Abstract
The aim of this study was to evaluate the effect of tulathromycin as a bovine respiratory disease (BRD) metaphylactic treatment on rumen fluid parameters in feedlot cattle in an intensive livestock production farm. One hundred beef cattle, immediately after housing, were divided in 2 equal groups: 50 animals with metaphylactic treatment against BRD (treated group; tulathromycin at 2.5 mg/kg BW) and 50 animals with placebo treatment (control group). Rumen fluid samples were collected from each animal by rumenocentesis in 3 periods: 1 d (T1), 8 d (T8), and 15 d (T15) after treatment. Rumen pH was determined by ruminal fluid using portable pH meter. Total volatile fatty acids (total VFA) were evaluated by high performance liquid chromatography (HPLC). All animals were singularly weighed at T1 and T15. Two-way analysis of variance (ANOVA) was applied to determine significant effects of treatment (treated group versus control group) and period (T1, T8, and T15) on rumen fluid parameters and body weight. No clinical signs of BRD or other related diseases were recorded during the periods of study from any animal. Statistically significant differences (P < 0.05) were found between treated group and control group for mean values of ruminal pH (6.02 versus 5.89) and total VFA (5.84 versus 5.13) at 8 d after treatment. The weight gain (Δ) showed an average increase of 8.6 kg in treated group (P < 0.05). The trends of ruminal pH and VFA values suggest an effect of tulathromycin as BRD metaphylactic treatment on the modulation of rumen fermentation, particularly 8 d after administration.
Résumé
L’objectif de cet étude a été l’évaluation des effets de l’utilisation de la tulathromycine comme traitement metaphylactique contre le syndrome «Bovine Respiratory Disease» (BRD) sur les paramètres du liquide ruminal chez les veaux d’engraissement en un élevage intensif. Cent veaux de boucherie ont été divisés en deux groups juste après la stabulation: 50 animaux ont reçu le traitement metaphylactique contre le BRD (group traité; 2,5 mg/kg PC de tulathromycine) et 50 animaux ont reçu un traitement placebo (group control). Les échantillons de liquide ruminal ont été prélevés sur chaque animal par ruminocentèse en trois moments : le premier jour (T1), 8 jours (T8) et 15 jours (T15) après le traitement. Le pH du rumen a été déterminé sur le liquide ruminal en utilisant un pH-mètre portable. Les acides gras volatiles totaux (AGV totaux) ont été évalués par chromatographie liquide à haute performance (CLHP). Tous les animaux ont étés pesés singulièrement au T1 et au T15. Les effets statistiquement significatifs du traitement (group traité versus group control) et du temps (T1, T8 et T15) sur les paramètres du liquide ruminal et sur le poids corporel ont été déterminés en appliquant l’analyse de la variance à deux facteurs (ANOVA). Pendant toutes les périodes d’étude aucun animal a montré de signes cliniques de BRD ou d’autres maladies. Différences statistiquement significatives (P < 0,05) ont étés trouvées entre le group traité et le group control en ce qui concerne les valeurs moyennes du pH ruminal (6,02 versus 5,89) e des AGV totaux (5,84 versus 5,13) 8 jours après le traitement. Le group traité a montré une augmentation moyenne de 8,6 kg du gain de poids (Δ) (P < 0,05). Les tendances des valeurs du pH ruminal et des AGV totaux suggèrent un effet de la tulathromycine comme traitement metaphylactique contre la BRD sur la modulation de la fermentation ruminal, surtout 8 jours après l’administration.
(Traduit par les auteurs)
Introduction
The intensive production system in the livestock breeding of beef cattle is commonly used in Europe and, in particular, Italy. Generally, the farming method includes the importation of beef cattle from abroad, aged 15 to 18 months and weighing between 350 and 400 kg, which are taken to the finishing stage and slaughter. A critical phase in a livestock production system is the restocking period after transportation and the first 20 to 30 d following housing. At this time, the animals often present health issues due to transport management and interactions with other animals (1,2). The growth of animals is subject to many stressors (different conditions of temperature, humidity, environment, transport, the change from an acclimation diet rich in fiber to a growth diet rich in concentrate, social interactions, and diseases), which change the physiological homeostasis and compromise the health status in restocking phase (1,2). The main issues that characterize this phase in raising beef cattle are respiratory diseases, digestive disorders, articular diseases, parasitic infections, metabolic diseases, and mineral deficiency status (2).
