Abstract
Regular physical activity in healthy individuals prevents development of chronic musculoskeletal pain; however, the mechanisms underlying this exercise-induced analgesia are not well understood. Interleukin-10(IL-10), an anti-inflammatory cytokine which can reduce nociceptor sensitization, increases during regular physical activity. Since macrophages play a major role in cytokine production and are present in muscle tissue, we propose that physical activity alters macrophage phenotype to increase IL-10 and prevent chronic pain. Physical activity was induced by allowing C57BL/6J mice free access to running wheels for 8 weeks and compared to sedentary mice with no running wheels. Using immunohistochemical staining of the gastrocnemius muscle to label regulatory (M2, secretes anti-inflammatory cytokines) and classical (M1, secretes proinflammatory cytokines) macrophages, the percentage of M2-macrophages increased significantly in physically active mice (68.5±4.6% of total) compared to sedentary mice (45.8±7.1% of total). Repeated acid injections into the muscle enhanced mechanical sensitivity of the muscle and paw in sedentary animals that does not occur in physically active mice; no sex differences occur in either sedentary or physically active mice. Blockade of IL-10 systemically or locally prevented the analgesia in physically active mice, i.e. mice developed hyperalgesia. Conversely, sedentary mice pretreated systemically or locally with IL-10 had reduced hyperalgesia after repeated acid injections. Thus, these results suggest that regular physical activity increases the percentage of regulatory macrophages in muscle and that IL-10 is an essential mediator in the analgesia produced by regular physical activity.
Keywords: pain, exercise, analgesia, physical activity, IL-10, cytokine, anti-inflammatory, macrophage, immune
I. Introduction
Large population studies show physically active individuals have a significantly lower risk for development of chronic pain [26;27] suggesting that regular physical activity prevents the development of chronic pain. Similarly, we previously show that 8 weeks of running wheel activity in mice prevents development of chronic non-inflammatory, but not acute inflammatory, pain [57]. Further, regular physical activity improves chronic pain in people with a variety of chronic pain conditions [3;11;16;20;30;32;50;60;70]. In healthy individuals, regular physical activity alters cytokine profiles with decreases in expression of pro-inflammatory cytokines, such as TNFα and IL-6, and increases in IL-10, an anti-inflammatory cytokine [18;42]. It is unclear if this increase in anti-inflammatory cytokines is responsible for the prevention and reduction of pain with regular physical activity.
The balance between pro- and anti-inflammatory cytokines is a key concept in understanding the effect the immune system has on muscle pain. Macrophages are immune cells playing a variety of roles in local tissues, including phagocytosis, antigen presentation, wound healing, and immune regulation following infection. Macrophages show remarkable plasticity changing their phenotype in response to environmental signals, and therefore, are able to take on either pro-inflammatory or anti-inflammatory roles in the immune system [34]. Classically activated (M1) macrophages are characterized by their ability to secrete high levels of pro-inflammatory cytokines (IL-1, IL-6, TNFα, and IL-23) which are key components in host immune defense [34;46]. Conversely, regulatory M2 macrophages are characterized by their ability to secrete anti-inflammatory cytokines (IL-10, IL-4) which are involved with the end stages of immune response dampening inflammation and promoting healing [34]. Whereas pro-inflammatory cytokines result in nociceptor activation and sensitization, IL-10 is able to reduce nociceptor sensitization and produce analgesia [1;31;44]. Animal studies examining exercise effects on the immune system show that physical activity alters phagocytosis, chemotaxis, and antigen presentation capacity of tissue macrophages indicating that regular physical activity can change resident macrophage function and phenotype [22;68].
Tissue resident macrophages are of embryologic origin and are distinct from blood monocyte derived macrophages (MDMs)[12]. Recent work suggests resident macrophages self-renew, are maintained through adulthood, and respond to mild insults without infiltration from blood monocytes. Tissue resident macrophages while of common origin have distinct roles in different tissues [10]. Exercising muscle secretes factors (protons, lactate, ATP) [19;23;29;40] that can directly activate macrophages [34;64] and change phenotype in response to external stimuli. For example, extracellular ATP enhances pro-inflammatory cytokine release [41;69], and promotes conversion to an M2 phenotype [5;6] while protons enhance endocytosis and secretion of IL-10 from macrophages [25].
Therefore, we hypothesized that regular physical activity (exercise) changes macrophage phenotype from pro-inflammatory M1 to the anti-inflammatory M2, which in turn increases IL-10 release to prevent development of chronic muscle hyperalgesia. To determine this, we examined: 1) if macrophage phenotypes are different in the muscle of sedentary vs. physically active mice; 2) if blocking IL-10 receptors prevents the protective effects of regular physical activity and; 3) if administering IL-10 either systemically or locally in sedentary mice mimics the protective effects of regular physical activity.
II. Methods and Materials
Animals
All experiments were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Experiments performed in male C57BL/6 mice (Jackson Laboratories) and started when the mice were 8–12 weeks old. All animals were housed individually for the duration of the experiment so that running wheel groups and control groups were housed in similar conditions. An additional set of male and female C57BL/6 animals tested for sex differences before and after induction of CMP, and between exercised and non-exercised animals. Animals were housed in the Animal Care Unit and had free access to food and water ad libitum throughout the experimental period.
Physical Activity Protocol
All experiments were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the NIH Guide for the Care and Use of Laboratory Animals. For the physical activity group, 8–12 week old male C57BL/6 mice were placed in cages with running wheels and given free access to running wheels in their home cages to increase physical activity levels for 8 weeks. For the sedentary group, mice were placed in cages without running wheels for the same 8 weeks duration. Both the running wheel and the sedentary groups were housed individually for the duration of the experiment. We specifically chose to use running wheels instead of treadmill running to minimize stress since stress can modulate nociception [4;24;35] to enhance or reduce pain behavior, thus confounding interpretation. Running wheels allow the animal to choose the timing, speed and duration of an exercise bout. Wheel running by normal rats does not induce increases in chronic stress markers: hypertrophy of the adrenal gland or increase the catecholamine content in the left ventricle of the heart [47]. Mice had free access to running wheels for the 8 weeks prior to induction of the model, i.e. injection 1 of pH 4.0 saline. Once the model was induced, running wheels were removed from their cages for the duration of the experiment. Animals continued to be housed individually. It is possible that stopping the running wheel activity and removing it from the cage could by itself cause stress by changing the environment.
Muscle tissue preparation and macrophage immunohistochemistry
Male C57BL6 mice were perfused with 4% paraformaldehyde and left gastrocnemius muscles were removed. Muscle tissues were then frozen using a Stand-Alone Gentle Jane Snap Freezer. Microscope slides were prepared by taking one 20µm section per 200µm of tissue using Leica Cryocut 1800 at −20°C for a total of 25–30 sections of tissue per gastrocnemius muscle. Macrophages in the muscle tissue were stained using primary antibody rat anti-mouse F4/80 (1:250, AbD Serotec, Hercules, CA) and secondary antibody donkey anti-rat Dylight488 (1:500, Jackson ImmunoResearch, West Grove, PA). Macrophages were also co-stained to detect the integrin CD11c (an M1 macrophage marker) or the mannose receptor CD206 (an M2 macrophage marker). Labeling of CD11c was done using hamster anti-mouse CD11c primary antibody (1:100, BioLegend, San Diego, CA) and secondary antibody goat anti-Armenian hamster Rhodamine-Red-X-conjugated (1:100, Jackson ImmunoResearch, Hercules, CA). Labeling of CD206 was done using biotinylated rat anti-mouse CD206 (1:200, BioLegend, San Diego, CA) and streptavidin, Alexafluor 568 conjugate (1:100, Jackson ImmunoResearch, Hercules, CA). Slides were coverslipped with VectaShield and stored at −20°C until analysis.
