Summary
Toll-like receptors (TLRs), first identified as pattern recognition receptors, are now recognized to serve as a key interface between innate and adaptive immunity. Systemic Lupus Erythematosus (SLE) is characterized by both continuous and cyclic stimulation of the innate and adaptive immune system by endogenous nucleic acids released from apoptotic or necrotic cells. TLR7 and TLR9 function as innate sensors of viral infection as their ligands are ssRNA and dsDNA, respectively. Recognition of self nucleic acids by endosomal TLRs in B cells and pDCs is thought to be an important step in the pathogenesis of SLE, generating anti-nuclear antibodies and producing type I IFN. In this review, we take a specific look at how TLR7, noncoding RNA, and SSA/Ro60 can contribute to clinical autoimmunity and organ damage in the context of neonatal lupus (NL). Although fifteen times less common than SLE, NL provides a unique opportunity to study two different aspects of autoimmunity: passively acquired tissue injury in a developing fetus and clinical progression of disease in an asymptomatic mother found to have anti-Ro60 autoantibodies only after identification of heart block/rash in a child. Finally, we discuss hydroxychloroquine (HCQ) use by asymptomatic subjects which may forestall the clinical expression of autoimmunity.
Keywords: TLR7, autoimmunity, anti-SSA/Ro60, neonatal lupus, lupus
Overview
Toll-like receptors (TLRs), first identified as pattern recognition receptors, bind pathogen associated molecular patterns (PAMPs), including proteins, nucleic acids, and lipoproteins and assume critical importance in the host defense against infections agents via elicitation of innate and adaptive immune responses. Among the more than 10 TLRs identified in humans, TLRs 3, 7, 8, and 9 are expressed in endosomes and recognize pathogen-derived and synthetic nucleic acids (1). Upon interaction with their respective ligands, these receptors signal through adapter proteins, the most common of which is Myeloid Differentiation Primary Response 88 (MyD88). However, TLR3 alternatively signals via TIR-domain-containing adapter-inducing-interferon-β (TRIF). These adapter proteins recruit additional molecules to commence signaling cascades which ultimately eventuate in the production of proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and type I interferon (2–4). The consequences of misregulated TLR activation including the participation of autoimmune-related genetic influences on PAMP-derived signaling (e.g. lower activation threshold) or settings involving host-derived ligands (activation by self), may manifest as an autoimmune disease.
Both TLR7 and TLR9 function as innate sensors of viral infection as their ligands are single-stranded RNA (ssRNA) and double-stranded DNA (dsDNA), respectively (4). Although TLR7 and 9 are expressed in intracellular compartments and do not normally have access to nucleic acid ligands, evidence has shown that receptors expressed by B cells (surface IgM), dendritic cells (DCs) (FcγR), or other antigen-presenting cells can facilitate the trafficking of nucleic acids down a pathway that eventually intersects the relevant intracellular TLR-containing compartment (5).
Systemic Lupus Erythematosus (SLE) is characterized by both continuous and cyclic stimulation of the innate and adaptive immune system by endogenous nucleic acids released from apoptotic or necrotic cells. This pathologic process is facilitated by circulating autoantibodies against nucleic antigens such as nucleosomes, histones, DNA, and ribonucleoproteins, a hallmark of disease. Recognition of self nucleic acids by endosomal TLRs on B cells and pDCs is thought to be an important step in the pathogenesis of SLE, generating anti-nuclear antibodies and the production of type I IFN. Following their activation by self-nucleic acid-associated immune complexes, PDCs migrate to the tissues. Immune complexes containing host-derived nuclei acids have been shown to induce inflammatory responses mediated through TLR7, 8, and 9. Autoantibody dependent, FcγR-mediated uptake permits nucleic acids to activate dendritic cells and macrophages through the ligation of endosomal TLRs (TLR) (6, 7).
In this review, we discuss endosomal TLR receptors, with a specific focus on TLR7, noncoding RNA, and SSA/Ro60 (Ro60), and how these factors contribute to disease. Although far more rare than SLE, neonatal lupus (NL) provides a unique opportunity to study two different aspects of autoimmunity: passively acquired tissue injury in a developing fetus and clinical progression of disease in the mother. The collateral insight of studying neonatal lupus is the unprecedented opportunity provided by asymptomatic mothers often found to have anti-Ro60 autoantibodies only after identification of heart block/rash in a child. Based on continued follow-up of asymptomatic NL mothers, nearly half progress, albeit few develop SLE (8). At the present time approximately 45% of the mothers are totally asymptomatic or have insufficient criteria to be classified as SLE or Sjogren’s Syndrome (SS), 30% have SS, 16% have SLE, and 9% have SLE/SS. Moreover, evidence that autoantibodies target Ro60 at an early stage of initiation and expansion of hyperactive immune systems is based on studies initiated at the Oklahoma Medical Research Foundation. By leveraging preclinical data of seemingly asymptomatic individuals who later develop SLE, these investigators showed that serological positivity for anti-Ro autoantibodies appears years before clinical diagnosis (9).
A goal is to leverage the phenotype to identify whether a property of TLR7 and the in vivo generation of small noncoding ribonucleic acid (the TLR7 ligand) in part underlie disease progression in otherwise healthy women whose anti-SSA/Ro “status” was identified solely based on the detection of neonatal lupus in her child.