Bovine respiratory disease (BRD) is a multifactorial syndrome influenced by many subjective factors (environment and management), that affect the local and systemic immune system of the animal which favors the rapid proliferation of different pathogens that can act individually or synergistically (3). The etiological agents involved in the syndrome are viral (bovine herpes virus, type 1; bovine respiratory syncytial virus; bovine adenovirus; parainfluenza virus 3; bovine viral diarrhoea virus), bacterial (Mannheimia haemolytica, Pasteurella multocida, Histophilus somni) and Mollicutes (Mycoplasma spp.) (4). This disease is one of the main causes affecting the profitability of intensive beef cattle breeding and can result in mortality, reduced production performance, and financial burden in the high costs for drugs both for prevention and therapy (5,6).
The overall incidence of BRD in the feedlot, which was defined as beef cattle that had lung lesions, was variable ranging from 5% (7) to 46% (8) or 64.4% (9). Because of the frequency with which BRD occurs in beef cattle, metaphylaxis treatments are commonly used in the housing or acclimation period and at the onset of respiratory symptoms (8).
Digestive disorders result from concentrated feed in the diet, which causes an increase of fermentation in the rumen and higher production of volatile fatty acids (VFA) and propionic acid. The VFA that are overproduced accumulate due to absorption failure of the rumen walls (10). In addition, the diet of these animals is low in long fiber food, so the time needed for mastication and saliva production is lower than normal. The negative effect on the rumen pH results from inadequate saliva production (11). The lower production of saliva associated with a high accumulation of VFA causes pH in the rumen to fall below 6. A pH of 6 is necessary for the production of abundant propionic acid (12) and the development of ruminal acidosis (13).
Ruminal acidosis is a metabolic disorder that negatively affects ruminal fermentation, animal health, production, and corporate profit (14). Acidosis is a multifactorial disease, but diet is the main factor that influences the lowering of the pH in the rumen. Diet is important both in terms of quantity (especially in starchy sources like corn, wheat, barley) and quality, and size of the food itself (15).
Tulathromycin is a macrolide that acts as bacteriostatic agent by reversible binding to 50S subunits of the ribosome and by inhibiting the transpeptidation and translocation process, which results in premature detachment of incomplete polypeptide chains (16). Macrolides have pharmacodynamic properties beyond their antimicrobial effects, including anti-inflammatory and immunomodulatory properties that are perceived to be clinically beneficial (16,17,18). In vitro tulathromycin acts against Gram-negative bacteria and Gram-positive bacteria that are commonly associated with respiratory disease in cattle and pigs, with in vitro MIC90 values of 1–4 mg/mL for bacteria isolated in respiratory cattle in Europe (19) and the USA (20).
However, tulathromycin exerts a prokinetic effect in milk-fed calves. The prokinetic effect suggests an additional potential therapeutic advantage in the treatment of infectious diseases in adult cattle beyond the elimination of infection. The tulathromycin treatment may also mitigate gastrointestinal tract hypomotility that is commonly associated with anorexia in diseased cattle (21).
The aim of this study was to evaluate the metaphylactic effect of tulathromycin treatment on rumen fluid parameters in feedlot cattle during the restocking period.
Materials and methods
Farm conditions and animals
One hundred beef cattle of Charolais breed were selected from a farm of 2500 feedlot cattle located in Eraclea, Italy (45°35′N; 12°41′E). The animals were imported from France and had an average weight of 434.05 ± 3.44 kg and an average age of 17.0 ± 0.6 mo.