Images were captured a Bio-Rad 1024 Confocal Microscope with a 20× objective lens and processed with Image J (NIH). All images for a given stain were taken under the same conditions and pictures were stored for off-line analysis. We counted F4/80 alone and the merged image for the specific stain (CD11c or CD206 + F4/80). To ensure similar sampling between groups and across the muscle, multiple sections were systematically taken across the entire muscle. Specifically one 20 µm section was taken every 200 µm for each gastrocnemius muscle for a total of 20–25 cross sections, and thus we analyzed 20–25 sections for each muscle for both the M1 and the M2 analysis. The entire muscle section was analyzed for macrophage content. We counted the total number of macrophages (F4/80) and the percentage that were double-labeled for the M1 (CD11c) or the M2 (αCD206) marker. An average of 123 (±43 SEM) macrophages per animal was counted in the muscle from physically active mice, and 188 (±43 SEM) macrophages per animal were counted in muscle from sedentary mice. A final total of 4 sedentary and 3 running wheel mice were used in these experiments. One animal was removed from the running wheel mice due to poor perfusion leading to inadequate immunohistochemistry staining. Power analysis showed significance of p<0.05 for the M2 immunohistochemistry differences resulted in 81% with the current data. Power analysis for the M1 sample showed to obtain a p<0.05 and 80% power we would need 16 animals per group. Thus, we did not increase sample size in this set of data.
Chronic Muscle Pain Model
Chronic pain was induced using acidic saline injections previously developed and characterized by our laboratory in rats and mice [56;58]. This model produces widespread mechanical hyperalgesia of the paw, muscle and viscera without tissue damage or infiltration of immune cells [15]. Male C57BL/6 mice were given two 20µl pH 4.0±0.1 preservative-free saline injections into the left gastrocnemius muscle 5 days apart. Mice were anesthetized briefly (<1min) with isoflurane (2–4%) during the injection. The pH was adjusted with HCl within 0.1 pH unit prior to injection. Decreases in muscle withdrawal thresholds normally occur 24 hours after the second injection in sedentary animals [56;58].
Pain Behavior and hyperalgesia
Muscle hyperalgesia was assessed using a calibrated force-sensitive tweezers to apply pressure across the gastrocnemius muscle as previously described [54;67;71]. Mice were placed head first into a gardener’s glove with the hind limb held in extension. Measurements of muscle withdrawal thresholds were taken at the minimum pressure required to cause hind limb withdrawal, and a total of three measurements were taken on each limb during each test which was then averaged. Mice were acclimated to this procedure with twice daily 5-minute sessions over a 2-day period prior to the first saline injection. Decrease in muscle withdrawal thresholds was interpreted as hyperalgesia; an increase in withdrawal threshold was interpreted as analgesia. In some mice the number of responses to repeated stimulation of the paw with an 0.4 mN von Frey filament was tested as we previously describe [57]. The number of paw withdrawals, out of 5, were counted, and 10 trials were averaged at each time period. An increase in the number of responses was interpreted as hyperalgesia.
IL-10 receptor blockade
To determine the effects of IL-10 and regular physical activity on musculoskeletal pain, mice which had undergone 8 weeks of regular physical activity or sedentary control mice were given local intramuscular (0.67µg/µL antibody, 15µL injection) into the left gastrocnemius muscle or intraperitoneal (i.p.) injections (2.5µg/µL antibody, 100µL injection) of either IL-10 receptor antibody or control IgG antibody (n=7 for sedentary local control IgG group, n=10 for physically active local aIL-10R group, n=8 for all other groups, BioXCell, West Lebanon, NH). All mice were administered two pH 4.0 saline injections 5 days apart as previously described. For mice given injections into the left gastrocnemius muscle, IL-10R or control IgG antibody was given 30 minutes prior to the second to the second pH 4.0 saline injection. For mice given systemic intraperitoneal injections, IL-10R or control IgG antibody was given one day prior to the second pH 4.0 injection. Muscle withdrawal threshold were then tested one day after the second pH 4.0 saline injection. The same protocol for local injections was also repeated for local injection of IL-10R on the right (contralateral) gastrocnemius muscle in sedentary and exercise mice (n=4 for each group).
IL-10 administration in sedentary mice
To determine if IL-10 plays a role in reducing musculoskeletal pain, sedentary mice were given either local or systemic doses of IL-10 cytokine and compared to vehicle. For local administration, 20µL of IL-10 (0.375ng/µL, R&D Systems, Minneapolis, MN) or PBS were administered locally into the left gastrocnemius muscle daily over 10 days (n=8 for each group). pH 4.0 saline injections were given on Day 5 and Day 10. Systemic doses of IL-10 or PBS (n=6 for each group) were administered at a constant rate of 2µg/day (0.33µg/µL at a rate of 0.25µL/hr) with an osmotic minipump (Alzet, Model 2007, Cupertino, CA) implanted subcutaneously for 9 days. pH 4.0 saline injections were given on Day 4 and Day 9.
Statistical Analysis
Data are presented as the mean ± S.E.M. The total number of macrophages, and macrophage percentages were analyzed with a one-way ANOVA for differences between groups. Behavioral tests were analyzed with a repeated measures ANOVA for differences across time and between groups. A post-hoc Tukey’s test examined for differences between groups. A two way (sex, running wheel) repeated measures ANOVA compared differences across time (before and after CMP). P<0.05 was considered statistically significant.
III. Results
R.1 Long-term physical activity alters macrophage phenotype populations in muscle
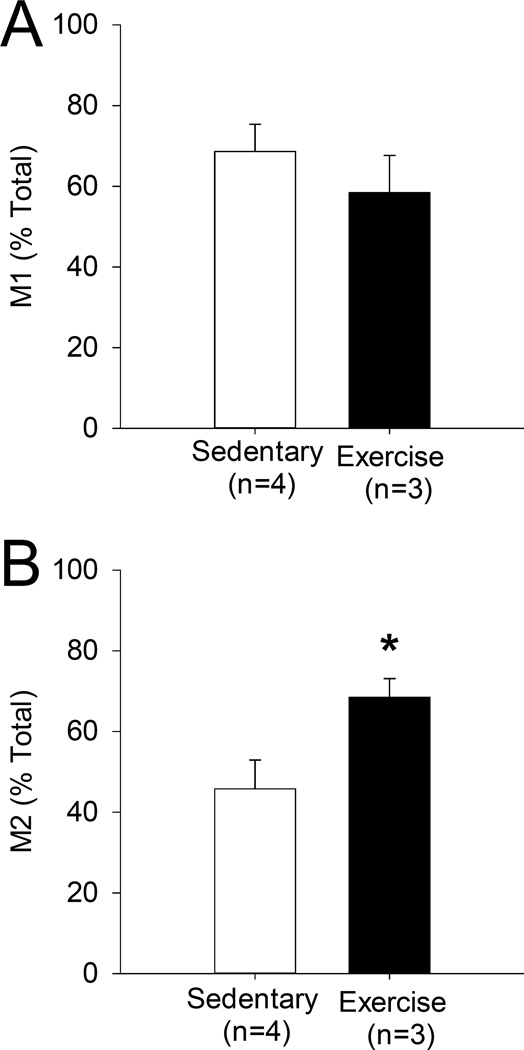
To determine if long-term physical activity influences macrophage phenotype in muscle, macrophages expressing the M2 and M1 markers, CD206 and CD11c, respectively, were quantified in the gastrocnemius muscle of mice that had undergone 8 weeks of physical activity and compared to mice that did not have access to running wheels. An average of 123 (±43 SEM) macrophages per animal were counted from muscle of physically active mice and 188 (±43 SEM) macrophages were counted from in muscle of sedentary mice. There were no significant differences between the total number of macrophages between groups (p=0.38, t-test). Representative images stained to detect the pan macrophage marker F4/80 along with CD206 or CD11c are shown for both physically active and sedentary mice (Figure 1,2). In muscles from physically active mice, there was an increase in the proportion of M2 macrophage phenotype within muscle, with 68.5% (±4.6% SEM) in physically active muscle compared to 45.8% (±7.1% SEM) CD206+ macrophages in sedentary muscle (p=0.02, t-test) (Figure 3). No significant difference was observed in M1 macrophage populations between muscles from physically active mice compared to muscles from sedentary mice (58.5% ±9.2 SEM and 68.6% ±6.8 SEM in physical activity and sedentary muscle respectively) (Figure 3).