A model to predict how factors directly and indirectly related to TLR and noncoding RNAs may evoke ANA in a susceptible subject
Among the more exciting TLR-related discoveries are recently described scenarios linking TLRs and onset of autoantibody positivity by researchers at UCSF. A single mutation (E613R) in the phosphatase CD45, which is an essential regulator of antigen receptor signaling, was shown to cause a lupus-like phenotype in mixed 129/Sv and C57BL/6 mice. The phenotype CD45E613R is extremely sensitive to genetic context (10). On the F10 B6 genetic background, the mutation was tolerant and the mice did not develop ANAs. In contrast, backcross of CD45E613R to the BALB/c genetic background resulted in the development of detectable ANAs and anti-dsDNA antibodies. However, the mice did not develop proteinuria or histopathologic evidence of glomerulonephritis or any other end organ disease. Forward genetics identified loci cooperating with the CD45E613R mutation that were responsible for the phenotype. A significant LOD score for anti-dsDNA antibody production was found for a locus on chromosome 9 identified as Wam1. When comparing SNPs in this locus between the B6 and BALB/c genetic backgrounds, Tlr9 was the only gene with nonsynonymous coding changes within the leucine-rich repeats of the ectodomain and immediately adjacent to the intracellular toll/IL-1R domain (both domains linked to TLR function (11)). Interestingly, knock out of TLR9 totally ablated the ANA in CD45E613R.BALB/c mice while increasing the occurrence of ANA in CD45E613R.B6 mice. For the later, ANA specificity revealed that both TLR9+/− and TLR9−/− CD45E613R.B6 mice failed to develop high-titer anti-dsDNA Abs. However, there was a significant increase in anti-RNP IgG autoantibodies in TLR9 CD45E613R.B6 mice. Taken together, these data clearly demonstrate that TLR9 negatively regulates autoantibody production in a gene dosage–dependent manner in CD45E613R.B6 mice. In addition, a contrast to the widely described solely tolerogenic role of TLR9 was highlighted by reporting that TLR9 may have a dual nature with distinct alleles conferring opposing effects on the development of ANAs.
A point worthy of re-emphasis was that genetic ablation of one copy of TLR9 in CD45E613R.B6 resulted in a higher frequency of anti-RNP autoantibodies, possibly due to dysregulation of TLR7 after loss of one of its checkpoints (Table 1). Also, recent literature support that TLR7 is held in check at varied stages involving Unc93B1, an ER-resident protein which interacts with TLR9 and predominates over TLR7 (12, 13) in a scenario involving a preferential Unc93B1-dependent transportation of TLR9 to endosomes. In the absence of TLR9, an Unc93B1-TLR7 dyad results in excessive TLR7 activation of immune cells. Autoimmune-prone mice that do not have functional TLR9 develop more severe clinical disease (14) and a recent study identified key underpins of TLR7 to drive systemic immunity by suggesting that the path involves a bifurcation of B cell fates (15). Specifically, B cell receptor (BCR)/TLR9 and BCR/TLR7 co-engagement result in distinct survival and functional phenotypes (15). For survival, BCR/TLR9 and not BCR/TLR7 varied with regard to BlyS dependence. For the functional phenotype, immune-complex stimulated B cells of co-engaged BCR/TLR7 but not of BCR/TLR9 resulted in upregulation of IRF4, a transcription factor of plasma cells. One interpretation is that TLR7 signaling by B cells is “hard wired” to promote plasma cell formation. In support of this concept, in B6 mice levels of anti-RNA autoantibodies are correlated with the TLR7 copy number (16) and in the pristine-induced murine lupus, TLR7 is a major driver of plasma cell differentiation (17).
Table 1.
Properties and phenotypes of knock out mice with a focus on the role of TLR7 in health and disease
| Candidate | Chromosome in humans |
Location | Phenotype of KO |
reference |
|---|---|---|---|---|
| TLR9 | 3p21 | Endosomes | More severe disease in autoimmune mice | (57) |
| TLR7 | Xp22, one inactive copy in female cells | Endosomes | Attenuation of disease in and autoimmune mice | (14) |
| UNC93B1 | 11q13 | Endosomes | Manifestations of disease | (12, 13) |
| Ro60 | 1q31 | Diffuse at cytoplasmic and nuclear sites | Manifestation of disease | (23) |
Related to these findings is the fact that while corticosteroids attenuate anti-dsDNA autoantibodies, anti-Ro autoantibodies e resistant. PBMCs, particularly the plasmacytoid dendritic cells (pDCs), while taking up apoptotic cells complexed with autoantibody containing IgG, have been proposed as a key source of aberrant IFN production and a major driver of SLE progression (18), a response that involves TLR7 and -9 activation. Activated pDCs become resistant to glucocorticoids, underlying the limited efficacy of these drugs in SLE (19) and treatment of lupus prone mice with TLR7 and -9 dual antagonists ameliorates disease. Clinical studies demonstrate decreased anti-dsDNA with Belimumab (20) but the effect of this therapy on anti-Ro/SSA has not been systematically evaluated. Rituximab does not significantly lower serum levels of anti-Ro/SSA and anti-La/SSB antibodies(21).
Taken together, anti-Ro autoantibodies may be difficult to therapeutically achieve because of limitations of these therapies to reach “entrenched” TLR7 signaling.
