Abstract
Overexpression of matrix metalloproteinases (MMPs) has been associated with increased tumor aggressiveness and metastasis dissemination. We investigated whether the contrasting metastatic behavior of feline and canine osteosarcoma is related to levels and activities of MMP2 and MMP9. Zymography and immunohistochemistry were used to determine expression levels of MMP2 and MMP9 in canine and feline osteosarcoma. Using immunohistochemistry, increased MMP9 levels were identified in most canine osteosarcomas, whereas cat samples more often displayed moderate levels. High levels of pro-MMP9, pro-MMP2, and active MMP2 were detected by gelatin zymography in both species, with significantly higher values for active MMP2 in canine osteosarcoma. These findings indicate that MMP2 is probably involved in canine and feline osteosarcoma and their expression and activity could be associated with the different metastatic behavior of canine and feline osteosarcoma.
Résumé
La surexpression de métalloprotéases de matrice (MPMs) a été associée avec une augmentation de l’agressivité des tumeurs et de la dissémination métastasique. Nous avons cherché à savoir si le comportement métastasique contrastant d’ostéosarcomes félin et canin est relié aux quantités et à l’activité de MPM2 et MPM9. La zymographie et l’immunohistochimie ont été utilisées afin de déterminer les niveaux d’expression de MPM2 et de MPM9 dans des ostéosarcomes canins et félins. En utilisant l’immunohistochimie, des quantités augmentées de MPM9 ont été identifiées dans la plupart des ostéosarcomes canins, alors que les échantillons félins montraient plus souvent des quantités modérées. Des niveaux élevés de pro-MPM9, pro-MPM2, et de la MPM2 active ont été détectés par zymographie sur gélatine chez les deux espèces, avec des valeurs significativement plus élevées pour de la MPM2 active dans les ostéosarcomes canins. Ces données indiquent que MPM2 est probablement impliquée dans les ostéosarcomes canins et félins et que leur expression et activité pourraient être associé avec le comportement métastasique différent des ostéosarcomes canins et félins.
(Traduit par Docteur Serge Messier)
Introduction
Osteosarcoma (OS) is the most common bone tumor in both the canine and feline species. It accounts for approximately 80% of primary bone tumors in dogs and 70% in cats (1–3). While canine and feline OS share the same clinical indicators and exhibit similar histological features, tumor behavior is considerably different in dogs and cats (4). Dogs die from the consequences of lung metastases in most cases. Therefore, the prognosis is poor for canine OS, the recurrence rate is high, and the survival time is low (5,6). The median survival time ranges from 3 mo to 1 y (7). In contrast, cats show increased long-term survival compared to dogs, ranging from approximately 13 to 64 mo (8). In addition to the decreased meta-static rate of 5% to 10%, the overall incidence of OS is notably lower in cats than in dogs (9,10). As the reasons for these differences are unclear, further knowledge of molecular mechanisms affecting OS metastatic outgrowth is badly needed.
Matrix metalloproteinases 2 (MMP2) and MMP9 belong to the MMP (matrix metalloproteinase) family, several members of which have been shown to contribute to development of OS in humans (11,12). This protein family is divided into different subgroups according to substrate specificities, such as collagenases, gelatinases (MMP2 and MMP9), stromelysins, matrilysins, MT-MMPs (membrane-type matrix metalloproteinases), and others (13,14). Both MMP2 (gelatinase A) and MMP9 (gelatinase B) are type-IV collagenases that are expressed by multiple cell types as well as cells associated with bone tissue (15,16). Most MMPs are secreted as latent precursors and activated by means of a proteolytic mechanism, followed by an autocatalytic cleavage of the cysteine-zinc bond (14,17). The activity of MMPs is regulated at the transcriptional level (18) or at the protein level by RECK (reversion-inducing-cysteine-rich protein with Kazal motifs), α2 M (α2 macroglobulin), endostatin, or TIMP (tissue inhibitor of metalloproteinases), which are the most often described natural MMP inhibitors responsible for controlling the degradation of extracellular matrix (ECM) (19–21).
High levels of MMP2 and MMP9 expression and activity have been shown to be associated with tumor aggressiveness, metastasis dissemination, and poor survival time in human and mouse models (22–24). Their importance in cancer progression is due to their ability to degrade components of the ECM, especially basement membrane components, including type-IV (25) and type-V (26) non-fibrillary collagens, type-VII collagen (27), type-X collagen (28), elastin (29), and fibronectin (30), which in turn facilitate tumor cell invasion and metastatic progression (22,31,32). Tumor progression with detaching of cells from the primary site, migration through the basement membrane into the circulatory system, and subsequent spread into the lung to form a metastatic colony is a process that occurs regularly in human osteosarcoma patients (33,34) and requires the active participation of gelatinases. Consistent with this model, there is considerable evidence that MMPs contribute to osteosarcoma metastasis development (22,35,36). Although significantly enhanced levels of MMP2 and MMP9 have been reported in human and canine osteosarcoma (31,37,38), MMP2 and MMP9 levels in feline OS tumor samples have not yet been investigated.
