Abstract
Background
Increased aortic stiffness and reduced coronary flow reserve (CFR) independently predict adverse outcomes. But information about relationships between arterial properties and CFR in subjects without obstructive coronary artery disease (CAD) is limited.
Methods
CFR was measured (Doppler flow wire and intracoronary adenosine) in 50 women (age 53±11 years) with symptoms and signs of myocardial ischemia without obstructive CAD. Aortic pulse wave velocity (aPWV), a measure of aortic stiffness, was obtained via catheter pullback; radial artery pressure waves were measured by applanation tonometry and central aortic pressure synthesized.
Results
Overall, CFR (mean 2.61 ± 0.47) was significantly correlated with aPWV (r = −0.51), pulse wave amplification (r = 0.45), augmented pressure (AP, r = −0.48), augmentation index (AIx, r = −0.44), aortic systolic pressure (r = −0.49), left ventricular wasted energy (LVEw, r = −0.47) (all P < 0.001), systolic pressure time index (r = −0.37, P < 0.008), and rate pressure product (r = −0.29, P < 0.04). In the multiple regression model including aPWV, CFR was still significantly correlated with aPWV (P < 0.008) and aortic systolic pressure (P < 0.01). No other measures contributed significant additional information.
Women with CFR ≤2.5 vs. those with CFR >2.5 had greater aPWV (894 ± 117 vs. 747 ± 93 cm/sec, P < 0.001), AP (14 ± 4.9 vs. 11 ± 4.1 mmHg, P < 0.008), AIx (32 ± 6.6 vs. 27 ± 6.6%, P < 0.003), LVEw (30 ± 12 vs. 21 ± 10 dyne-sec/cm2 × 102, P < 0.02) and reduced pulse pressure amplification (1.20 ± .07 vs. 1.26 ± .10, P < 0.008) and pressure wave travel time (133 ± 7.3 vs. 138 ± 6.9 msec, P < 0.04).
Conclusions
Among symptomatic women without obstructive CAD, CFR was inversely related to aortic systolic pressure and indices of aortic stiffness. These changes in arterial properties increase LV afterload requiring the ventricle to generate additional, but wasted, energy that increases indices of myocardial oxygen demand, reduces CFR and increases vulnerability to ischemia.
Keywords: coronary microvascular dysfunction, coronary flow reserve, aortic pressure waveform, pulse wave analysis, pulse wave velocity, wave reflections, aortic stiffness
Introduction
Cardiovascular disease is the leading cause of death among women and has been linked to multiple factors including increased aortic stiffness and wave reflections.1-5 A non-compliant aorta increases left ventricular (LV) afterload and places an additional workload on the ventricle that increases myocardial oxygen demand and may lead to hypertrophy and myocardial dysfunction.6 To accommodate for this increased oxygen demand, the myocardial microcirculation autoregulates to maintain coronary blood flow.7 An increase in myocardial oxygen demand causes an unfavorable imbalance in the myocardial oxygen supply-demand relationship that decreases coronary flow reserve (CFR) and promotes myocardial ischemia.8-10 These and other factors (i.e., increased heart rate, particularly among women, and increased cross-sectional area of perfused myocardium) augment resting (or basal) coronary flow and limit CFR.11-18
We have previously found evidence for increased pulse wave reflection and ejection duration in a sample of women with symptoms and signs of myocardial ischemia in the absence of obstructive coronary artery disease (CAD) from the Women's Ischemia Syndrome Evaluation (WISE).5 However, associations between properties of capacitance vessel stiffness and CFR have not been previously reported in such women. Accordingly, the purpose of this investigation was to assess relationships between CFR and measures of elastic properties and wave reflection characteristics of the systemic arterial system in a subgroup of women in the WISE study.
Methods
The WISE design, methods, and principal results have been published previously.19-25 The present ancillary study was conducted in a sample enrolled at the University of Florida (UF). Briefly, women undergoing clinically indicated coronary angiography to evaluate chest pain and/or signs of myocardial ischemia were enrolled. Those with angiographic-documented obstructive CAD (≥50% diameter stenosis by core lab), pregnancy, serum creatinine ≥2.0, and systolic blood pressure (BP) <100 mmHg, in addition to other WISE exclusions19, were not included. The UF Institutional Review Board approved both the overall WISE and this ancillary study, and each participant provided informed written consent.
Clinical protocol
After medical history recording, physical examination, and laboratory testing, coronary angiography with provocative studies were completed.20-23 Vasodilator medications were interrupted: long-acting calcium antagonists for ≥48 hours, short-acting calcium antagonists and long-acting nitrates for ≥24 hours, and sublingual nitrates for ≥2 hours. Coronary reactivity testing was performed in a stenosis-free area of the left anterior descending coronary artery. A Doppler-tipped flow guide wire (0.014 inch FloWire; JOMED/Cardiometrics, Mountain View, CA) was advanced through the diagnostic catheter. When a stable velocity signal was obtained, baseline recordings were collected. Intracoronary adenosine was administered into the left main coronary artery.24 At least three injections were made and average peak coronary flow velocity was obtained, with return to baseline documented before each bolus. Time-averaged peak velocity was calculated from pulsed-wave Doppler flow spectra.
The core lab analyzed recordings masked to all other data. CFR was defined as the ratio of average peak velocity after adenosine to average baseline velocity just prior to adenosine. Since this measure correlated closely (r = 0.87, P < 0.001) with volumetric flow among similar women in the WISE, it was used to represent CFR.25 Aortic stiffness was derived from aortic pulse wave velocity (aPWV) and wave reflection characteristics were obtained using pulse wave analysis (PWA) of central aortic pressure waves. The aPWV, measured in 43 women of the 50 women, was obtained invasively during cardiac catheterization, using the delay in pressure wave foot and distance travelled by the wave as the catheter was withdrawn from the ascending aorta to the femoral artery.26
Cuff BP measurements
Noninvasive data were collected ≥2 hours after eating, drinking coffee, or smoking, with subjects supine in the same quiet, temperature-controlled room after resting ≥15 minutes. Before angiography and CFR measurements, brachial systolic (bSP) and diastolic (DP) BP were measured in the left arm using a validated, automatic oscillometric BP monitor (Omron R3, Omron Healthcare, Kyoto, Japan) with appropriately-sized cuff. Three measurements were taken ≥2 minutes apart: the latter two were averaged for this analysis. Brachial pulse pressure (bPP) was calculated as the difference (bSP–DP). Hypertension was defined as bSP ≥140 and/or DP ≥90 mmHg. Diabetes was defined as fasting glucose >6.9 mmol/l.
