Abstract
Feral pigeons (Columbia livia) can harbor a range of zoonotic pathogens. A transversal study was undertaken to estimate the prevalence of feral pigeons infected by various pathogens in public areas in Montreal, Quebec. Cloacal swabs from captured birds were cultured for Salmonella spp. and Campylobacter spp. and tested by real-time polymerase chain reaction (RT-PCR) for the detection of Coxiella burnetii. An oropharyngeal swab was also submitted to real-time reverse-transcription polymerase chain reaction (RRT-PCR) for the detection of Newcastle disease virus. Among the 187 pigeons tested from 10 public areas, 9.1% (95% CI: 3.0 to 15.2) were positive for Campylobacter spp. with all strains identified as Campylobacter jejuni. The Campylobacter status of birds was not associated with individual characteristics of birds, with the exception of body score. None of the pigeons tested positive for the other pathogens. Direct or indirect contacts with feral pigeons may constitute a potential risk for Campylobacter infection in humans.
Résumé
Les pigeons sauvages (Columbia livia) peuvent être porteurs d’une variété d’agents pathogènes zoonotiques. Une étude transversale a été réalisée dans le but d’estimer la prévalence de pigeons sauvages infectés par différents agents pathogènes dans des aires publiques de la ville de Montréal, Québec (Canada). Des écouvillons cloacaux d’oiseaux capturés ont été cultivés pour Salmonella spp. et Campylobacter spp. et testés par une réaction en chaîne par polymérase en temps réel (RT-PCR) pour la détection de Coxiella burnetii. Des écouvillons oropharyngés ont également été testés par une réaction en chaîne par polymérase en temps réel après transcription inverse (RRT-PCR) pour la détection du virus de la maladie de Newcastle. Parmi les 187 pigeons testés provenant de 10 aires publiques, 9,1 % (IC 95 % : 3,0–15,2) étaient positifs à Campylobacter spp.; toutes les souches ont été identifiées en tant que Campylobacter jejuni. L’infection par Campylobacter n’était pas associée aux caractéristiques individuelles des oiseaux, à l’exception de l’état de chair. Aucun pigeon n’était positif aux autres agents pathogènes. Le contact direct ou indirect avec des pigeons sauvages peut représenter un risque potentiel pour les infections à Campylobacter jejuni chez l’humain.
(Traduit par les auteurs)
Feral pigeons living in urban areas can harbor many pathogens that are infectious to humans and thus might pose a public health risk (1). This includes Campylobacter spp. and Salmonella spp., which are associated with severe acute gastroenteritis in humans, and Coxiella burnetii, the causal agent of “Q fever” in humans. Virulent strains of avian paramyxovirus type 1 (APMV-1), responsible for Newcastle disease, can also be found in pigeons mostly constituting a threat to domestic poultry flocks (2). However, in humans, infection due to this virus has been reported in laboratory or farm workers, causing a self-limiting acute conjunctivitis. In 2007, a lethal case of pneumonia was attributed to this infection in a human patient with an underlying medical condition (3). This study was conducted in the city of Montreal, Quebec, to estimate the prevalence of feral pigeons infected with Coxiella burnetii, Campylobacter spp., Salmonella spp., and Newcastle disease virus, and to describe pigeon characteristics associated with infection.
Public sites located in Montreal where pigeons usually gathered and were accessible for research purposes were visited. Sites were selected if they sheltered at least 10 pigeons at the time of the visit and were located at least 1 km (Euclidean distance) from other sampled sites. The target sample size was 20 pigeons per site, for a total of 10 sites. This was calculated to get a total sample of 200 pigeons, which enabled the detection of infection in at least 1 pigeon at a 95% confidence level assuming an overall prevalence of at least 1.5%. In the absence of prior information, no clustering effect by site of capture was assumed in the estimation of the sample size. Pigeons were baited with crushed corn and bread, and captured using a nylon net, a cage with a unidirectional door, or a single-catch closing net bird trap. Pigeons were kept in a cage for the time of data collection (≤ 1 h) and then released. They were tagged with a site-specific color leg ring. Age was determined by iris and cere color (4). The color morph was noted. Body score was estimated by palpating the protuberance and development of the breast muscles alongside the ventral ridge of the keel using a scoring system from 1 (very thin) to 5 (very fat). Pigeons were weighed and each metatarsus was measured in triplicate. Dehydration state was evaluated based on the presence of filaments while opening the beak. External lesions were noted. Two cloacal samples and 1 oropharyngeal sample were taken using sterile swabs. One of the cloacal swabs was placed in a collection and transport device (BBL™ CultureSwab™ Stuart Medium; Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA) for culture. The oropharyngeal swabs were transferred to 2.5 mL of viral transport media (GIBCO® Hank’s balanced salt solution with glycerol and antimicrobials; Thermo Fisher Scientific, Waltham, Massachusetts, USA). Swabs were sent on ice to the laboratory within 24 h of collection. All procedures were approved by the Ethics boards of the University of Montreal.