In addition to studies focusing on TLRs and their downstream effectors, a very recent advance suggests that requisite ANA provoking factors may include a subset of noncoding RNAs that activate TLR7 with particularly high efficacy. Retroelement RNAs are short interspersed nuclear elements with restriction enzyme sensitivity (i.e. Alu, Arthrobacter luteus), the so-called Alu SINES that reside in the host genome. Hung and coworkers, using individual nucleotide cross linking and immunoprecipitation (iCLIP), show that a sizable percentage of RNA motifs bound by Ro60 are in fact derived from Alu elements (as well as Y RNAs) (22). Further, while the expression of Alu RNAs increases slightly when the human cell line GM12878 (an EBV transformed B-lymphocyte cell line) is treated with IFN-α, their expression is dramatically magnified in GM12878 Ro60 KO cells. In fact, Ro60 KO mice develop a mild autoimmune syndrome with the early onset of a positive ANA (23). Importantly, when Alu RNAs were transfected directly into human PBMCs from normal subjects using a lipid-based transfection reagent, TNF-α, IL-6, and IFN-α were expressed, readouts which were completely attenuated by co-treatment with chloroquine or IRS954, implicating endosomal TLRs in the response to Alu RNA. In contrast, co-incubation of the transfected PBMCs with BX795, an inhibitor of TBK1/IκKε (24) which mediates signaling downstream of cytosolic RNA receptors RIG-I, MDA-5 and TLR3, had no effect on Alu-mediated cytokine production. Taken together, the intracellular ribonuclear binding protein Ro60 may serve as a natural sponge of noncoding RNA with a physiological role to prevent the sequestered retroelement RNAs from reaching their target, TLR7 (Table 1). Genetics of autoantibodies include HLA and others factors, as candidates representing heritable and necessary attributes to provoke an ANA (Table 2). However, there is a lack of strong SNP associations, suggesting that other types of genetic variation or non-genetic factors are important, supporting the speculation that environmental exposure affecting the axis of TLR-small noncoding RNAs may have an impact on susceptibility to the onset of autoantibodies.
Table 2.
Association of genes and susceptibility to autoantibodies against DNA*
| Gene | Chromosome (SNP) | Comment | OR* |
|---|---|---|---|
| HLA-DRB3 | 6 (rs2187668) | Confers autoantibodies even in asymptomatics | 2.23 |
| STAT4 | 2 (rs7574865) | Autoantibody propensity loci | 1.77 |
| IRF5 | 7 (rs10488631) | Only non-HLA conferring autoantibodies even in asymptomatics | 1.92 |
| ITGAM | 16 (rs9888739) | Autoantibody propensity loci | 1.80 |
Case versus control; from Chung et al, 2011 (58)
The consequences of anti-Ro60 and Ro60 with bound RNA on apoptotic cells are TLR7 dependent
Ongoing research has revealed ever more complex and context specific roles for the macrophage in the afferent and efferent arms of the immune response. For example, while in murine models the normal clearing of apoptotic cells by macrophages is pivotal to tolerance and prevents a potentially detrimental immune response (25), anti-Ro60 antibodies thwart this process by binding to apoptotic cells and mimicking a pathogenic immune complex, resulting in a pro-inflammatory macrophage phenotype. Given the intracellular localization of Ro, its translocation to the surface of apoptotic cells, observed by Casciola-Rosen et al. (26), provided the first possible scenario explaining the nexus between anti-Ro antibodies and injury. Clearly accessibility matters. The link between apoptosis, Ro60, and Ro60’s recognition by anti-Ro autoantibodies has undergone scrutiny over the course of a decade of study. In recent study designed to probe the link between Ro trafficking and apoptosis for a wide range of cells types, there was support of a model in which maternal antibody populations against Ro60 apoptopes act to increase the risk of tissue damage in anti-Ro associated CHB. For models employing human fetal cardiocytes undergoing apoptosis, Ro translocates to the cell surface (27, 28) and this also occurs in models employing Jurkat T cells (29), HeLa cells (29), and murine fibroblasts (30). In contrast, anti-U1-RNP (31) and anti-lamin B1 (32) do not bind apoptotic cells. For the latter, it was previously demonstrated that although lamin B1 is redistributed to blebs of apoptotic surfaces, it is not bound by anti-Ro (33). These findings support discordance in the final cellular destination of translocated nuclear autoantigens during apoptosis, suggesting that Ro60’s exposure to antibody is a specific process. Additionally, there is a different temporal expression of immunodominant Ro60, Ro52, and La48 apoptopes.
Ro60 is a ring-shaped protein that binds Y RNAs and misfolded, defective small RNAs, suggesting a role in quality control (34–36).
The properties of Ro60 on an apoptotic particle were recently demonstrated using stable Ro60 knockout fibroblasts expressing wild-type or mutant FLAG3-Ro60 with supporting approaches employing siRNA to deplete Y3 or Y1 RNA. Prior studies have shown that mutant FLAG3-Ro60 displays varied Y binding (37). For example, FLAG3-Ro60(K170A R174A) binds Y RNA, whereas FLAG3-Ro60 (H187S) does not bind Y3 RNA. Fibroblasts expressing these constructs showed equivalent intracellular expression of Ro60 (flow cytometry and immunoblot (30)). In contrast, apoptotic fibroblasts (annexin V-positive, PI-negative) expressing FLAG3-Ro60(K170A R174A) were bound by anti-Ro60, whereas in FLAG3-Ro60(H187S) cells, there was no surface expression of Ro60. RNA interference of mY3 RNA in wild-type murine fibroblasts was associated with an inhibition of the apoptosis-induced surface translocation of Ro60. Interestingly, although siRNA was effective at depletion of mY1 RNA, this treatment did not diminish Ro60 surface expression on the apoptotic cells. This line of investigation provides evidence that Ro60 accessible to extracellular antibody during apoptosis is the result of intracellular transport and not simply the random extrusion of Ro60 and other pieces secondary to fragmentation of a dying cell. For the former, there were molecular attributes influencing this novel function, including Ro60 mutations and levels of mY3 RNA. Accordingly, we speculate that inference with a Ro60 chaperone molecule would inhibit available antigen to circulating autoantibodies. This would effectively attenuate the formation of immune complexes and the downstream inflammatory and profibrosing events which result in organ injury and loss of function.