The farm was subject to the risk of BRD. Serological testing for BRD diagnosis was done at each importation of animals. Bovine respiratory syncytial virus, bovine adenovirus, parainfluenza virus 3, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis were found on-farm.
Prophylactic protocol for prevention of BRD was carried out on-farm on the day of housing. Vaccinations were given to all animals as 2 mL IM dose of polyvalent vaccine for infectious bovine rhinotracheitis (IBR), para-influenza type 3 (PI3), bovine viral diarrhea (BVD-MD), and bovine respiratory syncytial virus (BRSV) (Cattlemaster 4; Zoetis, Italy). The treatment was repeated 16 d after arrival. All animals were treated with a 0.5 mg/kg body weight (BW) dose of anti-parasitic broad-spectrum drug (Cydectin Pour-on; Fort Dodge Animal Health S.p.a., Italy) at the same time.
Beef cattle arriving on-farm were placed on a higher long fiber acclimation diet prior to the growth diet for 5 d. After this period, the feed ingredients (Table IA) and the chemical composition (Table IB) of the total mixed ration (TMR) with high concentrate diet were the same for all animals. The stall had a separate feed bunk and watering point. Diet was provided daily as TMR ad libitum based on 10% feed refusal (as-fed basis). Dry matter intake (DMI) mean values were recorded for all beef cows during the period of study (DMI: 18 ± 1.5 kg per animal; DM: 9.78 ± 0.8 kg per animal).
Table I.
Feed ingredients (A) and chemical composition (B) of total mixed ratio (TMR) used for all animals in study
| % of total dry matter (DM) | |
|---|---|
| (A) Feed ingredients | |
| Maize silage | 33.2 |
| Corn mash | 13.63 |
| Corn gluten feed | 9.14 |
| Maize meal | 10.78 |
| Soybean meal 44 | 2.76 |
| Sugar beet pulps | 9.29 |
| Wheat straw | 9.2 |
| Protein, vitamin, and mineral premixa | 12 |
| (B) Chemical composition | |
| DM (%) | 54.02 |
| CP | 13.85 |
| EE | 3.29 |
| Ash | 5.84 |
| NDF | 37.22 |
| NFC | 31.15 |
Protein, vitamin, and mineral premix: vitamin A (45 000 IU/kg), vitamin D3 (4500 IU/kg), vitamin E (54 mg/kg), vitamin PP (45 mg/kg), choline (194.60 mg/kg), manganous sulphate (277.20 mg/kg), copper sulphate (141.48 mg/kg), selenium (0.99 mg/kg), zinc sulfate (792 mg/kg), ferrous carbonate (372.60 mg/kg), calcium (5.54 mg/kg), urea (37 240 mg/kg).
CP — crude protein; EE — ether extract; Ash — acid detergent fiber; NDF — neutral detergent fiber; NFC — non-fiber carbohydrates.
Immediately after housing all animals were divided randomly in 2 equal groups: 50 animals were treated with a single subcutaneous administration of tulathromycin (Draxxin; Zoetis, Italy) 2.5 mg/kg BW (treated group) as metaphylactic treatment; 50 animals were treated with a single subcutaneous administration of 2.5 mL of 0.9% NaCl solution (control group).
All animals were housed and divided into groups of 10 inside individual concrete-floor tie stalls within an enclosed barn. Health status was monitored for all animals daily. Bulls were singularly weighed at the beginning (T1) and at the end (T15) of the study period.
Rumenocentesis and rumen fluid analysis
A sample of fluid from the rumen was taken for each animal by rumenocentesis, method as described by Nordlund and Garrett (22) and modified by Gianesella et al (23). Rumenocentesis is the technique that provides accurate pH results (11,24,25). The collection of rumen fluid samples was carried out between 4 and 7 h after TMR administration because in this range the rumen pH reaches the peak acidity (11,24).
