Abstract
Osteoporosis is characterized by the progressive loss of bone mass and the micro-architectural deterioration of bone tissue, leading to bone fragility and an increased risk of fracture. Gallium has demonstrated efficacy in the treatment of several diverse disorders that are characterized by accelerated bone loss. Osteoblasts orchestrate bone degradation by expressing the receptor activator of NF-κB ligand (RANKL), however they additionally protect the skeleton by secreting osteoprotegerin (OPG). Therefore, the relative concentration of RANKL and OPG in bone is a key determinant of bone mass and strength. The current study demonstrated that gallium nitrate (GaN) is able to counteract bone loss in an experimental model of established osteoporosis. Ovariectomized (OVX) rats exhibited significantly increased bone mineral density following GaN treatment for 4 and 8 weeks by 19.3 and 37.3%, respectively (P<0.05). The bone volume of the OVX + GaN group was increased by 40.9% (P<0.05) compared with the OVX group. In addition, the current study demonstrated that GaN stimulates the synthesis of OPG however has no effect on the expression of RANKL in osteoblasts, as demonstrated by RT-qPCR, western blotting and ELISA, resulting in an increase in the OPG/RANKL ratio and a reduction in osteoclast differentiation in vivo and in vitro.
Keywords: osteoporosis, gallium nitrate, osteoblast, ovariectomized rats, osteoprotegerin, receptor activator of nuclear factor-κB ligand
Introduction
Osteoporosis is characterized by the progressive loss of bone mass and the micro-architectural deterioration of bone tissue, resulting in bone fragility and an increased risk of fracture (1). Gallium is a group IIIA metal and was first identified in 1875 by Paul-Émile Lecoq de Boisbaudran in France (2). Gallium has been demonstrated to be efficacious in the treatment of a number of diverse disorders that are characterized by accelerated bone loss, including cancer-associated hypercalcemia, bone metastases, Paget's disease, myeloma and fatal cage-layer osteoporosis (2–9). However, the adverse effects associated with gallium limits its use therapeutically for the treatment of osteoporosis (10).
Osteoblasts are key cells involved in bone remodeling. Osteoblasts orchestrate bone remodeling via the expression of receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) in response to osteoclast-stimulating hormones and cytokines, including parathyroid hormone, tumor necrosis factor and interleukin-1, however, in addition they protect the skeleton by secreting osteoprotegerin (OPG) (11). Osteoclasts are important cells in the process of bone resorption, and are predominantly regulated by RANKL and OPG, which are secreted by osteoblasts (12). Therefore, the relative concentrations of RANKL and OPG in bone are key determinants of bone mass and strength. OPG protects bone from excessive resorption by binding to RANKL and preventing it from binding to receptor activator of NF-κB (RANK) (13). Thus, it is suggested that the balance between RANKL and OPG serves an important role in the homeostasis of bone metabolism.
Considering the important role of OPG and RANKL in the regulation of osteoclasts and the inhibitory effects of gallium nitrate (GaN) on ovariectomized (OVX)-induced bone loss (5), the current study hypothesized that GaN may regulate the expression of OPG and RANKL in osteoblasts. Therefore, the aim of the present study was to investigate whether GaN is able to reduce RANKL and stimulate the expression of OPG in osteoblasts in vivo and in vitro, and thereby inhibit the differentiation of osteoclasts and prevent bone loss in OVX rats. The current study aimed to provide novel insight into the mechanisms of the effect of GaN on osteoporosis.
Materials and methods
Chemicals, experimental animals and treatments
GaN was purchased from BetaPharma Co., Ltd. (Shanghai, China). A total of 45 adult Sprague-Dawley female rats (Experimental Animal Center, Shengjing Hospital of China Medical University, Shenyang, China) at 8–10 weeks of age and 180–200 g in weight were used. Rats were randomly divided into three groups (15 rats/group), all to receive a supplement diet. The rats of two of the groups were to become OVX rats and those in the remaining group underwent a sham operation (control). Within 1 week of their arrival, rats were anesthe-tized with an intraperitoneal injection of 10% chloral hydrate (3.0 ml/kg; Tianjin Kermel Chemical Reagent Co., Ltd., Tianjin, China) and underwent either bilateral ovariectomy or a sham operation. Animals were kept separately in stainless-steel cages under controlled laboratory conditions (22–25°C, 40–60% relative humidity, 13 h light/11 h dark cycle) with standard rat chow and water available ad libitum for 2 months. Following this, group 1 (n=15, sham) and group 2 (n=15, OVX) were treated with the vehicle (HyClone™ phosphate-buffered saline; Thermo Fisher Scientific, Inc., Waltham, MA, USA) by intraperitoneal injection, and group 3 (n=15, OVX + GaN) was treated with GaN injected intraperitoneally (120 µg/kg/day) for 8 weeks.
All animal experiments were performed in accordance with the international standards for animal experimentation (14). All procedures were reviewed and approved by the Ethical Committee of China Medical University (Shenyang, China).
