Abstract
The oncolytic effects of reovirus in various cancers have been proven in many clinical trials in human medicine. Oncolytic virotherapy using reovirus for canine cancers is being developed in our laboratory. The objective of this study was to examine the synergistic anti-cancer effects of a combination of reovirus and low doses of various chemotherapeutic agents on mammary gland tumors (MGTs) in dogs. The first part of this study demonstrated the efficacy of reovirus in canine MGTs in vitro and in vivo. Reovirus alone exerted significant cell death by means of caspase-dependent apoptosis in canine MGT cell lines. A single injection of reovirus impeded growth of canine MGT tumors in xenografted mice, but was insufficient to induce complete tumor regression. The second part of this study highlighted the anti-tumor effects of reovirus in combination with low doses of paclitaxel, carboplatin, gemcitabine, or toceranib. Enhanced synergistic activity was observed in the MGT cell line treated concomitantly with reovirus and in all the chemotherapeutic agents except toceranib. In addition, combining reovirus with paclitaxel or gemcitabine at half dosage of half maximal inhibitory concentration (IC50) enhanced cytotoxicity by activating caspase 3. Our data suggest that the combination of reovirus and low dose chemotherapeutic agents provides an attractive option in canine cancer therapy.
Résumé
Les effets oncolytiques des reovirus dans divers cancers ont été prouvés lors de plusieurs essais cliniques en médecine humaine. La virothérapie oncolytique pour les cancers canins utilisant des reovirus est présentement en développement dans notre laboratoire. L’objectif de cette étude était d’examiner les effets synergiques anticancéreux d’une combinaison de reovirus et de faibles doses d’agents chimio-thérapeutiques variés sur les tumeurs des glandes mammaires (TGM) chez les chiens. La première partie de l’étude a démontré l’efficacité du reovirus sur des TGM in vitro et in vivo. Les reovirus utilisés seuls ont produit une quantité significative de mort cellulaire dans des lignées cellulaires canines de TGM via l’apoptose dépendante de la caspase. Une injection unique de reovirus interféra avec la croissance de TGM canines chez des souris ayant reçu une xénogreffe, mais était insuffisante pour induire une régression complète de la tumeur. La deuxième partie de cette étude a mis en évidence les effets anti-tumoraux des reovirus en combinaison avec de faibles doses de paclitaxel, de carboplatin, de gemcibatine, ou de toceranib. Une activité synergique augmentée fut observée dans la lignée cellulaire TGM traitée de manière concomitante avec du reovirus et tous les agents chimio-thérapeutique sauf le toceranib. De plus, en combinant le reovirus avec du paclitaxel ou de la gemcibatine à la moitié de la dose de la moitié de la concentration inhibitrice maximale (IC50) on augmenta la cytotoxicité en activant la caspase 3. Nos données suggèrent que la combinaison de reovirus et de faibles doses d’agents chimio-thérapeutiques fournie une option intéressante pour le traitement de cancer canin.
(Traduit par Docteur Serge Messier)
Introduction
Canine mammary gland tumor (MGT) is the most common neoplasm in female dogs and its biological features are similar to such tumors in humans (1). Ovariohysterectomy in female dogs at an early age before the first estrous cycle reduces the risk of MGT development by approximately 0.5% (2). The incidence of canine MGT in the United States has therefore been reported to be lower than in some European countries due to the common practice of pet neutering (3). Due to the aggressiveness of this disease, however, it remains one of the leading causes of death in female dogs. Complete surgical resection is the best treatment available for canine MGT as no other effective systemic therapy has yet been established. Although anthracycline or taxane is used as adjunctive chemotherapy for human breast cancer (4), doxorubicin, docetaxel, and gemcitabine have not yet been proven effective against advanced stages of MGT in dogs (5,6). This highlights the dire need for novel therapies that are effective against this canine cancer.
Oncolytic virotherapy is one of the new approaches in cancer treatment. Adenovirus, herpes simplex virus, and reovirus are among the few viruses known for their oncolytic properties (7). Unlike adenovirus and herpes simplex virus, which are genetically modified, reovirus is a naturally occurring, non-enveloped double-stranded ribonucleic acid (dsRNA) virus originally isolated from the human respiratory and gastrointestinal tract (8). Pathogenicity of reovirus is low as most adults are seropositive, while not showing any clinical symptoms (9,10).
Despite the lack of pathogenicity in humans, reovirus infects and kills oncogenic Ras-transformed cells without harming non-transformed normal cells (11,12). The major difference in cell susceptibility to reovirus lies in the ability of those cells to phosphorylate PKR (dsRNA-activated protein kinase). In susceptible cells infected by reovirus, Ras activation inhibits the phosphorylation of PKR and allows the expression of viral proteins before the release of viral progeny. It has also been reported, however, that reovirus can exert oncolysis independent of this pathway in some tumor cells (13). Oncolytic virotherapy using reovirus has been tested in many clinical trials for human cancers, such as breast cancer, malignant melanoma, multiple myeloma, and colorectal cancer (14), but studies of reovirus in veterinary oncology are still limited at the pre-clinical stages.
Our laboratory has previously reported that canine mast cell tumor is highly susceptible to reovirus-induced cell death, followed by canine MGT, canine malignant melanoma, and canine lymphoma (15–17). This suggests that these types of tumors are potential candidates for oncolytic virotherapy using reovirus. Recently, the combination of reovirus with various chemotherapeutic agents in human clinical trials has shown more profound anti-tumor effects (18–20). The objective of this study was to examine the synergistic anti-cancer effects of a combination of reovirus and chemotherapeutic agents in canine MGT cell lines in order to develop new treatments applicable in clinical settings in the future.
Materials and methods
Cell cultures, reovirus, and chemotherapeutic agents
Canine MGT cell lines, CHMp-13a, and CHMp-5b, which were established by Dr. Takayuki Nakagawa (University of Tokyo), were used in this study. Mouse L929 fibroblastic cell line was obtained from the Cell Resource Center for Biomedical Research (Institute of Development, Aging and Cancer, Tohoku University, Sendai, Japan) and was used in the titration of viral progeny. The human Burkitt’s lymphoma cell line, Raji, which was used as a positive control in the Ras glutathione S-transferase (GST) pull-down assay, was also obtained from the Cell Resource Center for Biomedical Research. All cell lines were grown in RPMI-based complete medium [RPMI1640 supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 55 μM 2-mercaptoethanol] and were maintained at 37°C in a humidified 5% carbon dioxide (CO2) incubator.
