies and Proportion of Studies Within Each Characteristic
| Characteristic | No. of studies (% of all reviewed studies) |
||
|---|---|---|---|
| Country | US | 2418–26,30,32–40,42–46 (77%) |
|
| Non-US | 76,17,27–29,31,41 (23%) |
||
| Setting | Healthcare system | All | 246,19–26,29–31,33–41,43–45 |
| VA system | 321–23 | ||
| Community-based | All | 717,18,27,28,32,42,46 | |
| Worksite | 227,28 | ||
| Control group | No control group | 529,31,38,40,42 (16%) |
|
| Control received usual care | 256,17–28,30,32–37,39,41,43–45 (81%) |
||
| Treated control | 146 (3%) |
||
| Publication period | 1980s | 419,27,28,41 (13%) |
|
| 1990s | 531,32,36,39,43 (16%) |
||
| 2000s | 146,17,18,20,24,26,29,30,33,37,38,40,42,45 (45%) |
||
| 2010s | 821–23,25,34,35,44,46 (26%) |
||
VA, U.S. Department of Veterans Affairs
Table 2.
Included Studies by Type of Economic Analysis
| Type of economic analysis | Number of Studies | |
|---|---|---|
| Intervention cost | 206,17–35 | |
| Healthcare cost | 2117,22,24,27–29,32–46 | |
| Both intervention and healthcare cost | 717,27,28,32–35 | |
| Cost-benefit or Net benefit | 217,24 | |
| Cost effectiveness | Cost per mmHg SBP reduced | 1018,20,21,23,25,29,30,33–35 |
| Cost per mmHg DBP reduced | 918,20,23,27–30,33,35 | |
| Cost per additional person achieving BP control |
116,19–24,28,30,34,35 | |
| Cost per life year saved | 222,34 | |
| Cost per QALY gained | 16 | |
BP, blood pressure; DBP, diastolic blood pressure; QALY, quality-adjusted life year; SBP, systolic blood pressure
Intervention Cost
This review identified three components of cost to implement TBC interventions: provider time, patient time, and rent and utilities. Studies that reported including two or more components provided “reasonably complete” accounting of intervention cost.
Table 3 shows the components included in the estimates of intervention cost reported in 20 included studies6,17–35; two studies26,33 measured it as the post minus pre cost for the intervention group; the remaining studies measuring it as the incremental cost over usual care. Confidence in the estimates was enhanced because most studies included important components of intervention cost and appropriately measured incremental cost. Further, the present review calculated medians and interquartile intervals to draw attention to the central tendency rather than to the range.
Table 3.
Intervention Cost: Components
| Study | Components | No. of components reported |
||
|---|---|---|---|---|
| Provider time |
Patient time |
Rent and utilities |
||
| Artinian 200118 | Y | — | — | 1 |
| Bertera 198119 | Y | — | — | 1 |
| Bosworth 2009b20 | Y | — | Y | 2 |
| Bosworth 201121 | Y | — | Y | 2 |
| Cote 200317 | Y | Y | — | 2 |
| Datta 201022 | Y | — | Y | 2 |
| Edelman 201023 | Y | — | — | 1 |
| Isetts 200824 | Y | — | Y | 2 |
| Katon 201025 | Y | — | Y | 2 |
| Litaker 200326 | Y | — | — | 1 |
| Logan 198127 | Y | Y | Y | 3 |
| Logan 198328 | Y | Y | — | 2 |
| Lowey 200729 | Y | — | — | 1 |
| Ma 200930 | Y | — | Y | 2 |
| Mason 20056 | Y | — | Y | 2 |
| McGhee 199431 | Y | Y | — | 2 |
| Munroe 199732 | — | — | — | NR |
| Okamoto 200133 | Y | — | — | 1 |
| Reed 201034 | Y | Y | Y | 3 |
| Wertz 201235 | — | — | — | NR |
| Total | 18 | 5 | 9 | |
NR, not reported; Y, yes
Table 4 summarizes estimates for intervention cost per person per year from the included studies. Based on 29 observations from 20 studies, the median intervention cost of TBC was $284 per person per year (interquartile interval [IQI]: $153 to $670). The median intervention cost was $359 for studies6,17,20–22,24,25,27,28,30,34 that were reasonably complete in their accounting of intervention cost, $198 per person per year for studies17–22,27,28,31,33,34 that focused solely on BP, and $225 for studies17,20–22,27,28,34 with both features. The cost of intervention was smaller where the focus was on BP control compared to interventions with one or more additional objective(s).