For Campylobacter detection, cloacal samples were plated on a Campylobacter CVA (cefoperazone, vancomycin, and amphotericin B) agar with 5% sheep blood (Bio-Media Unlimited, Woodbridge, Ontario) and incubated at 42 ± 1°C in a microaerobic atmosphere for 48 to 96 h. Curved Gram-negative rods on Gram stain were subcultured and identified using routine biochemical testing (5). For Salmonella, cloacal swabs were plated directly on brilliant green with novobiocin agar and on xylose-lysine-tergitol 4 agar (Bio-Media Unlimited) and incubated at 35 ± 1°C for 18 to 24 h. In addition, tetrathionate broth (Bio-Media Unlimited) was used as an enrichment broth, incubated at 42 ± 1°C for 20 to 24 h and subcultured on brilliant green with novobiocin agar and on xylose-lysine-tergitol 4 agar. Colonies resembling Salmonella spp. were submitted to additional standard biochemical tests (5). For Coxiella burnetii, a real-time polymerase chain reaction (RT-PCR) was done on cloacal samples as previously described (6). Oropharyngeal swabs were tested for Newcastle matrix gene using the detection of avian paramyxovirus type I (APMV-1) by Matrix and Fusion real-time reverse transcription polymerase chain reaction (RRT-PCR) assay [National Centre for Foreign Animal Disease (NCFAD) part of the Canadian Food Inspection Agency], based on the USDA-validated M-gene assay protocol (7). All samples yielding non-negative results were forwarded to NCFAD for confirmatory testing. The assay is capable of detecting APMV-1 (class II genotype 1, 2, 3, 4, and 5) subtypes. It does not detect wildlife class I genotype 6, APMV-2 to APMV-4 or APMV-7 types.
Prevalence of positive pigeons with 95% confidence intervals (95% CI) was estimated for each pathogen and adjusted for site clustering and sampling probabilities using computer software (SAS software, version 9.3; SAS Institute, Cary, North Carolina, USA). When all samples were negative for a given pathogen, the maximal possible prevalence in the population at a 95% CI was estimated using computer software (Winepiscope, version 2.0.; Epidecon, http://www.clive.ed.ac.uk/winepiscope). Individual characteristics of pigeons (Table I) associated with the infection status were only investigated for Campylobacter as it was the only pathogen detected. A multivariable logistic regression model with pigeon status (positive, negative) as the outcome was built (SAS, version 9.3; SAS Institute) using the Glimmix procedure with Laplace estimation, including characteristics of pigeons as fixed effects and the site as a random effect. Variables were selected using a backward procedure with a P > 0.05 (type 3 analysis, i.e., variable as a whole) as criterion for rejection; however, variables were kept in the model as potential confounders if their removal led to more than 30% change in the coefficient of other statistically significant variables in the model. The normality of residuals at the site level was evaluated visually. The Hosmer and Lemeshow goodness-of-fit test was also done on the final model (without the random effect).
Table I.
Characteristics of 187 pigeons captured in public sites in Montreal, Quebec, according to Campylobacter status, between June and July 2011
| Characteristics | Number of Campylobacter-positive pigeons/total number of pigeons (%) | |
|---|---|---|
| Age | ||
| Adult | 14/167 | (8.4) |
| In between | 2/14 | (14.3) |
| Juvenile | 1/6 | (16.7) |
| Color | ||
| Black | 2/29 | (6.9) |
| Blue-bar | 0/22 | (0.0) |
| Checker | 9/86 | (10.5) |
| Pied | 6/50 | (12.0) |
| Hydration status | ||
| Normal | 15/160 | (9.4) |
| Dehydration | 2/27 | (7.4) |
| Body score indexa,b | ||
| ≤ 1.5 | 7/64 | (10.9) |
| 2 | 3/78 | (3.9) |
| 2.5 | 3/33 | (9.1) |
| ≥ 3 | 4/12 | (33.3) |
| Body condition indexb,c | ||
| ≤ 8.3 | 8/46 | (17.4) |
| 8.4–9.2 | 5/47 | (10.6) |
| 9.3–10.1 | 2/47 | (4.3) |
| ≥ 10.2 | 2/47 | (4.3) |
| Metatarsus measure (mm)b | ||
| ≤ 32.8 | 3/44 | (6.8) |
| 32.9–34.3 | 6/53 | (11.3) |
| 34.3–35.4 | 4/46 | (8.7) |
| ≥ 35.5 | 4/44 | (9.1) |
| Presence of visible pathology | ||
| Absence | 15/152 | (9.9) |
| Active diseased | 1/11 | (9.1) |
| Chronic diseasee | 1/24 | (4.2) |
Using a scoring system from 1 (very thin) to 5 (very fat) based on palpation of the protuberance of the keel, development of the breast muscles immediately alongside the ventral ridge of the keel, and convexity or concavity of the breast muscle contour.