Measurements of bone mineral density (BMD) using dual-energy X-ray absorptiometry
Dual energy X-ray absorp-tiometry (XR-600; Norland Medical Systems, Inc., Tustin, CA, USA) was used to scan the left tibia of all rats to determine the level of BMD under chloral hydrate (3.0 ml/kg) anesthesia (for ~20 min, once per month.
Preparation of tissue
At the time of sacrifice, blood was collected from the dorsal aorta under ether anesthesia (Tianjin Kermel Chemical Reagent Co., Ltd.). Briefly, after the blood was collected, the rats were sacrificed by administering an overdose of the anesthetic. Following centrifugation at 1,800 × g at 4°C for 10 min, serum was harvested and stored at −20°C until analysis. The left tibiae were processed for histology. The right tibiae were processed for immunohistochemistry. The left femurs were processed for reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The right femurs were processed for western blot analysis. All bone tissues were immediately frozen at −80°C.
Bone histomorphometry
The tibia tissues were fixed in 4% formaldehyde (Tianjin Kermel Chemical Reagent Co., Ltd.) for 16 h at 4°C for histological evaluation. Following fixation, tibiae were decalcified in 10% ethylene diamine tetraacetic acid (Tianjin Kermel Chemical Reagent Co., Ltd.), dehydrated, embedded in paraffin (Beyotime Institute of Biotechnology, Shanghai, China), cut to 5 µm sections and stained with hematoxylin and eosin (H&E; Beyotime Institute of Biotechnology). The tibial analysis was conducted in the proximal metaphysis beginning adjacent to the epiphyseal growth plate. The callus total area, callus bony area and cartilage area were measured using Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Immunohistochemistry
For immunohistochemistry, sections were incubated overnight at 4°C with a polyclonal goat anti-rat OPG (cat. no. orb11189) and polyclonal goat anti-rat RANKL (cat. no. orb11190; 1:100; Biorbyt Ltd., Cambridge, UK). Goat serum (Solarbio, Beijing, China) was used as the blocking agent. The primary antibodies were detected by incubation (30 min at 37°C) with an anti-goat IgG secondary antibody conjugated with horseradish peroxidase (HRP; cat. no. A0227, 1:200; Beyotime Institute of Biotechnology, Haimen, China). Antibody complexes were visualized using a digital microscope (DP73; Olympus, Tokyo, Japan) with 3,3-diaminobenzine solution containing hydrogen peroxide (Beyotime Institute of Biotechnology) and then counterstained using hematoxylin. The integrated optical density was measured using Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc.).
Osteoblast cell culture
Osteoblasts were isolated from the calvariae of four male, 1-day old Sprague-Dawley rats (Experimental Animal Center, Shengjing Hospital of China Medical University) by sequential 0.25% trypsin and digestion with 0.1% type II collagenase (HyClone™; Thermo Fisher Scientific, Inc.). Cells released in the second and third digests were pooled and grown in HyClone™ Dulbecco's modified Eagle's medium-low glucose medium supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin (HyClone™; Thermo Fisher Scientific, Inc.) at 37°C in humidified 5% CO2 air. The cells were regularly trypsinized and subcultured to prevent cell confluence.
Osteoblast cytotoxicity test of GaN
The cytotoxicity of GaN against osteoblasts was investigated using a Cell Counting Kit-8 assay (CCK-8; Beyotime Institute of Biotechnology). In brief, cells were seeded into 96-well flat-bottomed plates at a density of 5×103 cells/well and then placed in serum-starved conditions for a further 6 h. Subsequently, cells were treated with GaN at increasing concentrations (0, 10−11, 10−10, 10−9, 10−8, 10−7, 10−6 and 10−5 mol/l) in the presence of 10% FBS for 24 h. Following the 24 h incubation, 10 µl CCK-8 dye was added to each well and incubated for 1 h according to the manufacturer's instructions. The absorbance of each well was measured at 450 nm using a microplate reader (Synergy H4; BioTek Instruments, Inc., Winooski, VT, USA).
Enzyme-linked immunosorbent assay (ELISA)
Primary osteoblasts were serum starved for 12 h, followed by GaN (10−9 mol/l) stimulation for 24 h. The supernatants of the osteoblasts were then collected. For in vivo and in vitro experiments, the supernatants of the osteoblasts and the serum from the rats were analyzed to measure the concentration of OPG and RANKL using the OPG and RANKL ELISA kits respectively (R&D Systems Inc., Minneapolis, USA), according to the manufacturer's instructions.
RNA extraction and RT-qPCR
The left femurs were placed in liquid nitrogen, bone chips were collected and gently mashed using a mallet. The bone tissues were then ground into a powder in liquid nitrogen using a pestle. For the in vivo and in vitro experiments, the total RNA was extracted from the pulverized bone powder and cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. The RNA concentration was determined by measuring absorbance at 260 nm, and 1 µg total RNA was transcribed to cDNA using the PrimerScript™ RT Reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's instructions. The primers used were as follows: OPG, forward 5′-GAC CCC AGA GCG AAA CAC G-3′ and reverse 5′-GGC ACA GCA AAC CTG AAG AA-3′; RANKL, forward 5′-CAT CGG GTT CCC ATA AAG-3′ and reverse 5′-GAA GCA AAT GTT GGC GTA-3′; β-actin, forward 5′-GGA GAT TAC TGC CCT GGC TCC TAG C-3′ and reverse 5′-GGC CGG ACT CAT CGT ACT CCT GCT T-3′. RT-qPCR was conducted using the Exicycler™ 96 (Bioneer Corporation, Daejeon, Korea) using the following cycling conditions: 95°C denaturation step for 10 min followed by 40 cycles of 95°C for 10 sec, 60°C annealing for 20 sec and extension at 72°C for 30 sec. The detection of the fluorescent product was conducted at the end of the 72°C extension period. The housekeeping gene β-actin was used to normalize the quantities of the target genes. Data were analyzed using the 2−ΔΔCq method (15), normalizing levels against those of β-actin. To evaluate the mean gene expression level, RT-qPCR was performed in triplicate for each sample.