The objective of this study was to determine and compare the levels and activity of MMP2 and MMP9 in canine and feline OS by means of gelatin zymography to find out whether the contrasting tumor behaviors in canines and felines are correlated with different levels of gelatinase activity. Immunohistochemistry for MMP2 and MMP9 was conducted to localize their distribution within the corresponding OS tumor tissue sample.
Materials and methods
A total of 24 OS samples (17 canine, 7 feline) were collected over a period of 4 y (2009 to 2013). Tissue samples were obtained by surgery during therapeutic interventions or by necropsy, according to the rules of the local ethical committee. Fresh samples were aliquoted for gelatin zymography, shock frozen in liquid nitrogen, and stored at −80°C until use. Samples for pathohistological examination, including tumor grading according to Kirpensteijn et al (6) and immunohistochemistry, were fixed in buffered 4% formaldehyde, embedded in paraffin (FFPE samples), and stained with hematoxylin and eosin (H&E). Decalcification of tissue samples using 8% ethylenediamine tetra-acetic acid (EDTA) was carried out for histology when necessary.
Animal data
Clinical and pathohistological data, including breed, gender, age, tumor localization, subtype of OS, and tumor grade, are summarized in Tables Ia and Ib.
Table Ia.
Clinical data from dogs with primary osteosarcoma (OS)
| Case number | Canine OS | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Subtype | Grade | Age (y) | Breed | Gendera | Localization | |
| 1 | Fibroblastic | 3 | 8 | Great Dane | m/n | Appendicular |
| 2 | Fibroblastic | 3 | 5 | Boxer | f/n | Appendicular |
| 3 | Chondroblastic | 2 | 13 | Mixed | f | Appendicular |
| 4 | Chondroblastic | 2 | 12 | Sheep dog | m | Extraskeletal |
| 5 | Telangiectatic | 3 | 7 | Boxer | f | Lung metastasis |
| 6 | Osteoblastic | 2 | 8 | Saint Bernard | f | Appendicular |
| 7 | Osteoblastic | 3 | 6 | Saint Bernard | f/n | Appendicular |
| 8 | Osteoblastic | 2 | 10 | Mixed | f/n | Appendicular |
| 9 | Osteoblastic | 2 | 9 | Mixed | f/n | Appendicular |
| 10 | Osteoblastic | 2 | 15 | Pinscher | f | Extraskeletal |
| 11 | Osteoblastic | 3 | 11 | Munsterlander | f | Axial |
| 12 | Osteoblastic | 2 | 7 | Leonberger | f | Appendicular |
| 13 | Osteoblastic | 2 | 11 | Dachshund | m/n | Appendicular |
| 14 | Osteoblastic | 2 | 12 | Pinscher | f/n | Appendicular |
| 15 | Osteoblastic | 2 | 9 | American Stafford | f/n | Appendicular |
| 16 | Mixed | 2 | 1 | Rhodesian Ridgeback | m | Appendicular |
| 17 | Poorly differentiated | 3 | 7 | Mixed | m | Appendicular |
Gender: f/n — female, neutered; m/n — male, neutered.
Table 1b.
Clinical data from cats with primary osteosarcoma (OS)
| Case number | Feline OS | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Subtype | Grade | Age (y) | Breed | Gendera | Localization | |
| 1 | Fibroblastic | 1 | 10 | Europ. short hair | f | Axial |
| 2 | Fibroblastic | 2 | 15 | Europ. short hair | m/n | Appendicular |
| 3 | Osteoblastic | 2 | 12 | Europ. short hair | m/n | Appendicular |
| 4 | Osteoblastic | 2 | 4 | Europ. short hair | m/n | Appendicular |
| 5 | Osteoblastic | 1 | 6 | Europ. short hair | f/n | Axial |
| 6 | Mixed | 1 | 13 | Europ. short hair | f/n | Appendicular |
| 7 | Mixed | 2 | 12 | Europ. short hair | f | Appendicular |
Gender: f/n — female, neutered; m/n — male, neutered.
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) tissue sections (5 μm thickness) were mounted on glutaraldehyde-activated 3-aminopropyl-triethoxysilane (APES)-coated slides. Dehydration was followed by removal of endogenous peroxidases with 0.6% hydrogen peroxide (H2O2) in 40% methanol. Unspecific binding of the primary antibody was minimized by incubating the tissue sections with 1.5% normal goat serum (PAA, Pasching, Austria) for 30 min. The sections were pretreated for epitope retrieval in a steamer for 30 min in 0.1 M citric acid buffer (pH 6.0) and subsequently cooled down to room temperature for 20 min.
Immunohistochemical staining for MMP2 and MMP9 was done using polyclonal antibodies (rabbit anti-MMP2, dilution 1:100; Abcam, Cambridge, United Kingdom and rabbit anti-MMP9, dilution 1:100; Abnova, Heidelberg, Germany) overnight at 4°C. After washing with phosphate-buffered saline (PBS), the tissue sections were incubated with the secondary antibody (Bright Vision Poly-HRP-anti-rabbit; Immunologic, Duiven, Netherlands) for 30 min at room temperature. Sections were then washed in PBS and developed with 10 mg DAB (3,3′-diaminobenzidine; Sigma-Aldrich, Vienna, Austria) in 50 mL Tris-hydrochloride (HCl) buffer pH 7.4 with 50μL 30% H2O2 for 10 min at room temperature. Finally, sections were counterstained with hemalumn, dehydrated, and mounted in xylene-soluble mounting medium (Consul Mount; Thermo Scientific, Waltham, Massachusetts, USA).