Categorization was done according to quartiles.
Estimated as the weight in grams divided by the average metatarsus measures.
Including ruffled/dirty feathers (n = 6), injury of the eye (n = 2), irritated skin with missing feathers (n = 2), injured wing (n = 1).
Including missing toes (n = 9), entanglement of toes with wire (n = 5), deformity of the wishbone (n = 3), induration on the leg (n = 3), crusted beak (n = 3), scar (hole) on the leg (n = 1).
A total of 187 feral pigeons were captured in 10 different public areas of Montreal between June 2nd 2011 and July 19th 2011. Characteristics of pigeons are presented in Tables I and II. The geographical distribution and characteristics of the 10 sampling sites is shown in Figure 1. Between 25 and 50 pigeons per site were observed at the time of selection, with an average of 39. None of the pigeons initially captured in one specific site were observed or recaptured in another site.
Table II.
Descriptive statisticsa of morphometric measures of 187 pigeons captured in public sites in Montreal, Quebec, between June and July 2011
| Mean | Median | Standard deviation | Minimum | Maximum | |
|---|---|---|---|---|---|
| Body weight (g) | 312 | 313 | 50 | 142 | 455 |
| Tarsus length (mm) | 34 | 34 | 2.0 | 29 | 41 |
| Body condition index (g/mm) | 9.1 | 9.2 | 1.4 | 4.2 | 12.3 |
Data were normally distributed according to visual assessment apart from a slight skew to the right for the tarsus length and a slight skew to the left for the tarsus length and body condition index.
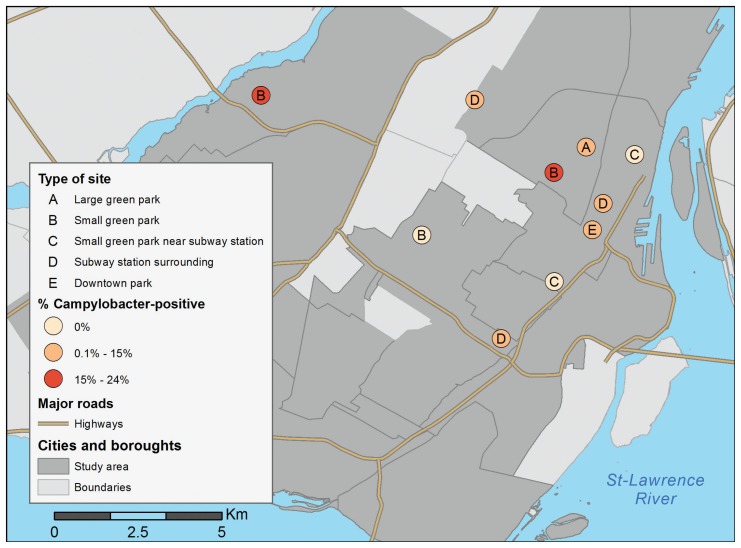
Figure 1.
Geographical distribution of sites according to the proportion of Campylobacter jejuni positive birds by sites, Montreal, Quebec, between June and July 2011.
Campylobacter spp. was isolated from 17 out of the 187 pigeons tested, with all isolates identified as Campylobacter jejuni. This gives a prevalence estimate of Campylobacter-positive pigeons of 9.1% (95% CI: 3.0 to 15.2). At least one pigeon that tested positive for Campylobacter spp. was found in 7 of the 10 sites. The prevalence in positive sites ranged from 5% to 24% (average of 13%). Only the body score was statistically significantly associated with Campylobacter status (P = 0.03). Post-hoc analysis with Bonferroni adjustment showed that pigeons with body scores ≥ 3 were more at risk of being Campylobacter positive compared to pigeons with body score of 2 [odds ratio (OR) = 12.5 (95% CI with Bonferroni adjustment: 1.3 to 125), P = 0.02]. No evidence of confounding or collineraity was observed during model building based on observation of coefficient estimates and standard errors. Clustering at the site level was negligible (covariance parameter estimate = 0.03 with standard error = 0.25). The model adequately fitted the data based on visual inspection of residuals and Hosmer and Lemeshow test (P = 1.00). None of the 187 pigeons was positive for Salmonella spp., Coxiella burnetii, or APMV-1. This gives a maximal possible prevalence in the population of 1.6% at a 95% confidence level for those 3 pathogens.