One of the strongest clinical associations with autoantibody directed to components of the Ro/La ribonucleoprotein complex is the development of permanent cardiac disease in an offspring, an alarming prospect facing all pregnant women with these reactivities. Injury to the fetal heart most often occurs during the 18–24th wk of gestation and is presumed to be dependent on the FcγRn-mediated transplacental passage of pathogenic maternal IgG autoAb (38) (reviewed in (39)). The signature cardiac disease typically manifests as CHB which can be of varied degree, but is most often complete (40). In addition to conduction disease, 10–15% of affected offspring will have a life-threatening cardiomyopathy (40). As awareness of this condition continued, it became evident that the maternal health accompanying the production of the putative autoAb is not a risk factor for fetal disease per se since many mothers are clinically asymptomatic at the pregnancy during which CHB is detected and only then are Ab sought and identified (8, 41).
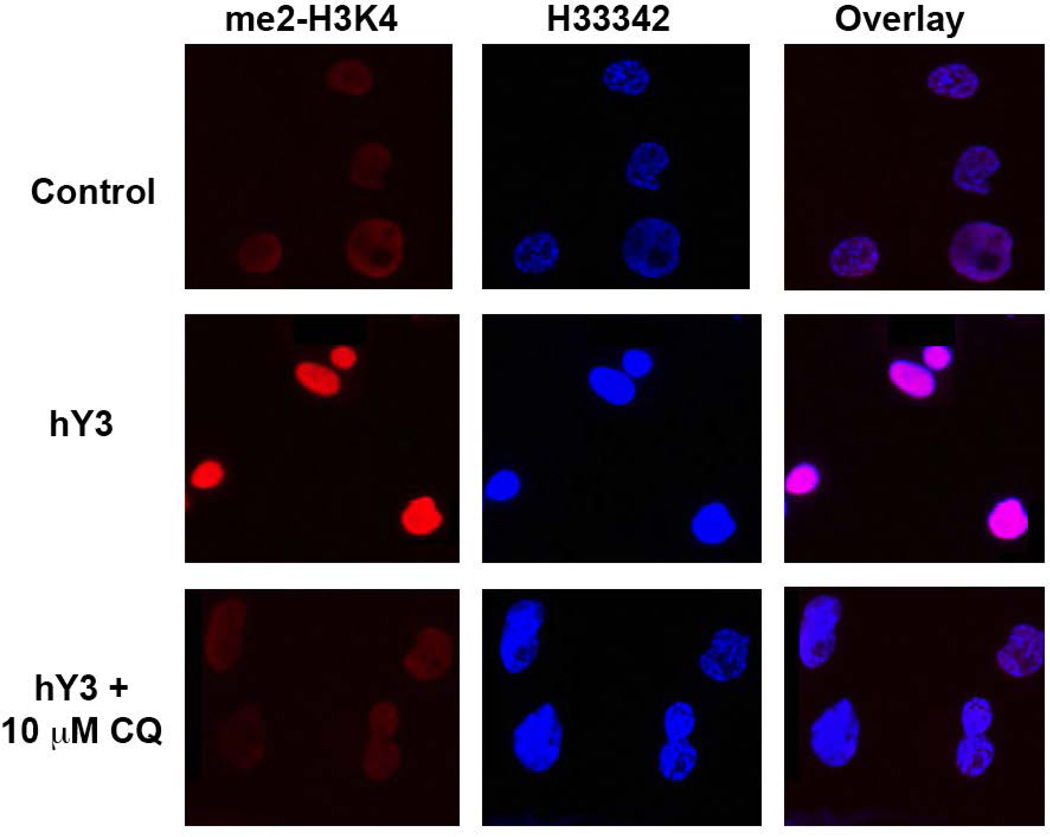
In a scenario involving the pathogenesis of CHB involving Ro60 antigen arriving at a stage where it is accessible to autoantibody, an immune complex containing hY3 may be responsible for instigating a powerful inflammatory response upon activation of TLR7 in tissue macrophages. In a recent study, human PBMC-derived macrophages treated with either an immune complex of anti-Ro60/Ro60/hY3 RNA or hY3 RNA delivered alone via a lipid-based transfection reagent highly upregulated TNF and IL6 expression (42). Preincubation with HCQ (5µM) decreased the hY3 stimulated TNF and IL6 gene expression. Because transcriptional activities of NF-κB and STAT1 were enhanced, further evaluation of hY3 dependent epigenetic modifications were performed. Ligation of TLR7/8 resulted in increased histone methylation as measured by increased H3K4me2 (Figure 1), a requirement for binding of NF-κB at certain promoters, specifically the kB1 region in the TNF-α promoter (ChIP-qPCR), which was significantly decreased by HCQ.
Figure 1. Differential in vitro expression of H3K4me2 antigen in THP-1 macrophages.
Resting and hY3 (a proxy of anti-Ro60 immune complex) stimulated macrophages with and without chloroquine (10 uM) were probed with an α-H3K4me2 antibody (red) and nuclei counterstain (blue). Note that from merged images, magenta indicates an overlap of H3K4me2 and the nucleus.
IFN-α treated macrophages were found to upregulate IL6, an event which was refractory to HCQ. An interpretation of this effect is that HCQ attenuates cellular production of IFN-α when mediated by TLR ligation but not the downstream effects resulting from subsequent stimulation by IFN-α and the elevations in IFN-α responsive genes tracking disease activity in SLE (6).