Table 4.
Intervention Cost per Person per Year
| All studies | Studies with ≥2 components of intervention costa |
Studies with only BP focus |
Studies with only BP focus and ≥2 components of intervention costa |
|
|---|---|---|---|---|
|
No. of studies (observations) |
206,17–35 (29) |
116,17,20–22,24,25,27,28,30,34 (17) |
1117–22,27,28,31,33,34 (18) |
717,20–22,27,28,34 (11) |
| Median (IQI) | $284 (IQI: $153 to $670) |
$359 (IQI: $198 to $722) |
$198 (IQI: $138 to $606) |
$225 (IQI: $187 to $664) |
Components of intervention cost were provider time, patient time, and rent and utilities.
BP, blood pressure; IQI, interquartile interval
Impact on Healthcare Cost
Five components of healthcare cost were identified for analysis of the impact of TBC: outpatient visits, hospital inpatient stays, emergency room visits, medications, and patient time. The accounting of healthcare cost was considered reasonably complete when studies included three or more of these components. Table 5 shows the 20 studies17,22,24,27–29,32–45 that reported the components included in estimates for cost of healthcare, with more than half of the studies including at least three components. Five studies24,29,38,40,42 measured the change as post intervention healthcare cost minus pre for the intervention group; the remaining studies measured it as the incremental healthcare cost experienced by the intervention group over that experienced by the control group.
Table 5.
Healthcare Cost: Components
| Study | Components | No. of components reported |
||||
|---|---|---|---|---|---|---|
| Out patient |
In patient |
ER | Drugs | Patient time |
||
| Bogden 199836 | Y | Y | Y | Y | — | 4 |
| Borenstein 200337 | Y | — | — | Y | — | 2 |
| Bunting 200838 | Y | Y | Y | Y | — | 4 |
| Carter 199739 | Y | — | — | Y | — | 2 |
| Cote 200317 | Y | Y | — | Y | Y | 4 |
| Datta 201022 | Y | Y | — | Y | — | 3 |
| Devine 200940 | — | — | — | Y | — | 1 |
| Eckerlund 198541 | Y | — | — | Y | Y | 3 |
| Fedder 200342 | Y | Y | Y | Y | — | 4 |
| Forstrom 199043 | — | — | — | Y | — | 1 |
| Isetts 200824 | Y | Y | Y | Y | — | 4 |
| Kulchaitanaroaj 201244 | Y | — | — | Y | — | 2 |
| Logan 198127 | Y | Y | — | Y | Y | 4 |
| Logan 198328 | Y | — | — | Y | Y | 3 |
| Lowey 200729 | — | — | — | Y | — | 1 |
| Munroe 199732 | — | — | — | Y | — | 1 |
| Murray 200445 | Y | Y | — | — | — | 2 |
| Okamoto 200133 | Y | Y | Y | Y | — | 4 |
| Reed 201034 | Y | Y | — | — | — | 2 |
| Wertz 201235 | Y | Y | Y | Y | — | 4 |
| Total | 16 | 11 | 6 | 18 | 4 | |
ER, emergency room; Y, Yes
Table 6 summarizes estimates of healthcare cost impacts associated with the TBC intervention. One study46 reported cost per person per year to be $4,316 higher for the intervention group compared to usual care. This was considered an outlier and not included in Table 6 because the TBC involved post-acute home-based care of high-risk patients. Across 23 observations of healthcare cost impacts from 20 studies, the median was $65 per person per year (IQI: –$235 to $318). Ten of the estimates from 10 studies17,24,35,36,38,40–43,45 were negative, indicating healthcare cost savings. With the focus on 11 studies17,22,24,27,28,33,35,36,38,41,42 that provided reasonably complete accounting, the median was a healthcare cost saving of $77 per person per year (IQI: –$436 to $98). Seven17,24,35,36,38,41,42 of these studies reported estimated healthcare cost-savings from TBC.
Table 6.
