Abstract
Growth differentiation factor 11 (GDF11) regulates cell growth and differentiation in both embryonic and adult tissues. Circulating GDF11 levels have recently been reported to be significantly lower in aging mice and restoration of GDF11 reversed age-related cardiac hypertrophy in old mice. Here, we evaluated the potential of serum levels of GDF11 as a circulating biomarker in dogs at different stages of heart failure, due to chronic mitral valve insufficiency (CMVI). We found no significant differences in serum GDF11 levels between dogs at different stages of CMVI-associated heart failure. Furthermore, the circulating levels of GDF11 did not correlate with age, body weight, echocardiographic variables, and the severity of CMVI-induced heart failure in dogs.
Résumé
Le facteur de différenciation de croissance 11 (GDF 11) régule la croissance cellulaire et la différenciation dans les tissus embryonnaires et adultes. Les quantités de GDF 11 circulant ont récemment été rapportées comme étant significativement plus faibles chez les souris vieillissantes et un rétablissement de GDF 11 renverse l’hypertrophie cardiaque reliée à l’âge chez les vieilles souris. Nous avons évalué le potentiel des quantités sériques de GDF 11 comme un biomarqueur circulant chez les chiens à différents stades de défaillance cardiaque due à une insuffisance mitrale chronique (IMC). Nous n’avons pas trouvé de différence significative dans les quantités de GDF 11 sériques entre les chiens à différents stades de défaillance cardiaque associée à l’IMC. De plus, les quantités de GDF 11 en circulation ne corrélaient pas avec l’âge, le poids corporel, les variables échographiques, et la sévérité de la défaillance cardiaque induite par l’IMC chez les chiens.
(Traduit par Docteur Serge Messier)
Growth differentiation factor 11 (GDF11), also known as bone morphogenetic protein 11 (BMP-11), is a member of the transforming growth factor beta superfamily known to regulate cell growth and differentiation in both embryonic and adult tissues (1). A recent study that used parabiosis procedures between young and old mice reported that restoration of GDF11 in older mice reversed age-related cardiac hypertrophy through regeneration of cardiomyocytes and reduction of brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) (2). The study also found a significant correlation between GDF11 and improved myocardial diastolic function, including SERCA-2 upregulation, an enzyme necessary for myocardial relaxation (2).
Chronic mitral valve insufficiency (CMVI) is the most common cardiac disease in dogs, characterized by chronic myxomatous mitral valvular degeneration resulting in mitral regurgitation (3). Chronic mitral valve insufficiency has been reported in ~ 50% Cavalier King Charles spaniel dogs at 6- to 7-years-old and ~ 50% Dachshund dogs at 10-years-old (4). Although many studies found the genetic etiology of CMVI, aging-related factors are the major cause of disease development and progression (5). The deteriorative regurgitation of CMVI frequently causes volume overload in both the left atrium (LA) and ventricle (LV), which in turn, causes pressure overload in the LA mainly due to increased end diastolic volume in the LV. Therefore, myocardial hypertrophy by LA pressure and volume overload, are the classic features of CMVI-induced heart failure (6).
In this study, we hypothesized that circulating GDF11 levels may be lower in dogs with advanced heart failure from CMVI, contributing to disease progression. In this case, circulating GDF11 could be a good prognostic biomarker during cardiac disease progression in dogs with CMVI. To test our hypothesis, we evaluated serum levels of GDF11 in dogs with CMVI, in order to assess and delineate the correlation of circulating GDF11 with age, body weight, echocardiographic variables, and the severity of heart failure.
Prior to the study, we obtained the approval of the animal ethics committee of Kangwon National University to obtain blood samples from clinically normal dogs for serum GDF11 assays. Informed written consents for sample collection, including information pertinent to our investigation, were obtained from dog owners prior to the commencement of our study. The animal testing programs, including animal care, euthanasia, and disposal of dead animals, were done in strict adherence to the guidelines of the National Research Council of Korea. Our study population consisted of 2 groups: clinically normal control and CMVI. The control group consisted of 11 clinically normal, small-breed dogs (2.7 to 12.0 kg), between 7 and 14 y of age without any evidence of cardiovascular disease and/or other systemic diseases on physical examination, as well as complete blood (cell) count (CBC), chemistry panel, chest radiography, and echocardiography.