Western blot analysis
The pulverized bone powder was homogenized in radioimmunoprecipitation assay (RIPA) lysis buffer [50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS); Beyotime Institute of Biotechnology, Shanghai] overnight at 4°C. Cell pellets were lysed directly in the culture bottles using the RIPA buffer. The lysates were collected and then centrifuged at 16,000 × g for 30 min at 4°C. The supernatant was then collected and the total protein levels were measured using a bicinchoninic acid assay kit (Beyotime Institute of Biotechnology). Equal amounts of protein (40 µg) were separated by SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane at 4°C (Beyotime Institute of Biotechnology, Shanghai). Following blocking in 5% fat-free milk for 1 h, the blots were incubated with the following primary antibodies: Anti-OPG (cat. no. orb11189; 1:200, Beyotime Institute of Biotechnology), anti-RANKL (cat. no. orb11190; 1:500, Beyotime Institute of Biotechnology) and anti-β-actin (cat. no. WL0001, 1:1,000; WanLei Life Sciences, Shenyang, China) at 4°C overnight. Membranes were then washed and incubated with HRP-conjugated secondary antibodies (anti-goat IgG; cat. no. A0277; 1:5,000) at 37°C for 2 h. Proteins were detected using enhanced chemiluminescence (Synoptics Ltd., Cambridge, UK). Blots were repeated at least three times for each condition. Following development, the band intensities were quantified using Gel-Pro-Analyzer software, version 4.0 (Media Cybernetics, Inc.)
Statistical analysis
Values are presented as the mean ± standard deviation and analyzed using the one-way analysis of variance and Student's t-test in IBM SPSS 19.0 software (IBM SPSS, Armonk, MY, USA). All experiments were repeated a minimum of three times, and representative experiments are presented throughout. P<0.05 was considered to indicate a statistically significant difference.
Results
BMD measurements
The average BMD of rats in each group throughout the experimental period is presented in Fig. 1A. Following ovariectomy, the BMD of animals was significantly reduced at 4 and 8 weeks (P<0.05), by 16.5 and 34.1%, respectively. However, the administration of GaN to OVX rats significantly increased BMD at 4 and 8 weeks (P<0.05), by 19.3 and 37.3%, respectively, compared with the OVX rats.
Figure 1.
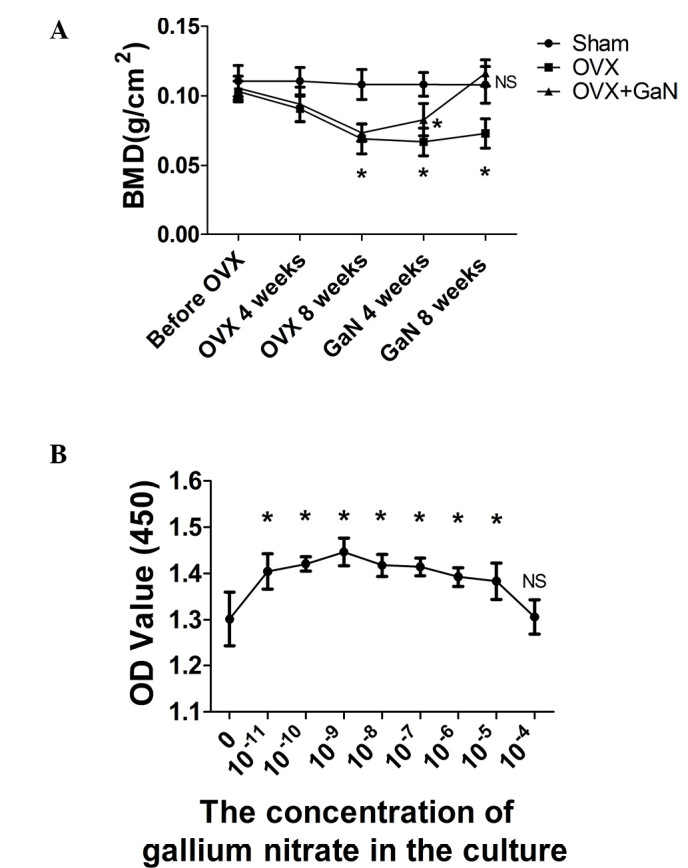
(A) BMD alterations during the experimental period. BMD measurements are from the sham, OVX and OVX + GaN groups, n=15 for each group at each time-point. *P<0.05, compared with the Sham group. (B) The GaN concentration of 10–9 mol/l resulted in the greatest OD value. Values are presented as the mean ± standard deviation. *P<0.05, compared with 0 mol/l. BMD, bone mineral density; NS, not significant; OVX, ovariectomized rats; GaN, gallium nitrate; OD, optical density.