The Dearing strain of reovirus serotype 3 (Reolysin, clinical grade reovirus; GMP) was obtained from Oncolytics Biotech (Calgary, Alberta). Paclitaxel, carboplatin, and gemcitabine were obtained from Sigma-Aldrich (St. Louis, Missouri, USA) and toceranib (SU11654) was obtained from Adooq Bioscience (Irvine, California, USA).
Cell proliferation assay
Inhibition of cell growth by reovirus was assessed by methylthiazole tetrazolium (MTT) assay (15). Briefly, both cell lines were seeded at 3000 cells in 96-well plates and mock-infected or infected with reovirus at a multiplicity of infection (MOI) of 0.1, 1.0, 10, 100, and 1000 plaque-forming units (PFUs) per cell in triplicate. Cells were cultured for 72 h before MTT reagent was added to the culture during the final 4 h before cell proliferation was assessed by measuring the absorbance in the plates. All experiments were repeated at least 3 times.
Cytotoxicity assay in reovirus-infected cell lines
Both of the cell lines were seeded at 3000 cells in 96-well plates. After 12 h of culture, cells were mock-infected or infected with reovirus at MOI of 70 PFUs per cell in triplicate. At 72 h post-infection (hpi), live and dead cells were counted after staining with 0.25% trypan blue. All experiments were repeated at least 3 times.
Viral progeny
Supernatant of the samples from the cytotoxicity assay was collected and kept at −80°C until analysis. Viral progeny in the samples was measured using the 50% tissue culture infectious dose (TCID50) assay on L929 cells, as described in a previous report (21), with some modifications. L929 cells were seeded at 10 000 cells per well in 96-well plates. Serial 10-fold dilutions of the stock virus or the culture supernatant from the cytotoxicity assay were prepared before being added to the wells. After 144 h of incubation, cytopathogenic effects (CPE) in the wells were recorded and TCID50 was calculated. The viral progeny was titrated using all the supernatant from the 3 repeats of the cytotoxicity assay.
Reovirus infectivity
Reovirus infectivity of the cell lines was quantified by flow cytometry. Both cell lines were infected with reovirus at MOI of 70 and the infected cells were harvested at 48 hpi. Cells were resuspended in phosphate-buffered saline (PBS) before being fixed and permeabilized with BD Cytofix/Cytoperm Kit (BD Biosciences, San Diego, California, USA). Intracellular viral proteins were stained with rabbit anti-reovirus polyclonal antibody, which was produced in our laboratory and was proven to be anti-reovirus specific by Western blotting in previous studies (15,16). The staining of primary antibody was followed by incubation with anti-rabbit immunoglobulin (IgG) PE antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA). Serum from healthy rabbit (Life Technologies, Tokyo, Japan) was used as the isotype control. The stained cells were analyzed using a BD Accuri C6 Flow Cytometer (BD Biosciences, San Jose, California, USA) and the results were analyzed using FlowJo software (Tree Star, San Carlos, California, USA).
Evaluation of apoptosis with flow cytometric analysis
The CHMp-13a and CHMp-5b cells were treated with reovirus at MOI of 70 for 24 and 48 h. Adherent cells were collected, washed with cold PBS, and 3 × 105 cells were resuspended in 30 μL of PBS. Cells were then incubated for 15 min at room temperature in the dark in cold 1× binding buffer containing Annexin V-FITC antibody, according to the manufacturer’s instructions (Miltenyi Biotech, Auburn, California, USA). The cells were pelleted and resuspended in cold 1× binding buffer. Cells were stained with 1.5 μL of propidium iodide (PI) at 1 μg/mL and analyzed using a BD Accuri C6 Flow Cytometer (BD Biosciences). Analyses were conducted with FlowJo software (Tree Star).
Measurement of inhibition of apoptosis
The CHMp-13a and CHMp-5b cells were seeded at 3000 cells in 96-well plates. The irreversible pan-caspase inhibitor, Z-VAD-FMK (caspase inhibitor I; Calbiochem, Billerica, Massachusetts, USA) was added to wells in triplicate at a concentration of 50 μM for 1 h before the cells were infected with reovirus at MOI of 70. After 72 hpi, cell viability was determined by the trypan blue exclusion test.
In-vitro synergy assay
Experiments were carried out as described in the in-vitro cell proliferation assay using 0.25, 0.5, 1, 2, and 4 times the IC50 (half maximal inhibitory concentration) dose as calculated by CompuSyn software (Paramus, New Jersey, USA) using a combination of reovirus and paclitaxel, carboplatin, gemcitabine, or toceranib.
The synergistic effects of reovirus and the chemotherapeutic agents on cell proliferation were assessed by calculating the combination-index (CI) values using CompuSyn software. Using the Chou-Talalay method, CI provides a quantitative measure of the degree of interaction between 2 or more agents (22,23). The CI value offers quantitative definitions that depict synergism (CI < 1), additive effect (CI = 1), and antagonism (CI > 1) (20,21).
Western blotting
The CHMp-13a and CHMp-5b cells were seeded at 2.5 × 105 cells and mock-infected or infected with reovirus at MOI 70. In order to evaluate the synergistic effects of the combination treatments, CHMp-5b cells were seeded at 2.5 × 105 cells and treated 0.5 times the calculated IC50 dose of the single or a combination of the cytotoxic agents. In order to confirm the inhibition of caspase 3 activation, both the cell lines were seeded at 2.0 × 105 cells in 6-well plates. Z-VAD-FMK was added at 50 μM for 1 h before the cells were infected at MOI 70. After 48 or 72 hpi, cells were harvested and lysed with NP40 lysis buffer [1% NP40, 10 mM Tris hydrogen chloride (HCl) (pH 7.5), 150 mM sodium chloride (NaCl), 1 mM ethylenediamine tetra-acetic acid (EDTA)], which was supplemented with complete, mini EDTA-free protease inhibitor mixture (Roche Diagnostics K.K., Tokyo, Japan) or 1× protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blotting. Rabbit anti-PARP [poly (ADP-ribose) polymerase] antibody (NeoMarkers, Fremont, California, USA) or rabbit anti-caspase 3 antibody (Cell Signaling Technologies, Danvers, Massachusetts, USA) were used as primary antibodies, followed by secondary labeling using goat anti-rabbit IgG horseradish peroxidase (HRP) (Zymed Laboratories, San Francisco, California, USA). After protein detection, the membranes were stripped and hybridized with mouse anti-beta-actin (Sigma-Aldrich Japan K.K., Tokyo, Japan) as loading control, followed by secondary labeling using goat anti-mouse IgG HRP (Zymed Laboratories).