HCQ is effective at therapeutic levels which are achievable in patients taking the drug as highlighted in current work on adherence to this medication and disease activity in SLE (43). Furthermore, 400mg hydroxychloroquine is currently being evaluated as a preventive approach to reduce the recurrence rate of congenital heart block. Considering the most likely mode of action, since endosomal TLR activity is dependent on the acidic environment of the endosome, pharmacologic approaches to attenuate TLR-dependent readouts have utilized bafilomycin, a macrolide antibiotic inhibitor of vacuolar-type H+ATPase, causing an increase in the pH of endosomal compartments and antimalarials, nonspecific inhibitors of endosomal acidification (6, 44). The mechanism of the latter was recently challenged in a study demonstrating that the inhibitory effect of HCQ is secondary to its direct binding to nucleic acids when concentrated within the endosome, thereby preventing ligands from binding to TLRs (45, 46).
Proxies of TLR7, which are unmasked by interrogations of sera of anti-Ro asymptomatic neonatal lupus mothers
In order to foster connections of in vitro models and the neonatal lupus mothers, there was a mining of medical records and clinical information on mothers enrolled in the RRNL and access to individuals’ stored specimens. Of importance, plasma has been isolated from the peripheral blood of approximately 50% of mothers from multiple visits. The goal is to utilize this special cohort in the pursuit of identifying associated mediators preceding clinical SLE. This identification is based on evaluating the changing plasma concentrations of these mediators.
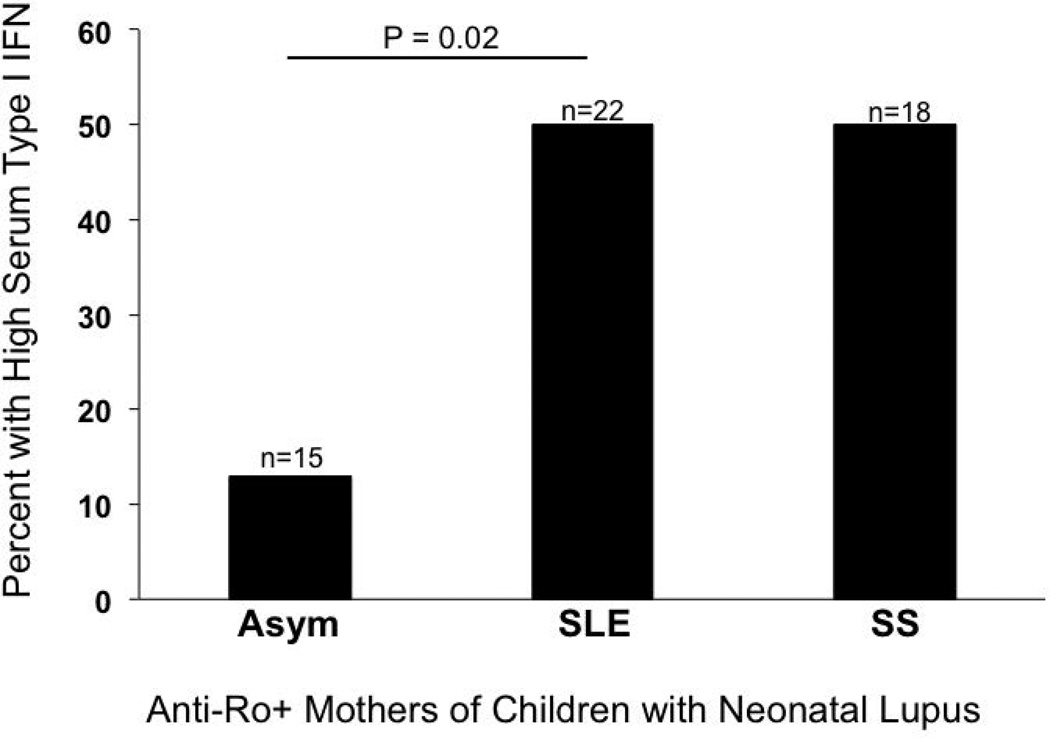
Despite high titers of circulating antibodies to Ro/SSA and a child with neonatal lupus, type I IFN gene expression using a functional reporter assay was not elevated in those women who were clinically asymptomatic, only those diagnosed with SLE or Sjogren’s Syndrome (Figure 2 (47)). This was not expected based on in vitro data demonstrating that sera containing these autoantibodies can promote type I IFN generation (48). Acordingly, this study suggests that anti-SSA/Ro antibodies alone are not sufficient for high levels of type I IFN in vivo and that other factors related to the background disease processes in SLE are important.
Figure 2. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro–positive mothers of children with neonatal lupus.
Using antibody-positive sera from mothers with manifestations of neonatal lupus, a functional reporter cell assay did not detect serum type I IFN in the anti-Ro positive aymptomatic group.
In autoimmune disease phenotypes, preclinical disease may be reflected by biomarkers whose presence may then predict tipping of the scales toward a clinical presentation of overt disease. Recently, soluble inflammatory mediators and regulatory mediators were evaluated in the sera of asymptomatic neonatal lupus women (49). Certain cytokines seem to reflect a skewing of the acquired immune system towards Th2 responses, including IL-6, IL-21 (50), and IL-4 (51). BlyS, a B cell factor of emerging interest due to its role in B cell homeostasis also appears to be temporally important in the transition to clinical SLE (52). In contrast, manifestations causally related to immune dysfunction may be forestalled by regulatory mediators such as IL1RA, TGF-β, and PAI-1 (53). The spectrum of subclinical disease may involve the expansion of injury which is secondary to TLR7 signaling. Identification of serum factors associated with immune dysfunction could offer novel insights into at-risk populations and uncover the potential for therapeutic intervention with hydroxychloroquine.