Healthcare Cost Impact per Person per Year
| All studies | Studies with ≥3 components of healthcare costa |
With only BP focus |
With only BP focus and ≥3 components of healthcare costa |
|
|---|---|---|---|---|
|
No. of studies (observations) |
2017,22,24,27–29,32–45 (23) |
1117,22,24,27,28,33,35,36,38,41,42 (11) |
1317,22,27,28,33,34,36,37,39,41,43–45 (16) |
717,22,27,28,33,36,41 (7) |
|
No. of studies (observations) with healthcare cost saving |
1017,24,35,36,38,40–43,45 (10) |
717,24,35,36,38,41,42 (7) |
517,36,41,43,45 (5) |
317,36,41 (3) |
| Median (IQI) | $65 (IQI: –$235 to $318) |
−$77 (IQI: –$436 to $98) |
$110 (IQI: –$46 to $446) |
$25 (IQI: –$53 to $123) |
Components of healthcare cost were for outpatient visits, medications, hospital inpatient stays, emergency room (ER) visits, and patient time.
BP, blood pressure; IQI, interquartile interval
Overall, evidence for TBC reducing healthcare cost was mixed, though most (64%) studies17,24,35,36,38,41,42 with a reasonably complete accounting of healthcare cost components indicated that TBC resulted in healthcare cost savings (Table 6).
For seven studies17,27,28,32–35 that reported both intervention cost and healthcare cost, the sum of the costs was computed as an estimate of the total cost of TBC, producing a median cost per person per year of $329 (IQI: $190 to $658).
Cost Per Unit Reduction of Blood Pressure and Cost Per Additional Person with Controlled Blood Pressure
Ten studies18,20,21,23,25,29,30,33–35 with 14 pairs of observations of intervention cost and reduction in SBP showed a median cost per unit of mmHg reduction in SBP of $87 (IQI: $52 to $202; Table 7). The median cost-effectiveness of TBC in reducing DBP was $102 per unit (IQI: $51 to $123) based on 11 observations from nine studies.18,20,23,27–30,33,35 The health benefit from reduced BP is very likely positive for SBP/DBP ≤140/90 as recommended by JNC-7 (www.nhlbi.nih.gov/health-pro/guidelines/current/hypertension-jnc-7) and previous studies have shown that the benefit becomes minimal for SBP and DBP below certain thresholds.47 A positive intervention cost and no possibility of health benefit from reducing BP below these thresholds warrants further comment. Of ten studies, the mean SBP after the effect of intervention was >140 in three studies,18,23,29 115–140 in seven studies,20,21,25,30,33–35 and <115 in no studies. Of nine studies, the mean DBP after the effect of intervention was >90 for two studies,27,28 70–90 in six studies,18,23,29,30,33,35 and at 68.8 in one study.20 Based on these means, it is likely that the reductions achieved in SBP/DBP from the interventions in this review fell within the beneficial range.
Table 7.
Cost-effectiveness of Reducing Systolic and Diastolic Blood Pressure, Measured as Cost per Millimeter of Mercury
| Systolic BP | Diastolic BP | |||
|---|---|---|---|---|
| All studies | Studies with only BP focus and ≥2 components of intervention costa |
All studies | Studies with only BP focus and ≥2 components of intervention costa |
|
|
No. of studies (observations) |
1018,20,21,23,25,29,30,33–35 (14) |
320,21,34 (5) |
918,20,23,27–30,33,35 (11) |
320,27,28 (3) |
|
Median (IQI) $/mmHg |
$87 (IQI: $52 to $202) |
$188 (IQI: $104 to $344) |
$102 (IQI: $51 to $123) |
$55 (IQI: NA) |
Components of intervention cost were for provider time, patient time, and rent and utilities.
BP, blood pressure; IQI, interquartile interval; NA, not applicable
Several studies reported the incremental percentage of people in the TBC intervention group who achieved controlled BP. This measure of impact is important from a public health and healthcare organization perspective because it provides a reading on population status with respect to uncontrolled BP. (Table 8 shows cost-effectiveness derived from this outcome.) The median incremental cost per additional person achieving BP control was $3,316 (IQI: $2,047 to $5,422), based on 16 observations from 11 studies.6,19–24,28,30,34,35 The thresholds for BP control were DBP<90 in two studies19,28 from the 1980s, SBP/DBP<140/80 in one6 based on a diabetic population , and SBP/DBP<140/90 in the others. The cost per additional person achieving BP control may appear large in comparison to the median intervention cost or the cost per unit of BP reduction. However, two factors should be considered. First, it is the cost per additional person achieving BP control compared to usual care. Second, the intervention is not 100% effective and only part of the intervention group will achieve BP control. For example, even a large reduction in SBP starting from a high baseline may not indicate controlled BP.