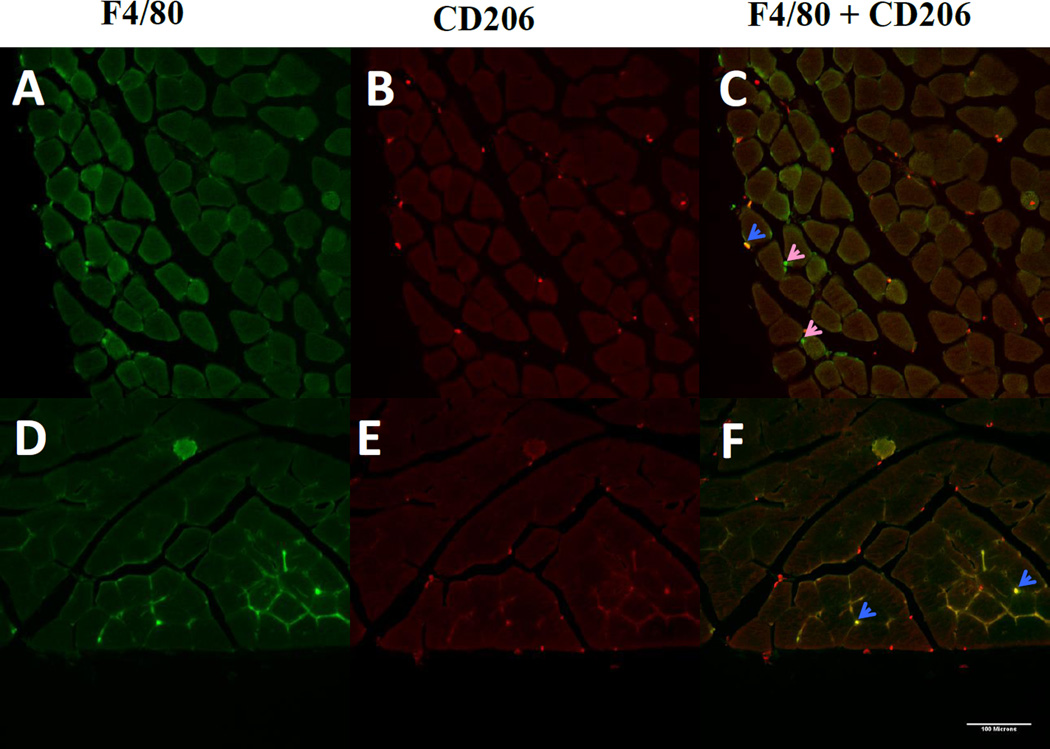
Figure 1. Immunofluorescent detection of regulatory macrophages.
Macrophages in gastrocnemius of muscle after 8 weeks of sedentary lifestyle (A–C) or 8 weeks of running wheel activity (D–F). Total macrophages were detected using antibodies to the pan-macrophage marker F4/80, shown in green (A,D). Sections were co-stained to detect the M2 maker CD206, shown in red (B,E). Co-localization of F4/80 and CD206 (C,F) shown in areas of yellow indicate regulatory macrophages (blue arrows). Pink arrows indicate F4/80+ macrophages that are CD206-negative.
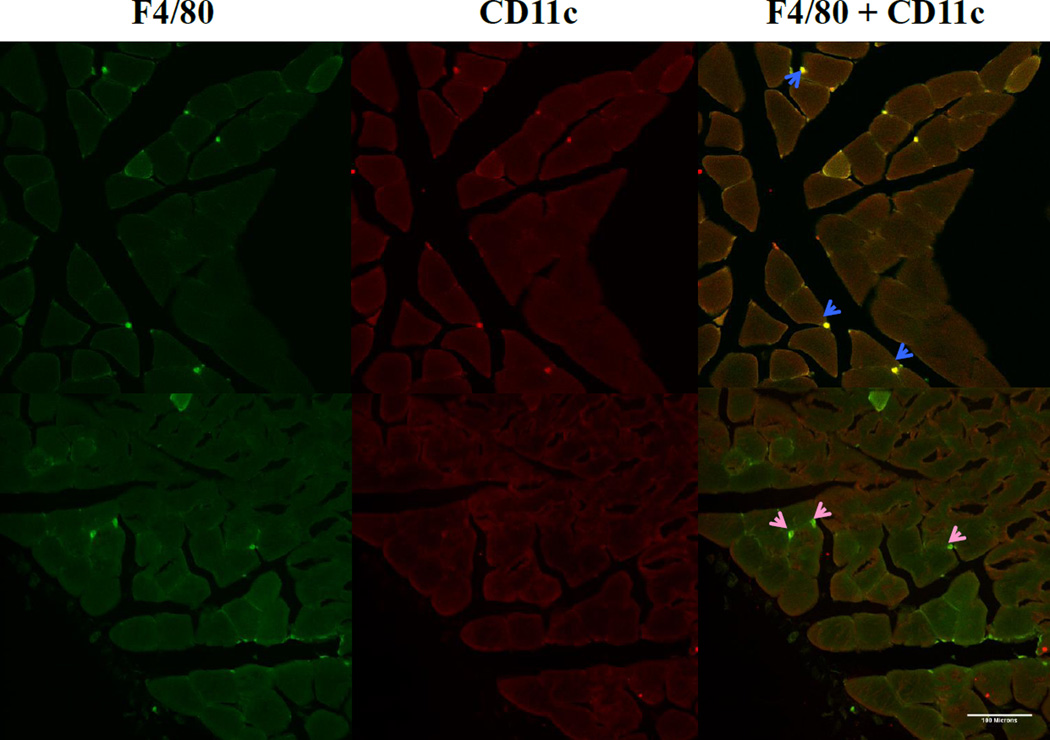
Figure 2. Immunofluorescent detection of classical macrophages.
Staining of macrophages in gastrocnemius of muscle after 8 weeks of sedentary lifestyle (A–C) or 8 weeks of running wheel activity (D-F). Sections were double-stained to detect total macrophages (F4/80, shown in green) (A,D) and the M1 marker CD11c, shown in red (B,E). Co-localization of F4/80 and CD11c markers (C,F) shown in areas of yellow indicate classically-activated macrophages (blue arrows). Pink arrows indicate F4/80+ macrophages that do not express CD11c.