Histological sections of canine and feline placenta were used as positive tissue controls for MMP2 and MMP9 immunohistochemistry. Negative controls were conducted by substituting the primary antibody with PBS. According to the manufacturer’s datasheet, the antibodies used are not suitable for distinguishing between latent and active forms. The expression of MMP2 and MMP9 in tumor tissue was semi-quantitatively assessed using 2 histological values, the area of positively stained cells and the signal intensity. Distinct brown-labeled cells were regarded as positive. The staining pattern was evaluated independently by 2 investigators (CG and IW). The area of positively stained cells of either MMP2 or MMP9 was scored on a scale from 1 to 3 (1: < 25%; 2: 25% to 50%; 3: > 50%) and the average staining intensity was scored on a scale from 1 to 3 (1: mild, 2: moderate, 3: marked). For calculating the immunopositivity score (IPS), the percentage area of positive cells was multiplied with the staining intensity (39). The final integer values were scored as IPS I (1 to 2), IPS II (3 to 4), and IPS III (6 to 9).
Gelatin zymography
Zymography was carried out according to Walter et al 2005 (40). Gelatinases (MMP2, MMP9) in their precursor and active forms were detected by means of gelatin zymography. Fresh frozen OS tissue was cut into small pieces (2 to 3 mm2), then homogenized in 500 μL EDTA buffer [10 mM Tris-HCl pH 7.5, 140 mM sodium chloride (NaCl), 5 mM EDTA, 1 mM phenylmethylsulphonyl fluoride (PMSF), 1 mM dithiothreitol (DTT), 1% Triton X-100] using a TissueRuptor (Qiagen, Hilden, Germany). The tissue homogenate was mixed with an equal volume of Tris/glycine/SDS sample buffer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Protein concentrations of the lysates were determined using the Bradford assay and aliquots of 10 μg protein lysate were loaded on non-reducing 4% polyacrylamide stacking gels and 10% resolving gels (gel thickness 1.0 mm), containing 0.1% gelatin (Sigma-Aldrich) as a substrate.
Electrophoresis was conducted for 30 min at 130 V, then 90 min at 100 V with Tris/glycine/SDS running buffer (Thermo Fisher Scientific) in a Mini-Protean Cell (Bio-Rad, Hercules, California, USA). After electrophoresis, gels were washed with renaturing buffer (Thermo Fisher Scientific) for 45 min and incubated with developing buffer (Thermo Fisher Scientific) overnight at 37°C. Gels were stained with 0.5% Coomassie Brilliant Blue R-250 for 2 h and destained with a mix of methanol, acetic acid, and water, at a volume ratio of 4:1:5, until gelatinases became clearly visible as white bands. Gels were scanned with an Image Scanner III (GE Healthcare Life Sciences, Munich, Germany) and quantified by densitometry analysis using Quantity One software (Version 4.4.0; Bio-Rad). For each lane/sample, band intensities of active and pro forms of MMP2 or MMP9 were expressed as relative percentage of overall activity of the respective gelatinase. Human recombinant gelatinases were used as a standard of pro- and active MMP2 (cat no. PF037 and PF023; Calbiochem/EMD Millipore, Billerica, Massachusetts, USA) and MMP9 (cat no. PF038).
A zymography inhibition assay was conducted by supplementing the developing buffer with 20 mM EDTA, which resulted in a complete absence of bands. The results obtained for pro- and active MMP2 and MMP9 were analyzed statistically using Student’s t-test; values of P ≤ 0.05 were considered significant.
Results
Immunohistochemistry
Matrix metalloproteinase 2 (MMP2) and MMP9 were detected in all canine and feline OS samples (24/24) using immunohistochemistry. In addition to tumor cells, multiple other cell types, such as osteoblasts, chondroblasts, fibroblasts, macrophages, or endothelial cells of blood vessels, showed positive staining for MMP2 and MMP9. The immunoreactivity was mainly cytoplasmic and, in rare cases, weak staining of the ECM, mainly osteoid matrix, was detected (Figure 1). The IPS scores for MMP2 and MMP9 expression in canine and feline OS are given in Tables IIa and IIb. Most (71%) canine OS samples displayed high MMP9 values (IPS III), whereas far fewer samples rated as IPS I and IPS II were detected (6% and 23%, respectively). In contrast, a noticeably lower proportion of the feline OS samples were rated as IPS III (43%) and the MMP9 values of more samples scored as IPS II (57%) compared with canine cases (23%). Immunoscoring for MMP2 revealed that almost half of the canine OS samples (47%), scored as IPS III, a lower number scored as IPS I (35%), whereas only 18% scored as IPS II. After MMP2 immunostaining in feline OS, most samples were scored as IPS II or equally distributed between IPS I and IPS III. As a result, most canine OS samples exhibited IPS III values for MMP9 and MMP2, whereas feline OS samples were mostly scored as IPS II for both MMPs.