Pulse wave analysis
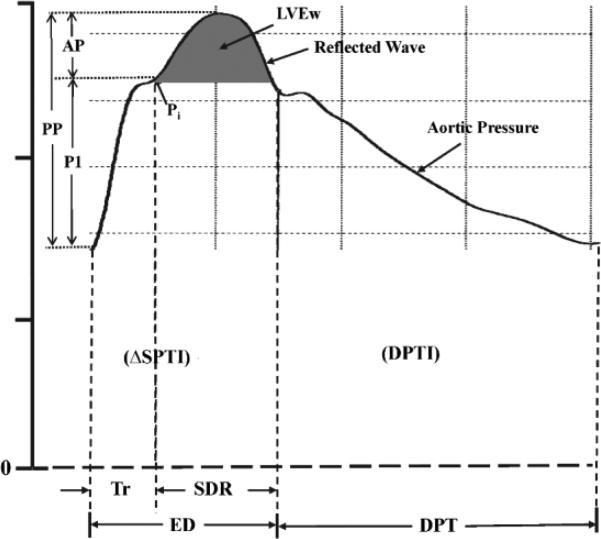
Wave reflection characteristics and event timing were assessed non-invasively (SphygmoCor system, AtCor Medical, Sydney, Australia). Radial artery pressure waveforms were recorded at the wrist, using applanation tonometry with a high-fidelity micromanometer (Millar Instruments, Houston, TX). After 20 sequential waveforms were acquired and averaged, a validated generalized mathematical transfer function was used to synthesize the corresponding central aortic pressure wave.6,27 Indices of pulsatile LV afterload and myocardial oxygen demand were derived from aortic pressure wave using PWA.5,6,28,29 The mechanical “afterload” imposed by the systemic circulation to the pumping LV is composed of a static (or steady) and a dynamic (pulsatile) component and is an important determinant of normal cardiovascular function and a key pathophysiologic factor in various cardiac and vascular disease states. The steady component depends on mean arterial pressure and the peripheral resistance, which in turn depends on arteriolar caliber, the total number of arterioles that are present “in parallel” and blood viscosity. The pulsatile component depends upon the elastic properties (arterial stiffness) and wave reflection characteristics of the arterial tree. LV afterload can be assessed in the frequency domain from the aortic input impedance spectrum (calculated from the harmonic components of central aortic pressure and flow waves) or estimated in the time domain from the central aortic pressure wave. 6 The merging point of forward (or incident) traveling and reflected (or backward traveling) pressure waves (inflection point, Pi) was identified (Figure 1). P1 is the amplitude of the forward traveling pressure wave created by LV ejection, and AP is the amplitude of the reflected (or backward traveling) pressure wave (augmented pressure) from the lower body. The forward pressure wave is related to central elastic artery stiffness and is not influenced by wave reflection. In general, acute changes in elastic artery stiffness occur passively (i.e., with change in distending pressure) while chronic changes occur over time (i.e., with changes in arterial wall thickness, collagen, and elastin content).6 Aortic augmentation index (AIx), a measure of the contribution of wave reflection to central pulse pressure (cPP), is defined as AP/cPP and is expressed as a percentage. Indices of aortic wave reflection are strongly predictive of major adverse cardiovascular events and all-cause mortality.1,2 Lower values of AIx indicate reduced wave reflection and/or delayed return of the reflected wave from the lower body as a result of decreased arterial stiffness (and PWV) and vice versa. AP and AIx are dependent upon elastic properties of the entire arterial tree (elastic and muscular arteries), transmission velocity of the reflected wave and distance to the major reflecting site.6 Acute reduction in AIx is due, primarily, to relaxation of smooth muscle in lower body arteries causing dilation and a decrease in stiffness and PWV. Time from onset aortic pressure upstroke to reflected wave upstroke, beginning at the inflection point, is the round-trip travel time (Tr) of the pressure wave to and from the major reflecting site in the lower body. Increased aortic stiffness and aPWV decrease Tr that is associated with increased reflected wave amplitude. When the reflected wave returns during systole (Figure 1) the aortic pressure wave is augmented, therefore, the LV must generate sufficient energy to overcome this boost in pressure that opposes emptying. This energy (LVEw), which takes into account both amplitude and duration (SDR) of the reflected wave, is wasted since it does not contribute to blood flow and is obtained as area under the systolic portion of the reflected wave.5,28-30 Furthermore, LVEw is deleterious, since it causes reduction in LV ejected blood during flow deceleration. Pulse pressure amplification, an approximation of arterial stiffness, was calculated as (bPP/cPP).6
Figure 1.
Central aortic pressure waveform synthesized from a radial pressure waveform. Pi indicates the merging (or inflection) point of the forward traveling and reflected (or backward traveling) waves. The early part of the ascending aortic pressure (i.e., forward traveling) wave with amplitude (P1) is generated by left ventricular (LV) ejection. The later part of the pressure wave with amplitude (AP) is the reflected wave arriving during systole and adding to the forward traveling pressure wave. Thus, pulse pressure (PP) = P1 + AP and augmentation index (AIx) = AP/PP. Tr is the sum of the travel time of the forward traveling wave from the LV to the periphery and the backward traveling reflected wave from the periphery to the LV; SDR is systolic duration of the reflected wave; ED is ejection duration (or systolic pressure time, SPT); DPTI is diastolic pressure time integral (or index) and DPT is diastolic pressure time. The area under the systolic portion of the reflected wave (dark shaded area) is defined as LV wasted energy (LVEw). Systolic pressure time index (SPTI) = ΔSPTI + LVEw.
The study group consisted of 50 women with core lab acceptable data: coronary angiography documenting no obstructive CAD (<50% diameter stenosis); CFR measurements: and high-quality recordings of aPWV measured by pressure catheter withdrawal, considered the reference standard6, 26, and radial artery pressure waveforms recorded noninvasively by applanation tonometry and derived aortic pressure waves. Data were analyzed using aortic pressure wave PWA, and high-quality recordings were defined as quality index >80% (derived from an algorithm including average pulse height, pulse height variation, diastolic variation, and maximum rate of rise of peripheral waveform) plus acceptable waveforms on visual inspection.
Statistical analysis
Statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). Data for continuous variables are summarized as mean±standard deviation (SD) or percentages where appropriate. Relationships between two continuous variables (e.g., AIx and CFR) were determined by simple linear regression analysis and Pearson's correlation coefficient was calculated. As previous studies10, including those from the WISE 20,24,25, have shown an association between CV abnormalities and low CFR, women were also divided by CFR ≤2.5 and >2.5. We also compared these data with data from a reference group of healthy women studied in our lab under the same conditions.5 Continuous data were compared using an unpaired 2-tailed t test, categorical data by chi-square test (expected frequency in all cells ≥5), or Fisher's exact test (expected frequency in at least 1 cell <5). A P <0.05 value was considered significant.