Evidence of natural infection for Salmonella spp., Coxiella burnetii, and Newcastle disease virus in feral pigeons has been previously reported (8–10). The absence of Salmonella spp. or Coxiella burnetii could be explained by the fact that the sampled sites were not near farms, which have been considered a likely source of these infections in pigeons (9,11). In Canada, Newcastle disease virus infection was previously diagnosed in feral pigeons from Ontario (12). Clinical signs compatible with this infection were not observed in the study; however, not all strains of the virus are virulent for pigeons. Most of the previous reports of avian paramyxovirus infection in pigeons involved the type 1 (i.e., Newcastle disease virus). Type 7, which is apathogenic for Columbidae, has also been reported (13) but was not tested in this study.
Campylobacter jejuni was detected in 9.1% of pigeons. A potential source of contamination could be the consumption of different types of food, including sealed garbage bags, as previously reported in pigeons in Montreal (11,14). Pigeons could also be contaminated through droppings of other wild bird species. In fact, within many bird species, apparently healthy individuals are frequent carriers of Campylobacter spp, including ring-billed gulls (Larus delawarensis) (15,16). Environmental water, as well as cat and dog feces, could also represent a source of infection (17). In Montreal, pigeons were reported to limit their movements within a small area, with very little mixing between groups (18). This is congruent with our observation that none of the pigeons initially captured was observed or recaptured in another site, and suggestive of a local source of bird contamination with Campylobacter spp. As a study limitation, our prevalence estimate might not be valid for other periods of the year, as seasonal variations in the prevalence of Campylobacter carriage in urban wild birds, including pigeons, were reported in other countries (19,20).
The age and morphological characteristics of individual pigeons was not significantly associated with the Campylobacter jejuni status, as previously reported (19). However, pigeons with higher body score index (≥ 3) were more at risk of infection. This association might be driven by healthier or dominant pigeons having access to a larger variety of food sources. As well, Campylobacter jejuni infection was not associated with any indicator of poor health status, substantiating the hypothesis that pigeons are asymptomatic reservoirs of the bacteria (19).
Presence of Campylobacter jejuni in urban pigeons constitutes a potential risk for human health, as environmental exposure to bird droppings is a consistent risk factor for human campylobacteriosis (21). Many sites investigated were occupied by homeless people and some of them were feeding pigeons with their hands. They may constitute a high-risk population due to poor health status, lower access to hand-washing facilities, and higher rates of infection by immunosuppressive diseases (22). Children playing outside are also particularly at risk of such environmental transmission. In conclusion, direct or indirect exposure to feral pigeons should be considered as a potential source for campylobacteriosis in urban areas.
Acknowledgments
The authors acknowledge the city of Montreal and participating boroughs for giving permission to conduct the study in their public areas. We also acknowledge Marie-Ève Turcotte, Jonathan Cyr, Ivany Rodrigues de Moraes, Ariane Santamaria-Bouvier, and Jacques Dancoste for their contributions. This study was funded by the “Fonds vert” of the Ministery of Health and Social Affairs of Quebec in the context of the Action 21 of the Action plan 2006–2012 on climate changes (PACC). Appreciation is extended to the Science Branch, CFIA, National Centre for Foreign Animal Disease for support concerning the detection of APMV-1 by Matrix and Fusion Real-Time RT-PCR Assay, and to the “Laboratoire d’épidémiosurverillance animale du Québec” technicians for their assistance in this project.