The CMVI age-matched (Table I) group included 69 small breed dogs (2.4 to 11.2 kg) aged between 7 and 17 y. All dogs with CMVI had been presented for a cardiology consultation with a heart murmur, followed by clinical signs of cardiovascular disorders including cough and/or exercise intolerance. All dogs underwent physical examination, CBC, chemistry panel, chest radiography, and echocardiography, as was done for the control group. We excluded dogs that had any other clinically relevant systemic diseases, such as renal failure or hypertension. The diagnosis of CMVI was made on the basis of clinical signs (i.e., heart murmur, coughing, ascites, and exercise intolerance), chest radiography, and echocardiographic findings, according to the canine CMVI diagnostic guidelines (3). The dogs with CMVI were also subgrouped according to the criteria proposed by the International Small Animal Cardiac Health Council (ISACHC) for the functional classification of heart disease (7). Some dogs with symptomatic CMVI were being medicated for heart disease, depending on its severity. Prescribed drugs include enalapril, furosemide, spironolactone, pimobendan, digoxin, and amlodipine.
Table I.
Serum growth differentiation factor 11 (GDF11) concentrations and demographic characteristics of the study population
| ISACHCa | ||||
|---|---|---|---|---|
|
|
||||
| Control (n = 11) | I (n = 17) | II (n = 29) | III (n = 20) | |
| Age | ||||
| Median age (y) | 8.4 | 11.6 | 11.6 | 11.8 |
| Age range (y) | 4.3 to 11.6 | 7 to 15 | 5.7 to 16 | 7.9 to 16 |
| Mean ± SD | 8.02 ± 2.32 | 10.99 ± 2.29 | 11.31 ± 2.46 | 11.36 ± 2.30 |
| Gender | ||||
| Male (% CM) | 4 (100%) | 8 (75%) | 13 (85%) | 13 (92%) |
| Female (% SF) | 7 (86%) | 9 (78%) | 16 (75%) | 7 (57%) |
| Body weight (kg) | 4.70 ± 2.69 | 4.44 ± 1.75 | 5.21 ± 2.54 | 4.54 ± 1.25 |
| VHS | 9.77 ± 0.37 | 10.18 ± 0.53 | 11.01 ± 0.95 | 11.89 ± 1.13 |
| Transmitral E-peak | 0.66 ± 0.17 | 0.74 ± 0.18 | 0.92 ± 0.24 | 1.37 ± 0.43 |
| LA:Ao | 1.15 ± 0.09 | 1.46 ± 0.18 | 1.79 ± 0.39 | 2.45 ± 0.67 |
| LVIDd:Ao | 1.43 ± 0.14 | 1.81 ± 0.48 | 2.00 ± 0.43 | 2.78 ± 0.65 |
| sBUN (mg/dL) | 17.27 ± 6.75 | 21.46 ± 11.19 | 30.75 ± 20.47 | 36.08 ± 25.75 |
| sCreatinine (mg/dL) | 0.67 ± 0.22 | 0.78 ± 0.32 | 0.96 ± 0.46 | 1.19 ± 0.55 |
| sGDF11 (pg/mL) | 111.6 ± 135.0 | 93.8 ± 131.6 | 92.1 ± 190.4 | 98.8 ± 127.8 |
Data were represented as mean ± standard deviation.
ISACHC — the International Small Animal Cardiac Heart Council; CM — castrated male; SF — spayed female; VHS — vertebral heart scale; LA:Ao — left atrial to aorta ratio; LVID:Ao — left ventricular internal dimension at diastole to aorta ratio; sBUN — serum blood urea; sCreatinine — serum creatinine; GDF11 — Growth differentiation factor 11.