GST pull-down assay for Ras status
Ras activation status of the cell lines was evaluated with the glutathione S-transferase (GST) pull-down assay as reported in a previous study (15). Briefly, a vector that expresses GST fused with Ras-binding domain (RBD) of Raf-1 was constructed. Then, Escherichia coli competent cell JM109 was transformed with this vector and GST-RBD was extracted with lysis buffer. Extracted proteins from the cells were mixed with glutathione-sepharose 4B beads (GE Healthcare, Tokyo, Japan) for 1 h before washing with lysis buffer. Ras activation status of the cell lines was evaluated after GST pull-down. Western blotting was carried out as previously described using mouse anti-pan-Ras (Calbiochem) and goat anti-mouse IgG HRP (Zymed Laboratories).
Subcutaneous tumor xenograft models in NOD/SCID mice
Eight to 9-week-old NOD/ShiJic-scid [nonobese diabetic/severe combined immunodeficient (NOD/SCID)] mice were obtained from Kyudo (Saga, Japan) and studies were conducted in a specific pathogen-free area in accordance with the Yamaguchi University Animal Care and Use Guidelines. CHMp-5b cells (1.0 × 107 in 50 μL PBS) were implanted subcutaneously into the right flank of the mice under general anesthesia (day 1). 1.0 × 108 PFUs of live reovirus (experimental group; 3 male and 5 female mice) or UV-inactivated reovirus (control group; 2 male and 5 female mice) in 20 μL PBS were injected intratumorally 14 d after tumor transplantation. Tumor growth was determined by measuring the tumor volume with a caliper every other day until euthanasia at day 21. Tumors were fixed in 10% neutral buffered formalin and embedded in paraffin before staining with hematoxylin and eosin (H&E) for histopathological analysis. For immunohistochemical staining, deparafinized samples were treated with Target Retrieval Solution (Dako, Glostrup, Denmark) before treatment with 3% hydrogen peroxidase and Protein Block (Dako). Sections were incubated with rabbit anti-reovirus polyclonal antibody (1:500 dilution; produced in our laboratory), followed by Histofine Simple Stain MAX-PO (Nichirei Biosciences, Tokyo, Japan). Slides were subjected to 3,3-diamino-benzidine tetrachloride (Roche Diagnostics K.K.) staining before counterstaining with Meyer’s hematoxylin.
Results
Reovirus is highly infective and induces cell death in canine MGT cell lines
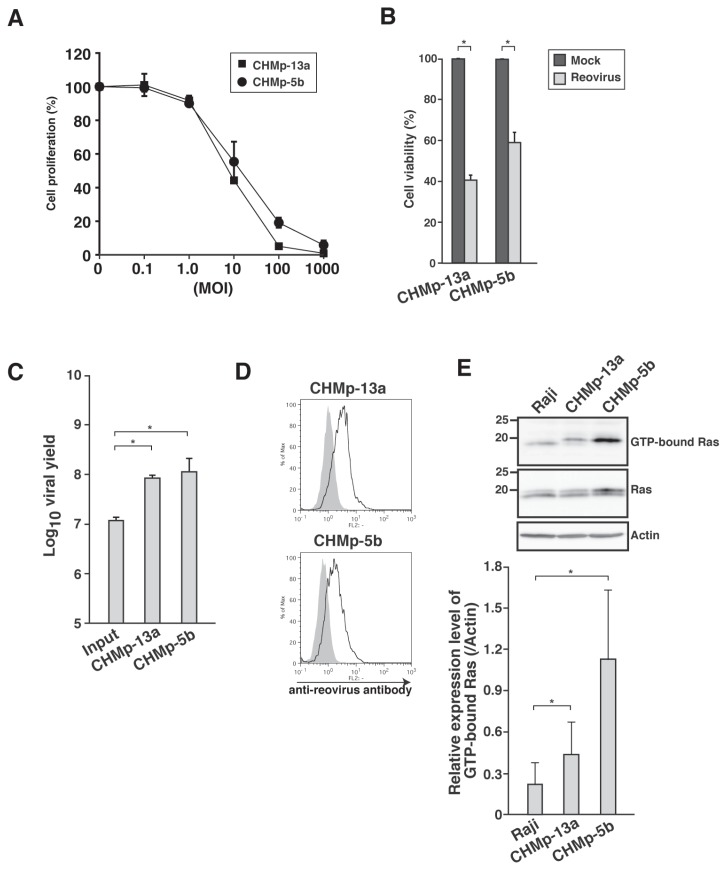
Our previous report (17) focused on various canine MGT cell lines as potential candidates for reovirus therapy. In this study, 2 canine MGT cell lines, CHMp-13a and CHMp-5b, were chosen due to the published biological behavior of these cell lines in xenotransplanted mice (24). Firstly, the susceptibility of the cell lines to reovirus was examined. CHMp-13a and CHMp-5b showed more than 50% of inhibition of cell proliferation by reovirus at MOI of 100 and inhibition was affected in a dose-dependent manner (Figure 1A). In addition to MTT assay, trypan blue exclusion test revealed that reovirus induced cell death in both the cell lines (Figure 1B). Viral progeny was measured by TCID50 assay to determine reovirus replication (Figure 1C). Both the cell lines had increments of viral progeny titer, which was consistent with the detection of viral proteins using flow cytometric analysis (Figure 1D). These results indicated that CHMp-13a and CHMp-5b are highly susceptible to reovirus as observed in the parental CHMp cells (17).
Figure 1.
Evaluation of reovirus susceptibility in cell lines of canine mammary gland tumor (MGT). A — Two canine MGT cell lines in triplicate wells were mock-infected or infected with reovirus at the indicated multiplicity of infection (MOI). At 72 h post-infection (hpi), cell proliferation was quantified by methylthiazole tetrazolium (MTT) assay. Mean ± SD of 3 independent experiments is shown. B — Cell lines in triplicate wells were mock-infected or infected with reovirus at MOI 70. At 72 hpi, cell viability was quantified by trypan blue exclusion test. Mean ± SD of 3 independent experiments is shown. * indicates P < 0.05. C — Supernatant of reovirus-infected (MOI 70) cell lines was harvested at 72 hpi and virus titers were determined by TCID50 assay. Input indicates the initial virus titer used in the infection of cells. Mean ± SD of 3 independent experiments is shown. * indicates P < 0.05. D — Cell lines were infected with reovirus at MOI 70. Reovirus-infected cell lines were harvested at 48 hpi. Cells were permeabilized, followed by staining with rabbit anti-reovirus polyclonal antibody and analyzed by flow cytometry. The shaded histogram and open histogram indicate staining with isotype control and anti-reovirus antibody, respectively. Results shown represent 2 repeats. E — Ras-GTP from cell lysates of Raji and canine MGT cell lines was affinity-precipitated with GST-RBD protein. The affinity-precipitated Ras-GTP was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blotting with anti-Ras antibody. Actin was used as control for protein loading. Results shown represent 3 repeats. Quantification of GTP-Ras by densitometry is shown below images. Mean ± SD of 3 independent experiments is shown. * indicates P < 0.05.