Figure 3. Macrophage phenotypes in sedentary vs. physically active muscle.
After immunohistochemical staining, macrophages in the muscle were counted and F4/80+/CD11c+ (A) and F4/80/CD206+ (B) macrophage populations were compared between sedentary and physically active muscle. Exercise muscle showed significantly higher populations of M2 macrophages (*p=0.02). There were no differences in the proportion of M1 macrophages. No changes in total macrophage
R.2. Effects of chronic muscle pain model and running wheel activity in male and female mice
We previously show that repeated intramuscular injections of acidic saline produces bilateral decreases in muscle withdrawal threshold and bilateral increases in the number of paw withdrawals to noxious mechanical stimuli [57;58]. Similarly, Table 1 shows in male and female mice there is a significant decrease in the muscle withdrawal threshold bilaterally 24h after induction of the chronic muscle pain model (p=0.0001 ipsilateral and contralateral). There is also a significant increase in the number of responses to noxious mechanical stimuli bilateral 24h after induction of the chronic muscle pain model (p=0.0001 ipsilateral and contralateral). Our prior study showed that 8 weeks of running wheel activity prevented the development of hyperalgesia in a model of chronic muscle pain induced by repeated intramuscular acid injections [57]. We similarly show that in both male and female mice the decrease in muscle withdrawal thresholds and increase in number of responses to mechanical stimulation does not occur and are significantly different than sedentary male and female mice (Table 1)(p=0.0001). There was no effect for sex. Male and female sedentary mice both show bilateral increased sensitivity (i.e. hyperalgesia) to mechanical stimulation of the muscle and paw after acid injections, and male and female physically active mice both show no development of hyperalgesia after induction of chronic muscle pain.
Table 1.
Muscle withdrawal threshold and reponses to mechanical stimulation before and after induction of chronic muscle pain and in sedentary mice compared to those that performed 8 weeks of running wheel activity.
| Outcome Measure | Side | Non-runner | Runner | ||
|---|---|---|---|---|---|
| Male (n=5) |
Female (n=5) |
Male (n=10) |
Female (n=12) |
||
| Baseline muscle withdrawal threshold (mN) | Ipsilateral | 1713±33 | 1764±45 | 1788±34 | 1720±20 |
| Contralateral | 1806±57 | 1808±30 | 1858±31 | 1784±23 | |
| Baseline paw withdrawal threshold (number of responses, 0–5) | Ipsilateral | .42±.16 | .32±.08 | .63±.11 | .58±.13 |
| Contralateral | .28±.11 | .42±.11 | .40±.09 | .50±.12 | |
| CMP muscle withdrawal threshold (mN) | Ipsilateral | 1154±38 | 1097±64 | 1782±43 | 1682±23 |
| Contralateral | 1406±58 | 1223±25 | 1789±37 | 1753±30 | |
| CMP paw withdrawal threshold (number of responses, 0–5) | Ipsilateral | 2.66±.24 | 2.8±.26 | .62±.11 | .71±.14 |
| Contralateral | 2.28±.42 | 2.48±.41 | .48±.13 | .68±.13 | |
R.3 Blockade of IL-10 receptors prevents the protective effects of physical activity
Our prior study showed that 8 weeks of running wheel activity prevented the development of hyperalgesia in a model of chronic muscle pain induced by repeated intramuscular acid injections [57]. Since IL-10 is released by M2 macrophages and produces analgesic effects, we compared muscle withdrawal thresholds from mice treated with Il-10 receptor blocking antibody or control IgG after 8 weeks of running wheel activity and repeated acid injections.
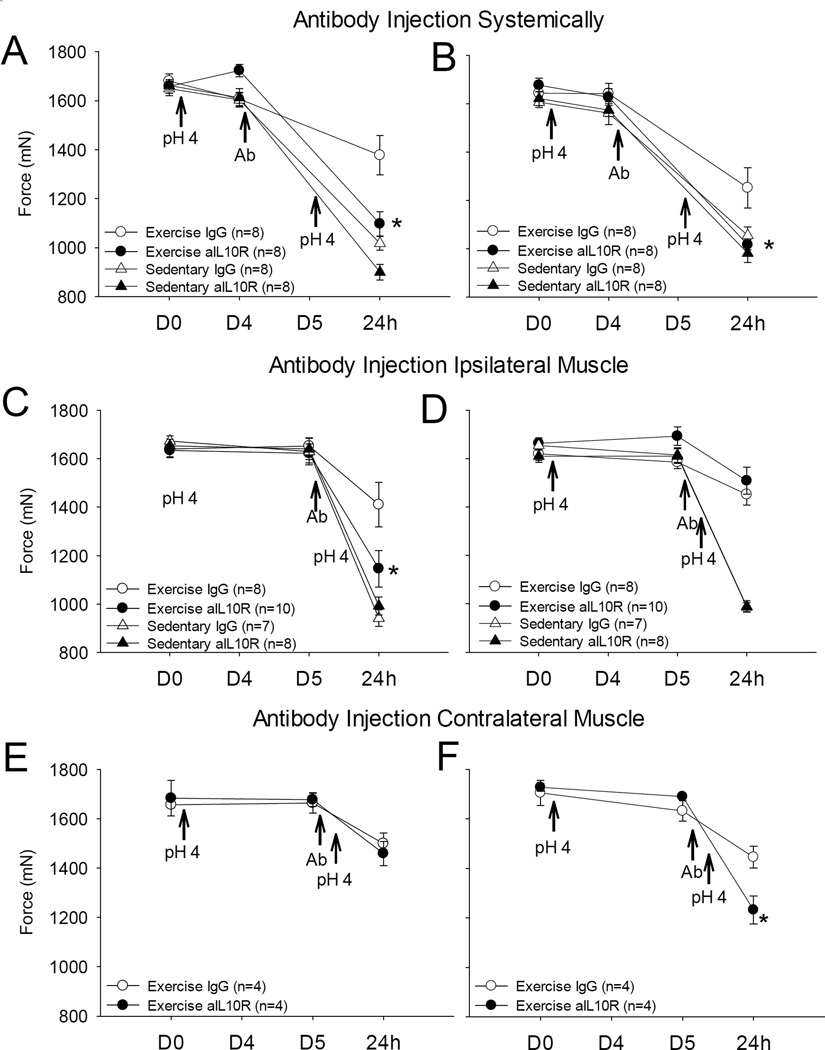
Sedentary mice injected with the control antibody showed a significant decrease in withdrawal thresholds bilaterally. In contrast, 8 weeks of running wheel activity prevented this decrease in withdrawal threshold. Systemic blockade of IL-10R prevented the analgesia produced by 8 weeks of running wheel activity (p < 0.005). Muscle withdrawal thresholds of physically active mice given systemic IL-10 receptor antibody were 1097 mN (± 49 SEM) and 1016 mN (± 21 SEM) in ipsilateral (left) and contralateral (right) hind limbs respectively, while in mice given control injections had muscle withdrawal thresholds of 1377 mN (± 80 SEM) and 1252 mN (± 84 SEM) in left and right hind limbs respectively (Figure 4). The withdrawal thresholds and hyperalgesia were similar and were not significantly different from to sedentary mice given either IL-10 receptor antibody or control antibody (IL-10R block left hind limb 899 mN ± 32 SEM, Control left hind limb 1017 mN ± 29 SEM; IL-10R block right limb 982 mN ± 38 SEM, Control right hind limb 1056 mN ± 35 SEM). (Figures 4)
Figure 4. IL-10 receptor blockade.