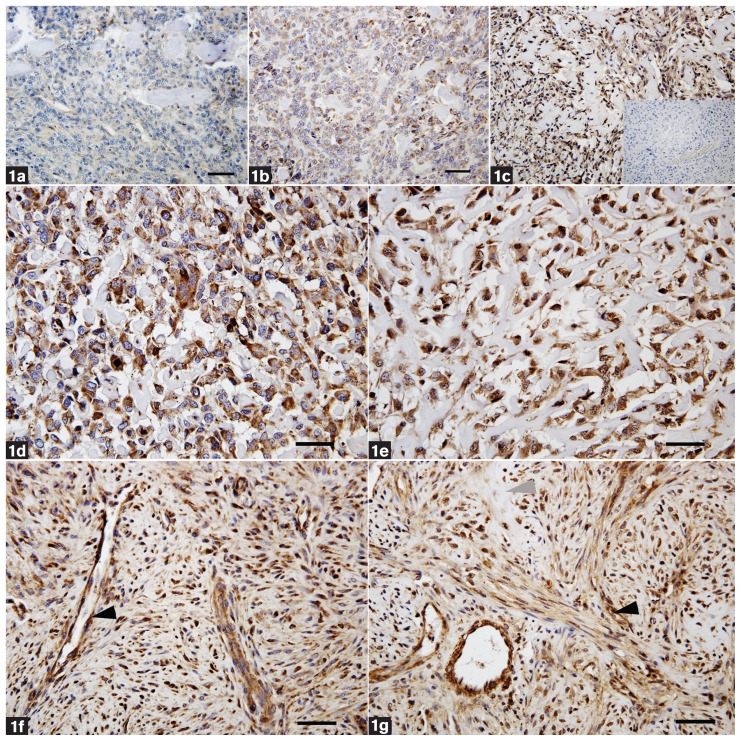
Figure 1.
Distribution of the different immunostaining levels scored from 1 to 3 (1 = mild, 2 = moderate, 3 = marked) (a to c). Mild staining intensity (a), moderate staining intensity (b), and marked staining intensity (c), including the negative control for MMP9 (inset). Canine osteoblastic OS (case no. 6) with marked staining intensity for MMP2 (d) and MMP9 (e) were scored as IPS III. Typical osteoblast-like cells producing osteoid matrix are shown. Feline osteoblastic OS (case no. 21) with marked staining intensity for MMP2 (f) and MMP9 (g) were scored as IPS III. The latter 2 panels show a heterogeneous cell pattern indicating whirl formation of spindle-shaped cells. Note also the intense staining of tumor cells and blood vessels, particularly in the area of tumor cell whorls (black arrowhead). Area of osteoid matrix surrounded by tumor cells is marked (grey arrowhead). Bar = 50 μm.
Table IIa.
Immunopositivity score (IPS) for MMP9 expression in canine and feline osteosarcoma (OS)
| MMP9 | ||||
|---|---|---|---|---|
|
|
||||
| Subtype | Case numbers | IPS I | IPS II | IPS III |
| Canine OS | ||||
| Fibroblastic | 1, 2 | — | 1 | 1 |
| Chondroblastic | 3, 4 | — | 1 | 1 |
| Telangiectatic | 5 | — | — | 1 |
| Osteoblastic | 6 to 15 | 1 | 2 | 7 |
| Mixed | 16 | — | — | 1 |
| Poorly differentiated | 17 | — | — | 1 |
| Total (% integer values) | 6 | 23 | 71 | |
| Feline OS | ||||
| Fibroblastic | 18, 19 | — | 2 | — |
| Osteoblastic | 20 to 22 | — | 2 | 1 |
| Mixed | 23, 24 | — | — | 2 |
| Total (% integer values) | — | 57 | 43 | |
Table IIb.
Immunopositivity score (IPS) for MMP2 expression in canine and feline osteosarcoma (OS)
| MMP2 | ||||
|---|---|---|---|---|
|
|
||||
| Subtype | Case numbers | IPS I | IPS II | IPS III |
| Canine OS | ||||
| Fibroblastic | 1, 2 | — | 2 | — |
| Chondroblastic | 3, 4 | 1 | — | 1 |
| Telangiectatic | 5 | — | — | 1 |
| Osteoblastic | 6 to 15 | 5 | — | 5 |
| Mixed | 16 | — | — | 1 |
| Poorly differentiated | 17 | — | 1 | — |
| Total (% integer values) | 35 | 18 | 47 | |
| Feline OS | ||||
| Fibroblastic | 18, 19 | 1 | 1 | — |
| Osteoblastic | 20 to 22 | — | 2 | 1 |
| Mixed | 23, 24 | 1 | — | 1 |
| Total (% integer values) | 29 | 43 | 29 | |
Due to the low number of cases per tumor subtype, it was not possible to correlate MMP2 or MMP9 immunohistochemistry with the different tumor subtypes. In the canine osteoblastic OS group (n = 10), however, most of the samples were scored as IPS III for MMP9, whereas for MMP2, they were assessed equally as IPS I and IPS III.