Bivariate correlations were computed between CFR and variables measuring aortic stiffness/wave reflection. Multivariable regression analysis was conducted to further develop relationships between CFR and vessel stiffness/wave reflection measurements adjusting for demographic and historical factors. Main predictors of interest are indices of vessel stiffness (e.g., aPMV, time travel of wave, and amplification) as well as indices of wave reflection (e.g., reflected wave amplitude, augmentation index and LV wasted energy). Covariates are age, history of hypertension, history of diabetes, BMI, and aortic systolic pressure (SP) recorded during these studies. The following multiple linear regression models were built sequentially by adding one group of variables at a time. Model 1 included all covariates (age, hypertension, diabetes, BMI, and aortic SP). Model 2 included all covariates plus indices of vessel stiffness. Model 3 included all covariates, indices of vessel stiffness, and indices of wave reflection. Original coefficients are computed for predictors in their original units. Standardized coefficients are standardized so that their means are equal to zero and SDs are equal to 1 to allow comparison of relative effect size between predictors independent of original units.
Results
Baseline characteristics
Pertinent clinical characteristics are summarized in Table I for all study women and subgrouped by CFR catagory. Mean age (±SD) was 53±11 years, half were postmenopausal, most were overweight or obese, about a quarter had a history of hypertension, 12% had a history of diabetes, and 39% had history of smoking. CFR ranged from 1.7-3.6 (mean 2.61±0.47), with 24 women in the group with CFR ≤2.5 and 26 women with CFR >2.5. CFR subgroups were similar by age, height, weight, body mass index, waist circumference, heart rate, and other characteristics, and were also similar to another sample from the WISE.5
Table I.
Pertinent baseline clinical characteristics of the study group.
| Characteristic | All Women (n=50) | CFR ≤2.5 (n=24) | CFR >2.5 (n=26) | P Value |
|---|---|---|---|---|
| Age (years) | 53±11 | 55±8.8 | 52±10 | 0.26 |
| Race/Ethnicity - white (%) | 87 | 89 | 85 | |
| Height (cm) | 165±7.0 | 164±6.3 | 165±8.0 | 0.41 |
| Weight (kg) | 83±22 | 79±18 | 87±22 | 0.19 |
| Body mass index (kg/m2) | 30.3±6.9 | 29.4±5.8 | 32±7.5 | 0.18 |
| Waist circumference (inches) | 35.8±4.9 | 35.3±5.1 | 36.2±4.7 | 0.38 |
| Heart rate (beats/minute) | 71±9.9 | 70±8.5 | 72±9.5 | 0.49 |
| Medical history, % | ||||
| Hypertension | 27 | 33 | 22 | |
| Diabetes | 12 | 11 | 12 | |
| Cigarette smoking (ever) | 39 | 44 | 36 | |
| Dyslipidemia | 26 | 28 | 24 | |
| Family history of coronary artery disease | 42 | 56 | 32 | |
| Postmenopausal | 51 | 50 | 52 | |
| Medications, % | ||||
| Hormone replacement therapy (ever) | 37 | 39 | 36 | |
| Birth control pills (ever) | 53 | 56 | 52 | |
| Vasodilator medication | 44 | 44 | 44 | |
| Statin (current) | 33 | 39 | 28 |
Data are presented as mean ± standard deviation or as a percentage. P value is coronary flow reserve (CFR) ≤2.5 vs. CFR >2.5.
Brachial and central aortic BP
Hemodynamic variables are summarized by CFR subgroup and compared with those from the reference group in Table II. Linear regression analysis for the entire group showed inverse correlation between CFR and bSP (r = −0.40; P <0.004), DP (r = −0.35; P <0.01), mean pressure (r = −0.47; P <0.001), cSP (r = −0.49; P <0.001) and cPP (r = −0.35; P <0.01) (Table III). Data from CFR subgroups (≤2.5 vs. >2.5) indicate that bSP (137±16 vs. 131±14 mm Hg; P <0.21), DP (83±8.5 vs. 80±9.9 mm Hg; P <0.21), and mean pressure (103±10 vs. 98±10 mm Hg; P <0.21) were similar. Also cSP (128±14 vs. 121±14 mm Hg; P <0.07) and cPP (45±11 vs. 41±9.3 mm Hg; P < 0.17) were similar in women with CFR ≤2.5 vs. >2.5.
Table II.
Hemodynamic variables for women with CFR ≤2.5 vs. >2.5 and the reference group.
| Sub Group | P values (P<) | |||||
|---|---|---|---|---|---|---|
| Variable | Reference N=59 | CFR≤2.5 n=24 | CFR>2.5 n=26 | CFR≤2.5 vs Ref | CFR>2.5 vs Ref | CFR≤2.5 vs >2.5 |
| CFR | 3.32±0.3 | 2.23±0.20 | 3.03±0.26 | NA | NA | 0.001 |
| Brachial Systolic Pressure (mmHg) | 125±15 | 137±16 | 131±14 | 0.003 | 0.05 | 0.21 |
| Diastolic Pressure (mmHg) | 78±11 | 83±8.5 | 80±9.9 | 0.01 | 0.27 | 0.21 |
| Mean Pressure (mmHg) | 95±10 | 103±10 | 98±10 | 0.002 | 0.16 | 0.07 |
| Brachial Pulse Pressure (mmHg) | 47±9.7 | 54±13 | 52±11 | 0.05 | 0.12 | 0.53 |
| Aortic Systolic Pressure (mmHg) | 115±15 | 128±14 | 121±14 | 0.001 | 0.07 | 0.07 |
| Aortic Pulse Pressure (mmHg) | 36±7.9 | 45±11 | 41±9.3 | 0.002 | 0.04 | 0.17 |
| Forward Pressure Wave Amplitude (P1) (mmHg) | 27±5.4 | 30±7.4 | 30±7.3 | 0.13 | 0.09 | 0.96 |
| Reflected Pressure Wave Amplitude (AP) (mmHg) | 9.3±4.3 | 14±4.9 | 11±4.1 | 0.001 | 0.21 | 0.008 |
| Aortic Augmentation Index (%) | 24±8.7 | 32±6.6 | 27±6.6 | 0.001 | 0.20 | 0.003 |
| Ejection Duration (msec) | 309±23 | 331±17 | 327±23 | 0.001 | 0.002 | 0.47 |
| Systolic Duration of Reflected Wave (SDR) (msec) | 173±23 | 198±17 | 190±23 | 0.001 | 0.004 | 0.16 |
| Round Trip Travel Time of Wave to and from the periphery (Tr) (msec) | 137±14 | 133±7.3 | 138±6.9 | 0.22 | 0.52 | 0.04 |
| Diastolic Pressure Time Index (DPTI) (mmHg · sec) × 102 | 33±6.2 | 35±3.3 | 33±4.7 | 0.04 | 0.94 | 0.09 |
| Systolic Pressure Time Index (SPTI) (mmHg · sec) × 102 | 23±4.1 | 27±4.4 | 25±2.8 | 0.002 | 0.005 | 0.24 |
| Subendocardial Viability Ratio (DPTI/SPTI) | 1.5±0.22 | 1.36±0.24 | 1.33±0.23 | 0.008 | 0.001 | 0.65 |
| Systolic Pressure Time (msec) | 309±23 | 331±17 | 327±23 | 0.001 | 0.002 | 0.47 |
| Diastolic Pressure Time (msec) | 544±112 | 539±112 | 522±101 | 0.83 | 0.35 | 0.59 |
| Pulse Pressure Amplification (dimensionless) | 1.3±0.13 | 1.20±0.07 | 1.26±0.10 | 0.001 | 0.05 | 0.008 |
| Wasted LV Energy (dyne-sec/cm2) × 102 | 18±8.9 | 30±11 | 22±10 | 0.001 | 0.08 | 0.001 |
| Aortic Pulse Wave Velocity (cm/sec) | 667±101 | 894±117 | 747±93 | 0.01 | 0.05 | 0.001 |
| Heart Rate × Aortic Systolic Pressure (mmHg × beats/min) × 102 | 81±13 | 90±16 | 86±10 | 0.07 | 0.24 | 0.34 |
Data presented are mean±standard deviation or P values; CFR = coronary flow reserve; Ref = reference group.