All dogs were fasted for 12 h before the collection of blood samples, which were drawn from either the cephalic or jugular veins to determine serum levels of GDF11. Blood samples were drawn directly into sterile vacutainer tubes (BD Vacutainer; Becton, Dickinson and Company, New Jersey, USA) and then centrifuged at 1500 × g for 10 min at 4°C. The supernatants were stored at −80°C. The serum GDF11 levels were measured according to the manufacturer’s instructions using a commercial ELISA based kit (Canine Growth Differentiation Factor 11 ELISA Kit; MyBioSource, San Diego, California, USA). The GDF11 ELISA kit applies the competitive enzyme immunoassay technique utilizing a monoclonal anti-GDF11 antibody and a GDF11-HRP conjugate. For validation of the assay using canine samples, serum from 1 of the control dogs was spiked into the same amount of controls with increasing concentrations of GDF11. Linearity and parallelism were assessed using spiked canine samples and manufacturer’s controls for 5 different concentrations. Recovery was assessed as the percentage of recovered GDF11-spiked canine samples versus expected GDF11 at 6 different concentrations. All samples were run in duplicate, and the mean values were used for further statistics.
Echocardiographic examinations were conducted in accordance with recommended standards for dogs. M-mode, Doppler, and 2-dimensional (2-D) echocardiography (X300; Simens, Germany) were done in left and right lateral recumbency. M-mode echocardiography was used to measure left ventricular internal dimension at systole (LVIDs) and diastole (LVIDd). 2-dimensional echocardiography was used to measure LA and proximal aortic (Ao) diameter from the right parasternal short axis at the aortic valve level. Pulsed-wave Doppler echocardiography was used to measure transmitral E-peak velocity from the left parasternal apical 4 chamber plane at the tips of the mitral valve when open. These measurements were used to determine the LA to proximal Ao diameter (LA/Ao) ratio and LVIDd to Ao diameter (LVIDd/Ao) ratio.
Statistical analyses were done using commercially available statistical software (SPSS Statistics 21; IBM, New York, New York, USA). Descriptive statistics were calculated for quantitative variables by study group and analyzed for normality using the Kolmogorov-Smirnov test. Differences in serum GDF11 concentrations among groups were evaluated using Kruskal-Wallis one way analysis of variance (ANOVA). Pearson’s coefficient of bivariate correlation analysis was used to test the strength of correlation between serum GDF11 levels and disease severity based on echocardiographic indices, age, and body weight. A probability value of P < 0.05 was considered statistically significant, unless stated otherwise.
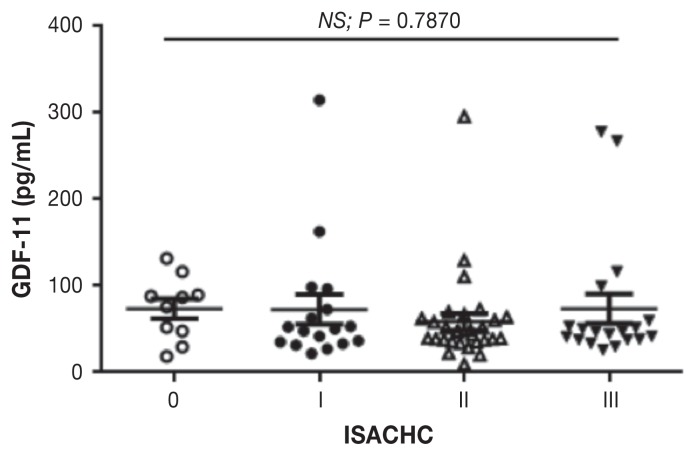
The mean age, body weight, gender, and breed of each group are summarized in Table I. The median serum GDF11 concentration in this study population was 111.6 ± 135.0 pg/mL (mean ± standard deviation; range: 27.9 to 438.1) in control, 93.8 ± 131.6 pg/mL (range: 21.8 to 612.3) in ISACHC I, 92.1 ± 190.4 pg/mL (range: 9.5 to 1052.0) in ISACHC II, and 98.8 ± 127.8 pg/mL (range: 20.1 to 550.1) in ISACHC III group. The median serum GDF11 concentration between controls and dogs with CMVI were not significantly different (Figure 1). No statistically significant difference was found between dogs with CMVI and different stages of heart failure (P > 0.05; Figure 1). Serum GDF11 levels did not correlate with age (r = 0.1011) and body weight (r = 0.0411) using univariate analysis. No significant correlation was observed between serum GDF11 and serum creatinine (r = 0.0204), blood urea (r = 0.0398), transmitral E-peak (r = 0.0188), LA to Ao (r = 0.001), LVIDd to Ao (r = 0.1954), and vertebral heart scale (r = 0.0538).