Gelatin zymography
The results of the zymography experiments are summarized in Table III. All canine and feline OS samples investigated by zymography were positive for both MMP2 and MMP9 (24/24). The pattern showed white bands on the blue gel with an apparent molecular weight ranging from 62 kDa to 92 kDa, as confirmed by co-migrating human recombinant protein standards (Figure 2). MMP9 was detected at 92 kDa (pro-MMP9) and 83 kDa (active MMP9). Bands of MMP2 were present at 72 kDa (pro-MMP2), as well as at 66 kDa (active MMP2) and rarely as an additional active MMP2 band at 62 kDa. Significantly lower levels (P < 0.01) of active MMP9 than the inactive form were observed in all canine and feline tumor samples (Figures 3a and 3b). Mean active MMP9 values, given as percentage of total amount of the enzyme, ranged from 6.0% to 15.0% in the canine OS and from 5.0% to 12.0% in the feline OS samples (Table III). The highest relative value of active MMP9 was observed in the poorly differentiated subtype of canine OS, with the lowest value in the telangiectatic OS case. In the cat, mixed-subtype OS samples exhibited the lowest active MMP9 amount (5%) of all tumor subtypes.
Table III.
Detection of levels of pro- and active MMP2 and MMP9 in canine and feline osteosarcoma (OS) samples using gelatin zymography
| Mean (%) ± SD | |||||
|---|---|---|---|---|---|
|
|
|||||
| Subtype | Case numbers | Pro-MMP9 | Active MMP9 | Pro-MMP2 | Active MMP2 |
| Canine OS | |||||
| Fibroblastic | 1, 2 | 89.0 ± 5.7 | 11.0 ± 5.7 | 74.5 ± 2.1 | 25.5 ± 2.1 |
| Chondroblastic | 3, 4 | 92.0 ± 2.8 | 8.0 ± 2.8 | 67.0 ± 24.0 | 33.0 ± 24.1 |
| Telangiectatic | 5 | 94.0 ± — | 6.0 ± — | 57.0 ± — | 43.0 ± — |
| Osteoblastic | 6 to 15 | 91.1 ± 4.9 | 8.9 ± 4.9 | 53.3 ± 16.4 | 46.7 ± 16.4 |
| Mixed | 16 | 89.0 ± — | 11.0 ± — | 58.0 ± — | 42.0 ± — |
| Poorly differentiated | 17 | 85.0 ± — | 15.0 ± — | 86.0 ± — | 14.0 ± — |
| Feline OS | |||||
| Fibroblastic | 18, 19 | 90.0 ± 7.1 | 10.0 ± 7.1 | 84.5 ± 3.5 | 15.5 ± 3.5 |
| Osteoblastic | 20 to 22 | 88.0 ± 7.0 | 12.0 ± 7.0 | 75.7 ± 10.3 | 24.3 ± 10.3 |
| Mixed | 23, 24 | 95.0 ± 1.4 | 5.0 ± 1.4 | 73.0 ± 4.2 | 27.0 ± 4.2 |
SD — standard deviation.
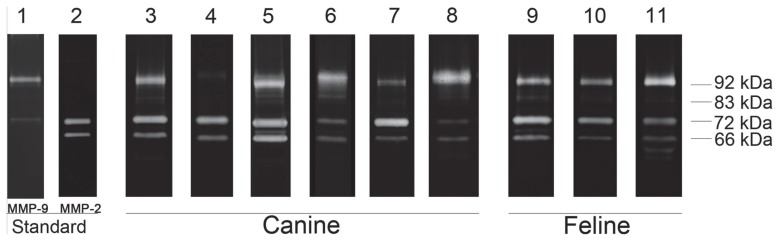
Figure 2.
Examples of gelatin zymography pattern of canine and feline osteosarcoma (OS). Lane 1: human pro-MMP9 standard. Lane 2: human pro- and active MMP2 standard. Canine tumor samples are shown from lane 3 to 8. Lane 3: osteoblastic OS (case no. 6); lane 4: fibroblastic OS (case no. 2); lane 5: mixed OS (case no. 16); lane 6: chondroblastic OS (case no. 4); lane 7: poorly differentiated OS (case no. 17); lane 8: telangiectatic subtype (lung metastasis, case no. 5). Feline tumor samples are shown from lane 9 to 11. Lane 9: osteoblastic OS (case no. 21); lane 10: fibroblastic OS (case no. 18); and lane 11: mixed OS (case no. 23). The molecular weights of pro- and active MMPs are indicated from 62 to 92 kDa.
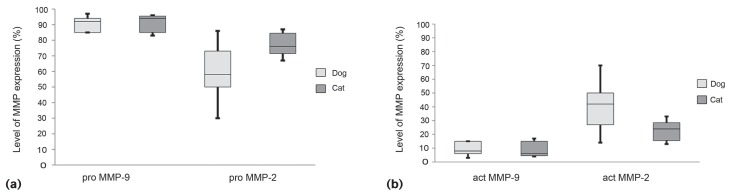
Figure 3.
Levels of pro- and active MMP2 and MMP9 as assessed by gelatin zymography. Pro-MMP9 was expressed at significantly (P < 0.01) higher levels than pro-MMP2 in canine and feline samples (a). In both species, the levels of active MMP2 were significantly (P < 0.01) higher than active MMP9 (b). In feline OS samples, a significantly lower amount of active MMP2 was expressed (P < 0.01).