Table 8.
Cost-effectiveness per Additional Person Achieving Blood Pressure Controla
| All studies | Studies with only BP focus and ≥2 components of intervention costb |
|
|---|---|---|
|
No. of studies (observations) |
116,19–24,28,30,34,35 (16) |
520–22,28,34 (7) |
| Median (IQI) | $3316 (IQI: $2047 to $5422) |
$5327 (IQI: $2046 to $7154) |
Thresholds for BP control : two19,28 studies from the 1980s were based on DBP<90, one6 based on SBP/DBP<140/80 for a diabetic population , and the remaining were based on SBP/DBP<140/90.
Components of intervention cost were provider time, patient time, and rent and utilities.
BP, blood pressure; IQI, interquartile interval
Cost-Benefit Studies and Cost-Utility Studies
Cost-benefit or cost per QALY outcomes are needed to draw conclusions on the economic value of an intervention. Few included studies reported these outcomes: two studies17,24 reported benefit-to-cost ratios, two22,34 provided cost per life year saved, and one6 estimated cost per QALY gained.
Cost-benefit studies
Two studies reported the ratio of the monetized value of intervention benefit to intervention cost as 12.1:124 and 10:1,17 respectively, indicating that TBC was cost-saving, but several caveats apply. The first study24 had multiple objectives beyond BP control, and healthcare cost estimates were for patients selected from a pool of high utilizers. The second study17 underestimated the cost of software development and deployment by simply dividing the fixed cost from the trial by the much larger number of people with high BP in the Quebec region, without considering issues of scalability.
Cost-effectiveness studies
One study6 reported a cost per QALY of $4,763, which is far below the $50,000 threshold for cost-effectiveness. The estimate was based partly on measurements of intervention cost and SBP reduction from an actual intervention; modeling of QALYs saved was based on the relationship between observed reduction in SBP and QALYs saved from a large population-based RCT.
Two other cost-effectiveness studies reported cost per life year saved, one22 ranging from $48,995 to $100,744 and the other34 from $23,299 to $64,832. Finally, another study29 reported the cost of TBC in terms of observed averted health events, namely $64,610 per cardiovascular event averted and $118,873 per chronic heart disease event averted. There is no standard threshold against which to compare these estimates of cost per averted health events to reach a determination about their economic value.
Cost-effectiveness studies (cost per QALY): SBP to QALY converted studies
Ten included studies provided estimates for reductions in SBP but did not evaluate the long-term effects on morbidity and mortality. The team translated these results into two sets of cost-effectiveness estimates based on formulae relating SBP reduction to QALY gained in two reference studies.6,7
Fourteen observations of SBP reductions from the ten included studies18,20,21,23,25,29,30,33–35 were converted to QALYs (Table 9), with associated intervention costs discounted at 3% and summed over a 20-year expected lifetime. Applying the method from one of the reference studies6 to these data resulted in an estimated median cost per QALY gained of $13,992 (IQI: $8,339 to $32,292). Two observations from one study21 produced estimates that were above the conservative cost-effectiveness threshold of $50,000 per QALY, with one just over $50,000 and one just under $60,000. Applying the formula from the second reference study7 to the same data resulted in a median estimated cost per QALY gained of $9,716 (IQI: $5,791 to $22,425). Based on this formula, all 14 observations produced estimates that were below the threshold. Keeping in mind that the $50,000 threshold is based on intervention cost plus the change in healthcare cost, an additional set of estimates for net cost per QALY gained was derived for three studies33–35 that reported intervention cost, healthcare cost, and reduction in SBP. Net cost per QALY gained estimates were $3,641,33 $37,071,34 and $5,49135 based on the method of the first reference study6 and $2,529,33 $25,744,34 and $3,81335 based on the second reference study.7
Table 9.