The baseline guanosine triphosphate-(GTP)-loading status of Ras was determined in both the cell lines in order to investigate the involvement of Ras activation as the molecular determinant for reovirus susceptibility. Ras activity of the cell lines was higher than Raji (Figure 1E). Raji was used as a control because it is moderately susceptible to reovirus-induced cell death and has a certain level of Ras activation as shown in a previous study (25).
Reovirus induces caspase-dependent apoptosis in canine MGT cell lines
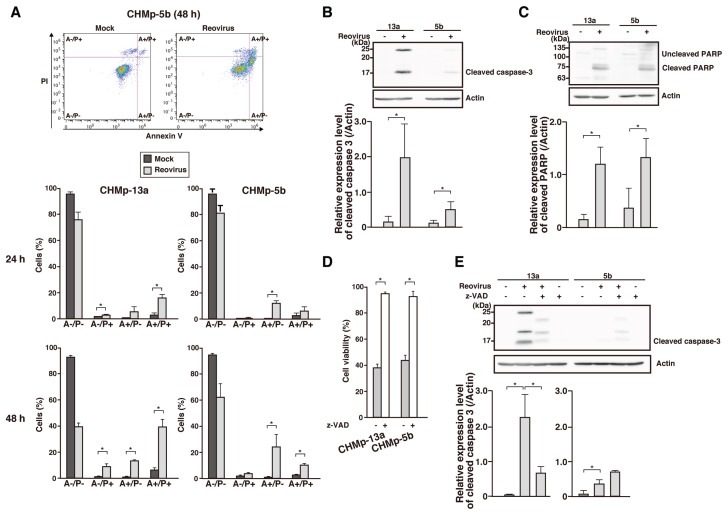
Previous studies have indicated that caspase-dependent apoptosis is one of the mechanisms of reovirus-induced cell death in susceptible cell lines (15,16). Therefore, Annexin V/PI assay was carried out to assess the amount of apoptotic cells in reovirus-infected culture. In both the cell lines, the percentage of early apoptotic cells (A+/P−) increased over time after reovirus infection compared to mock-infected cells (Figure 2A). At the same time, the percentage of necrotic cells (A−/P+) also increased in CHMp-13a after reovirus infection.
Figure 2.
Reovirus-induced cell death in canine MGT cell lines by means of the caspase-dependent apoptosis pathway. A — Reovirus-infected (MOI 70) cell lines were harvested at 24 h or 48 h post-infection (hpi) and stained with Annexin V/PI assay before flow cytometric analysis. The population of living (A−/P−), necrotic (A−/P+), early apoptotic (A+/P−), and late apoptotic/necrotic (A+/P+) cells was summarized. Dot plots shown represent CHMp-5b at 48 hpi. Mean ± SD of 3 independent experiments is shown. * indicates P < 0.05. B,C — Cell lysates of reovirus-infected (MOI 70) cell lines at 48 hpi were prepared before proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Caspase 3 activation (B) and poly (ADP-ribose) polymerase (PARP) cleavage (C) were detected using anti-caspase 3 antibody and anti-PARP antibody, respectively. Actin was used as protein-loading control. Bar graphs shown are densitometric quantifications of the relative expression level of each protein relative to actin. Mean ± SD of 3 independent experiments is shown. * indicates P < 0.05. D — Cell lines in triplicate wells were pre-treated with DMSO or 50 μM of Z-VAD-FMK (Z-VAD) for 1 h at 37°C in incubator before being infected with reovirus at MOI 70. Cell viability was quantified by trypan blue exclusion test at 72 hpi. Mean ± SD of 3 independent experiments is shown. * indicates P < 0.05. E — Cell lines were pre-treated with DMSO or 50 μM of Z-VAD for 1 h at 37°C in incubator before infection with reovirus at MOI 70. At 72 hpi, lysates of harvested cells were prepared before proteins were separated using SDS-PAGE. Caspase activation was detected using anti-caspase 3 antibody. Actin was used as protein-loading control. Bar graphs shown are densitometric quantifications of the relative expression levels of cleaved caspase 3 relative to actin. Mean ± SD of 3 independent experiments is shown. * indicates P < 0.05.
In order to prove that apoptosis of reovirus-infected cells occurred by means of caspase 3 activation, Western blot analysis was carried out using anti-caspase 3 and anti-PARP antibodies. As shown in Figures 2B and 2C, cleavage of caspase 3 and PARP was shown in both the cell lines at 48 hpi. Furthermore, both of the cell lines were pre-treated with Z-VAD-FMK before reovirus infection and cell viability was determined by trypan blue dye exclusion at 72 hpi. Cytotoxicity of reovirus infection was completely inhibited by Z-VAD-FMK in the cell lines (Figure 2D). In order to confirm that caspase 3 activation was inhibited by Z-VAD-FMK, the cleavage of caspase 3 was also examined using Western blotting (Figure 2E). Cleaved caspase 3 decreased in the Z-VAD-FMK-treated CHMp-13a, but an unexpected increment was observed in the Z-VAD-FMK-treated CHMp-5b. These results indicated that reovirus-induced cell death in CHMp-13a and CHMp-5b cell lines is predominantly due to apoptosis related to a caspase inhibitor-sensitive pathway.