Pro- and active MMP2 was variably distributed in canine OS samples (Figures 3a and 3b). The portion of active MMP2 ranged from 14.0% to 46.7% in canine OS samples and from 15.5% to 27.0% in feline OS samples. The highest values of active MMP2 were detected in the osteoblastic and telangiectatic OS subtypes; the lowest amount was evaluated in poorly differentiated OS. In the feline species, the mixed-subtype samples showed the highest relative active MMP2 value. Canine samples revealed overall significantly (P < 0.01) higher levels of active MMP2 than those of cats.
When comparing both methods of MMP detection, gelatin zymography and immunohistochemistry, results shared similarities according to the total amount of gelatinases. Summarizing immunohistochemistry revealed higher relative IPS scores for MMP2 and MMP9 in canine than in feline samples and zymography showed significantly higher amounts of active MMP2 levels in OS of the canine species. The tumor grades of the analyzed feline and canine OS samples were explicitly divergent between cats and dogs. Cats expressed low and moderate tumor grade levels, whereas dogs displayed significantly higher grades (Table I). No relationship was identified when comparing MMP2 and MMP9 levels with tumor grade values of feline and canine OS samples.
Discussion
In the present study, the distribution and expression levels of pro- and active MMP2 and MMP9 in canine and feline OS samples were determined and compared. It has previously been demonstrated that MMPs play a major role in OS tumor invasion and metastasis development (22,41–43). Although expression of MMP2 and MMP9 in canine OS has been studied before, no data have been reported on MMP expression in feline OS. Considering the significantly differing metastasis rates between the 2 species, we expected different levels of MMP2 and MMP9 in canine than in feline OS. In canine OS samples, we detected higher MMP9 immunostaining scores (IPS) than those for MMP2. Immunohistochemistry was applied to assess the distribution of MMP2 and MMP9 in tissue sections of feline and canine tumors, but the antibodies used cannot distinguish between active and pro-forms of these MMPs. Nonetheless, it is important to determine the activity levels of MMPs, as they represent the functional form of the enzyme, whereas the general expression of some MMPs is almost ubiquitous (43–45).
Gelatin zymography is the state-of-the-art technique to quantify MMP2 and MMP9 with a high specificity and sensitivity (19). As expected, we could detect MMP2 and MMP9 in their precursor and active forms in all OS samples. Significantly higher amounts of active MMP2 were detected in canine OS samples than in feline samples. One tumor sample (mixed feline OS, case no. 23) displayed an additional MMP2 form at the apparent molecular weight of 62 kDa. It has been previously reported that the detection of this low molecular weight form of active MMP2 is associated with high malignancy and metastasis development in canine OS tissue (31,41). In contrast, the feline case in our study was a grade I OS, which means that we cannot suggest a correlation with malignancy and metastasis activity.
The results discussed here are in line with previous reports for canine primary OS samples, particularly the generally low expression of active MMP9 (31). The question of whether both gelatinases are important in tumor progression and metastasis, or if only 1 is the main player, is controversial and still under discussion. It has been reported that high MMP2 expression is significantly associated with poor prognosis and lower survival time in human OS patients (46). Our experiments revealed that the canine OS samples showed a higher relative amount of active MMP2 than the feline samples, which might be related to the higher metastatic rate observed in dogs. This differing result might also be due to the generally lower OS tumor grade in felines, as Loukopoulus et al (31) reported a connection between the amount of MMP and tumor grade in canine OS. These researchers assumed that MMP9 does not play a major role in the primary tumor or established metastasis environment, as the active form of MMP9 was only present in 3 out of 76 canine tumor tissues examined. Unlike this previous study (31), we found active MMP9 in all OS samples examined, albeit at a minor expression level. While we did not find a significant difference in the incidence of active MMP9 between cats and dogs, MMP9 may also have a significant function in tumor malignancy, including OS. It has been reported that MMP9 is an important indicator of tumor progression in feline mammary tumors (47). The results of a meta-analysis report propagate MMP9 as an effective OS biomarker for predicting the survival chance in human patients affected by OS (48). Other studies reported that overexpression of MMP9 was associated with increased metastatic potential in rats (18). This study showed that MMP2 was not overexpressed in transplantable rat OS with high metastatic potential and that active MMP2 was not detectable in all cases and therefore concluded that MMP2 is not involved in the metastatic process (18). It has to be considered that these results are based on studies of different tumors and species, and substantial species-specific differences cannot be excluded.
Our experimental approach revealed significant differences between the canine and feline species regarding active MMP2, but not MMP9 in OS samples. It is therefore concluded that the striking differences in canine and feline OS tumor behavior might be associated with the expression and activity of MMP2. As the experiments were carried out on only a small number of feline OS cases, however, further investigations are needed to increase knowledge about the regulation of MMPs in OS, as well as their connection with clinical prognosis.
Acknowledgments
This study was funded by the Austrian Science Fund (FWF: P 23336-B11). The authors thank Claudia Höchsmann, Waltraud Tschulenk, and Alexander Tichy for their technical support and Jim Hutchins for editing the English manuscript.