Cost per QALY Gained Based on Conversion of Reduced SBP Attributable to Intervention
| Conversion method 16 | Conversion method 27 | |
|---|---|---|
|
No. of studies reporting intervention cost and SBP reduction |
10 studies18,20,21,23,25,29,30,33–35 | |
|
20-year intervention cost per person Median (IQI) |
$9,299 (IQI: $4,838 to $11,110) | |
|
Reduction in SBP (mmHg) Median (IQI) |
6.70 (IQI: 3.90 to 7.43) | |
|
20-year QALY gained Median (IQI) |
0.623 (IQI: 0.363 to 0.691) | 0.897 (IQI: 0.522 to 0.995) |
|
20-year cost per QALY gained Median (IQI) |
$13,992 (IQI: $8339 to $32,292) | $9,716 (IQI: $5791 to $22,425) |
| Type of team member added |
||
| Nurse18,20,21,25,30,34 | $24,042 (IQI: $8836 to $44,752) | $16,696 (IQI: $6136 to $31,077) |
| Pharmacist and Other23,29,30,33,35 |
$10,244 (IQI: $1934 to $13,992) | $7114 (IQI: $1343 to $9716) |
| Baseline SBP | ||
| >14018,23,29,33 | $5587 (IQI: $1334 to $9693) | $3880 (IQI: $927 to $6731) |
| ≤14020,21,25,30,34,35 | $20,564 (IQI: $11,381 to $41,826) | $14,280 (IQI: $7903 to $29,045) |
IQI, interquartile interval; QALY, quality-adjusted life years; SBP, systolic blood pressure
Several characteristics of the interventions were explored as explanatory variables for the variation observed in cost per QALY gained: implementation in a health system or community setting, type of team member added to usual care, management of medication, compared to usual care or not, and whether the baseline SBP was high or low relative to the 140 threshold. All the studies included medication management and/or medication counseling. All studies had a control group that received usual care and all but two18,35 were implemented in healthcare settings. The results of the categorical analyses based on the remaining variables are presented in Table 9. It may appear that teams that added pharmacists and others had lower cost per QALY gained than those that added nurses. However, this difference in cost-effectiveness may also be explained by different baseline rates for SBP because the same studies that used pharmacists and others had higher baseline SBP.
Conclusion
Summary of Findings
Evidence of cost per QALY gained from this economic review indicates that TBC is cost-effective in improving BP control.
Discussion
The major caveat in this review is that the formulae for the relationship between SBP and QALY were drawn from the experience of people with diabetes and comorbid high BP. It is not clear whether, and to what extent, limitation to people with diabetes leads to an overestimation or underestimation of the relationship between SBP and QALYs; even though it is possible that the overall QALY may be worse for diabetic patients compared to hypertensive patients, the relative impact of SBP reduction on the overall QALY of diabetic patients compared to that of hypertensive patients is uncertain.
The cost of intervention and the impact on healthcare cost are key estimates reported in the included studies from which the present review drew findings about cost-effectiveness and the cost impact of TBC on utilization of healthcare resources. The studies varied in completeness of accounting for components of these costs and in whether the increment in cost was measured relative to a control group. Despite these variations, the finding of cost-effectiveness is credible given that most studies were reasonably complete in accounting for components of intervention cost and all but two of the 28 cost per QALY estimates were below the conservative $50,000 threshold. Net cost per QALY saved could be calculated only for 3 studies, but all three estimates were below the conservative threshold of $50,000.
The generalizability of the review’s results to practice is not seamless because a substantial proportion of the evidence is drawn from studies implemented in research settings. Also, the review’s overall cost-effectiveness conclusion is based on modeled long-term outcomes. The incentives and protocols that bound provider and patient together as a collaborative team in research must be replicated or replaced with alternatives to implement the intervention in practice. Hence, multiple reimbursement systems (e.g., for pharmacy and medical benefits) will have to be coordinated and new services and providers may also have to be added to reimbursement systems. The change necessary in the organization, delivery, and reimbursement for care is feasible and sustainable, as demonstrated by the success of the Kaiser Permanente Northern California hypertension program.48
By extrapolating reductions in BP found in trials to 20-year horizons of QALY gained, the present review implicitly assumed that the TBC intervention is sustained and paid for over the same period. Over this long term, members of the team must receive compensation to ensure their continued participation. From a financial standpoint, the insurers/payers must find it in their interest to make a long-term commitment to support these teams and to pay individual providers, some of whom they had not dealt with directly. Arrangements by health plans to reimburse teams were far from prevalent during the periods covered in the included studies. In contrast, recent health care reform in the U.S. and the Affordable Care Act may promote TBC through Accountable Care Organizations (ACOs) by encouraging the formation of patient-centered teams and improving care coordination among clinicians.49,50
Finally, it may be argued that individuals don’t stay in health plans long enough for the plans to reap the financial benefits of some types of prevention,51 but members with high BP are well-served by TBC to bring their BP under control and manage any relapse.