Conclusion
Under physiological conditions, the activation of TLR7 is tightly regulated. In a scenario whereby there is release of endogenously generated small noncoding nucleic acids, ligands of TLR7, the intracellular ribonucleoprotein Ro60 may provide a safeguard by serving as a barrier between noncoding RNA and TLR7. One model predicts that unbound ribonucleic acid (not associated with Ro60) may penetrate the endosome resulting in an untoward activation of TLR7 (Figure 3). In a scenario of macrophages treated with anti-Ro autoantibodies in vitro, TLR7 serves to anchor a two-receptor paradigm (FcγR, TLRs (6, 7)) involving a wide spectrum of immune cells, with antibodies, inflammatory cytokines, and the production of IFN-α.
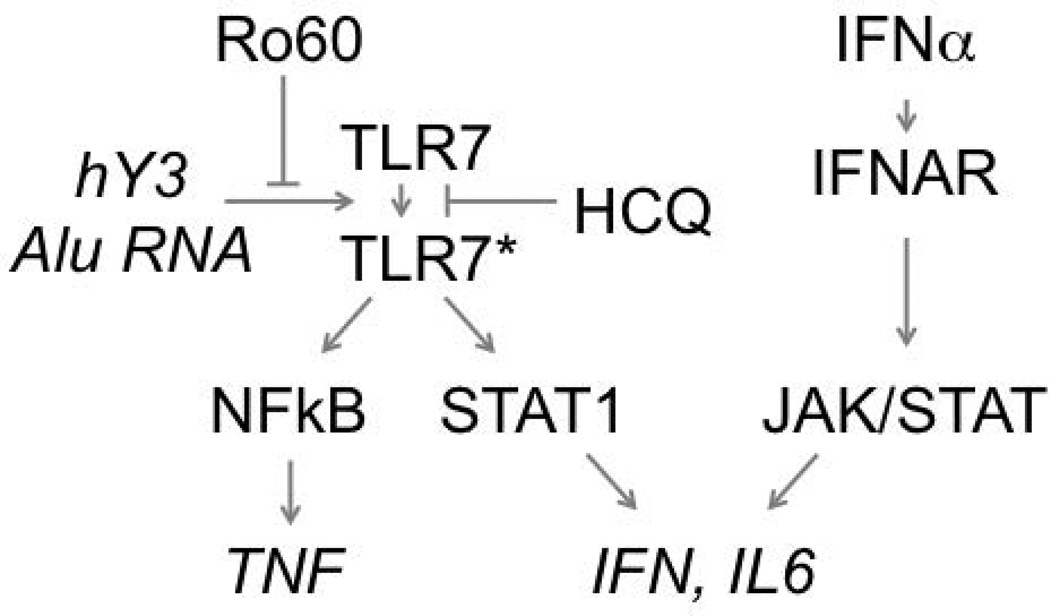
Figure 3. Ro60 and hydroxychloroquine act to attenuate the inflammatory pathway of Toll-like receptor 7 (TLR7) and its cognate ligand noncoding RNA.
Within B cells, dendritic cells, and macrophages, the engagement of TLR7 with ligands, hY3 and Alu RNA, in the context of the two-receptor hypothesis (not shown) results in activation of pathways driven by epigenetic modifications influencing the transcription factors STAT1 and NF-κB. Intracellular Ro60 will attenuate this response by reducing the available pool of hY3, Alu RNA, and other noncoding RNAs. In addition, hydroxychloroquine inhibits TLR-dependent epigenetic modifications and cytokine production. HCQ is specific to the endosome and does not block downstream autocrine-paracrine cytokine signaling. *TLR7 that has completed the transition from latent to an active form.
Based on murine studies, engagement of BCR/TLR7 resulted in a loss of regulation of TLR7, a scenario that may account for a substantial portion of risk for developing autoantibodies, such as anti-Ro60. In addition, we speculate that for subjects who transition to clinical disease, the risk is also conferred by the associations of the two receptor signaling and the “hair triggered” TLR7 (Figure 4). In support of this concept, male BXSB (Yaa) mice, which have a second active copy of the TLR7 gene on the Y chromosome, develop increased autoreactive B cell responses with a spontaneous ANA and manifestations involving glomerulonephritis (54).
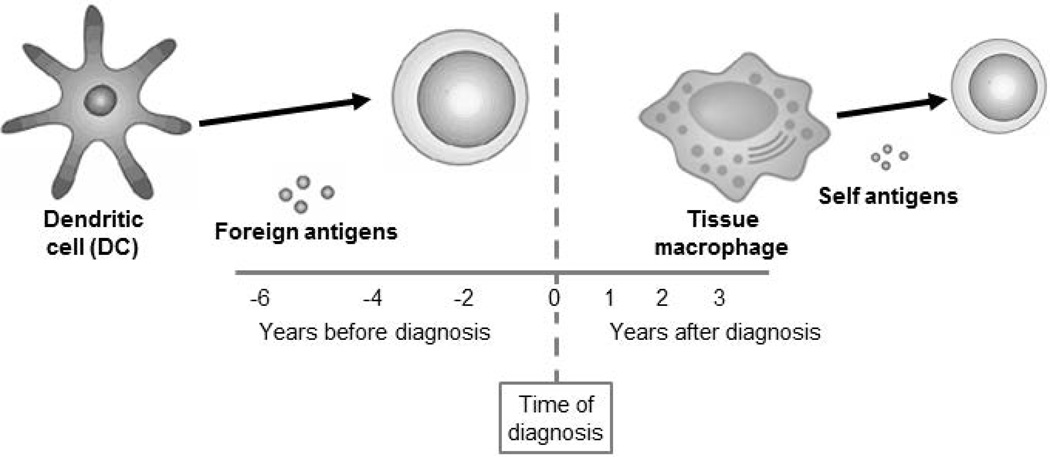
Figure 4. Towards understanding the associations of a “Hair trigger” TLR7 and sequelae involving dendritic cells and macrophages in the context of the appearance of the first clinical manifestation of SLE.
