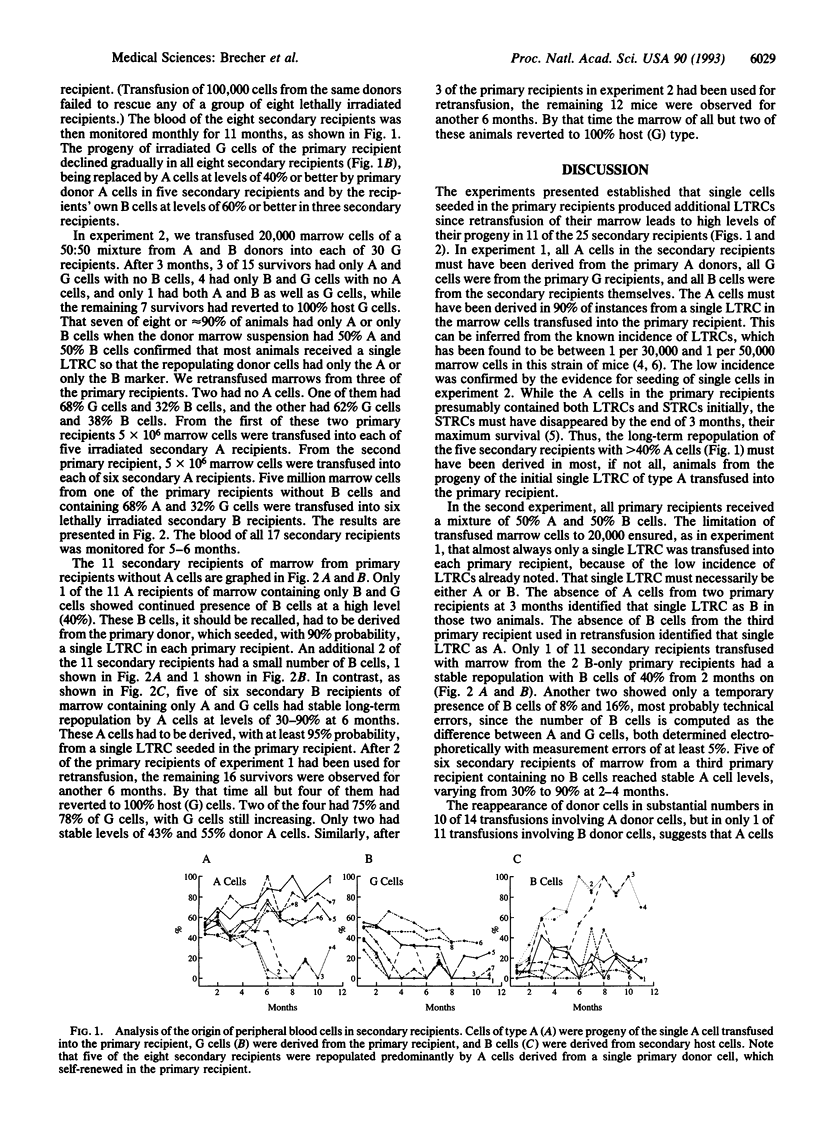
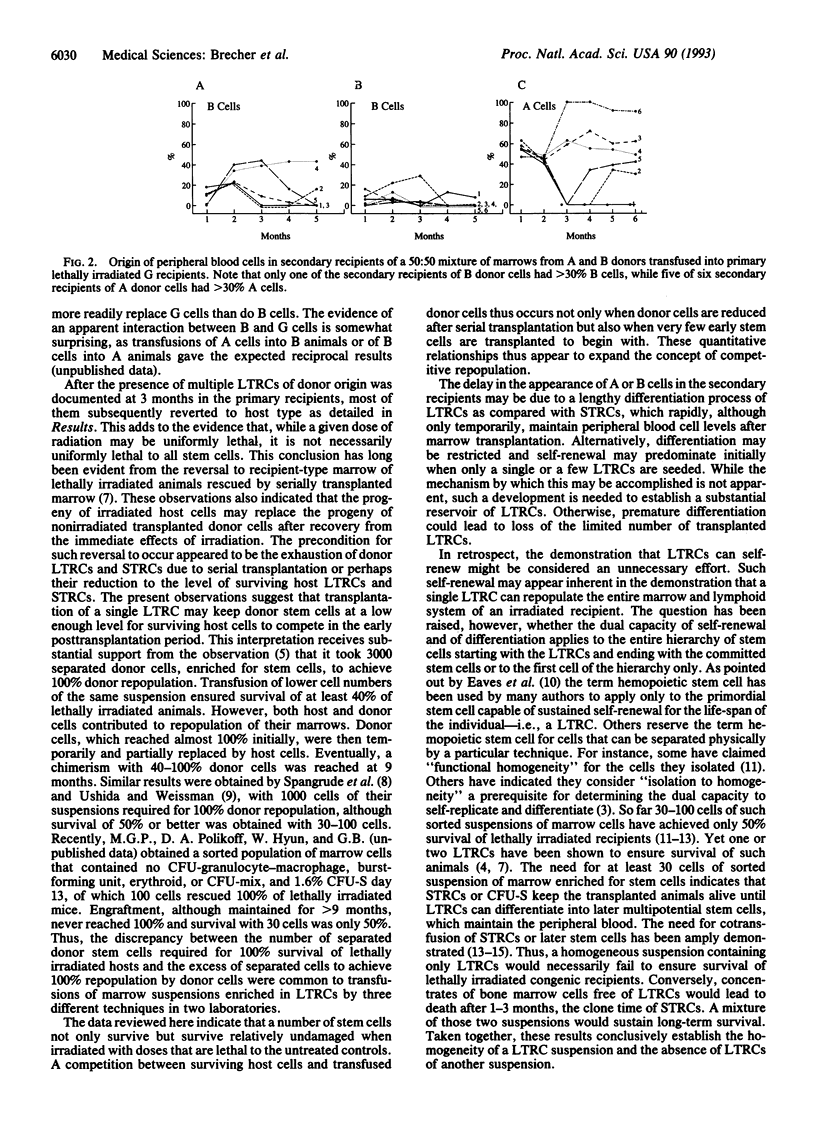
Abstract
Self-renewal implies maintenance of all attributes of the original in the offspring and is considered characteristic of the hemopoietic stem cells. Yet, it has been questioned whether one of the most primitive hemopoietic stem cells, the long-term repopulating cell (LTRC), has that capacity. The present experiments demonstrate that single LTRCs can repopulate the lymphohemopoietic system of a lethally irradiated mouse and that the progeny of a single LTRC in a primary recipient again contains LTRCs capable of repopulating lethally irradiated secondary recipients. The transfusion of very small numbers of marrow cells (10,000-20,000 cells containing one or no LTRCs) unexpectedly provided insight into competitive marrow repopulation. At these low levels of stem cells, irradiated host stem cells or their progeny competed successfully with unirradiated donor cells. This parallels the known reemergence and marrow repopulation by host cells when the number of nonirradiated donor stem cells is reduced by serial transplantation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelmez S. H., Andrews R. G., Bernstein I. D. Uncovering the heterogeneity of hematopoietic repopulating cells. Exp Hematol. 1991 Oct;19(9):861–862. [PubMed] [Google Scholar]
- Brecher G., Neben S., Yee M., Bullis J., Cronkite E. P. Pluripotent stem cells with normal or reduced self renewal survive lethal irradiation. Exp Hematol. 1988 Aug;16(7):627–630. [PubMed] [Google Scholar]
- Czerniak B., Herz F., Wersto R. P., Koss L. G. Asymmetric distribution of oncogene products at mitosis. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4860–4863. doi: 10.1073/pnas.89.11.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves C. J., Sutherland H. J., Udomsakdi C., Lansdorp P. M., Szilvassy S. J., Fraser C. C., Humphries R. K., Barnett M. J., Phillips G. L., Eaves A. C. The human hematopoietic stem cell in vitro and in vivo. Blood Cells. 1992;18(2):301–307. [PubMed] [Google Scholar]