Evidence Gaps
More complete and comprehensive reporting of cost and its components is needed. Some evaluation studies simply reported an aggregate estimate for intervention cost or healthcare cost without discussing the components. Many evaluations reported the aggregate estimate and listed the components but did not provide values for the components, precluding an analysis of which components contributed the most to the aggregate estimate. An analysis of the components of cost or benefit reported in a body of evaluation studies would provide the data to determine what should be considered drivers of the estimated values.
More research is needed to see how variations in the TBC model (i.e., that employ different methods of patient engagement through differences in team structure and team activities) affect intervention cost and cost-effectiveness.
Associated costs of the intervention were not always reported. A number of studies reported only healthcare cost impact, probably with the objective of determining if TBC is healthcare cost-saving. However, a determination about economic value could not be drawn from these studies because the associated costs of intervention were not reported.
No evaluation study considered the impact of TBC on improved productivity at work. This review noted the omission of productivity considerations from intervention evaluations despite the fact that the magnitude of productivity losses attributable to CVD and stroke by themselves is highlighted in the burden literature.
Finally, a major gap in the economic evaluation literature for BP control is lack of a widely accepted standard relating reduced BP to QALY for the general population of people with high BP.
Acknowledgements
This review would not have been possible without the subject matter expertise and contributions of our coordination team in the Community Guide Branch at CDC, from other areas of CDC, and our external partners: David B. Callahan, MD, Office Of Public Health Preparedness And Response, CDC; Diane Dunet, MPA, PhD, Division Of Heart Disease And Stroke Prevention, CDC; external partners, Kimberly J. Rask, MD, PhD, Emory University, Atlanta; Daniel T. Lackland, DrPH, Medical University of South Carolina, Charleston; Barry Carter, PharmD, University of Iowa, Iowa City. The authors also acknowledge Randy W. Elder, PhD, Kate W. Harris, BA, Kristen D. Folsom, MPH, and Onnalee Gomez, MS, from the Community Guide Branch at CDC for their assistance throughout the review process.
The work of Krista Proia, Gibril Njie, and Ramona Finnie was supported with funds from the Oak Ridge Institute for Scientific Education (ORISE).
Footnotes
No author has any conflict of interest or financial disclosure.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of CDC.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: A policy statement From the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics—2014 Update A Report From the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: A Community Guide systematic review. Am J Prev Med. 2014;47(1):86–99. doi: 10.1016/j.amepre.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichler H-G, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–528. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller G, Cohen JT, Roehrig C. Cost-effectiveness of cardiovascular disease spending. J Am Coll Cardiol. 2012;60(20):2123–2124. doi: 10.1016/j.jacc.2012.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28(1):40. doi: 10.2337/diacare.28.1.40. [DOI] [PubMed] [Google Scholar]
- 7.McEwan P, Peters JR, Bergenheim K, Currie CJ. Evaluation of the costs and outcomes from changes in risk factors in type 2 diabetes using the Cardiff stochastic simulation cost-utility model (DiabForecaster) Curr Med Res Opin. 2005;22(1):121–129. doi: 10.1185/030079906X80350. [DOI] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1):293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 10.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 11.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 12.CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287(19):2542. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- 13.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM: I. Model construction and assumptions. Diabetes Care. 1997;20(5):725–734. doi: 10.2337/diacare.20.5.725. [DOI] [PubMed] [Google Scholar]