Footnotes
The authors declare that they have no conflict of interests.
References
- 1.Misdorp W, Van der Heul RO. Tumours of bones and joints. Bull World Health Organ. 1976;53:265–282. [PMC free article] [PubMed] [Google Scholar]
- 2.Covey JL, Farese JP, Bacon NJ, et al. Stereotactic radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Vet Surg. 2014;43:174–181. doi: 10.1111/j.1532-950X.2014.12082.x. [DOI] [PubMed] [Google Scholar]
- 3.Selvarajah GT, Bonestroo FA, Kirpensteijn J, et al. Heat shock protein expression analysis in canine osteosarcoma reveals HSP60 as a potentially relevant therapeutic target. Cell Stress Chaperones. 2013;18:607–622. doi: 10.1007/s12192-013-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimopoulou M, Kirpensteijn J, Moens H, Kik M. Histologic prognosticators in feline osteosarcoma: A comparison with phenotypically similar canine osteosarcoma. Vet Surg. 2008;37:466–471. doi: 10.1111/j.1532-950X.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 5.Castro J, Santalucia S, Nazareth W, et al. Axial osteosarcoma in dog — Case report. J Vet Adv. 2013;3:29–33. [Google Scholar]
- 6.Kirpensteijn J, Kik M, Rutteman GR, Teske E. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol. 2002;39:240–246. doi: 10.1354/vp.39-2-240. [DOI] [PubMed] [Google Scholar]
- 7.Selvarajah GT, Kirpensteijn J. Prognostic and predictive biomarkers of canine osteosarcoma. Vet J. 2010;185:28–35. doi: 10.1016/j.tvjl.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Bitetto WV, Patnaik AK, Schrader SC, Mooney SC. Osteosarcoma in cats: 22 cases (1974–1984) J Am Vet Med Assoc. 1987;190:91–93. [PubMed] [Google Scholar]
- 9.Liu SK, Dorfman HD, Patnaik AK. Primary and secondary bone tumours in the cat. J Small Animal Pract. 1974;15:141–156. doi: 10.1111/j.1748-5827.1974.tb05671.x. [DOI] [PubMed] [Google Scholar]
- 10.Quigley PJ, Leedale AH. Tumors involving bone in the domestic cat: A review of fifty-eight cases. Vet Pathol. 1983;20:670–686. doi: 10.1177/030098588302000603. [DOI] [PubMed] [Google Scholar]
- 11.Broadhead ML, Clark JCM, Myers DE, Dass CR, Choong PFM. The molecular pathogenesis of osteosarcoma: A review. Sarcoma. 2011:12. doi: 10.1155/2011/959248. Article ID 959248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark JM, Dass C, Choong PM. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134:281–297. doi: 10.1007/s00432-007-0330-x. [DOI] [PubMed] [Google Scholar]
- 13.Clark IM. Methods in Molecular Biology. Totowa: Humana Press; 2001. [Google Scholar]
- 14.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 15.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 16.Nyman JS, Lynch CC, Perrien DS, et al. Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J Bone Miner Res. 2011;26:1252–1260. doi: 10.1002/jbmr.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Kido A, Tsutsumi M, Iki K, et al. Overexpression of matrix metalloproteinase (MMP)-9 correlates with metastatic potency of spontaneous and 4-hydroxyaminoquinoline 1-oxide (4-HAQO)-induced transplantable osteosarcomas in rats. Cancer Lett. 1999;137:209–216. doi: 10.1016/s0304-3835(98)00368-1. [DOI] [PubMed] [Google Scholar]
- 19.Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38:73–83. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- 20.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi C, Sheng Z, Horan TP, et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci. 1998;95:13221–13226. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjornland K, Flatmark K, Pettersen S, Aaasen AO, Fodstad O, Maelandsmo GM. Matrix metalloproteinases participate in osteosarcoma invasion. J Surg Res. 2005;127:151–156. doi: 10.1016/j.jss.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Stamenkovic I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 24.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Olson MW, Toth M, Gervasi DC, Sado Y, Ninomiya Y, Fridman R. High affinity binding of latent matrix metalloproteinase-9 to the α2 (IV) chain of collagen IV. J Biol Chem. 1998;273:10672–10681. doi: 10.1074/jbc.273.17.10672. [DOI] [PubMed] [Google Scholar]
- 26.Morodomi T, Ogata Y, Sasaguri Y, Morimatsu M, Nagase H. Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochem J. 1992;285:603–611. doi: 10.1042/bj2850603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seltzer JL, Eisen AZ, Bauer EA, Morris NP, Glanville RW, Burgeson RE. Cleavage of type VII collagen by interstitial collagenase and type IV collagenase (gelatinase) derived from human skin. J Biol Chem. 1989;264:3822–3826. [PubMed] [Google Scholar]
- 28.Welgus HG, Fliszar CJ, Seltzer JL, Schmid TM, Jeffrey JJ. Differential susceptibility of type X collagen to cleavage by two mammalian interstitial collagenases and 72-kDa type IV collagenase. J Biol Chem. 1990;265:13521–13527. [PubMed] [Google Scholar]