Systemic and local injections of IL-10R antibody (aIL-10R) were given 24 hours (i.p., systemic) or 30 minutes (i.m. gastrocnemius, local) before the second pH 4.0 saline injection in sedentary and physically active groups. The exercise group given aIL-10R systemically showed a decrease in withdrawal threshold on (A) ipsilateral and (B) contralateral hind limbs that was less than the group that received the control IgG injection. Local injections of aIL-10R into ipsilateral (side of pH injection) gastrocnemius (C and D) showed a decrease in threshold only on the (C) side of antibody injection and was significantly lower than the group that received the control IgG injection. Contralateral gastrocnemius injection of antibody (E and F) also showed a decrease in threshold only on the side of antibody injection and was significantly lower than the group that received the control IgG injection (F). (*p<0.01 vs. physically active IgG control).
To assess if local blockade of IL-10 receptors in the muscle prevents the analgesia produced by 8 weeks of running wheel activity, IL-10R antibody was administered intramuscularly prior to the second pH 4.0 saline injection There was a significant decrease in muscle withdrawal threshold in the left (ipsilateral) hind limb of physical activity mice administered IL-10R blocking antibody (1146 mN ± 76 SEM) compared to the left hind limb of physically active mice injected with the control IgG antibody (1410 mN ± 92 SEM) (p < 0.001). No differences were observed in right (contralateral) limb muscle withdrawal thresholds which did not receive IL-10R blocking antibody; muscle withdrawal thresholds were 1508 mN (± 56 SEM) and 1452 mN (± 44 SEM) in mice given IL-10R antibody vs those given the control IgG antibody, respectively (Figures 4C and 4D). Administration of IL-10R blocking antibody had no effect on muscle withdrawal thresholds in sedentary mice in both left (992 mN ± 38 SEM and 941 mN ± 34 SEM in mice given IL-10R antibody vs. control respectively) and right (986 mN ± 18 SEM and 989 mN ± 24 SEM, IL-10R antibody vs. control respectively) hind limbs. We then tested if the effects of the IL-10R antibody given in the muscle had a systemic effect by applying the IL-10R blocking antibody to the contralateral muscle. Injection of the IL-10R antibody into the contralateral muscle prevented the analgesia observed in the muscle injected with the antibody, but not the ipsilateral muscle that received the pH 4.0 saline injection. Decreases in muscle withdrawal thresholds were observed in the limb of physically active mice only on the side injected with the IL-10R antibody and were significantly different from those that received the control IgG antibody (1232 mN ± 55 SEM in hind limb given IL-10R antibody injection, 1445 mN ± 43 SEM in hind limb given control injection) (p = 0.003) (Figures 4E and 4F).
We further tested if IL-10R antibody blocked the effects of IL-10 in mice that received pH 4.0 saline injections. In the group that received a control IgG antibody (n=4) 24h prior to injection of IL-10, a single injection of 15 ng/20µl IL-10 into ipsilateral muscle increased the muscle withdrawal threshold from 984 + 29 mN to 1517 + 84 mN (15 minutes post injection) and this increased remained significant for 1h. This increase was prevented by prior injection of the IL-10 receptor blocking antibody (n=8) with decreases in withdrawal threshold before IL-10 averaging 995 ± 54 mN before IL-10 injection and 1121 ± 64 mN after IL-10 injection and was significantly lower than those receiving control IgG injection (p=0.01). Thus, these data show that IL-10 was able to reverse the hyperalgesia produced by repeated acid injections, and that the IL-10 receptor antibody blocks the effects of IL-10.
R.4 IL-10 administration in sedentary mice reproduces the protective effects of long-term physical activity in chronic pain
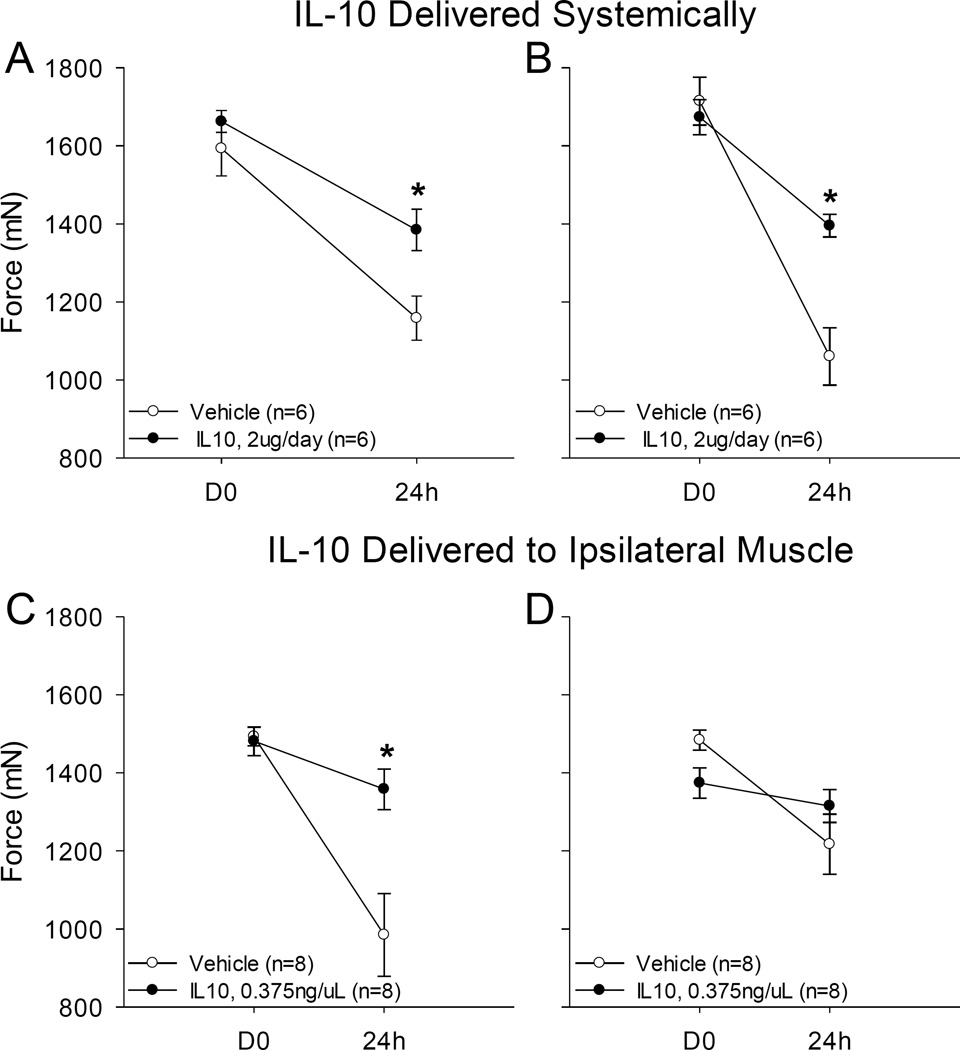
To determine whether systemic administration of IL-10 mimics the effects of running wheel activity and thus prevents the development of hyperalgesia after repeated intramuscular acid injections, IL-10 was administered systemically at a constant dose over nine days by osmotic mini-pumps to sedentary mice. Mice treated with systemic IL-10 had significantly less hyperalgesia compared to mice that received vehicle. The average ipsilateral hind limb muscle withdrawal threshold of IL-10-treated mice was 1384 mN (± 53 SEM) compared to the average muscle withdrawal threshold from vehicle-treated mice (1159 mN (± 56 SEM)) (p=0.015). Similarly, the contralateral average muscle withdrawal threshold of IL-10-treated mice was 1395 mN (± 29 SEM) compared to 1060 mN (±73 SEM) from vehicle-treated mice (Figure 5). No significant changes were observed in muscle withdrawal threshold of IL-10 or vehicle-treated mice 3 days after insertion of the mini-pump prior to the first pH 4.0 saline injection.