Rumen fluid was collected using a 13G 105-mm needle (Intralune PP, Vygon, France) and a 50 mL syringe from a 20 × 20 cm disinfected area in the left flank, from the ventral sac of the rumen, approximately 15 to 20 cm caudal and ventral to the costocondral junction of the last rib. Fifteen milliliters of rumen fluid were collected from each animal. Rumen fluid samples were taken at 3 different times: 1 d (T1), 8 d (T8), and 15 d (T15) after treatment.
The rumen fluid pH was determined using a digital portable pH meter (Zetalab PC70; XSintruments, Padua, Italy). In order to determine VFA, an aliquot of 8 mL of rumen fluid was immediately acidified with 2 mL of hydrogen chloride (HCl 0.6 M) and stored at 4°C until samples arrived at the laboratory where they were stored at −20°C until subsequent analysis.
The quantitative determination of the VFA was done in one run by using high performance liquid chromatography (HPLC). The VFA found were: acetic acid, propionic acid, iso-butyric acid, n-butyric acid, iso-valeric acid, and n-valeric acid.
Statistical analysis
Data obtained were analyzed by analysis of variance (ANOVA) for repeated measures to verify the effect of treatment using the Proc Mixed procedure (SAS, version 9.2; SAS Institute, Cary, North Carolina, USA). All dependent variables measured over time periods were evaluated using the following model:
where: Yijk = dependent variable, μ = overall mean; Treatmenti = main effect of tulatromycin or control treatment, Periodj = main effect of time period, and eijk = residual error term. Significance was determined at P ≤ 0.05, unless otherwise indicated. All results were expressed as mean values ± standard deviation (± SD).
Results
Table II shows the mean values (± SD) with the related statistically significant differences (P < 0.05) of all ruminal parameters obtained in treated and control groups during the different periods (T1, T8, and T15). Statistically significant differences (P < 0.05) were found between the treated group and the control group for mean values (± SD) of acetic acid, propionic acid, and n-valeric acid 8 d after treatment. There were also statically significant differences (P < 0.05) between the treated group and the control group for mean values (± SD) of iso-butyric acid, iso-valeric acid, and n-valeric acid 15 d after treatment.
Table II.
Mean values ± standard deviation (SD) of ruminal fluid parameters divided into groups (treated group, control group) in different periods (T1, T8, and T15)
| Time 1 | Time 8 | Time 15 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Control | Treated | Control | Treated | Control | Treated | |
| Ruminal pH | 5.83 ± 0.09 | 5.81 ± 0.09 | 5.89 ± 0.09a | 6.02 ± 0.09b | 5.94 ± 0.09 | 5.96 ± 0.09 |
| Acetic acid (mg/mL) | 3.19 ± 0.11 | 3.01 ± 0.11 | 3.18 ± 0.11a | 2.86 ± 0.11b | 2.76 ± 0.11 | 2.80 ± 0.11 |
| Propionic acid (mg/mL) | 1.37 ± 0.09 | 1.22 ± 0.09 | 1.45 ± 0.09a | 1.24 ± 0.09b | 1.54 ± 0.09 | 1.40 ± 0.09 |
| iso-butyric acid (mg/mL) | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.11 ± 0.01a | 0.05 ± 0.01b |
| n-butyric acid (mg/mL) | 0.93 ± 0.06 | 0.78 ± 0.06 | 0.96 ± 0.06 | 0.84 ± 0.06 | 1.05 ± 0.06 | 1.04 ± 0.06 |
| iso-valeric acid (mg/mL) | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.12 ± 0.01 | 0.09 ± 0.01 | 0.14 ± 0.01a | 0.09 ± 0.01b |
| n-valeric acid (mg/mL) | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.01a | 0.04 ± 0.01b | 0.11 ± 0.01a | 0.08 ± 0.01b |
| Total VFA (mg/mL) | 5.64 ± 0.20 | 5.37 ± 0.20 | 5.84 ± 0.20a | 5.13 ± 0.20b | 5.68 ± 0.20 | 5.60 ± 0.20 |
| Acetic/propionic ratio | 2.38 ± 0.02 | 2.40 ± 0.02 | 2.23 ± 0.02a | 2.47 ± 0.02b | 2.13 ± 0.02 | 2.24 ± 0.02 |
Different letters on the same line indicate statistically significant differences (P < 0.05) in the same period between the 2 groups.