- Hellman S., Botnick L. E., Hannon E. C., Vigneulle R. M. Proliferative capacity of murine hematopoietic stem cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):490–494. doi: 10.1073/pnas.75.1.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. J., Wagner J. E., Celano P., Zicha M. S., Sharkis S. J. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990 Sep 13;347(6289):188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- KAY H. E. HOW MANY CELL-GENERATIONS? Lancet. 1965 Aug 28;2(7409):418–419. doi: 10.1016/s0140-6736(65)90763-4. [DOI] [PubMed] [Google Scholar]
- Micklem H. S., Lennon J. E., Ansell J. D., Gray R. A. Numbers and dispersion of repopulating hematopoietic cell clones in radiation chimeras as functions of injected cell dose. Exp Hematol. 1987 Mar;15(3):251–257. [PubMed] [Google Scholar]
- Neben S., Redfearn W. J., Parra M., Brecher G., Pallavicini M. G. Short- and long-term repopulation of lethally irradiated mice by bone marrow stem cells enriched on the basis of light scatter and Hoechst 33342 fluorescence. Exp Hematol. 1991 Oct;19(9):958–967. [PubMed] [Google Scholar]
- Necas E., Znojil V., Vácha J. Stem cell number versus the fraction synthesizing DNA. Exp Hematol. 1988 Mar;16(3):231–234. [PubMed] [Google Scholar]
- Novak J. P., Stewart C. C. Stochastic versus deterministic in haemopoiesis: what is what? Br J Haematol. 1991 Jun;78(2):149–154. doi: 10.1111/j.1365-2141.1991.tb04409.x. [DOI] [PubMed] [Google Scholar]
- Rosendaal M., Hodgson G. S., Bradley T. R. Haemopoietic stem cells are organised for use on the basis of their generation-age. Nature. 1976 Nov 4;264(5581):68–69. doi: 10.1038/264068a0. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Scollay R. A simplified method for enrichment of mouse hematopoietic stem cells. Exp Hematol. 1990 Sep;18(8):920–926. [PubMed] [Google Scholar]
- Spangrude G. J., Smith L., Uchida N., Ikuta K., Heimfeld S., Friedman J., Weissman I. L. Mouse hematopoietic stem cells. Blood. 1991 Sep 15;78(6):1395–1402. [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2520–2524. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy S. J., Lansdorp P. M., Humphries R. K., Eaves A. C., Eaves C. J. Isolation in a single step of a highly enriched murine hematopoietic stem cell population with competitive long-term repopulating ability. Blood. 1989 Aug 15;74(3):930–939. [PubMed] [Google Scholar]
- Uchida N., Weissman I. L. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin- Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992 Jan 1;175(1):175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]