Table III.
Correlations between coronary flow reserve and pertinent study variables.
| Variable | r | P |
|---|---|---|
| Age (years) | –0.29 | 0.04 |
| Brachial systolic pressure (mmHg) | –0.40 | 0.004 |
| Diastolic pressure (mmHg) | –0.35 | 0.01 |
| Mean pressure (mmHg) | –0.47 | 0.001 |
| Brachial pulse pressure (mmHg) | –0.22 | 0.11 |
| Aortic systolic pressure (mmHg) | –0.49 | 0.001 |
| Aortic pulse pressure (mmHg) | –0.35 | 0.01 |
| Forward pressure wave amplitude (P1)(mmHg) | –0.12 | 0.42 |
| Reflected pressure wave amplitude (AP) (mmHg) | –0.48 | 0.001 |
| Aortic augmentation index (%) | –0.44 | 0.001 |
| Ejection duration (msec) | –0.18 | 0.21 |
| Systolic duration of reflected wave (SDR)(msec) | –0.29 | 0.04 |
| Round trip travel time of wave to and from the periphery (Tr)(msec) | 0.29 | 0.04 |
| Diastolic pressure time index (DPTI)(mmHg · sec) × 102 | –0.38 | 0.006 |
| Systolic pressure time index (SPTI)(mmHg · sec) × 102 | –0.37 | 0.008 |
| Subendocardial viability ratio (DPTI/SPTI) | 0.007 | 0.96 |
| Systolic pressure time (msec) | 0.04 | 0.78 |
| Diastolic pressure time (msec) | –0.07 | 0.58 |
| Pulse pressure amplification (dimensionless) | 0.45 | 0.001 |
| Wasted LV energy (dyne-sec/cm2) × 102 | –0.47 | 0.001 |
| Aortic pulse wave velocity (cm/sec) | –0.51 | 0.001 |
| Heart rate × aortic systolic pressure (mmHg × beats/min) × 102 | –0.29 | 0.04 |
r = Pearson's correlation coefficient, LV = left ventricular
Aortic pulse wave velocity and pulse pressure amplification
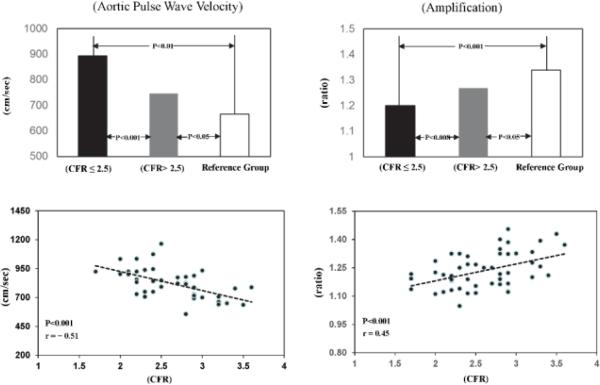
Aortic stiffness as aPWV was measured in 43 women. For the entire study group, linear regression analysis identified correlations between CFR and aPWV (r = −0.51; P <0.001) and between CFR and pulse wave amplification (r = 0.45; P <0.001) (Figure 2, Table III).
Figure 2.
Above: Average values of aortic pulse wave velocity (N = 43) and pulse pressure amplification (N = 50) in WISE women with CFR ≤2.5 (dark bar) compared with those with CFR >2.5 (grey bar) and a reference group of women (N = 59) (open bar). P values are shown for CFR ≤2.5 vs Reference Group; CFR ≤2.5 vs CFR >2.5; CFR >2.5 vs Reference Group.
Below: Scattergrams and linear regression lines for aortic pulse wave velocity and pulse pressure (PP) amplification brachial PP/aortic PP plotted against CFR in all WISE women. The same format is used for figures 3 and 4.
Women with CFR ≤2.5 vs. those with CFR >2.5 had greater aPWV (894±117 vs. 747±93 cm/sec; P <0.001); both are above normal (Figure 2,) indicating stiffer aortas.6 Also, pulse pressure amplification was less in women with CFR ≤2.5 (1.20±0.07 vs. 1.26±0.10; P <0.008).