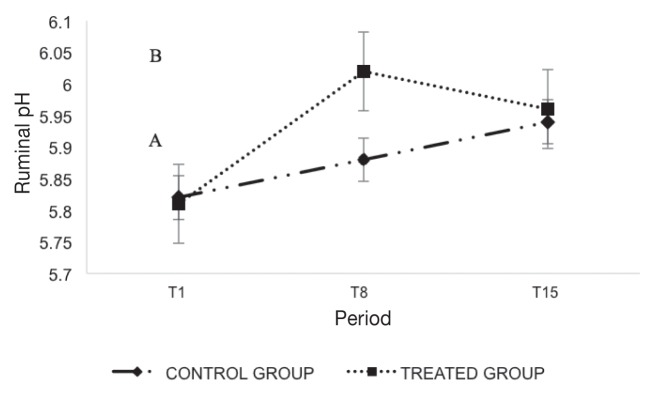
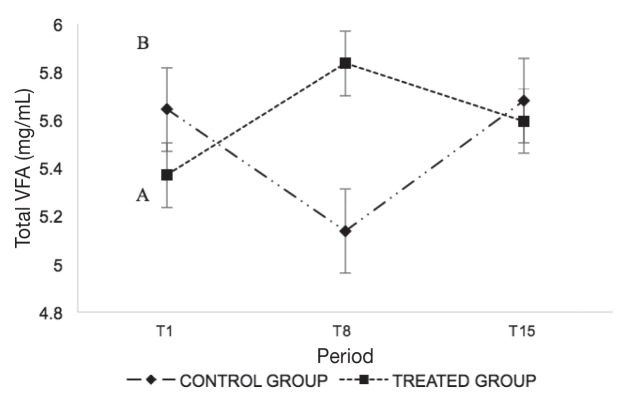
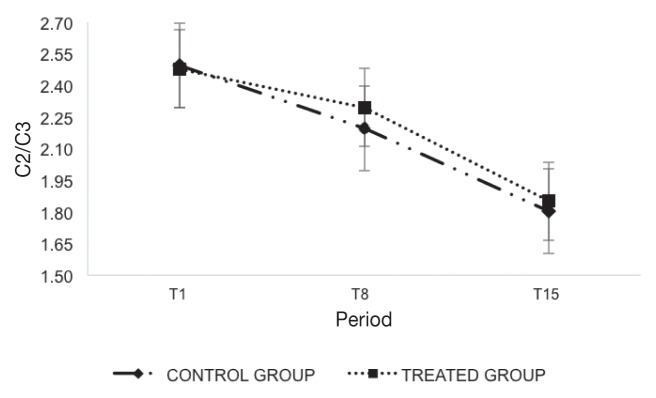
Ruminal pH values results were comparable between the 2 groups at T1 and T15, while statistically significant differences (P < 0.05) were recorded in T8 (Figure 1). Figure 2 shows statistically significant differences (P < 0.05) of the VFA values between groups after T8. The acetic/propionic ratio (C2/C3) showed a significant effect (P < 0.05) as shown in Figure 3. A statistically significant difference (P < 0.05) was found in the weight gain (Δ) showing an average increase of 8.6 kg in treated group (Table III).
Figure 1.
Mean values ± standard deviation (SD) of ruminal pH divided by the sampling period (T1, T8, and T15) and groups (treated group, control group).
A,B Different letters indicate statistically significant differences (P < 0.05) in the same period between the 2 groups.
Figure 2.
Mean values ± standard deviation (SD) of total VFA divided by the sampling period (T1, T8, and T15) and groups (treated group, control group).
A,B Different letters indicate statistically significant differences (P < 0.05) in the same period between the 2 groups.
Figure 3.
Mean values ± standard deviation (SD) of the acetic/propionic acid ratio (C2/C3) divided by sampling period (T1, T8, and T15) and groups (treated group, control group).