Reovirus inhibits growth of canine MGT engrafted in NOD/SCID mice
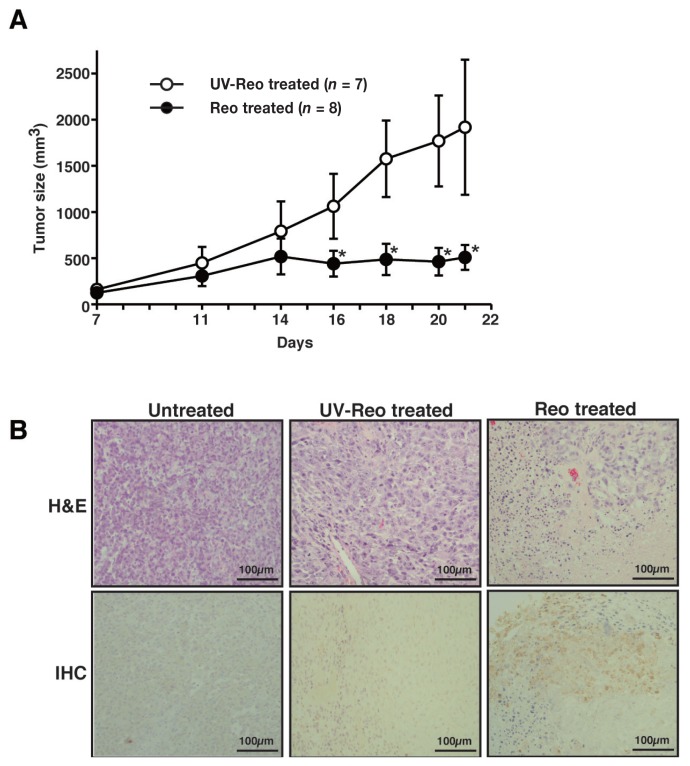
In order to assess the oncolytic potential of reovirus in vivo, subcutaneous xenograft models were created using NOD/SCID mice. In a previously published study, tumor growth of CHMp-13a cells stopped a week after inoculation, indicating the difficulty of creating the subcutaneous CHMp-13a mouse models compared to the subcutaneous CHMp-5b tumors that increased in size over time (24). Therefore, the CHMp-5b subcutaneous xenograft models were chosen and treated with a single intratumoral injection of reovirus. All mice were treated with reovirus or UV-inactivated reovirus 14 d post-transplantation of CHMp-5b. Tumor growth was significantly limited by reovirus 21 d post-transplantation of CHMp-5b compared to the UV-inactivated reovirus-treated group (Figure 3A). Hematoxylin and eosin (H&E)-stained histopathological samples from the CHMp-5b xenograft models showed extensive necrotic lesions within the reovirus-treated tumors, in contrast to the untreated tumors and UV-inactivated reovirus-treated tumors (Figure 3B). Immunohistochemical staining using the anti-reovirus antibody demonstrated the presence of reovirus proteins in the reovirus-treated tumors (Figure 3B). These results confirmed that reovirus infected the tumor cells and suppressed the growth of tumors in vivo.
Figure 3.
Reovirus-suppressed tumor growth in xenograft mouse models of canine MGT. CHMp-5b (1.0 × 107 cells) was implanted subcutaneously at the right flank of mice (day 1). After 14 d, the tumors were treated with a single intratumoral injection of 1.0 × 108 PFUs of reovirus or UV-inactivated reovirus (control). Tumor size was measured with a caliper (A). Data represents the mean ± SD of each treatment group. * indicates P < 0.05. UV-inactivated reovirus did not show any infectivity to L929 cells. B — Hematoxylin and eosin (H&E) and immunohistochemical (IHC) results from representative mice with no treatment, UV-inactivated reovirus, or reovirus 21 d post-transplantation are shown. Antibody used for IHC is targeted at the reoviral μ and σ proteins (15,16).
Reovirus cytotoxicity is enhanced in CHMp-5b when combined with chemotherapeutic agents
Reovirus treatment regressed tumor growth in NOD/SCID mice engrafted with CHMp-5b cells, but the effects were weaker than expected. Therefore, we hypothesized that the synergistic effects of other anti-cancer agents can enhance the effects of reovirus therapy. This led us to examine the effects of reovirus in combination with chemotherapeutic agents, mainly paclitaxel, carboplatin, gemcitabine, and toceranib, in CHMp-5b cells. Firstly, drug-induced cytotoxicity in CHMp-5b was assessed as a single agent (data not shown). The sensitivity of CHMp-5b to the agents varied and was dose-dependent. Based on these results, the IC50 of each of the chemotherapeutic agents in CHMp-5b was determined (Table I). Similarly, the IC50 was determined for reovirus. In order to evaluate the synergistic effects of reovirus and the chemotherapeutic agents, CHMp-5b was treated with reovirus, chemotherapeutic agents, or a combination of reovirus and the chemotherapeutic agents, at doses representing 4, 2, 1, 0.5, and 0.25 times the calculated IC50 in a constant ratio, according to previously reported methods (22,23).
Table I.
Synergistic interaction of reovirus and chemotherapeutic agents on CHMp-5b
| Mean of CI values ± SD* | ||||
|---|---|---|---|---|
|
|
||||
| IC50 ± SD | ED50 | ED75 | ED95 | |
| Reovirus | MOI 24 ± 5.7 | |||
| Paclitaxel | 0.60 ± 0.04 (μM) | 0.54 ± 0.04 | 0.58 ± 0.04 | 0.66 ± 0.03 |
| Carboplatin | 34 ± 7.9 (μM) | 0.48 ± 0.04 | 0.52 ± 0.04 | 0.57 ± 0.03 |
| Gemcitabine | 0.39 ± 0.03 (μM) | 0.51 ± 0.05 | 0.54 ± 0.05 | 0.60 ± 0.05 |
| Toceranib | 7.6 ± 1.9 (μM) | 0.81 ± 0.06 | 0.83 ± 0.08 | 0.86 ± 0.10 |
Data is calculated from 3 independent experiments at the ED (effective dose) indicated.
CI — combination index.