Amplitude and timing of wave reflection indices
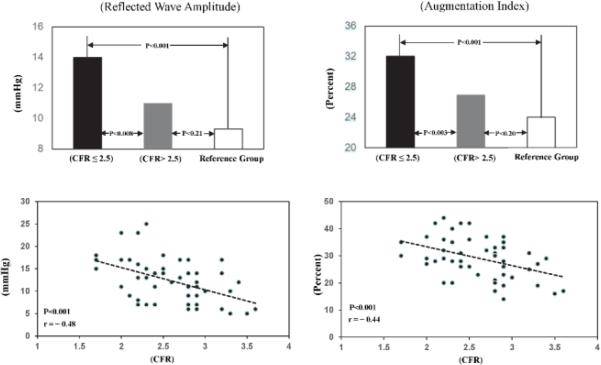
Reflected pressure wave amplitude, AP (14±4.9 vs 11±4.1 mm Hg; P <0.008) and augmentation index, AIx (32±6.6 vs 27±6.6 %; P <0.003) (Figure 3) were greater in women with CFR ≤2.5 vs. those with CFR >2.5 (Table III), while the systolic duration (SDR) of the reflected pressure wave was similar. Also, linear regression analysis for the entire group showed a significant correlation between CFR and AP (r = −0.48; P <0.001), AIx (r = −0.44; P <0.001) (Figure 3) and SDR (r = −0.29; P <0.04). Thus, increases in wave reflection amplitude and AIx resulted from increased wave reflection due to increased arterial stiffness, which is supported by increased aPWV and reduced pulse pressure amplification.
Figure 3.
Above: Average values of reflected wave amplitude and augmentation index in WISE women with CFR ≤2.5 compared with those with CFR >2.5 and the reference group.
Below: Scattergrams and linear regression lines for reflected wave amplitude and augmentation index plotted against CFR in all WISE women.
Indices of myocardial oxygen demand
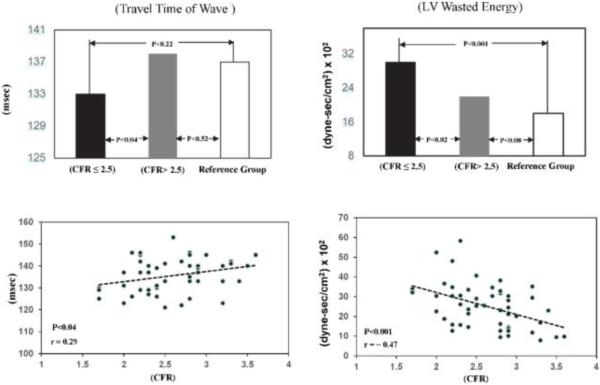
Linear regression analysis for the entire study group showed inverse correlations between CFR and indices of myocardial oxygen demand as LVEw (r = −0.47; P <0.001, Figure 4), SPTI (r = −0.37; P <0.008), and rate pressure product (r = −0.29; P <0.04). Differences in arterial wall properties and wave reflection characteristics indicate a significant increase in pulsatile LV afterload in women with CFR ≤2.5 vs. CFR>2.5. Travel time of the wave, Tr, to and from the periphery was less in women with CFR ≤2.5 (133±7.3 vs. 138±6.9 msec; P <0.04). This represents an increase in pulsatile afterload (i.e., wave reflection amplitude) and increase in LVEw (30±11 vs. 22±10 × 102 dyne-sec/cm2; P <0.001) (Figure 4). Finally, diastolic pressure time index (DPTI) and systolic pressure time index (SPTI) and their ratio (subendocardial viability ratio or supply/demand ratio) were similar in the two study groups.
Figure 4.
Above: Average values of travel time of pressure wave to and from periphery and left ventricular (LV) wasted energy in WISE women with CFR ≤2.5 compared with those with CFR >2.5 and the reference group.
Below: Scattergrams and linear regression lines for travel time of pressure wave to and from periphery and LV wasted energy plotted against CFR in all WISE women.
In the entire study group, no significant correlations were noted between CFR and forward pressure wave amplitude (P1), subendocardial viability ratio (DPTI/SPTI), bPP, systolic pressure time, diastolic pressure time (DPT), or ejection duration.
Multivariable Modeling
In the multiple regression model including aPWV, CFR was significantly correlated with aPWV (P < 0.008) and aortic systolic pressure (P < 0.01). (Table IV). Interestingly, age (×10 years), history of hypertension, history of diabetes, BMI (×10), and aortic SP (×10 mmHg) contributed only about 24% of the variance in CFR. Adding the indices of vessel stiffness (aPWV, travel time of wave, and pulse pressure amplification) explained ~50% of the variance in CFR. However, adding indices of wave reflections did not contribute significantly to increasing the R2.
Table IV.
Multivariable regression model
| Model | Variable | Coefficient | Standardized Coefficient | Model R2 (add in order) | ΔR2 (add in order) | P-value |
|---|---|---|---|---|---|---|
| Model 1: Covariates | Age (×10) | −0.04 (0.07) | −0.10 | 0.13 | 0.13 | 0.008 |
| Hypertension | −0.01 (0.13) | −0.01 | 0.13 | 0.0003 | 0.90 | |
| Diabetes | −0.07 (0.17) | −0.07 | 0.13 | 0.004 | 0.62 | |
| BMI (×10) | −0.10 (0.10) | −0.15 | 0.13 | <0.0001 | 0.98 | |
| Aortic SP (×10) | −0.01 (0.06) | −0.02 | 0.24 | 0.11 | 0.01 | |
| Model 2: Covariates + Vessel stiffness | aPWV | −0.002(0.0005) | −0.50 | 0.42 | 0.18 | 0.002 |
| Travel Time of Wave | 0.01(0.01) | 0.15 | 0.46 | 0.04 | 0.14 | |
| Amplification | 1.23(0.81) | 0.27 | 0.50 | 0.04 | 0.14 | |
| Model 3: Covariates + vessel stiffness + Wave reflection* | - | - | 0.52 | 0.02 | 0.76 |
Models (left column), variables included in the model, coefficient, standardized coefficient, Model R2 are the R2 of the multivariable regression model, and ΔR2 of a predictor is the change of model R2 when the predictor is added to the model. The P-value tests whether the ΔR2 is significantly different from zero.
Discussion
We have extended our observations to evaluate the influence of aortic stiffness and wave reflections on CFR in a sample of women with signs and symptoms of ischemia but non-obstructive CAD. Aortic stiffness, as aPWV and wave reflection information, was obtained from the synthesized central aortic pressure wave. Bivariate regression analysis was initially performed to investigate correlation between CFR and indices of aortic stiffness, wave reflections, and myocardial oxygen demand across the spectrum of CFR obtained. As in previous WISE reports, data were also summarized by subgroups with CFR ≤2.5 (linked with adverse outcomes)23 and CFR >2.5. Their age, height, weight, body mass index, heart rate, ejection duration, and DPT, conditions known to alter central aortic pressure waveform and indices of wave reflection6 as well as CFR12,16,31-35, were similar (Table I).