Figure 1.
Serum growth differentiation factor 11 (GDF11) concentrations in this study population. ISACHC — International Small Animal Cardiac Health Council
The aging process is associated with loss of tissue function through disrupted homeostatic and regenerative mechanisms (3,5). Among many diseases and disorders associated with advanced age, one of the most debilitating causes is the loss of normal cardiac function leading to heart failure as seen in CMVI in dogs. The regenerative decline of many tissues observed during aging is thought to be due to stem cell dysfunction, partly regulated by changes in the composition of blood-borne factors present in the vascular system (8). A recent study found that the circulatory level of GDF11 in young mice was high and declined with age (2). Using parabiosis procedures between young and old mice, the authors found reversal of age-related cardiomyopathy through youthful regeneration of cardiomyocytes and reduction of natriuretic peptides, providing evidence that GDF11 is involved in age-related cardiac degeneration. However, no study has evaluated the level of circulating GDF11 in dogs with age-related cardiac degeneration. Since CMVI is one of the most common age-related diseases in dogs, our cases of canine CMVI were appropriate to evaluate the potential of GDF11 as a circulating biomarker. Our findings were in stark contrast to what we expected. The levels of serum GDF11 in dogs with CMVI were not significantly different to the levels of age-matched healthy control dogs. In addition, the levels of circulating GDF11 did not indicate the severity of CMVI-associated heart failure. Furthermore, the levels of GDF11 had no correlation with age, body weight, renal function, and echocardiographic variables.
There were several limitations to this study. First, the study population was small and may not have provided sufficient statistical power to adequately reflect the correlation of the levels of GDF11 to the severity of heart failure in dogs with CMVI. The healthy control dogs enrolled in this study may have had subclinical degenerative diseases, which might not be efficiently detected by routine diagnostic examination. The potential limitations could be minimized by a bigger population size. Second, the influence of age on the level of GDF11 should be re-assessed in different age groups of dogs, because the level of GDF11 was only assessed in an aged population in the current study. Lastly, our findings may also imply a difference in cardiac pathophysiology between dogs and mice. Nevertheless, this is the first study to evaluate the serum levels of GDF11 as a circulating biomarker in dogs with age-related clinical diseases.
Acknowledgments
This study was supported by Kangwon National University.
References
- 1.Ge G, Hopkins DR, Ho WB, Greenspan DS. GDF11 forms a bone morphogenetic protein 1-activated latent complex that can modulate nerve growth factor-induced differentiation of PC12 cells. Mol Cell Biol. 2005;25:5846–5858. doi: 10.1128/MCB.25.14.5846-5858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Int Med. 2009;23:1142–1150. doi: 10.1111/j.1939-1676.2009.0392.x. [DOI] [PubMed] [Google Scholar]
- 4.Häggström J, Hansson K, Kvart C, Swenson L. Chronic valvular disease in the cavalier King Charles spaniel in Sweden. Vet Rec. 1992;131:549–553. [PubMed] [Google Scholar]
- 5.Buchanan JW. Chronic valvular disease (endocardiosis) in dogs. Adv Vet Sci Comp Med. 1977;21:75–106. [PubMed] [Google Scholar]
- 6.Kihara Y, Sasayama S, Miyazaki S, et al. Role of the left atrium in adaptation of the heart to chronic mitral regurgitation in conscious dogs. Circ Res. 1988;62:543–553. doi: 10.1161/01.res.62.3.543. [DOI] [PubMed] [Google Scholar]
- 7.International Small Animal Cardiac Health Council. Recommendations for the diagnosis and treatment of heart failure in small animals. Woodbridge, New Jersey: ISACHC Publication; 1994. p. 5. [Google Scholar]
- 8.Brack AS. Ageing of the heart reversed by youthful systemic factors! EMBO J. 2013;32:2189–2190. doi: 10.1038/emboj.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]