Table III.
Mean values ± standard deviation (SD) of the weight of animals divided into groups (treated group, control group) and periods (T1 and T15)
| Group | T1 | T15 | Δ |
|---|---|---|---|
| Treated | 431.3 ± 3.39 | 467.9 ± 4.26 | 36.6 ± 5.44a |
| Control | 436.8 ± 3.48 | 464.8 ± 3.95 | 28.0 ± 5.26b |
Different letters indicate statistically significant differences (P < 0.05) in the same period between groups.
Discussion
Our findings showed that administration of tulathromycin in beef cattle was able to modulate ruminal fermentation processes during the first part of the production cycle. The TMR and DMI did not vary over the time and between intakes of beef cattle in the 2 groups. No adverse reaction occurred after the 3 rumenocentesis procedures in any of the animals.
The different healthcare approach in the management of the 2 groups confirms the difference in performance during the 3 periods of study (T1, T8, and T15). Trends of ruminal pH and VFA values suggested that fermentation in the rumen is improved 8 d after administration of tulathromycin in the treated group.
Individual ruminal pH obtained in the field showed values in the physiological limits ranging between 5.8 and 6.7 (26), but highest values were recorded in the treated group 8 d after administration of tulathromycin.
The VFA trends were in accordance with other studies conducted on dairy cattle (27). The production of large amounts of VFA is derived from the administration of a diet rich in fermentable carbohydrates that promote high fermentation activity (23). The beef cattle included in this study received the same nutritional management and, therefore, it is conceivable that a treatment effect on the ruminal activity where tulathromycin leads to a modulation of bacterial populations (such as the growth of cellulolytic and amylolytic bacteria producing lactic acid) with consequently lower accumulation of VFA in the rumen (11) and an increase in the prokinetic effects (20).
The values of both isoforms of valeric acid (iso-valeric acid and n-valeric acid) were found to be lower in the treated group compared to control group. The n-valeric acid is derived from the catabolism of proteins and it may be toxic, even if the scientific evidence reported it to be present only in trace amounts in dairy cattle (11,23). However, the presence of this acid at levels of 0.11 mg/mL in the control group at 15 d after the treatment led us to consider further studies on the production of the n-valeric acid and on its possible effects on the health status of the animals.
The acetic and propionic acid ratio (C2/C3) suggests that the rumen activity is variable in the ruminant. Acetic acid (C2) represents approximately 65% to 75% of the VFA, while the propionic acid (C3) is 15% to 20%, as the ratio C2/C3 is 3 to 3.5 in diets rich in forage. If the diet is rich in starchy sources, the C2/C3 ratio decreases even more to 2, with C2 percentage of 55% to 60% and C3 percentage of 30% to 35%. Figure 3 reported that beef cattle showed VFA values that could be expected in a starchy rich diet (range: 2.13 to 2.47) (8). The C2/C3 ratio for both groups was similar in T1 and T15.
The increase in pH and the reduction of VFA concentration were found 8 days after the administration of tulathromycin in each treated animal. An interesting hypothesis may be the antimicrobial and anti-inflammatory effect of tulathromycin (2) on the amylolytic bacteria population and on the inflammatory response in the case of ruminal acidosis. In fact, changes in the physicochemical conditions of different body compartments as a consequence of disease may affect the selectivity of a drug for a tissue, disposal of the parent compound and its residues, and the efficacy of the drug. Another physicochemical feature that may dictate the movement of the drug across body compartments is that the tulathromycin molecule is more soluble in hydrophilic versus hydrophobic media (readily soluble in water at pH 8.0 or below) (20).
According to Tennant et al (6), the effect of administration of tulathromycin in feedlot cattle during the restocking period improves the welfare conditions and increases production performance during the first phase of growth (8.6 kg of mean weight gain in treated animals compared to control group). This weight gain may be further enhanced by improved modulation of rumen fermentation in animals treated by tulathromycin.