Using multivariable regression analysis we further developed relationships between CFR and vessel stiffness/wave reflection measurements adjusting for covariates (confounders) including age, history of hypertension, history of diabetes, BMI, aortic systolic pressure and aortic stiffness related variables. We found that these covariates are significantly correlated with CFR and explained about half of the total variance of CFR (sum of R2 for the final model R2 was 0.50). The aPWV appeared to contribute the most information to the model. Said another way, aortic stiffness appears to contribute more to CFR than the demographic and other lab variables studied previously in the absence of other conditions known to alter CFR. These observations indicate that alterations in arterial structure and function may partially exhaust CFR and contribute to vulnerability to myocardial ischemia. Our findings could help to explain the relationship between increased elastic artery stiffness and adverse outcomes reported by others1-3 and emphasize that multiple factors beyond flow limiting stenosis are likely involved in ischemic heart disease.
In healthy younger (age 39±12 years) men and women (n = 76)32 with CFR 3.32±0.3, heterogeneity in CFR was noted; however, in our women only 3 had a CFR >3.32. Therefore, the large majority of our women had reduced CFR, as expected since all had symptoms and/or signs of ischemic heart disease. It is important to recognize that different cardiovascular abnormalities may contribute to limit CFR. An increase in myocardial oxygen demand or increase in blood supply will decrease CFR.7 For example, a high grade coronary stenosis13,15, elevated basal coronary flow12,16,33, reduced DPT13,15, and reduced coronary microvascular dilatory capacity all reduce CFR. Therefore, it is often not possible to discriminate between coronary microvascular disease (CMD) and an increase in basal coronary flow due to increased myocardial oxygen demand that may also partially exhaust CFR.17 We found in our study that both elastic artery stiffness and wave reflection amplitude were inversely correlated with CFR but in multivariable analysis the wave reflection indices did not contribute significant additional information to CFR. Furthermore, a significant increase in adverse outcomes has been reported previously in WISE women with CFR≤2.5.23 This study, using direct measurements of aPWV, coronary blood flow velocity, and CFR, extends our initial observations5 that WISE women have detrimental changes in vessel stiffness characteristics and diastolic timing that increase indices of LV afterload, myocardial oxygen demand and wasted LV pressure energy to reduce coronary perfusion.7,13 These functional alterations contribute to an undesirable mismatch in myocardial oxygen supply/demand ratio that favors ischemia and angina.6,7
Our findings confirm and extend previous reports suggesting that increased arterial stiffness is inversely related to CFR in individuals with chest pain and non-obstructive coronary arteries.10 Also, both animal experiments16 and human studies10 have shown that increased aortic stiffness causes an increase in basal coronary flow which tends to decrease CFR since maximal stimulated flow does not change.17 Furthermore, acute experimental studies show that increased aortic stiffness causes an increase in myocardial oxygen demand16 and an increase in subendocardial ischemia14 and decrease in CFR even without epicardial coronary obstruction.15 Also, inelastic vascular prosthesis for the proximal aorta increases pulsatile arterial load and causes LV hypertrophy in chronic animal experiments without a change in mean arterial pressure or peripheral resistance.11 Such changes in cardiovascular properties and ventricular/vascular coupling cause a decrease in CFR.12,17 Less information is available on the effects of arterial properties and wave reflections on CFR in humans with normal coronary angiography, and none exclusively in women. An invasive study similar to ours found an inverse relation between CFR and aPWV in a small mixed group of patients with stable angina irrespective of the presence of a stenosis.21 In studies using non-invasive techniques in larger mixed groups of patients with no CAD, patients with CFR ≤2.5 had stiffer aortas than those with CFR >2.5.10 Also, two studies (one in mostly elderly Japanese men and the other in patients with heart failure [LV ejection fraction < 35%]) found an inverse relationship between AIx and CFR10,34, which is similar to the results of our study in women.
CFR varies according to age, sex32, heart rate33, and probably LV ejection duration, therefore it is essential to compare, as in our present study, CFR data collected in one patient group with those collected from a group with similar age, sex, heart rate, and ejection duration. Previous studies from our laboratory measured radial artery pressure waveforms using applanation tonometry, and the central aortic pressure waveform was synthesized using a generalized mathematical transfer function.5,28 Computer software was then used to obtain the various wave reflection indices from the aortic pressure waveform using PWA. In one study5, wave reflection characteristics and time intervals (systolic and diastolic) were investigated in a case-matched referenced sample of asymptomatic women and cases with chest pain and angiographically normal (or non-obstructed) epicardial coronary arteries from the WISE. Those results showed that the sample of women from the WISE had increased wave reflection characteristics including elevated LVEw and ejection duration and decreased pulse pressure amplification and DPT.
At least 50% of women undergoing coronary angiography for chest pain have non-obstructive epicardial coronary arteries. It is believed that CMD (likely due to endothelial and/or vascular smooth dysfunction), measured as CFR, is a predominant etiologic mechanism of ischemia (and angina) in this cohort of women. Earlier studies from WISE have observed that reduced CFR was associated with adverse outcomes (first occurrence of death, nonfatal myocardial infarction, nonfatal stroke, or heart failure hospitalization).23 These studies found an inverse relationship between event rate and CFR. Also, the recent study by Murthy et al36 in men and women without known CAD found that CFR was a powerful incremental predictor of adverse outcomes (cardiac death, nonfatal myocardial infarction, late revascularization, and heart failure hospitalization). In that study, CFR by positron emission tomography was inversely related to CMD, and CFR <2.0 was used to define CMD.