Towards understanding the associations of a “hair trigger” TLR7 and sequelae involving dendritic cells and macrophages in the context the first clinical manifestation of SLE. The time of the first appearance of any clinical criterion useful for the classification of systemic lupus erythematosus (clinical onset) are indicated by the dashed line. At 6 yrs before onset, the subject is asymptomatic and positive for anti-Ro60, an autoantibody which appears years before clinical diagnosis (9). Foreign antigens such as EBV (note that lupus patients are highly susceptible to EBV infection compared to the general population and that antibodies against Epstein–Barr virus nuclear antigen 1 [EBNA-1] cross-react with Ro60 [51]) may continue to drive lymphocytes at lymphoid tissue towards the formation of anti-Ro autoantibodies (59). At a stage involving clinically apparent disease, macrophages, residing at local sites produce cytokines and factors injuring host tissue. Within the spectrum of this response there may be associations of onset of clinical disease, TLR7, and a two-receptor paradigm (FcγR, TLRs) involving macrophages and the production of IFN-α.
Finally, we discuss hydroxychloroquine as a therapy to act at the level of endosomal TLR7 to extinguish the downstream cytokines and initial production of IFN-α. In addition, considering the therapeutic reach and the target population, because SLE has links to multiple downstream pathways, HCQ may be unable to dampen the immune storm once it progresses. In contrast, underscoring the importance of macrophage stimulation via TLR ligation in the pathogenesis of disease, both a case control retrospective review of anti-Ro antibody exposed fetuses of mothers with SLE and evaluation of subsequent pregnancies following the birth of a child with congenital heart block suggested that HCQ might have a role in both primary and secondary prevention (55, 56). In addition, there is support for HCQ use by asymptomatic subjects, such as anti-Ro60 positive mothers of children with neonatal lupus, to forestall the clinical expression of autoimmunity.
Acknowledgement
Study in the author’s laboratory was supported by a National Institutes of Health Merit Award (R37 AR042455, 3R37AR042455-21S1, 3R37AR042455-21S2 (J.P.B.)), the Research Registry for Neonatal Lupus (N01-AR-4-2220 (J.P.B.)), a Lupus Foundation of America Lifeline grant (J.P.B.) and the National Institutes of Health (R03 HD069986 and 1 R01 HD079951-01A1 (J.P.B.)).
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Horton CG, Pan ZJ, Farris AD. Targeting Toll-like receptors for treatment of SLE. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/498980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnare M, Rollinghoff M, Qureshi S. Toll-like receptors: sentinels of host defence against bacterial infection. International archives of allergy and immunology. 2006;139:75–85. doi: 10.1159/000090001. [DOI] [PubMed] [Google Scholar]
- 3.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and biophysical research communications. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Akira S. Toll-like receptors in innate immunity. International immunology. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 5.Lafyatis R, York M, Marshak-Rothstein A. Antimalarial agents: closing the gate on Toll-like receptors? Arthritis and rheumatism. 2006;54:3068–3070. doi: 10.1002/art.22157. [DOI] [PubMed] [Google Scholar]
- 6.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis and rheumatism. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 7.Clancy RM, Alvarez D, Komissarova E, Barrat FJ, Swartz J, Buyon JP. Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. Journal of immunology. 2010;184:2148–2155. doi: 10.4049/jimmunol.0902248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera TL, et al. Disease progression in mothers of children enrolled in the Research Registry for Neonatal Lupus. Annals of the rheumatic diseases. 2009;68:828–835. doi: 10.1136/ard.2008.088054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 10.Mills RE, et al. Unbiased modifier screen reveals that signal strength determines the regulatory role murine TLR9 plays in autoantibody production. Journal of immunology. 2015;194:3675–3686. doi: 10.4049/jimmunol.1500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nature reviews Immunology. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukui R, et al. Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA-but against RNA-sensing. The Journal of experimental medicine. 2009;206:1339–1350. doi: 10.1084/jem.20082316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukui R, et al. Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity. 2011;35:69–81. doi: 10.1016/j.immuni.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Nickerson KM, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. Journal of immunology. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nundel K, et al. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. Journal of immunology. 2015;194:2504–2512. doi: 10.4049/jimmunol.1402425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deane JA, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee PY, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. The Journal of experimental medicine. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. Journal of immunology. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 19.Guiducci C, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stohl W, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2012;64:2328–2337. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St Clair EW, et al. Rituximab therapy for primary Sjogren's syndrome: an open-label clinical trial and mechanistic analysis. Arthritis and rheumatism. 2013;65:1097–1106. doi: 10.1002/art.37850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung T, et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science. 2015 doi: 10.1126/science.aac7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue D, et al. A lupus-like syndrome develops in mice lacking the Ro 60-kDa protein, a major lupus autoantigen. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7503–7508. doi: 10.1073/pnas.0832411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark K, Plater L, Peggie M, Cohen P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. The Journal of biological chemistry. 2009;284:14136–14146. doi: 10.1074/jbc.M109.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117:5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 26.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. The Journal of experimental medicine. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda ME, et al. Accessibility of SSA/Ro and SSB/La antigens to maternal autoantibodies in apoptotic human fetal cardiac myocytes. Journal of immunology. 1998;161:5061–5069. [PubMed] [Google Scholar]