Figure 5. Systemic and local administration of IL-10.
(A, B) IL-10 (2 µg/day) was administered for 9 days with a subcutaneous mini-pump in sedentary mice. pH 4.0 saline injections were given in the left gastrocnemius muscles on days 3 and 8. Mice given systemic IL-10 had higher withdrawal thresholds than those that received vehicle for both (A) ipsilateral (*p=0.015) and (B) contralateral (*p=0.002) limbs. (C, D) Local L-10 injections in the gastrocnemius muscle were given daily with pH 4.0 saline administered on days 4 and 9. Mice given IL-10 had higher withdrawal thresholds than those that received vehicle on the side given IL-10 (C) while the contralateral side showed no change compared to saline control (D)(*p=0.001).
To examine if IL-10 prevented the decrease in withdrawal thresholds in sedentary mice by acting locally in the muscle IL-10 injections were given into the left (ipsilateral) gastrocnemius muscle over the course of 10 days prior to induction of the pH model. Mice that received IL-10 injections had significantly higher muscle withdrawal thresholds compared to mice that received vehicle injections (p = 0.001). Ipsilateral muscle withdrawal thresholds of IL-10-treated mice were 1358 mN (± 52 SEM) and 1315 mN (±42 SEM) compared to vehicle-treated mice (985 mN (±107 SEM) and 1217 mN (± 77 SEM) (Figure 5).
IV. Discussion
4.1 Physical activity alters resident muscle macrophage populations to favor regulatory macrophage phenotype
The current study shows that after long-term (8 weeks) physical activity, there was a significant increase in the percentage of macrophages that expressed CD206, indicating an increase regulatory macrophage phenotype. Macrophages play a role in innate immunity within the body, protect the organism against disease, and are located in nearly every tissue type including muscle [34]. Regulatory macrophages secrete anti-inflammatory cytokines and their main function is to dampen the immune response by removing infectious microbes, limiting inflammation, and promoting tissue repair [34]. Similar to the present study, exercise increases the number of regulatory macrophages in adipose tissue from obese mice [22;38]. Since one function of regulatory macrophages is to secrete anti-inflammatory cytokines, the increase of regulatory macrophages within physically active muscle suggests a concomitant increase in anti-inflammatory mediators. This correlates well with the fact that after regular physical activity IL-10 release increases whereas pro-inflammatory cytokine release diminishes [42].
Interestingly, however, we did not observe an associated decrease in the percentage of classical (pro-inflammatory) macrophages in muscle long-term physical activity, nor was there a significant difference in the number of macrophages in sedentary versus physically active muscle. Despite this, the current study does show a significant increase in the ratio of regulatory to classical macrophages after long-term physical activity. The increase in macrophages which co-expressed CD206 and F4/80 with no simultaneous decrease in macrophages which co-expressed CD11c and F4/80 in physically active muscle may represent a transition state as classical macrophages phenotypically switch over to the regulatory macrophage phenotype. This transition state, induced by regular physical activity, may be a so-called “mixed phenotype” state with both anti-inflammatory and pro-inflammatory characteristics. Previous studies in other clinical conditions show the coexistence of cells in different activation states or mixed M1/M2 phenotypes [43;53;66]. So while the overall number of macrophages that exhibit M1 macrophage characteristics stays the same, the number of macrophages which secrete anti-inflammatory cytokines is increased due to the increase in transition-state macrophages which have both anti-inflammatory and pro-inflammatory characteristics. Further studies to understand the time-course for the phenotypic switch will provide information on how quickly exercise can modulate the immune system and provide a general time-course for the beneficial effects of exercise.
4.2 IL-10 plays a key role in analgesia produced by regular physical activity
The current study shows that the analgesic effects of regular physical activity are prevented by blockade of IL-10 receptors given either systemically or locally in the muscle. Administration of the IL-10 receptor antibody systemically prevented the analgesia bilaterally, while local administration prevented analgesia in the muscle the blocking antibody was injected. Conversely, administration of IL-10 both systemically and locally in the muscle of sedentary mice mimics the effects of regular physical activity by preventing the development of hyperalgesia bilaterally or in the muscle IL-10 was administered. Further the hyperalgesia produced by muscle insult was reversed by local injection of IL-10. Thus, these data suggest that regular physical activity increases IL-10 in muscles systemically, and that IL-10 plays a critical role in the analgesia produced by regular physical activity.
Our data are in agreement with prior studies that show administration of IL-10 inhibits the development of hyperalgesia: prodynorphin-induced allodynia, endotoxin-induced hyperalgesia, and spinal cord injury-induced spontaneous pain [21;28;44]. Similar results have also been seen in microglia (which are macrophages of the central nervous system) where increasing expression of IL-10 in these cells reduces hyperalgesia after nerve injury [33;51]. Additionally, cytokine levels in patients with chronic widespread pain show lower expression of anti-inflammatory cytokines such as IL-10 and increases in inflammatory cytokines [63]; IL-10 is expected to reduce pro-inflammatory cytokines, IL-1β and/or TNF [21;28;44]. In fact, IL-10 reduces inflammatory cytokines in the spinal cord after intrathecal IL-10 injection, and in inflamed skin after systemic IL-10 injection. Similarly, over-expression of IL-10 peripherally (HSV viral vector expressing IL-10) [73;74] reduces HIV-induced hyperalgesia and inflammatory cytokines in the spinal cord, and formalin-induced nociceptive behaviors [75]. Studies in IL-10−/− mice are mixed. In uninjured animals, IL-10−/− mice have enhanced nociception as evidenced by an increase in paw licking response on the hot plate latency [62], the only nociceptive test used in the study. After spinal injury, IL-10−/− mice have increased spontaneous pain behaviors compared to controls [1]. Consistently, however, in animals with tissue injury, exogenous application of IL-10 reduces hyperalgesia and reduces factors associated with inflammation and injury. We extend these prior studies and show for the first time that IL-10 contributes to the analgesic effects of regular physical activity when given prior to induction of the hyperalgesia.
Of note, while IL-10 administration in sedentary animals decreased hyperalgesia in the hind limb in which it was administered, there was no change in hyperalgesia on the contralateral limb regardless of if IL-10 was administered in the same or opposite limb as the pH4.0 saline injections. Similarly, administration of IL-10 receptor blocking antibody locally into the hind limb of physically active mice resulted in hyperalgesia only in the limb given the antibody and not the contralateral limb. This would suggest IL-10 does not prevent the central sensitization that occurs after repeated acid injection [61], since contralateral hyperalgesia still persists despite local IL-10 injections. The data further suggest that the IL-10 increases after regular physical activity act on peripheral nociceptors making them less responsive to peripheral stimuli. Indeed IL-10 receptor is located on dorsal root ganglia neurons and IL-10 reduces Nav1.8, but not Nav1.9, sodium currents in DRG neurons. In fact, pretreatment with IL-10 resulted in a downregulation of the expression of Nav1.8 mRNA and protein in DRG in basal conditions and decreased TNF-alpha induced upregulation [52]. Thus, it is possible that regular exercise reduces excitability of nociceptors by downregulating Nav1.8 so that subsequent activation of a nociceptor to a noxious stimuli, e.g. squeezing the muscle, produces less input to the central nervous system.