Bone histomorphometry analysis
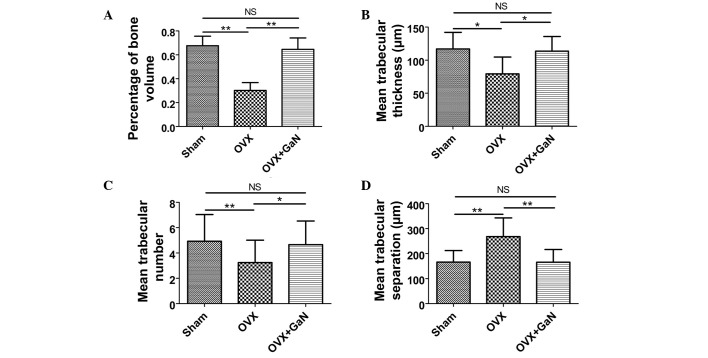
To investigate the effect of GaN on OVX-induced bone loss in vivo, H&E staining (Fig. 2) was conducted. The results of bone histomorphometry analysis were expressed as the percentage of bone volume (BV), mean trabecular thickness (Tb.Th), mean trabecular number (Tb.N) and mean trabecular separation (Tb.Sp). The BV in the tibia of the OVX + GaN group increased by 40.9% (P<0.05) compared with the OVX group (Fig. 3A). GaN significantly increased the BV in the tibia of OVX rats. The Tb.Th of the GaN treatment group was significantly increased by 43.3% (P<0.01) compared with the OVX group (Fig. 3B). The Tb.N exhibited an increase following GaN treatment of 43.4% (P<0.05) compared with the OVX group (Fig. 3C). In addition, treatment with GaN led to a reduction in the Tb.Sp by 38.2% (P<0.01) compared with the OVX treatment group (Fig. 3D).
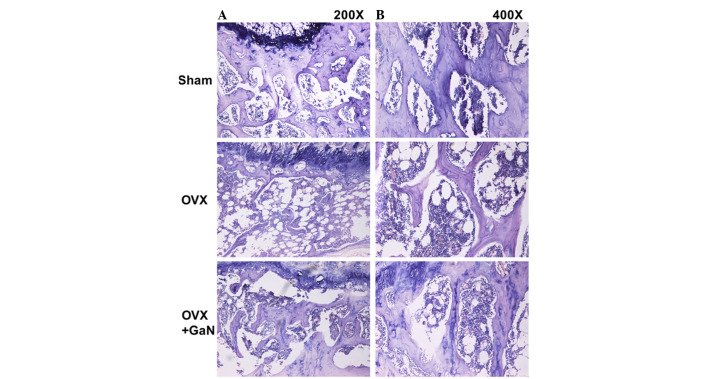
Figure 2.
GaN reduces bone loss in OVX rats. Hematoxylin and eosin staining of the metaphyseal region of the proximal tibiae in sham group rats and OVX group rats treated with or without 120 µg/kg/day GaN [magnification; (A) x200, (B) x400]. GaN, gallium nitrate; OVX, ovariectomized rats.
Figure 3.
Effects of GaN on bone histomorphometry. (A) The percentage of bone volume. (B) The mean trabecular thickness. (C) The mean trabecular number. (D) The mean trabecular separation. Values are presented as the mean ± standard deviaiton. *P<0.05, **P<0.01. GaN, gallium nitrate; OVX, ovariectomized rats; NS, not significant.
Immunohistochemical assessment
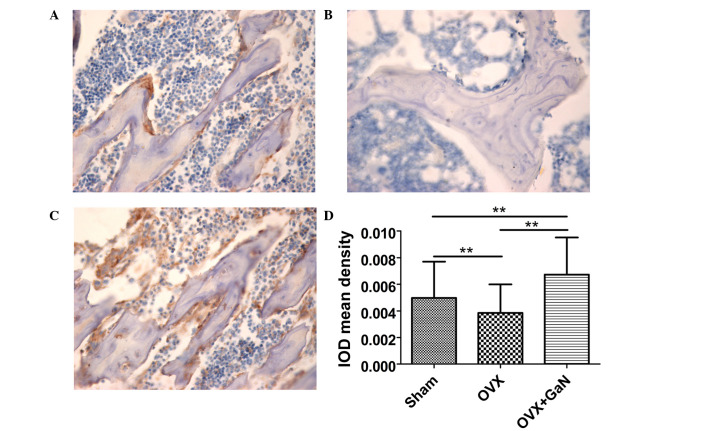
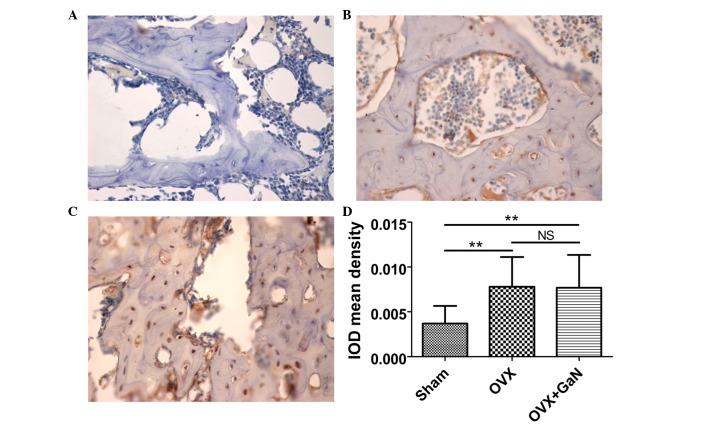
To confirm the preventative effect of GaN on OVX-induced bone loss in vivo, immunohistochemical assessment was conducted to examine the expression of OPG and RANKL in rats. The results demonstrated that GaN is able to enhance the expression of OPG (P<0.01) compared with the OVX group (Fig. 4), however without affecting the expression levels of RANKL compared with the OVX treatment group (Fig. 5).