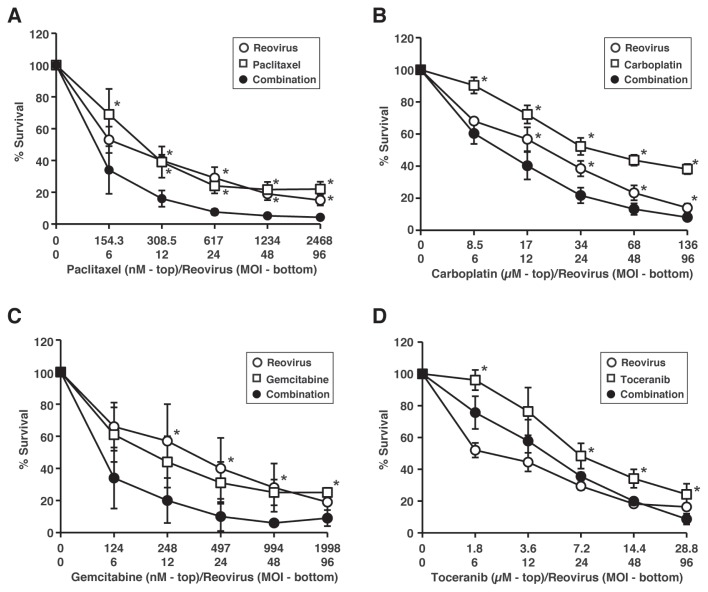
The combination of reovirus and chemotherapeutic agents enhanced the tumor cell-killing effects, compared to each of the agents alone except toceranib (Figure 4). Based on this data, we analyzed the combination index (CI) using the isobologram analysis to determine whether the enhanced cell death was synergistic. The CI provides a quantitative measurement of the degree of interaction between 2 or more cytotoxic agents. The combination of reovirus and paclitaxel, carboplatin, or gemcitabine demonstrated synergistic cytotoxic effects against CHMp-5b cells at the indicated concentrations (Table I). In contrast, the combination of reovirus and toceranib was shown to be less synergistic at those concentrations.
Figure 4.
Evaluation of synergistic effects of reovirus and chemotherapeutic agents in CHMp-5b. CHMp-5b cells were treated with reovirus alone (open circles), chemotherapeutic agent alone (open squares), or a combination of the 2 (black circles) at the indicated concentrations for 72 h. Cell proliferation was quantified by methylthiazole tetrazolium (MTT) assay. Mean ± SD of 3 independent experiments is shown. Statistical differences of percentage survival between single treatments (chemotherapeutic agent or reovirus) and combination treatments were evaluated using Dunnett’s method. * indicates P < 0.05.
Combination of reovirus and chemotherapeutic agents activates the caspase-pathway
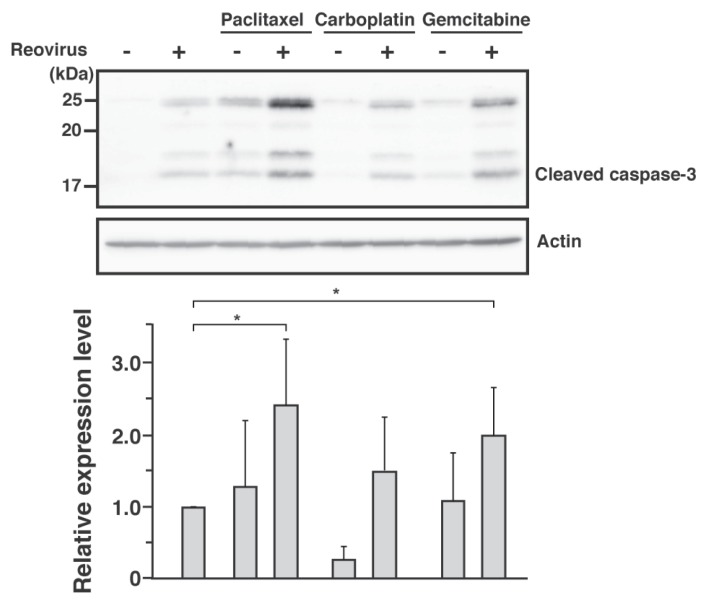
In order to assess the relationship between the synergistic cytotoxicity effects of reovirus-chemotherapeutic agents and the caspase pathway, the expression of cleaved caspase 3 was analyzed by Western blotting. Similar to reovirus, paclitaxel and gemcitabine alone induced cleavage of caspase 3 in CHMp-5b cells, while carboplatin did not. A combination of reovirus and either paclitaxel or gemcitabine, however, induced significant levels of cleaved caspase 3 compared with reovirus alone (Figure 5). The combination of reovirus and carboplatin showed a slight increment of cleaved caspase 3, but was not statistically significant against reovirus alone. Depending on the type of chemotherapeutic agents, these data suggested that the synergistic effects seen in CHMp-5b cells compared with reovirus are mediated by apoptosis.
Figure 5.
The combination of reovirus and chemotherapeutic agents enhanced cell death by means of caspase-dependent apoptosis. Whole cell lysates were prepared from CHMp-5b treated with the chemothera-peutic agents at half of IC50 for 48 h. Proteins were separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before the cleavage of caspase 3 was assessed using Western blotting with anti-caspase 3 antibody. Actin was used as protein-loading controls. Results shown represent 3 repeats. Bar graphs shown are densitometric quantifications of the relative expression levels of cleaved caspase 3 of chemotherapeutic agents with or without reovirus relative to cleaved caspase 3 of reovirus alone. Mean ± SD of 3 independent experiments is shown. Statistical analysis was carried out using t-test between reovirus alone and chemotherapeutic agents with or without reovirus. * indicates P < 0.05.
Discussion
As CHMp-13a and CHMp-5b are clones derived from CHMp (24), the susceptibility of these 2 cell lines to reovirus was similar to their parental cell line (17). Despite their common origin, CHMp-13a and CHMp-5b express different tumor-related proteins and exhibit distinctive cellular morphology and growth patterns. CHMp-5b expresses the mesenchymal genes and is concomitantly more malignant and tumorigenic in vitro and in vivo than the epithelial CHMp-13a. In our study, both cell lines showed similar susceptibility to reovirus, with more than 40% of the cells killed by reovirus infection (Figure 1B).
Annexin V/PI staining and the cleavage of caspase 3 and PARP confirmed that reovirus-induced cytotoxicity was due to caspase-dependent apoptosis. Moreover, treatment with a pan-caspase inhibitor, Z-VAD-FMK, completely inhibited the reovirus-induced cytotoxicity (Figure 2D), which was also consistent with studies of canine mast cell tumors and lymphoma cell lines (15,16). As expected, caspase 3 activation induced by reovirus decreased in CHMp-13a treated with Z-VAD-FMK (Figure 2E), although the opposite result was obtained in CHMp-5b. Although there is no evidence-based explanation, we speculate that this phenomenon in CHMp-5b might involve other caspases. Since Z-VAD-FMK is a pan-caspase inhibitor, it can also inhibit caspases other than caspase 3, such as caspase 8 and caspase 7. In CHMp-13a, reovirus-induced apoptosis is completely dependent on caspase 3, but reovirus-induced apoptosis in CHMp-5b might involve another caspase, such as caspase 7, which can compensate for the loss of caspase 3 in certain cells.