Alternatively, one could propose that regular physical activity increases muscle blood flow and buffering capacity [49] making the muscle less responsive to the acid injection, which would be manifested as an analgesic effect. However the fact that IL-10R blockade can readily prevent the analgesia argues against this by suggesting the sensitization pathways are being masked by IL-10 produced by physical activity.
4.3. Fatigue metabolites activate macrophages
Physical activity increases cytokines and fatigue metabolites locally and in plasma [17;45;48;76]. These fatigue metabolites (protons, lactate, ATP), secreted by exercising muscle [13;19;23;29;39;40], activate macrophages [34;64]. Extracellular ATP activates purinergic-7 receptors (P2×7) on macrophages enhancing release of pro-inflammatory cytokines[41;69], and promoting conversion to an M2 phenotype [5;6]. Protons activate ASIC3 on macrophages enhancing endocytosis and secretion of IL-10 [25]. Indeed, fatiguing exercise enhances hyperalgesia to muscle insult, and the enhanced hyperalgesia is prevent by removal of muscle macrophages [13]. ASIC3 plays a significant role in this hyperalgesia since ASIC3−/− mice do not develop hyperalgesia after fatiguing exercise, and blockade of ASIC3 in muscle tissue prevents development of hyperalgesia [13]; however, downregulation of ASIC3 in neurons innervating muscle has no effect on the hyperalgesia [13]. Lastly, acupuncture promotes a phenotypic switch with an increase in M2 and decrease in M1 macrophages, and this switch does not occur in IL-10−/− mice [7]. Thus, we propose that fatigue metabolites activate macrophages locally to release IL-10, and these substances promote phenotypic switching to an M2 phenotype in response to regular physical activity.
4.4 Clinical relevance and translation
Clinically, physically inactive individuals have a greater risk for development of chronic pain [26;27;72]. The current study similarly shows that physically inactive mice develop mechanical hyperalgesia of the paw and muscle, and this is similar between male and female mice. While females have a greater prevalence of chronic musculoskeletal pain [2], the conditions that promote these differences are not clear. Our prior study shows that fatiguing stimuli combined with a low-dose muscle insult can produce robust sex differences, but the type of stimuli (isometric or full body fatigue; carrageenan or acid muscle insult) results in different results (widespread vs local hyperalgesia; enhanced vs. longer-lasting hyperalgesia; estrogen dependent vs. non-estrogen dependent)[14;55;59]. Further, in physically inactive individuals with chronic musculoskeletal pain, a single bout of fatiguing exercise enhances pain [8;9;20;60;65]. In contrast, regular physical activity prevents and reduces pain in a variety of chronic musculoskeletal conditions [3;11;16;20;26;27;30;32;50;50;60;70]. The current study similarly shows that regular physical activity prevents development of chronic muscle pain, and this prevention occurs equally between male and female mice. The dichotomy between increasing and decreasing pain with exercise may be related to macrophage phenotypes in muscle and how they respond to the fatiguing stimuli so that under physically inactive conditions there is a greater release of inflammatory cytokines and under exercise conditions there is a greater release of anti-inflammatory cytokines. In support, people with fibromyalgia show greater circulating and evoked release of inflammatory cytokines when compared to healthy controls [36;37]; regular exercise reduces inflammatory cytokines, and increases release of IL-10 from monocytes in fibromyalgia subjects [36]. Thus, regular physical activity may modulate the state of the immune system so that the balance of anti-inflammatory to inflammatory cytokines increases, and thus prevent development of chronic pain.
Acknowledgements
Funded by NIH AR061371, a Doris Duke Fellowship (AL), T32NS045549 and T32GM007337 (NG). We thank Lynn Rasmussen, Jessica Danielson, and Sandra Kolker for excellent technical assistance.
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Abraham KE, McMillen D, Brewer KL. The effects of endogenous interleukin-10 on gray matter damage and the development of pain behaviors following excitotoxic spinal cord injury in the mouse. Neurosci. 2004;124:945–952. doi: 10.1016/j.neuroscience.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazelmans E, Bleijenberg G, Voeten MJ, van der Meer JW, Folgering H. Impact of a maximal exercise test on symptoms and activity in chronic fatigue syndrome. J Psychosom Res. 2005;59:201–208. doi: 10.1016/j.jpsychores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Green PG, Levine JD. Stress enhances muscle nociceptor activity in the rat. Neurosci. 2011;185:166–173. doi: 10.1016/j.neuroscience.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood. 2013;122:1935–1945. doi: 10.1182/blood-2013-04-496216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen HB, Mosser DM. Extrinsic and intrinsic control of macrophage inflammatory responses. J Leukoc Biol. 2013;94:913–919. doi: 10.1189/jlb.0413236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol. 2015;51:19–31. doi: 10.1007/s12035-014-8790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dailey DL, Keffala VJ, Sluka KA. Cognitive and physical fatigue tasks enhance pain, cognitive fatigue and physical fatigue in people with fibromyalgia. Arthritis Care Res (Hoboken) 2015;67:288–296. doi: 10.1002/acr.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damsgard E, Thrane G, Anke A, Fors T, Roe C. Activity-related pain in patients with chronic musculoskeletal disorders. Disabil Rehabil. 2010;32:1428–1437. doi: 10.3109/09638280903567877. [DOI] [PubMed] [Google Scholar]
- 10.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fransen M, McConnell S, Bell M. Therapeutic exercise for people with osteoarthritis of the hip or knee. A systematic review. J Rheumatol. 2002;29:1737–1745. [PubMed] [Google Scholar]
- 12.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 13.Gregory NS, Brito R, Fusaro MCGO, Sluka KA. ASIC3 is required for development of fatigue-induced hyperalgesia. Mol Neurobiol. 2015 doi: 10.1007/s12035-014-9055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. Pain. 2013;154:2668–2676. doi: 10.1016/j.pain.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An Overview of Animal Models of Pain: Disease Models and Outcome Measures. Journal of Pain. 2013;14:1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome - A systematic review. Eur J Pain. 2010;14:5–10. doi: 10.1016/j.ejpain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Hollander DB, Durand RJ, Trynicki JL, Larock D, Castracane VD, Hebert EP, Kraemer RR. RPE, pain, and physiological adjustment to concentric and eccentric contractions. Med Sci Sports Exerc. 2003;35:1017–1025. doi: 10.1249/01.MSS.0000069749.13258.4E. [DOI] [PubMed] [Google Scholar]
- 18.Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc. 2004;36:960–964. doi: 10.1249/01.mss.0000128186.09416.18. [DOI] [PubMed] [Google Scholar]
- 19.Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol. 2000;528(Pt 1):157–163. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Kanaan SA, Poole S, Saade NE, Jabbur S, Safieh-Garabedian B. Interleukin-10 reduces the endotoxin-induced hyperalgesia in mice. J Neuroimmunol. 1998;86:142–150. doi: 10.1016/s0165-5728(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 22.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 23.Keller P, Keller C, Carey AL, Jauffred S, Fischer CP, Steensberg A, Pedersen BK. Interleukin-6 production by contracting human skeletal muscle: autocrine regulation by IL-6. Biochem Biophys Res Commun. 2003;310:550–554. doi: 10.1016/j.bbrc.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 24.Konarzewski M, Sadowski B, Jozwik I. Metabolic correlates of selection for swim stress-induced analgesia in laboratory mice. Am J Physiol. 1997;273:R337–R343. doi: 10.1152/ajpregu.1997.273.1.R337. [DOI] [PubMed] [Google Scholar]