To our knowledge, our study is the first invasive investigation that directly measured aPWV and CFR exclusively in women without CAD. Our results indicate that CFR is inversely related to both aortic stiffness (Figure 2) and indices of wave reflection (Figures 3 and 4). Additionally, regression analysis showed an inverse relation between CFR and conventional indices of myocardial oxygen demand, namely SPTI and heart rate:aortic systolic pressure product, probably the result of increased aortic stiffness and reflected wave amplitude. Finally, the women displayed a significant inverse relationship between CFR and LVEw that implies an increase in myocardial mass30 and oxygen demand. It is well known that coronary flow is elevated in pathologic LV hypertrophy and that CFR is reduced12,32,33,35, which in subjects without obstructive atherosclerosis is suggestive of CMD. Furthermore, there is a positive relationship between myocardial oxygen demand and coronary flow.37 The underlying etiology for CMD is unknown. Although adenosine is mostly a direct vascular smooth muscle relaxing agent, there are adenosine receptors in the endothelium. Thus the reduced CFR observed in these women may include a component of endothelial dysfunction and reduced bioavailability of NO.38,39 Furthermore, we have shown in other studies that many women in the WISE study, when tested directly with intracoronary acetylcholine, have evidence for coronary endothelial dysfunction.19 Also, plasma levels of the endothelium-derived peptide endothelin-1, which is a potent vasoconstrictor, may be increased in this patient group.40 If this is the case, a treatment strategy that restores bioavailability of NO (e.g., phosphodiesterase type 5 inhibition) and inhibits endothelin-1 may improve CMD, increase CFR, and simultaneously decrease reflected wave amplitude.41 CMD in women with chest pain and other findings suggesting myocardial ischemia but non-obstructive CAD, along with increased reflected wave amplitude, are each known to be associated with major adverse cardiovascular events over follow up2,42-44, therefore successful treatment of the underlying vascular smooth muscle and endothelial cell dysfunction may be treatment targets to test in clinical trials aimed at improving outcomes. Indeed, Pauly et al20 showed that angiotensin-converting enzyme (ACE) inhibition increased CFR in women with non-obstructive coronary arteries. ACE inhibitors are vasodilators that are known to reduce wave reflection amplitude and lower central aortic pressure.6
Limitations
Invasive and non-invasive measurements of the various cardiovascular variables were not made at exactly the same time; however, they were performed within a very short time (2-4 hours) of each other. An earlier study found that LV ejection duration and systolic pressure time was greater in a group of WISE women versus an apparently healthy reference group.5 LV pulsatile afterload, heart rate × central systolic pressure product, and wasted energy (indicators of myocardial mass and oxygen demand) clearly are increased. However, DPT (an indicator of myocardial oxygen supply) was not different between women with CFR ≤2.5 and those with CFR >2.5; also, there was no relation between CFR and DPT in the entire group. The cause of these changes in arterial stiffness and wave reflection characteristics could be a change in wall properties of the elastic arteries or a change in smooth muscle tone of muscular arteries and arterioles (microcirculation). Vasodilator medication was similar in the two groups (44% with CFR ≤2.5 vs. 44% with CFR >2.5). Lastly, although our sample size was relatively small, these are the only such data yet to be reported exclusively in women. We believe that these women reflect a representative sample of the population of women with symptoms and/or signs of ischemia in the absence of epicardial CAD.
Conclusions
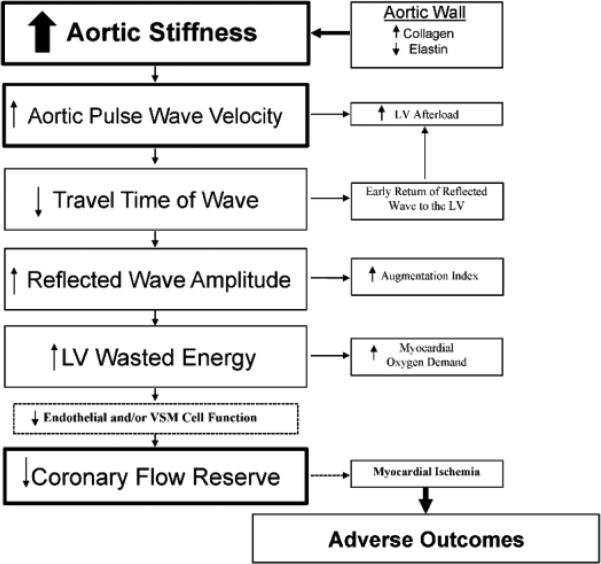
Among symptomatic women without obstructive CAD, increased aortic stiffness and, to a lesser degree, wave reflection characteristics increase LV afterload and are associated with increased wasted pressure energy and myocardial oxygen demand which may reduce NO production. These alterations in arterial structure and function are associated with decreased CFR and may lead to adverse outcomes (Figure 5). It is apparent that other factors, which were not measured in this study, may partially limit CFR and contribute to ischemic heart disease and related adverse outcomes.23,36
Figure 5.
Flow chart showing the progression and relationship between vessel stiffness/wave reflections and other factors (endothelial and/or vascular smooth muscle [VSM] cell dysfunction) and CFR.
Highlights.
Indices of aortic stiffness and wave reflections are increased in women with ischemia without obstructive CAD and reduced CFR.
The increase in these indices implies an increase in mechanical properties of the large elastic arteries.
These characteristics augment LV afterload requiring additional, but wasted, energy.
Accompanying increases in myocardial oxygen demand partially exhaust CFR.
Such changes in arterial properties and CFR increase vulnerability to myocardial ischemia.
Acknowledgments
Funding
This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR000064, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women's Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women's Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women's Health Research (SWHR), Washington, D.C., The Linda Joy Pollin Women's Heart Health Program, and the Erika Glazer Women's Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Dr. Nichols is a consultant for Millar Instruments, Inc (Houston, Texas).
References
- 1.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Zamani P, Jacobs DR, Jr, Segers P, et al. Reflection magnitude as a predictor of mortality: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2014;64:958–64. doi: 10.1161/HYPERTENSIONAHA.114.03855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–46. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutinho T. Arterial stiffness and its clinical implications in women. Can J Cardiol. 2014;30:756–64. doi: 10.1016/j.cjca.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Nichols WW, Denardo SJ, Johnson BD, et al. Increased wave reflection and ejection duration in women with chest pain and non-obstructive coronary artery disease: ancillary study from the Women's Ischemia Syndrome Evaluation. J Hypertens. 2013;31:1447–54. doi: 10.1097/HJH.0b013e3283611bac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols WW, O'Rourke MF, Vlachopoulos C. McDonald's Blood Flow in Arteries: Theoretic, Experimental and Clinical Principles. 6th edition Edward Arnold; London: 2011. [Google Scholar]
- 7.Hoffman JI, Buckburg GD. The myocardial oxygen supply:demand index revisited. J Am Heart Assoc. 2014;3:e000285. doi: 10.1161/JAHA.113.000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Namasivayam M, Adji A, O'Rourke MF. Influence of aortic pressure wave components determined noninvasively on myocardial oxygen demand in men and women. Hypertension. 2011;57:193–200. doi: 10.1161/HYPERTENSIONAHA.110.160200. [DOI] [PubMed] [Google Scholar]