References
- 1.Haag-Wackernagel D, Moch H. Health hazards posed by feral pigeons. J Infect. 2004;48:307–313. doi: 10.1016/j.jinf.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Dortmans JC, Koch G, Rottier PJ, Peeters BP. A comparative infection study of pigeon and avian paramyxovirus type 1 viruses in pigeons: Evaluation of clinical signs, virus shedding and seroconversion. Avian Pathol. 2011;40:125–130. doi: 10.1080/03079457.2010.542131. [DOI] [PubMed] [Google Scholar]
- 3.Goebel SJ, Taylor J, Barr BC, et al. Isolation of avian paramyxovirus 1 from a patient with a lethal case of pneumonia. J Virol. 2007;81:12709–12714. doi: 10.1128/JVI.01406-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kautz JE, Seamans TW. Estimating age of nestling and juvenile feral rock doves. J Wildl Manage. 1986;50:544–547. [Google Scholar]
- 5.Quinn PJ, Carter ME, Markey B, Carter GR, editors. Clinical Veterinary Microbiology. London, England: Wolfe Publishing-Mosby; 1994. p. 648. [Google Scholar]
- 6.Klee SR, Tyczka J, Ellerbrok H, et al. Highly sensitive real-time PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiol. 2006;6:2. doi: 10.1186/1471-2180-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise MG, Suarez DL, Seal BS, et al. Development of a real-time reverse-transcription PCR for detection of newcastle disease virus RNA in clinical samples. J Clin Microbiol. 2004;42:329–338. doi: 10.1128/JCM.42.1.329-338.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillehaug A, Jonassen CM, Bergsjo B, et al. Screening of feral pigeon (Colombia livia), mallard (Anas platyrhynchos) and graylag goose (Anser anser) populations for Campylobacter spp., Salmonella spp., avian influenza virus and avian paramyxovirus. Acta Vet Scand. 2005;46:193–202. doi: 10.1186/1751-0147-46-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.To H, Sakai R, Shirota K, et al. Coxiellosis in domestic and wild birds from Japan. J Wildl Dis. 1998;34:310–316. doi: 10.7589/0090-3558-34.2.310. [DOI] [PubMed] [Google Scholar]
- 10.Dovc A, Zorman-Rojs O, Rataj AV, Bole-Hribovsek V, Krape U, Dobeic M. Health status of free-living pigeons (Columba livia domestica) in the city of Ljubljana. Acta Vet Hung. 2004;52:219–226. doi: 10.1556/AVet.52.2004.2.10. [DOI] [PubMed] [Google Scholar]
- 11.Kapperud G, Rosef O. Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp., and Salmonella spp. in Norway. Appl Environ Microbiol. 1983;45:375–380. doi: 10.1128/aem.45.2.375-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston KM, Key DW. Paramyxovirus-1 in feral pigeons (Columba livia) in Ontario. Can J Vet. 1992;33:796–800. [PMC free article] [PubMed] [Google Scholar]
- 13.Marlier D, Vindevogel H. Viral infections in pigeons. Vet J. 2006;172:40–51. doi: 10.1016/j.tvjl.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Palameta B, Lefebvre L. The social transmission of a food-finding technique in pigeons Columba livia — What is learned? Anim Behav. 1985;33:892–896. [Google Scholar]
- 15.Waldenstrom J, Broman T, Carlsson I, et al. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl Environ Microbiol. 2002;68:5911–5917. doi: 10.1128/AEM.68.12.5911-5917.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quessy S, Messier S. Prevalence of Salmonella spp., Campylobacter spp. and Listeria spp. in ring-billed gulls (Larus delawarensis) J Wildl Dis. 1992;28:526–531. doi: 10.7589/0090-3558-28.4.526. [DOI] [PubMed] [Google Scholar]
- 17.Whiley H, van den Akker B, Giglio S, Bentham R. The role of environmental reservoirs in human campylobacteriosis. Inter J Environ Res Public Health. 2013;10:5886–5907. doi: 10.3390/ijerph10115886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levesque H, McNeil R. Abundance and activities of the rock dove Columba livia in the port of Montreal, Quebec, Canada. Can Field Nat. 1985;99:343–355. [Google Scholar]
- 19.Vazquez B, Esperon F, Neves E, Lopez J, Ballesteros C, Munoz MJ. Screening for several potential pathogens in feral pigeons (Columba livia) in Madrid. Acta Vet Scand. 2010;52:45. doi: 10.1186/1751-0147-52-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan V. Faeco-prevalence of Campylobacter jejuni in urban wild birds and pets in New Zealand. BMC research notes. 2015;8:1. doi: 10.1186/1756-0500-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domingues AR, Pires SM, Halasa T, Hald T. Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol Infect. 2012:1–12. doi: 10.1017/S0950268811002676. [DOI] [PubMed] [Google Scholar]
- 22.Badiaga S, Raoult D, Brouqui P. Preventing and controlling emerging and reemerging transmissible diseases in the homeless. Emerg Infect Dis. 14:1353–1359. doi: 10.3201/eid1409.082042. [DOI] [PMC free article] [PubMed] [Google Scholar]