In conclusion, tulathromycin treatment has an effect on the modulation of rumen fluid parameters 8 d after administration resulting in increased production performance. The improvement in health, welfare, and production performance in animals after treatment during the first phase of growth are demonstrated. However, further studies investigating clinical and biochemical parameters on the response after administration of tulathromycin in a larger sample size and with additional diagnostic techniques, such as the evaluation of the ruminal bacterial population, may be indicated.
Acknowledgment
The study was supported by Zoetis, Italy.
References
- 1.Cooke RF, Bohnert DW, Moriel P, Hess BW, Mills RR. Effects of polyunsaturated fatty acid supplementation on ruminal in situ forage degradability, performance, and physiological responses of feeder cattle. J Anim Sci. 2011;89:3677–3689. doi: 10.2527/jas.2010-3515. [DOI] [PubMed] [Google Scholar]
- 2.Stanton AL, Kelton DF, LeBlanc SJ, et al. The effect of treatment with long-acting antibiotic at postweaning movement on respiratory disease and on growth in commercial dairy calves. J Dairy Sci. 2010;93:574–581. doi: 10.3168/jds.2009-2414. [DOI] [PubMed] [Google Scholar]
- 3.Nickell JS, White BJ. Metaphylactic antimicrobial therapy for bovine respiratory disease in stocker and feedlot cattle. Vet Clin North Am Food Anim Pract. 2010;26:285–301. doi: 10.1016/j.cvfa.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Ellis JA. The immunology of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract. 2001;17:535–549. doi: 10.1016/S0749-0720(15)30005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirchhoff J, Uhlenbruck S, Goris K, Keil GM, Herrler G. Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Vet Res. 2014;45:20. doi: 10.1186/1297-9716-45-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tennant TC, Ives SE, Harper LB, Renter D, Lawrence TE. Comparison of tulathromycin and tilmicosin on the prevalence and severity of bovine respiratory disease in feedlot cattle in association with feedlot performance, carcass characteristics, and economic factors. J Anim Sci. 2014;92:5203–5213. doi: 10.2527/jas.2014-7814. [DOI] [PubMed] [Google Scholar]
- 7.Snowder GD, Van Vleck LD, Cundiff LV, Bennett GL. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J Anim Sci. 2006;84:1999–2008. doi: 10.2527/jas.2006-046. [DOI] [PubMed] [Google Scholar]
- 8.Galyean ML, Gunter SA, Malcolm-Callis KJ. Effects of arrival medication with tilmicosin phosphate on health and performance of newly received beef cattle. J Anim Sci. 1995;73:1219–1226. doi: 10.2527/1995.7351219x. [DOI] [PubMed] [Google Scholar]
- 9.Schneider MJ, Tait RG, Busby WD, Reecy JM. An evaluation of bovine respiratory disease complex in feedlot cattle: Impact on performance and carcass traits using treatment records and lung lesion scores. J Anim Sci. 2009;87:1821–1827. doi: 10.2527/jas.2008-1283. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraja TG, Titgemeyer EC. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J Dairy Sci. 2007;90:17–38. doi: 10.3168/jds.2006-478. [DOI] [PubMed] [Google Scholar]
- 11.Morgante M, Stelletta C, Berzaghi P, Gianesella M, Andrighetto I. Subacute rumen acidosis in lactating cows: An investigation in intensive Italian Dairy herds. J Anim Physiol Anim Nutr. 2007;91:226–234. doi: 10.1111/j.1439-0396.2007.00696.x. [DOI] [PubMed] [Google Scholar]
- 12.Bannink A, France J, Lopez S, et al. Modelling the implications of feeding strategy on rumen fermentation and functioning of the rumen wall. Anim Feed Sci Technol. 2008;143:3–26. [Google Scholar]