- 29.Senior RM, Griffin GL, Fliszar CJ, Shapiro SD, Goldberg GI, Welgus HG. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991;266:7870–7875. [PubMed] [Google Scholar]
- 30.Collier IE, Wilhelm SM, Eisen AZ, et al. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988;263:6579–6587. [PubMed] [Google Scholar]
- 31.Loukopoulos P, Mungall BA, Straw RC, Thornton JR, Robinson WF. Matrix metalloproteinase-2 and -9 involvement in canine tumors. Vet Pathol. 2003;40:382–394. doi: 10.1354/vp.40-4-382. [DOI] [PubMed] [Google Scholar]
- 32.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Himelstein BP, Asada N, Carlton MR, Collins MH. Matrix metalloproteinase-9 (MMP-9) expression in childhood osseous osteosarcoma. Med Pediatr Oncol. 1998;31:471–474. doi: 10.1002/(sici)1096-911x(199812)31:6<471::aid-mpo2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari C, Benassi M, Ponticelli F, et al. Role of MMP-9 and its tissue inhibitor TIMP-1 in human osteosarcoma: Findings in 42 patients followed for 1–16 years. Acta Orthop Scand. 2004;75:487–491. doi: 10.1080/00016470410001295-1. [DOI] [PubMed] [Google Scholar]
- 35.Kähäri V-M, Saarialho-Kere U. Trends in molecular medicine: Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31:3–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- 36.Kimura R, Ishikawa C, Rokkaku T, Janknecht R, Mori N. Phosphorylated c-Jun and Fra-1 induce matrix metalloproteinase-1 and thereby regulate invasion activity of 143B osteosarcoma cells. Biochim Biophys Acta (BBA) — Mol Cell Res. 2011;1813:1543–1553. doi: 10.1016/j.bbamcr.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Lana SE, Ogilvie GK, Hansen RA, Powers BE, Dernell WS, Withrow SJ. Identification of matrix metalloproteinases in canine neoplastic tissue. Am J Vet Res. 2000;61:111–114. doi: 10.2460/ajvr.2000.61.111. [DOI] [PubMed] [Google Scholar]
- 38.Tang N, Song W-X, Luo J, Haydon RC, He T-C. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beltran E, Matiasek K, De Risio L, de Stefani A, Feliu-Pascual AL, Matiasek LA. Expression of MMP-2 and MMP-9 in benign canine rostrotentorial meningiomas is not correlated to the extent of peritumoral edema. Vet Pathol. 2013;50:1091–1098. doi: 10.1177/0300985813481610. [DOI] [PubMed] [Google Scholar]
- 40.Walter I, Handler J, Miller I, Aurich C. Matrix metalloproteinase 2 (MMP-2) and tissue transglutaminase (TG 2) are expressed in periglandular fibrosis in horse mares with endometrosis. Histol Histopathol. 2005;20:1105–1113. doi: 10.14670/HH-20.1105. [DOI] [PubMed] [Google Scholar]
- 41.Loukopoulos P, O’Brien T, Ghoddusi M, Mungall BA, Robinson WF. Characterisation of three novel canine osteosarcoma cell lines producing high levels of matrix metalloproteinases. Res Vet Sci. 2004;77:131–141. doi: 10.1016/j.rvsc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Han J, Yong B, Luo C, Tan P, Peng T, Shen J. High serum alkaline phosphatase cooperating with MMP-9 predicts metastasis and poor prognosis in patients with primary osteosarcoma in Southern China. World J Surg Oncol. 2012;10:37. doi: 10.1186/1477-7819-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miya K, Misumi K, Miyoshi N, et al. Interpreting gelatinase activity in tumor tissue and serum as a prognostic marker of naturally developing canine tumors. J Vet Med Sci. 2005;67:769–775. doi: 10.1292/jvms.67.769. [DOI] [PubMed] [Google Scholar]
- 44.Shang HS, Chang JB, Lin JH, et al. Deguelin inhibits the migration and invasion of U-2 OS human osteosarcoma cells via the inhibition of matrix metalloproteinase-2/-9 in vitro. Molecules. 2014;19:16588–16608. doi: 10.3390/molecules191016588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang JJ, Emanuel BA, Mack WJ, Tsivgoulis G, Alexandrov AV. Matrix metalloproteinase-9: Dual role and temporal profile in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23:2498–2505. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Wen X, Liu H, Yu K, Liu Y. Matrix metalloproteinase 2 expression and survival of patients with osteosarcoma: A meta-analysis. Tumor Biol. 2014;35:845–848. doi: 10.1007/s13277-013-1116-1. [DOI] [PubMed] [Google Scholar]
- 47.Akkoc A, Inan S, Sonmez G. Matrix metalloproteinase (MMP-2 and MMP-9) and steroid receptor expressions in feline mammary tumors. Biotech Histochem. 2012;87:312–319. doi: 10.3109/10520295.2011.652173. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Zhang K, Liu LH, et al. A systematic review of matrix metalloproteinase 9 as a biomarker of survival in patients with osteosarcoma. Tumor Biol. 2014;35:1–5. doi: 10.1007/s13277-014-1717-3. [DOI] [PubMed] [Google Scholar]