Figure 4.
OPG immunohistochemical staining of proximal tibiae sections in OVX rats treated with or without 120 µg/kg/day of GaN (magnification, ×400). (A) Sham group, (B) OVX group, (C) OVX+GaN group and (D) the IOD mean density of OPG expression. Values are presented as the mean ± standard deviation. *P<0.05, **P<0.01. OPG, osteoprotegerin; OVX, ovariectomized rats; GaN, gallium nitrate; IOD, integrated optical density; NS, not significant.
Figure 5.
RANKL immunohistochemical staining of proximal tibiae sections in OVX rats treated with or without 120 µg/kg/day of GaN (magnification, x400). (A) Sham group, (B) OVX group, (C) OVX + GaN group and (D) the IOD mean density of RANKL expression. Values are presented as the mean ± standard deviation. *P<0.05, **P<0.01. RANKL, receptor activator of nuclear factor-κB ligand; OVX, ovariectomized rats; GaN, gallium nitrate; IOD, integrated optical density; NS, not significant.
Osteoblast cytotoxicity test
The optimal concentration of GaN was determined using a CCK-8 assay. For the control, the concentration of GaN was 0 mol/l. The optical density values of the osteoblasts were greatest following treatment with 10−9 mol/l GaN. Therefore, this concentration was selected as the optimal concentration of GaN for subsequent investigations (Fig. 1B).
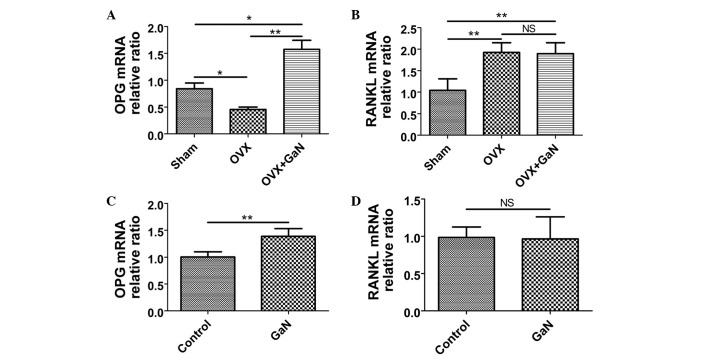
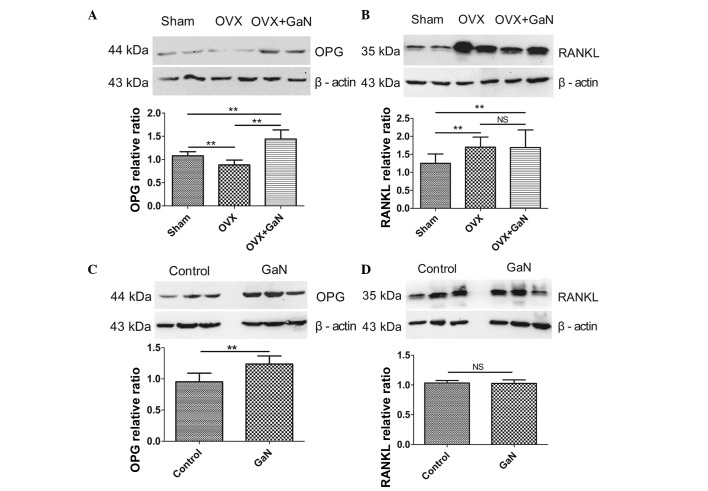
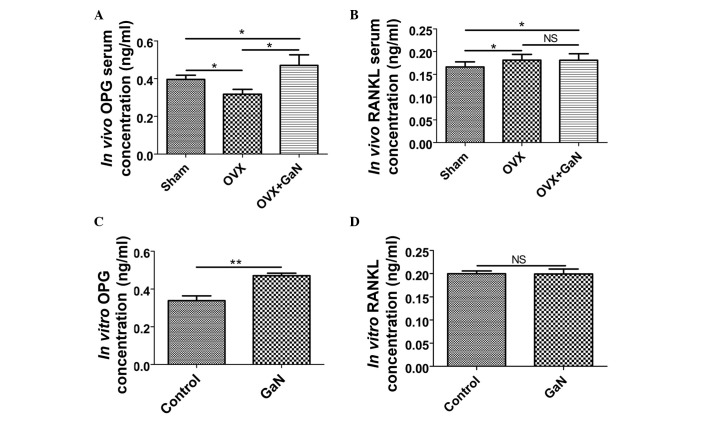
GaN affects the mRNA and protein expression ratio of OPG/RANKL in vivo and in vitro
To investigate the effect of GaN on OPG and RANKL expression in vivo and in vitro, OVX rats and osteoblasts were treated with GaN, and the mRNA and protein levels of OPG and RANKL in bone tissue were measured using RT-qPCR and western blot analysis, respectively (Figs. 6 and 7). In addition, the expression levels of OPG and RANKL in the supernatants of osteoblasts and the serum of rats were measured using ELISA (Fig. 8). Together, the results indicated that GaN increased the expression levels of OPG, however did not effect RANKL expression in GaN-treated OVX rats and osteoblast cells. These data demonstrate that GaN stimulates OPG however does not affect RANKL expression in vivo and in vitro.