- 13.Dijkstra J, Ellis JL, Kebreab E, et al. Ruminal pH regulation and nutritional consequences of low pH. Anim Feed Sci Technol. 2012;172:22–33. [Google Scholar]
- 14.Krause KM, Oetzel GR. Inducing subacute ruminal acidosis in lactating dairy cows. J Dairy Sci. 2005;88:3633–3639. doi: 10.3168/jds.S0022-0302(05)73048-4. [DOI] [PubMed] [Google Scholar]
- 15.De Nardi R, Marchesini G, Gianesella M, et al. Blood parameters modification at different ruminal acidosis conditions. Agriculturae Conspectus Scientificus. 2013;78:259–262. [Google Scholar]
- 16.Giguère S, Prescott JF, Baggot JD, Walker RD, Dowling PM. Antimicrobial Therapy in Veterinary Medicine. 4th ed. Ames, Iowa: Blackwell Publishing; 2006. Macrolides, azalides, and ketolides; pp. 191–206. [Google Scholar]
- 17.Hawkyard CV, Koerner RJ. The use of erythromycin as a gastrointestinal prokinetic agent in adult critical care: Benefits versus risks. J Antimicrob Chemother. 2007;59:347–358. doi: 10.1093/jac/dkl537. [DOI] [PubMed] [Google Scholar]
- 18.Buret AG. Immuno-modulation and anti-inflammatory benefits of antibiotics: The example of tilmicosin. Can J Vet Res. 2010;74:1–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Godinho KS. Susceptibility testing of tulathromycin: Interpretative breakpoints and susceptibility of field isolates. Vet Microbiol. 2008;129:426–432. doi: 10.1016/j.vetmic.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Evans NA. Tulathromycin: An overview of a new triamilide antibiotic for livestock respiratory disease. Vet Ther. 2005;6:83–95. [PubMed] [Google Scholar]
- 21.Rashnavadi M, Nouri M, Haji Hajikolaei MR, Najafzadeh H, Constable PD. Effect of spiramycin and tulathromycin on abomasal emptying rate in milk-fed calves. Can J Vet Res. 2014;78:61–67. [PMC free article] [PubMed] [Google Scholar]
- 22.Nordlund KV, Garrett EF. Rumenocentesis: A technique for collecting rumen fluid for the diagnosis of subacute rumen acidosis in dairy herds. Bovine Practitioner. 1994;28:109–112. [Google Scholar]
- 23.Gianesella M, Morgante M, Cannizzo C, et al. Subacute ruminal acidosis and evaluation of blood gas analysis in dairy cow. Vet Med Int. 2010 doi: 10.4061/2010/392371. pii: Article ID: 392371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrett EF, Pereira MN, Nordlund KV, Armentano LE, Goodger WJ, Oetzel GR. Diagnostic methods for the detection of subacute ruminal acidosis in dairy cows. J Dairy Sci. 1999;82:1170–1178. doi: 10.3168/jds.S0022-0302(99)75340-3. [DOI] [PubMed] [Google Scholar]
- 25.Duffield T, Plaizier JC, Fairfield A, et al. Comparison of techniques for measurement of rumen pH in lactating dairy cows. J Dairy Sci. 2004;87:59–66. doi: 10.3168/jds.S0022-0302(04)73142-2. [DOI] [PubMed] [Google Scholar]
- 26.Kolver ES, De Veth MJ. Prediction for ruminal pH from pasture-based diets. J Dairy Sci. 2002;85:1255–1266. doi: 10.3168/jds.S0022-0302(02)74190-8. [DOI] [PubMed] [Google Scholar]
- 27.Gianesella M, Piccione G, Cannizzo C, Casella S, Morgante M. Influence of temperature and humidity on rumen pH and fatty acids in dairy cows. J Environ Biol. 2012;33:1093–1096. [PubMed] [Google Scholar]
- 28.Fischer CD, Beatty JK, Zvaigzne CG, Morck DW, Lucas MJ, Buret AG. Anti-inflammatory benefits of antibiotic-induced neutrophil apoptosis: Tulathromycin induces caspase-3-dependent neutrophil programmed cell death and inhibits NF-{kappa} B signaling and CXCL8 transcription. Antimicrob Agents Chemother. 2011;55:338–348. doi: 10.1128/AAC.01052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]