- 28.Reed JH, Clancy RM, Purcell AW, Kim MY, Gordon TP, Buyon JP. beta2-glycoprotein I and protection from anti-SSA/Ro60-associated cardiac manifestations of neonatal lupus. Journal of immunology. 2011;187:520–526. doi: 10.4049/jimmunol.1100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed JH, et al. Different temporal expression of immunodominant Ro60/60 kDa-SSA and La/SSB apotopes. Clin Exp Immunol. 2007;148:153–160. doi: 10.1111/j.1365-2249.2007.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed JH, Sim S, Wolin SL, Clancy RM, Buyon JP. Ro60 Requires Y3 RNA for Cell Surface Exposure and Inflammation Associated with Cardiac Manifestations of Neonatal Lupus. Journal of immunology. 2013;191:110–116. doi: 10.4049/jimmunol.1202849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clancy RM, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. The Journal of clinical investigation. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clancy RM, et al. Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. Journal of immunology. 2002;169:2156–2163. doi: 10.4049/jimmunol.169.4.2156. [DOI] [PubMed] [Google Scholar]
- 33.Dieude M, et al. Association of autoantibodies to nuclear lamin B1 with thromboprotection in systemic lupus erythematosus: lack of evidence for a direct role of lamin B1 in apoptotic blebs. Arthritis and rheumatism. 2002;46:2695–2707. doi: 10.1002/art.10552. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien CA, Wolin SL. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994;8:2891–2903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Smith JD, Shi H, Yang DD, Flavell RA, Wolin SL. The Ro autoantigen binds misfolded U2 small nuclear RNAs and assists mammalian cell survival after UV irradiation. Curr Biol. 2003;13:2206–2211. doi: 10.1016/j.cub.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Shi H, O'Brien CA, Van Horn DJ, Wolin SL. A misfolded form of 5S rRNA is complexed with the Ro and La autoantigens. Rna. 1996;2:769–784. [PMC free article] [PubMed] [Google Scholar]
- 37.Sim S, Weinberg DE, Fuchs G, Choi K, Chung J, Wolin SL. The subcellular distribution of an RNA quality control protein, the Ro autoantigen, is regulated by noncoding Y RNA binding. Mol Biol Cell. 2009;20:1555–1564. doi: 10.1091/mbc.E08-11-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. Journal of immunology. 1996;157:3317–3322. [PubMed] [Google Scholar]
- 39.Buyon JP, Friedman DM. Neonatal lupus. In: Lahita RG, Tsokos G, Buyon JP, Koike T, editors. Systemic Lupus Erythematosus. 5th ed. San Diego: Academic Press; 2011. pp. 541–567. [Google Scholar]
- 40.Izmirly PM, et al. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124:1927–1935. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waltuck J, Buyon JP. Autoantibody-associated congenital heart block: outcome in mothers and children. Ann Intern Med. 1994;120:544–551. doi: 10.7326/0003-4819-120-7-199404010-00003. [DOI] [PubMed] [Google Scholar]
- 42.Clancy RM, Markham AJ, Reed JH, Blumenberg M, Halushka MK, Buyon JP. Targeting downstream transcription factors and epigenetic modifications following Toll-like receptor 7/8 ligation to forestall tissue injury in anti-Ro60 associated heart block. Journal of autoimmunity. 2015 doi: 10.1016/j.jaut.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costedoat-Chalumeau N, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2006;54:3284–3290. doi: 10.1002/art.22156. [DOI] [PubMed] [Google Scholar]
- 44.Kelly KM, et al. "Endogenous adjuvant" activity of the RNA components of lupus autoantigens Sm/RNP and Ro 60. Arthritis and rheumatism. 2006;54:1557–1567. doi: 10.1002/art.21819. [DOI] [PubMed] [Google Scholar]
- 45.Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. Journal of immunology. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 46.Lamphier M, et al. Novel small molecule inhibitors of TLR7 and TLR9: mechanism of action and efficacy in vivo. Molecular pharmacology. 2014;85:429–440. doi: 10.1124/mol.113.089821. [DOI] [PubMed] [Google Scholar]
- 47.Niewold TB, Rivera TL, Buyon JP, Crow MK. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis and rheumatism. 2008;58:541–546. doi: 10.1002/art.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes and immunity. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izmirly PMCR, Munroe Melissa, Rasmussen Sara, Saxena Amit, Scher Jose U, Thanou Aikaterini, Kamp Stan, Merrill Joan T, Buyon Jill P, James Judith. Elevated Regulatory Mediators and Interferon Gamma Associated Responses, but Not Interferon Alpha, BLyS or IP-10, Accompany High-Titer Anti-Ro Autoantibodies in Asymptomatic Mothers of Children with Neonatal Lupus Arthritis and rheumatism. 2014;9S:1605. (Abstract). [Google Scholar]
- 50.Frohlich A, et al. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- 51.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dillon SR, et al. B-lymphocyte stimulator/a proliferation-inducing ligand heterotrimers are elevated in the sera of patients with autoimmune disease and are neutralized by atacicept and B-cell maturation antigen-immunoglobulin. Arthritis research & therapy. 2010;12:R48. doi: 10.1186/ar2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine & growth factor reviews. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 54.Subramanian S, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Izmirly PM, et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Annals of the rheumatic diseases. 2010;69:1827–1830. doi: 10.1136/ard.2009.119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Izmirly PM, et al. Maternal Use of Hydroxychloroquine Is Associated With a Reduced Risk of Recurrent Anti-SSA/Ro-Antibody-Associated Cardiac Manifestations of Neonatal Lupus. Circulation. 2012;126:76–82. doi: 10.1161/CIRCULATIONAHA.111.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Chung SA, et al. Differential genetic associations for systemic lupus erythematosus based on anti-dsDNA autoantibody production. PLoS genetics. 2011;7:e1001323. doi: 10.1371/journal.pgen.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poole BD, Templeton AK, Guthridge JM, Brown EJ, Harley JB, James JA. Aberrant Epstein-Barr viral infection in systemic lupus erythematosus. Autoimmunity reviews. 2009;8:337–342. doi: 10.1016/j.autrev.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]