Figure 6.
mRNA expression levels of OPG and RANKL in bone tissue were measured by reverse transcription-quantitative polymerase chain reaction. Data were analyzed using the 2−ΔΔCq method and normalized to β-actin. (A) The OPG mRNA relative ratio in vivo. (B) The RANKL mRNA relative ratio in vivo. (C) The OPG mRNA relative ratio in vitro. (D) The RANKL mRNA relative ratio in vitro. Values are presented as the mean ± standard deviation. *P<0.05, **P<0.01. OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-κB ligand; OVX, ovariectomized rats; GaN, gallium nitrate; NS, not significant.
Figure 7.
Protein expression levels of OPG and RANKL in bone tissue were measured by western blotting. Data were normalized against β-actin. (A) The OPG protein relative ratio in vivo. (B) The RANKL protein relative ratio in vivo. (C) The OPG protein relative ratio in vitro. (D) The RANKL protein relative ratio in vitro. Values are presented as the mean ± standard deviation. *P<0.05, **P<0.01. OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-κB ligand; OVX, ovariectomized rats; GaN, gallium nitrate; NS, not significant.
Figure 8.
Expression levels of OPG and RANKL in the supernatants of osteoblasts and the serum of rats were measured by the enzyme-linked immunosorbent assay. (A) The serum concentration of OPG in vivo. (B) The serum concentration of RANKL in vivo. (C) The supernatant concentration of OPG in vitro. (D) The supernatant concentration of RANKL in vitro. Values are presented as the mean ± standard deviation. *P<0.05, **P<0.01. OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-κB ligand; OVX, ovariectomized rats; GaN, gallium nitrate; NS, not significant.
Discussion
Osteoporosis is the most common bone disorder in aging populations and is an important public health issue, with osteoporotic fractures having a key impact upon health, in terms of acute and long term disability and economic cost (16). The World Health Organization describes osteoporosis as a generalized metabolic disease characterized by progressive loss of the elements of bone tissue and a simultaneous deterioration of skeletal microarchitecture (17), leading to bone fragility and resulting in an increased risk of fractures. Epidemiological data worldwide have consistently demonstrated that the annual incidence of fragility fractures is increasing, in particular in ageing populations (18–23). Furthermore, awareness of osteoporosis is increasing, with this multifactorial disease regarded as a serious public health issue.
Elemental gallium, a group IIIA metal, alters the mineral, matrix and cellular properties of bone (24,25). In animal experiments, gallium has been demonstrated to affect OVX osteopenic rats by reducing serum mineral levels and increasing bone mineral content (26). X-ray diffraction analysis of bone powder from gallium-treated rats demonstrated alterations characteristic of an increase in the size or crystalline perfection of hydroxyapatite minerals (27). The current study demonstrated that GaN treatment resulted in an increase in tibia trabecular bone parameters, BMD and bone strength in OVX rats. The results of histomorphometric analysis indicate that GaN administration increased the BV and Tb.N, whilst reducing the Tb.Sp in the proximal tibia. These results are in accordance with previous studies which documented the effect of gallium on osteoporosis in OVX rats following short-term treatment (16,28). However, the adverse effects of gallium have limited its use as a therapeutic agent for the treatment of osteoporosis (10). A common adverse effect is hypocalcemia, which on occasion is sufficiently severe to result in transient tetany (29,30). Yeast may be used as an element carrier that is able to convert inorganic elements to organic species and thereby reduce element-associated toxicity (31–33). Previous studies have demonstrated that yeast-incorporated gallium not only reduces gallium-associated toxicity, however additionally maintains its therapeutic effect on improving bone loss and promoting fracture healing in OVX female rats (16,26,28).