- 25.Kong X, Tang X, Du W, Tong J, Yan Y, Zheng F, Fang M, Gong F, Tan Z. Extracellular acidosis modulates the endocytosis and maturation of macrophages. Cell Immunol. 2013;281:44–50. doi: 10.1016/j.cellimm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152:2241–2247. doi: 10.1016/j.pain.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 27.Landmark T, Romundstad PR, Borchgrevink PC, Kaasa S, Dale O. Longitudinal associations between exercise and pain in the general population--the HUNT pain study. PLoS One. 2013;8:e65279. doi: 10.1371/journal.pone.0065279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL. Cytokine involvement in dynorphin-induced allodynia. Pain. 2000;84:159–167. doi: 10.1016/s0304-3959(99)00195-5. [DOI] [PubMed] [Google Scholar]
- 29.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol. 2003;95:577–583. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- 30.Maquet D, Croisier JL, Renard C, Crielaard JM. Muscle performance in patients with fibromyalgia. Joint Bone Spine. 2002;69:293–299. doi: 10.1016/s1297-319x(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 31.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 32.Mengshoel AM, Vollestad NK, Forre O. Pain and fatigue induced by exercise in fibromyalgia patients and sedentary healthy subjects. Clinical & Experimental Rheumatol. 1995;13:477–482. [PubMed] [Google Scholar]
- 33.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716:106–119. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 34.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omiya Y, Goto K, Ishige A, Komatsu Y. Changes in analgesia-producing mechanism of repeated cold stress loading in mice. Pharmacol Biochem Behav. 2000;65:261–266. doi: 10.1016/s0091-3057(99)00215-4. [DOI] [PubMed] [Google Scholar]
- 36.Ortega E, Bote ME, Giraldo E, Garcia JJ. Aquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patients. Scand J Med Sci Sports. 2012;22:104–112. doi: 10.1111/j.1600-0838.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 37.Ortega E, Garcia JJ, Bote ME, Martin-Cordero L, Escalante Y, Saavedra JM, Northoff H, Giraldo E. Exercise in fibromyalgia and related inflammatory disorders: known effects and unknown chances. Exerc Immunol Rev. 2009;15:42–65. [PubMed] [Google Scholar]
- 38.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 41.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2×7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 42.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 43.Pettersen JS, Fuentes-Duculan J, Suarez-Farinas M, Pierson KC, Pitts-Kiefer A, Fan L, Belkin DA, Wang CQ, Bhuvanendran S, Johnson-Huang LM, Bluth MJ, Krueger JG, Lowes MA, Carucci JA. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J Invest Dermatol. 2011;131:1322–1330. doi: 10.103/jid.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- 45.Rahkila P, Hakala E, Alen M, Salminen K, Laatikainen T. Beta-endorphin and corticotropin release is dependent on a threshold intensity of running exercise in male endurance athletes. Life Sci. 1988;43:551–558. doi: 10.1016/0024-3205(88)90158-0. [DOI] [PubMed] [Google Scholar]
- 46.Rigamonti E, Zordan P, Sciorati C, Rovere-Querini P, Brunelli S. Macrophage plasticity in skeletal muscle repair. Biomed Res Int. 2014;2014:560629. doi: 10.1155/2014/560629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rupp H. Differential effect of physical exercise routines on ventricular myosin and peripheral catecholamine stores in normotensive and spontaneously hypertensive rats. Circ Res. 1989;65:370–377. doi: 10.1161/01.res.65.2.370. [DOI] [PubMed] [Google Scholar]
- 48.Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- 49.Saltin B, Gollnick PD. Compr Physiol. Supplement 27. Handbook of Physiology, Skeletal Muscle; 2011. Skeletal muscle adaptability: significance for metabolism and performanc; pp. 555–631. [Google Scholar]
- 50.Schachter CL, Busch AJ, Peloso PM, Sheppard MS. Effects of short versus long bouts of aerobic exercise in sedentary women with. Fibromyalgia: A randomized controlled trial. Physical Therapy. 2003;83:340–358. [PubMed] [Google Scholar]
- 51.Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, Liu XG. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol. 2013;247:466–475. doi: 10.1016/j.expneurol.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 52.Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, Liu XG. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol. 2013;247:466–475. doi: 10.1016/j.expneurol.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. Journal of Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Sluka KA, Danielson J, Rasmussen L, Dasilva LF. Exercise-Induced Pain Requires NMDA Receptor Activation in the Medullary Raphe Nuclei. Med Sci Sports Exerc. 2012;44:420–427. doi: 10.1249/MSS.0b013e31822f490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 57.Sluka KA, O'Donnell JM, Danielson J, Rasmussen LA. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol. 2013;114:725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 59.Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain. 2010;148:188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136:331–339. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tu H, Juelich T, Smith EM, Tyring SK, Rady PL, Hughes TK., Jr Evidence for endogenous interleukin-10 during nociception. J Neuroimmunol. 2003;139:145–149. doi: 10.1016/s0165-5728(03)00126-7. [DOI] [PubMed] [Google Scholar]
- 63.Uceyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 2006;54:2656–2664. doi: 10.1002/art.22026. [DOI] [PubMed] [Google Scholar]
- 64.Ulmann L, Hirbec H, Rassendren F. P2×4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 66.Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van dV, Amor S, Teunissen CE, van HJ, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 Play Different Roles in the Development of Hyperalgesia After Inflammatory Muscle Injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 69.Wewers MD, Sarkar A. P2×(7) receptor and macrophage function. Purinergic Signal. 2009;5:189–195. doi: 10.1007/s11302-009-9131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain. 2004;109:497–499. doi: 10.1016/j.pain.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 71.Yokoyama T, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 72.Zhang R, Chomistek AK, Dimitrakoff JD, Giovannucci EL, Willett WC, Rosner BA, Wu K. Physical Activity and Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Med Sci Sports Exerc. 2015;47:757–764. doi: 10.1249/MSS.0000000000000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng W, Huang W, Liu S, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. IL-10 mediated by herpes simplex virus vector reduces neuropathic pain induced by HIV gp120 combined with ddC in rats. Mol Pain. 2014;10:49. doi: 10.1186/1744-8069-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng W, Huang W, Liu S, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. Interleukin 10 mediated by herpes simplex virus vectors suppresses neuropathic pain induced by human immunodeficiency virus gp120 in rats. Anesth Analg. 2014;119:693–701. doi: 10.1213/ANE.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15:183–190. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zwetsloot KA, John CS, Lawrence MM, Battista RA, Shanely RA. High-intensity interval training induces a modest systemic inflammatory response in active, young men. J Inflamm Res. 2014;7:9–17. doi: 10.2147/JIR.S54721. [DOI] [PMC free article] [PubMed] [Google Scholar]