Even though further studies are necessary to elucidate the complete mechanism of reovirus-induced cell death in CHMp-5b, we strongly believe that reovirus-induced cell death occurs by means of caspase-dependent pathways. Necrosis is also involved in reovirus-induced cell death in CHMp-13a (Figure 2A), although it is still not clear how it contributes to this phenomenon. Besides apoptosis, studies of some human cancers have also shown that reovirus induces cell death through necrosis, necroptosis, or autophagy (26–28). The mechanism of reovirus-induced cell death in CHMp-13a may be partly associated with those pathways.
In addition to the in-vitro study, we also confirmed the susceptibility of reovirus in subcutaneous xenograft mouse models using CHMp-5b (Figure 3A). Although tumor growth was impeded, complete tumor regression was not observed in mice treated with a single injection of reovirus. In clinical cases, multiple injections of reovirus are warranted in order to exert prominent tumor lytic effects due to the additional antibody-neutralizing effects of treatment (29,30). Data from this study suggest that reovirus as monotherapy for canine solid tumor has a limited efficacy, unlike the previous study in which a single injection of reovirus completely regressed MCT in xenotransplanted mice (15).
New approaches using a combination of reovirus and chemotherapeutic agents have recently been tested in various human cancer cell lines of different origins and also in a number of clinical trials (31–36). In order to assess the enhancement of reovirus-induced cytotoxicity in CHMp-5b, we chose chemotherapeutic agents such as paclitaxel, carboplatin, and gemcitabine, which are commonly used to treat tumors, and toceranib, which is the first FDA-approved receptor tyrosine kinase inhibitor for treating cancer in dogs (37). These agents exert cytotoxic effects through different mechanisms of action. Paclitaxel works as a taxane, carboplatin is a pseudoalkylating agent, and gemcitabine is an anti-metabolite agent, while toceranib is a molecular-targeted drug that inhibits multiple tyrosine kinase. Combination analysis using the Chou-Talalay method (22) showed significant levels of synergistic effects between reovirus and the chemotherapeutic agents tested except toceranib. These results indicate that paclitaxel, carboplatin, and gemcitabine are suitable candidates for combination therapy with reovirus in canine MGT (Table I). Even though the CI value of toceranib and reovirus was < 1, it is the highest among the chemotherapeutic agents, which indicates that toceranib showed the least synergistic effects with reovirus.
The levels of canine maximum plasma concentration (Cmax) of paclitaxel, carboplatin, gemcitabine, and toceranib are 3.95 μg/mL (4.6 μM), 14.1 μg/mL (38 μM), 10.7 μg/mL (36 μM), and 109 ng/mL (0.27 μM), respectively. The IC50 of toceranib in the in-vitro studies is higher than the canine Cmax levels. When the canine Cmax levels are compared to the IC50, toceranib is the only agent in this study with a higher IC50 than the canine Cmax level. Even though the relationship of IC50 and canine Cmax levels for paclitaxel, gemcitabine, and carboplatin was not assessed in this study, the combination of reovirus and chemotherapeutic agents that demonstrated synergistic effects had IC50 that are either lower or equivalent to the Cmax levels. These results suggest that synergistic anti-tumor activities can also be achieved in tumor-bearing dogs when reovirus and the 3 chemotherapeutic agents are used in combination at the IC50 value.
Interestingly, the combination treatment of reovirus and paclitaxel or gemcitabine in CHMp-5b led to an increased activation of caspase 3 compared to reovirus treatment alone (Figure 5). Some studies showed that the combination treatment of reovirus and chemotherapeutic agents (paclitaxel, gemcitabine, and vinblastine) enhances cell death by means of the caspase pathway (20,36). On the other hand, cell death induced by the combination of reovirus and an alkylating agent (cisplatin) is not caspase-dependent (18,36). Similarly, in this study, the combination of reovirus and carboplatin did not noticeably enhance caspase activation, even though the CI for reovirus and carboplatin indicated a synergistic relationship. This suggests that the synergistic cell-killing of reovirus and carboplatin might involve other mechanisms, although concrete evidence has yet to be found.
In this study, we used immunocompromised mouse models engrafted with canine MGT cell lines to examine the direct cytotoxic effect of reovirus on canine MGT. In order to apply these results to actual canine cancer treatment, the involvement of host immune systems will need to be considered and these effects assessed in immunocompetent mouse models in future studies. Reovirus infection may be hindered by an existing neutralizing antibody in immunocompetent hosts. This can be overcome, however, as reovirus has an avoidance mechanism against those antibodies that involves hitchhiking onto peripheral blood mononuclear cells (PBMCs) and dendritic cells, as suggested by the results of human clinical trials (38).
In addition to the synergistic effects shown in this and previous studies (39,40), simultaneous usage of chemotherapeutic agents with reovirus may induce immunosuppressive effects on the host immune response, which could limit the production of anti-reovirus neutralizing antibodies. Furthermore, reovirus stimulates both the innate and adaptive immunity as an indirect mean against the targeted tumor (41–43). Even though we did not examine the efficacy of the combination of reovirus and chemotherapeutic agent in vivo, previous studies suggest that more profound effects can be expected from the combination therapy in immunocompetent models (41– 43).
Optimizing the effectual dose of a chemotherapeutic agent can be an exhausting process for both veterinarians and pet owners. The quality of life of a beloved pet is often jeopardized and there is a risk of an overdose. Based on the results of this study, the efficacy of reovirus as a monotherapy is limited in canine solid tumor. On the other hand, synergistic data obtained from this study showed that the combination of reovirus and a chemotherapeutic agent at a lower dosage enhanced the anti-tumor effects in canine MGT. This provides a particularly attractive alternative since it can reduce or even eliminate the adverse effects of chemotherapy. With continuous effort, it is expected that this novel therapeutic approach for canine MGT will be one of the forerunners of anti-cancer therapy in the veterinary field.
Acknowledgment
The authors extend deep appreciation to Dr. Takayuki Nakagawa (University of Tokyo) for providing the cell lines used in this study.