Bone formation and resorption are normally in physiological balance, maintaining a stable bone mass. At the cellular level, the beneficial effects of gallium are based on a dual effect on osteoblasts and osteoclasts, which control the process of bone remodeling. A previous report by Verron et al (34) indicates that gallium reduces osteoclastic resorption, differentiation and formation in vitro without affecting the viability or activity of primary and MC3T3-E1 osteoblasts. Osteoclasts are key cells in bone resorption, and their differentiation is predominantly regulated by RANKL and OPG. As such, the balance between RANKL and OPG serves a significant role in the homeostasis of bone metabolism (35–39). OPG protects bone from excessive resorption by binding to RANKL and preventing it from binding to RANK. Therefore, the relative concentration of RANKL and OPG in bone is a major determinant of bone mass and strength (13,40,41). The evidence from previous studies led to the current study, which investigated the mechanisms underlying this regulation using an in vivo experimental model of established osteoporosis and an in vitro cell culture system. The present study demonstrated the important role of GaN in the regulation of the OPG/RANKL axis and the inhibition of osteoclastogenesis in vivo and in vitro. These data support the view that GaN inhibits differentiation of osteoclasts by stimulating OPG without affecting RANKL production in osteoblasts, which may indicate the potential mechanism of GaN in the prevention of post-menopausal osteoporosis.
In conclusion, the current study demonstrates that GaN is able to counteract bone loss in an experimental model of established osteoporosis. In addition, the data have demonstrated that GaN stimulates the synthesis of OPG without affecting RANKL expression in osteoblasts, resulting in an increase in the OPG/RANKL ratio and a reduction in osteoclast differentiation in vivo and in vitro. These results suggest that GaN may upregulate the secretion of OPG in osteoblasts, and subsequently prevent bone loss and osteoporosis.
Acknowledgments
The current study was supported by grants from the National Natural Science Foundation of China (grant nos. 81370981 and 81300714).
References
- 1.Peck WA. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-E. [DOI] [PubMed] [Google Scholar]
- 2.Warrell RP, Jr, Bockman RS, Coonley CJ, Isaacs M, Staszewski H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J Clin Invest. 1984;73:1487–1490. doi: 10.1172/JCI111353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warrell RP, Jr, Skelos A, Alcock NW, Bockman RS. Gallium nitrate for acute treatment of cancer-related hypercalcemia: Clinicopharmacological and dose response analysis. Cancer Res. 1986;46:4208–4212. [PubMed] [Google Scholar]
- 4.Warrell RP, Jr, Israel R, Frisone M, Snyder T, Gaynor JJ, Bockman RS. Gallium nitrate for acute treatment of cancer-related hypercalcemia. A randomized, double-blind comparison to calcitonin. Ann Intern Med. 1988;108:669–674. doi: 10.7326/0003-4819-108-5-669. [DOI] [PubMed] [Google Scholar]
- 5.Warrell RP., Jr Gallium nitrate for the treatment of bone metastases. Cancer. 1997;80(Suppl):1680–1685. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1680::AID-CNCR19>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Warrell RP, Jr, Bosco B, Weinerman S, Levine B, Lane J, Bockman RS. Gallium nitrate for advanced Paget disease of bone: Effectiveness and dose-response analysis. Ann Intern Med. 1990;113:847–851. doi: 10.7326/0003-4819-113-11-847. [DOI] [PubMed] [Google Scholar]
- 7.Niesvizky R. Gallium nitrate in multiple myeloma: Prolonged survival in a cohort of patients with advanced-stage disease. Semin Oncol. 2003;30(Suppl 5):20–24. doi: 10.1016/S0093-7754(03)00172-6. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Jiang Z, Liu X. Biochemical mechanism of gallium on prevention of fatal cage-layer osteoporosis. Biol Trace Elem Res. 2010;134:195–202. doi: 10.1007/s12011-009-8467-x. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Wang C. Activity of gallium on prevention of fatal cage-layer osteoporosis. Biol Trace Elem Res. 2009;132:129–135. doi: 10.1007/s12011-009-8403-0. [DOI] [PubMed] [Google Scholar]
- 10.Foster BJ, Clagett-Carr K, Hoth D, Leyland-Jones B. Gallium nitrate: The second metal with clinical activity. Cancer Treat Rep. 1986;70:1311–1319. [PubMed] [Google Scholar]
- 11.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 12.Teitelbaum SL. Osteoclasts, integrins, and osteoporosis. J Bone Miner Metab. 2000;18:344–349. doi: 10.1007/s007740070007. [DOI] [PubMed] [Google Scholar]
- 13.Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 14.ICLA recommendations for the specification of the animals, the husbandry, and the techniques used in animal experimentation. International Committee on Laboratory Animals - Secretariat. Anat Anz. 1979;145:413–414. [PubMed] [Google Scholar]