- 14.Kothari V, Stevens RJ, Adler AI, et al. UKPDS 60 risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002;33(7):1776–1781. doi: 10.1161/01.str.0000020091.07144.c7. [DOI] [PubMed] [Google Scholar]
- 15.Stevens RJ, Kothari V, Adler AI, Stratton IM, Holman RR. United Kingdom Prospective Diabetes Study Group. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci. 2001;101(6):671–679. [PubMed] [Google Scholar]
- 16.Currie CJ, McEwan P, Peters JR, Patel TC, Dixon S. The routine collation of health outcomes data from hospital treated subjects in the Health Outcomes Data Repository (HODaR): descriptive analysis from the first 20,000 subjects. Value Health. 2005;8(5):581–590. doi: 10.1111/j.1524-4733.2005.00046.x. [DOI] [PubMed] [Google Scholar]
- 17.Cote I, Gregoire JP, Moisan J, Chabot I, Lacroix G. A pharmacy-based health promotion programme in hypertension: cost-benefit analysis. Pharmacoeconomics. 2003;21(6):415–428. doi: 10.2165/00019053-200321060-00005. [DOI] [PubMed] [Google Scholar]
- 18.Artinian NT, Washington OG, Templin TN. Effects of home telemonitoring and community-based monitoring on blood pressure control in urban African Americans: a pilot study. Heart Lung. 2001;30(3):191–199. doi: 10.1067/mhl.2001.112684. [DOI] [PubMed] [Google Scholar]
- 19.Bertera EM, Bertera RL. The cost-effectiveness of telephone vs clinic counseling for hypertensive patients: a pilot study. Am J Public Health. 1981;71(6):626. doi: 10.2105/ajph.71.6.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control. Ann Intern Med. 2009;151(10):687. doi: 10.1059/0003-4819-151-10-200911170-00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011;171(13):1173. doi: 10.1001/archinternmed.2011.276. [DOI] [PubMed] [Google Scholar]
- 22.Datta SK, Oddone EZ, Olsen MK, et al. Economic analysis of a tailored behavioral intervention to improve blood pressure control for primary care patients. Am Heart J. 2010;160(2):257–263. doi: 10.1016/j.ahj.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Edelman D, Fredrickson SK, Melnyk SD, et al. Medical clinics versus usual care for patients with both diabetes and hypertension. Ann Intern Med. 2010;152(11):689. doi: 10.7326/0003-4819-152-11-201006010-00001. [DOI] [PubMed] [Google Scholar]
- 24.Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc. 2008;48(2):203. doi: 10.1331/JAPhA.2008.07108. [DOI] [PubMed] [Google Scholar]
- 25.Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litaker D, Mion LC, Planavsky L, Kippes C, Mehta N, Frolkis J. Physician-nurse practitioner teams in chronic disease management: the impact on costs, clinical effectiveness, and patients' perception of care. J Interprof Care. 2003;17(3):223–237. doi: 10.1080/1356182031000122852. [DOI] [PubMed] [Google Scholar]
- 27.Logan AG, Milne BJ, Achber C, Campbell WP, Haynes RB. Cost-effectiveness of a worksite hypertension treatment program. Hypertens. 1981;3(2):211–218. doi: 10.1161/01.hyp.3.2.211. [DOI] [PubMed] [Google Scholar]
- 28.Logan AG, Milne BJ, Flanagan PT, Haynes RB. Clinical effectiveness and cost-effectiveness of monitoring blood pressure of hypertensive employees at work. Hypertens. 1983;5(6):828. doi: 10.1161/01.hyp.5.6.828. [DOI] [PubMed] [Google Scholar]
- 29.Lowey A, Moore S, Norris C, Wright D, Silcock J, Hammond P. The cost-effectiveness of pharmacist-led treatment of cardiac risk in patients with type 2 diabetes. Pharm World Sci. 2007;29(5):541–545. doi: 10.1007/s11096-007-9101-7. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Berra K, Haskell WL, et al. Case management to reduce risk of cardiovascular disease in a county health care system. Arch Intern Med. 2009;169(21):1988. doi: 10.1001/archinternmed.2009.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGhee SM, McInnes GT, Hedley AJ, Murray TS, Reid JL. Coordinating and standardizing long-term care: evaluation of the west of Scotland shared-care scheme for hypertension. Br J Gen Pract. 1994;44(387):441. [PMC free article] [PubMed] [Google Scholar]
- 32.Munroe WP, Kunz K, Dalmady-Israel C, Potter L, Schonfeld WH. Economic evaluation of pharmacist involvement in disease management in a community pharmacy setting. Clin Ther. 1997;19(1):113–123. doi: 10.1016/s0149-2918(97)80078-1. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto MP, Nakahiro RK. Pharmacoeconomic evaluation of a pharmacist-managed hypertension clinic. Pharmacotherapy. 2001;21(11):1337–1344. doi: 10.1592/phco.21.17.1337.34424. [DOI] [PubMed] [Google Scholar]