- 9.Erdogan D, Yildirim I, Ciftci O, et al. Effects of normal blood pressure, prehypertension, and hypertension on coronary microvascular function. Circulation. 2007;115:593–9. doi: 10.1161/CIRCULATIONAHA.106.650747. [DOI] [PubMed] [Google Scholar]
- 10.Saito M, Okayama H, Nishimura K, et al. Possible link between large artery stiffness and coronary flow velocity reserve. Heart. 2008;94:e20. doi: 10.1136/hrt.2007.126128. [DOI] [PubMed] [Google Scholar]
- 11.Morita S, Asou T, Kuboyama I, et al. Inelastic vascular prosthesis for proximal aorta increases pulsatile arterial load and causes left ventricular hypertrophy in dogs. J Thorac Cardiovasc Surg. 2002;124:768–74. doi: 10.1067/mtc.2002.124244. [DOI] [PubMed] [Google Scholar]
- 12.O'Gorman DJ, Thomas P, Turner MA, et al. Investigation of impaired coronary vasodilator reserve in the guinea pig heart with pressure induced hypertrophy. Eur Heart J. 1992;13:697–703. doi: 10.1093/oxfordjournals.eurheartj.a060237. [DOI] [PubMed] [Google Scholar]
- 13.Ferro G, Duilio C, Spinelli L, et al. Relation between diastolic perfusion time and coronary artery stenosis during stress-induced myocardial ischemia. Circulation. 1995;92:342–7. doi: 10.1161/01.cir.92.3.342. [DOI] [PubMed] [Google Scholar]
- 14.Nichols WW, Mehta JL, Donnelly WH, et al. Reduction in coronary vasodilator reserve following coronary occlusion and reperfusion in anesthetized dog: role of endothelium-derived relaxing factor, myocardial neutrophil infiltration and prostaglandins. J Mol Cell Cardiol. 1988;20:943–54. doi: 10.1016/s0022-2828(88)80148-2. [DOI] [PubMed] [Google Scholar]
- 15.Feldman RL, Nichols WW, Pepine CJ, Conti CR. Influence of aortic insufficiency on the hemodynamic significance of a coronary artery narrowing. Circulation. 1979;60:259–68. doi: 10.1161/01.cir.60.2.259. [DOI] [PubMed] [Google Scholar]
- 16.Saeki A, Recchia F, Kass DA. Systolic flow augmentation in hearts ejecting into a model of stiff aging vasculature. Influence on myocardial perfusion-demand balance. Circ Res. 1995;76:132–41. doi: 10.1161/01.res.76.1.132. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman JI. Maximal coronary flow and the concept of coronary vascular reserve. Circulation. 1984;70:153–9. doi: 10.1161/01.cir.70.2.153. [DOI] [PubMed] [Google Scholar]
- 18.O'Rourke MF. How stiffening of the aorta and elastic arteries leads to compromised coronary flow. Heart. 2008;94:690–1. doi: 10.1136/hrt.2007.134791. [DOI] [PubMed] [Google Scholar]
- 19.Denardo SJ, Wen X, Handberg EM, et al. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a Women's Ischemia Syndrome Evaluation (WISE) ancillary study. Clin Cardiol. 2011;34:483–7. doi: 10.1002/clc.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, non-obstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678–84. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bairey-Merz CN, Kelsey SF, Pepine CJ, et al. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–61. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 22.Cannon RO., III Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J Am Coll Cardiol. 2009;54:877–85. doi: 10.1016/j.jacc.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–75. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 25.Reis SE, Holubkov R, Conrad Smith AJ, et al. WISE Investigators Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 26.Weber T, Ammer M, Rammer M, et al. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27:1624–30. doi: 10.1097/HJH.0b013e32832cb04e. [DOI] [PubMed] [Google Scholar]
- 27.Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–7. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 28.Denardo SJ, Nandyala R, Freeman GL, et al. Pulse wave analysis of the aortic pressure waveform in severe left ventricular systolic dysfunction. Circ Heart Fail. 2010;3:149–56. doi: 10.1161/CIRCHEARTFAILURE.109.862383. [DOI] [PubMed] [Google Scholar]
- 29.Casey DP, Beck DT, Nichols WW, et al. Effects of enhanced external counterpulsation on arterial stiffness and myocardial oxygen demand in patients with chronic angina pectoris. Am J Cardiol. 2011;107:1466–72. doi: 10.1016/j.amjcard.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashimoto J, Nichols WW, O'Rourke MF, et al. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: influence of arterial wave reflection. Am J Hypertens. 2008;21:329–33. doi: 10.1038/ajh.2007.49. [DOI] [PubMed] [Google Scholar]
- 31.Rigo F. Coronary flow reserve in stress-echo lab. From pathophysiologic toy to diagnostic tool. Cardiovasc Ultrasound. 2005;3:8. doi: 10.1186/1476-7120-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chareonthaitawee P, Kaufmann PA, Rimoldi O, et al. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50:151–61. doi: 10.1016/s0008-6363(01)00202-4. [DOI] [PubMed] [Google Scholar]
- 33.Rossen JD, Winniford MD. Effect of increases in heart rate and arterial pressure on coronary flow reserve in humans. J Am Coll Cardiol. 1993;21:343–8. doi: 10.1016/0735-1097(93)90673-o. [DOI] [PubMed] [Google Scholar]
- 34.Snoer M, Monk-Hansen T, Olsen RH, et al. Insulin resistance and exercise tolerance in heart failure patients: linkage to coronary flow reserve and peripheral vascular function. Cardiovasc Diabetol. 2012;11:97. doi: 10.1186/1475-2840-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cecchi F, Olivotto I, Gistri R, et al. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–35. doi: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 36.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khouri EM, Gregg DE, Rayford CR. Effect of exercise on cardiac output, left coronary flow and myocardial metabolism in the unanesthetized dog. Circ Res. 1965;17:427–37. doi: 10.1161/01.res.17.5.427. [DOI] [PubMed] [Google Scholar]
- 38.Chilian WM, Kuo L, DeFily DV, et al. Endothelial regulation of coronary microvascular tone under physiological and pathophysiological conditions. Eur Heart J. 1993;14(Suppl I):55–9. [PubMed] [Google Scholar]
- 39.Lapu-Bula R, Ofili E. From hypertension to heart failure: role of nitric oxide-mediated endothelial dysfunction and emerging insights from myocardial contrast echocardiography. Am J Cardiol. 2007;99:7D–14D. doi: 10.1016/j.amjcard.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann E, Assennato P, Donatelli M, et al. Plasma endothelin-1 levels in patients with angina pectoris and normal coronary angiograms. Am Heart J. 1998;135:684–8. doi: 10.1016/s0002-8703(98)70286-8. [DOI] [PubMed] [Google Scholar]
- 41.Schofield RS, Edwards DG, Schuler BT, et al. Vascular effects of sildenafil in hypertensive cardiac transplant recipients. Am J Hypertens. 2003;16:874–7. doi: 10.1016/s0895-7061(03)01006-9. [DOI] [PubMed] [Google Scholar]
- 42.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 43.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 44.Leoncini G, Ratto E, Viazzi F, et al. Increased ambulatory arterial stiffness index is associated with target organ damage in primary hypertension. Hypertension. 2006;48:397–403. doi: 10.1161/01.HYP.0000236599.91051.1e. [DOI] [PubMed] [Google Scholar]