- 15.Liss B. Improved quantitative real-time RT-PCR for expression profiling of individual cells. Nucleic Acids Res. 2002:30e89. doi: 10.1093/nar/gnf088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Z, Fu Q. Therapeutic effect of organic gallium on ovariectomized osteopenic rats by decreased serum minerals and increased bone mineral content. Biol Trace Elem Res. 2010;133:342–349. doi: 10.1007/s12011-009-8445-3. [DOI] [PubMed] [Google Scholar]
- 17.Goodfellow LR, Earl S, Cooper C, Harvey NC. Maternal diet, behaviour and offspring skeletal health. Int J Environ Res Public Health. 2010;7:1760–1772. doi: 10.3390/ijerph7041760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanis JA, McCloskey EV. Epidemiology of vertebral osteoporosis. Bone. 1992;13(Suppl 2):S1–S10. doi: 10.1016/8756-3282(92)90189-4. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ, III, Lane AW, Cooper C, Eastell R, O'Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113–119. doi: 10.1007/BF01623271. [DOI] [PubMed] [Google Scholar]
- 20.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–522. doi: 10.1016/S8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 21.Van der Klift M, De Laet CE, McCloskey EV, Hofman A, Pols HA. The incidence of vertebral fractures in men and women: The Rotterdam Study. J Bone Miner Res. 2002;17:1051–1056. doi: 10.1359/jbmr.2002.17.6.1051. [DOI] [PubMed] [Google Scholar]
- 22.Felsenberg D, Silman AJ, Lunt M, Armbrecht G, Ismail AA, Finn JD, Cockerill WC, Banzer D, Benevolenskaya LI, Bhalla A, et al. European Prospective Osteoporosis Study (EPOS) Group Incidence of vertebral fracture in europe: Results from the European Prospective Osteoporosis Study (EPOS) J Bone Miner Res. 2002;17:716–724. doi: 10.1359/jbmr.2002.17.4.716. [DOI] [PubMed] [Google Scholar]
- 23.Lord SR. Hip fractures: Changing patterns in hospital bed use in NSW between 1979 and 1990. Aust N Z J Surg. 1993;63:352–355. doi: 10.1111/j.1445-2197.1993.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 24.Repo MA, Bockman RS, Betts F, Boskey AL, Alcock NW, Warrell RP., Jr Effect of gallium on bone mineral properties. Calcif Tissue Int. 1988;43:300–306. doi: 10.1007/BF02556640. [DOI] [PubMed] [Google Scholar]
- 25.Bockman RS, Boskey AL, Blumenthal NC, Alcock NW, Warrell RP., Jr Gallium increases bone calcium and crystallite perfection of hydroxyapatite. Calcif Tissue Int. 1986;39:376–381. doi: 10.1007/BF02555174. [DOI] [PubMed] [Google Scholar]
- 26.Bockman RS, Boskey AL, Blumenthal NC, Alcock NW, Warrell RP., Jr Gallium increases bone calcium and crystallite perfection of hydroxyapatite. Calcif Tissue Int. 1986;39:376–381. doi: 10.1007/BF02555174. [DOI] [PubMed] [Google Scholar]
- 27.Ma Z, Fu Q. Comparison of the therapeutic effects of yeast-incorporated gallium with those of inorganic gallium on ovariectomized osteopenic rats. Biol Trace Elem Res. 2010;134:280–287. doi: 10.1007/s12011-009-8472-0. [DOI] [PubMed] [Google Scholar]
- 28.Pei Y, Fu Q. Yeast-incorporated gallium promotes fracture healing by increasing callus bony area and improving trabecular microstructure on ovariectomized osteopenic rats. Biol Trace Elem Res. 2011;141:207–215. doi: 10.1007/s12011-010-8708-z. [DOI] [PubMed] [Google Scholar]
- 29.Bedikian AY, Valdivieso M, Bodey GP, Burgess MA, Benjamin RS, Hall S, Freireich EJ. Phase I clinical studies with gallium nitrate. Cancer Treat Rep. 1978;62:1449–1453. [PubMed] [Google Scholar]
- 30.Krakoff IH, Newman RA, Goldberg RS. Clinical toxicologic and pharmacologic studies of gallium nitrate. Cancer. 1979;44:1722–1727. doi: 10.1002/1097-0142(197911)44:5<1722::AID-CNCR2820440528>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Zhang B, He X, Zhang P, Chai Z. Selection of a high-biomass, chromium-rich yeast strain and optimization of cultivation conditions. J Ind Microbiol Biotechnol. 2001;27:195–198. doi: 10.1038/sj.jim.7000161. [DOI] [PubMed] [Google Scholar]
- 32.Han C, Yuan J, Wang Y, Li L. Hypoglycemic activity of fermented mushroom of Coprinus comatus rich in vanadium. J Trace Elem Med Biol. 2006;20:191–196. doi: 10.1016/j.jtemb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Han C, Cui B, Wang Y. Vanadium uptake by biomass of Coprinus comatus and their effect on hyperglycemic mice. Biol Trace Elem Res. 2008;124:35–39. doi: 10.1007/s12011-008-8120-0. [DOI] [PubMed] [Google Scholar]
- 34.Verron E, Masson M, Khoshniat S, Duplomb L, Wittrant Y, Baud'huin M, Badran Z, Bujoli B, Janvier P, Scimeca JC, et al. Gallium modulates osteoclastic bone resorption in vitro without affecting osteoblasts. Br J Pharmacol. 2010;159:1681–1692. doi: 10.1111/j.1476-5381.2010.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteopro-tegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 37.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 38.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, III, Frankel WN, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 39.American Society for Bone and Mineral Research President's Committee on Nomenclature Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. J Bone Miner Res. 2000;15:2293–2296. doi: 10.1359/jbmr.2000.15.12.2293. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]