References
- 1.Liu D, Xiong H, Ellis AE, et al. Molecular homology and difference between spontaneous canine mammary cancer and human breast cancer. Cancer Res. 2014;74:5045–5056. doi: 10.1158/0008-5472.CAN-14-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oostendorp L, Stalmeier P, Donders A. Efficacy and safety of palliative chemotherapy for patients with advanced breast cancer pretreated with anthracyclines and taxanes: A systematic review. Lancet Oncol. 2011;12:1053–1061. doi: 10.1016/S1470-2045(11)70045-6. [DOI] [PubMed] [Google Scholar]
- 3.Schneider R, Dorn CR, Taylor DO. Factors influencing canine mammary cancer development and postsurgical survival. J Natl Cancer Inst. 1969;43:1249–1261. [PubMed] [Google Scholar]
- 4.MacVean DW, Monlux AW, Anderson PS, Jr, Silberg SL, Roszel JF. Frequency of canine and feline tumors in a defined population. Vet Pathol. 1978;15:700–715. doi: 10.1177/030098587801500602. [DOI] [PubMed] [Google Scholar]
- 5.Marconato L, Lorenzo RM, Abramo F, Ratto A, Zini E. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumors in dogs. Vet Comp Oncol. 2008;6:90–101. doi: 10.1111/j.1476-5829.2007.00143.x. [DOI] [PubMed] [Google Scholar]
- 6.Simon D, Schoenrock D, Baumgärtner W, Nolte I. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J Vet Intern Med. 2006;20:1184–1190. doi: 10.1892/0891-6640(2006)20[1184:patimm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Vacchelli E, Eggermont A, Sautès-Fridman C, et al. Trial watch: Oncolytic viruses for cancer therapy. Oncoimmunology. 2013;2:e24612. doi: 10.4161/onci.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen L, Hovis JF, Mastrota FM, Bell JA, Huebner RJ. An outbreak of infection with a type 1 reovirus among children in an institution. Am J Hyg. 1960;71:266–274. doi: 10.1093/oxfordjournals.aje.a120109. [DOI] [PubMed] [Google Scholar]
- 9.Selb B, Weber B. A study of human reovirus IgG and IgA antibodies by ELISA and western blot. J Virol Methods. 1994;47:15–25. doi: 10.1016/0166-0934(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 10.Tai JH, Williams JV, Edwards KM, Wright PF, Crowe JE, Dermody TS. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J Infect Dis. 2005;191:1221–1224. doi: 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 12.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: Usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twigger K, Roulstone V, Kyula J, et al. Reovirus exerts potent oncolytic effects in head and neck cancer cell lines that are independent of signalling in the EGFR pathway. BMC Cancer. 2012;12:368. doi: 10.1186/1471-2407-12-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black AJ, Morris DG. Clinical trials involving the oncolytic virus, reovirus: Ready for prime time? Expert Rev Clin Pharmacol. 2012;5:517–520. doi: 10.1586/ecp.12.53. [DOI] [PubMed] [Google Scholar]
- 15.Hwang CC, Umeki S, Kubo M, et al. Oncolytic reovirus in canine mast cell tumor. PLoS One. 2013;8:e73555. doi: 10.1371/journal.pone.0073555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang CC, Umeki S, Igase M, et al. The effects of oncolytic reovirus in canine lymphoma cell lines. Vet Comp Oncol. 2014 doi: 10.1111/vco.12124. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Igase M, Hwang CC, Coffey M, Okuda M, Noguchi S, Mizuno T. The oncolytic effects of reovirus in canine solid tumor cell lines. J Vet Med Sci. 2015;77:541–548. doi: 10.1292/jvms.14-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandha HS, Heinemann L, Simpson GR, et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res. 2009;15:6158–6166. doi: 10.1158/1078-0432.CCR-09-0796. [DOI] [PubMed] [Google Scholar]
- 19.Karapanagiotou EM, Roulstone V, Twigger K, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–2089. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roulstone V, Twigger K, Zaidi S, et al. Synergistic cytotoxicity of oncolytic reovirus in combination with cisplatin-paclitaxel doublet chemotherapy. Gene Ther. 2013;20:521–528. doi: 10.1038/gt.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 22.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 23.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Murai K, Nakagawa T, Endo Y, et al. Establishment of a pair of novel cloned tumor cell lines with or without metastatic potential from canine mammary adenocarcinoma. Res Vet Sci. 2012;93:468–472. doi: 10.1016/j.rvsc.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Alain T, Kim M, Johnston RN, et al. The oncolytic effect in vivo of reovirus on tumor cells that have survived reovirus cell killing in vitro. Br J Cancer. 2006;95:1020–1027. doi: 10.1038/sj.bjc.6603363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda Y, Nishimura G, Yanoma S, Kubota A, Furukawa M, Tsukuda M. Reovirus oncolysis in human head and neck squamous carcinoma cells. Auris Nasus Larynx. 2004;31:407–412. doi: 10.1016/j.anl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Berger AK, Danthi P. Reovirus activates a caspase-independent cell death pathway. MBio. 2013;4:e00178–13. doi: 10.1128/mBio.00178-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thirukkumaran CM, Shi ZQ, Luider J, et al. Reovirus modulates autophagy during oncolysis of multiple myeloma. Autophagy. 2013;9:413–414. doi: 10.4161/auto.22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirasawa K, Nishikawa SG, Norman KL, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- 30.Yang WQ, Lun X, Palmer CA, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: Racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 31.Maitra R, Seetharam R, Tesfa L, et al. Oncolytic reovirus preferentially induces apoptosis in KRAS mutant colorectal cancer cells, and synergizes with irinotecan. Oncotarget. 2014;5:2807–2819. doi: 10.18632/oncotarget.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemann L, Simpson GR, Boxall A, et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer. 2011;11:221. doi: 10.1186/1471-2407-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin® (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther. 2012;20:1998–2003. doi: 10.1038/mt.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comins C, Spicer J, Protheroe A, et al. REO-10: A phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–5572. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twigger K, Vidal L, White CL, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res. 2008;14:912–923. doi: 10.1158/1078-0432.CCR-07-1400. [DOI] [PubMed] [Google Scholar]
- 36.Sei S, Mussio JK, Yang Q-E, et al. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 38.Adair RA, Roulstone V, Scott KJ, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roulstone V, Khan K, Pandha HS, et al. Phase I trial of cyclophosphamide as an immune modulator for optimizing oncolytic reovirus delivery to solid tumors. Clin Cancer Res. 2015;21:1305–1312. doi: 10.1158/1078-0432.CCR-14-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Errington F, White CL, Twigger KR, et al. Inflammatory tumor cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15:1257–1270. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Errington F, Steele L, Prestwich R, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 43.Prestwich RJ, Errington F, Diaz RM, et al. The case of oncolytic viruses versus the immune system: Waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]