- 34.Reed SD, Yanhong LI, Oddone EZ, et al. Economic evaluation of home blood pressure monitoring with or without telephonic behavioral self-management in patients with hypertension. Am J Hypertens. 2010;23(2):142–148. doi: 10.1038/ajh.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wertz D, Hou L, DeVries A, et al. Clinical and economic outcomes of the Cincinnati Pharmacy Coaching Program for diabetes and hypertension. Manag Care. 2012;21(3):44. [PubMed] [Google Scholar]
- 36.Bogden PE, Abbott RD, Williamson P, Onopa JK, Koontz LM. Comparing standard care with a physician and pharmacist team approach for uncontrolled hypertension. J Gen Intern Med. 1998;13(11):740–745. doi: 10.1046/j.1525-1497.1998.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23(2):209–216. doi: 10.1592/phco.23.2.209.32096. [DOI] [PubMed] [Google Scholar]
- 38.Bunting BA, Smith BH, Sutherland SE. The Asheville Project: clinical and economic outcomes of a community-based long-term medication therapy management program for hypertension and dyslipidemia. J Am Pharm Assoc. 2008;48(1):23–31. doi: 10.1331/JAPhA.2008.07140. [DOI] [PubMed] [Google Scholar]
- 39.Carter BL, Barnette DJ, Chrischilles E, Mazzotti GJ, Asali ZJ. Evaluation of hypertensive patients after care provided by community pharmacists in a rural setting. Pharmacotherapy. 1997;17(6):1274. [PubMed] [Google Scholar]
- 40.Devine EB, Hoang S, Fisk AW, Wilson-Norton JL, Lawless NM, Louie C. Strategies to optimize medication use in the physician group practice: the role of the clinical pharmacist. J Am Pharm Assoc. 2009;49(2):181–191. doi: 10.1331/JAPhA.2009.08009. [DOI] [PubMed] [Google Scholar]
- 41.Eckerlund I, Jonsson E, Ryden L, Rastam L, Berglund G, Isacsson SO. Economic evaluation of a Swedish medical care program for hypertension. Health Policy. 1985;5(4):299–306. doi: 10.1016/0168-8510(85)90047-8. [DOI] [PubMed] [Google Scholar]
- 42.Fedder DO, Chang RJ, Curry S, Nichols G. The effectiveness of a community health worker outreach program on healthcare utilization of West Baltimore City Medicaid patients with diabetes with or without hypertension. Ethn Dis. 2003;13(1):22–27. [PubMed] [Google Scholar]
- 43.Forstrom MJ, Ried LD, Stergachis AS, Corliss DA. Effect of a clinical pharmacist program on the cost of hypertension treatment in an HMO family practice clinic. Ann Pharmacother. 1990;24(3):304–309. doi: 10.1177/106002809002400318. [DOI] [PubMed] [Google Scholar]
- 44.Kulchaitanaroaj P, Brooks JM, Ardery G, Newman D, Carter BL. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32(8):772–780. doi: 10.1002/j.1875-9114.2012.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray MD, Harris LE, Overhage JM, et al. Failure of computerized treatment suggestions to improve health outcomes of outpatients with uncomplicated hypertension: results of a randomized controlled trial. Pharmacotherapy. 2004;24(3):324–337. doi: 10.1592/phco.24.4.324.33173. [DOI] [PubMed] [Google Scholar]
- 46.Pezzin LE, Feldman PH, Mongoven JM, McDonald MV, Gerber LM, Peng TR. Improving blood pressure control: Results of home-based post-acute care interventions. J Gen Intern Med. 2011;26(3):280–286. doi: 10.1007/s11606-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banach M, Aronow WS. Blood pressure J-curve: current concepts. Curr Hypertens Rep. 2012;14(6):556–566. doi: 10.1007/s11906-012-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310(7):699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taplin SH, Foster MK, Shortell SM. Organizational leadership for building effective health care teams. Ann Fam Med. 2013;11(3):279–281. doi: 10.1370/afm.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis K, Abrams M, Stremikis K. How the Affordable Care Act will strengthen the nation's primary care foundation. J Gen Intern Med. 2011;26(10):1201–1203. doi: 10.1007/s11606-011-1720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herring B. Suboptimal provision of preventive healthcare due to expected enrollee turnover among private insurers. Health Econ. 2010;19(4):438–448. doi: 10.1002/hec.1484. [DOI] [PubMed] [Google Scholar]