Abstract
Oxidative stress induced by ischemia and hypoxia in the livers of donors after brain death (DBD) is associated with poor organ function and low patient survival rates in those receiving DBD liver transplants. Peroxiredoxin 6 (Prdx6) can defend cells against liver damage induced by oxidative stress. The present study aimed to investigate the role of Prdx6 in ischemia- and hypoxia-induced liver damage in DBD livers. Liver tissue samples from ten DBD patients were collected. The control group constituted of six liver samples from patients with liver hemangioma that had accepted tumor excision surgery. Protein expression levels were determined by western blotting, cell viability was assessed using a CCK-8 assay, intracellular reactive oxygen species (ROS) levels were measured using a ROS assay kit, and phospholipase A2 (PLA2) activity was measured using a PLA2 assay kit. In DBD liver samples, Prdx6 expression was downregulated and the nuclear factor-κB (NF-κB) signaling pathway was activated. Furthermore, when human liver L02 cells were exposed to ischemia and hypoxia, the expression of Prdx6 was reduced, causing an increase in reactive oxygen species (ROS); this in turn activated NF-κB signaling and lowered cell viability (P<0.01). In agreement, overexpression of Prdx6 reduced ROS levels and improved cell viability. It was also demonstrated that inhibition of NF-κB increased Prdx6 expression, while inhibition of Prdx6 limited PLA2 activity, exacerbating ischemia- and hypoxia-induced cell damage. This data suggests that Prdx6 and its PLA2 activity have a protective role in DBD livers, the expression of which is regulated by NF-κB. Thus, Prdx6 may be a novel target to alleviate liver damage in DBD.
Keywords: peroxiredoxin 6, donors after brain death, liver transplant, oxidative stress, nuclear factor-κB, phospholipase A2
Introduction
Liver transplantation is an effective treatment strategy for a number of end-stage liver diseases including liver failure, alcohol liver disease and liver cancer (1–3). Due to the shortage of liver donors, transplantation from donors after brain death (DBD) has increased. From 2010 to 2014, there were 2,897 cases of voluntary post-mortem donations in China (4), the majority of which were DBD. However, compared with liver transplantation from a living donor, the DBD liver transplant has more grafting complications, and acute rejections of organs from DBD occur at a significantly higher rate compared with the organs of living donors 6–24 months following transplantation (5). The primary causes of this are: Massive catecholamine release leading to oxidative stress (6), release of inflammatory mediators leading to systemic inflammatory response (7,8) and pro-apoptotic caspase activation (9,10). Brain death is independent of hemodynamic stability, however, liver macro- and microcirculation decreases, causing hepatic oxidative stress to increase and liver cells to become ischemic-hypoxic (11). Oxidative stress has a major role in organ transplant complications (12); however, the mechanism that induces liver damage during brain death is not fully understood.
Peroxiredoxins are a widely distributed superfamily of peroxidases that can eliminate reactive oxygen species (ROS) (13). According to the number of redox-active cysteines, peroxiredoxins can be classified as 1-Cys or 2-Cys peroxiredoxin (Prdx). Prdx6 is a unique mammalian member of the 1-Cys Prdx family (14). Prdx6 is a bifunctional protein that exhibits peroxidase, as well as phospholipase A2 (PLA2), activities (14). Prdx6 is expressed in the liver (15) and protects cells from damage by ROS induced by ischemia-reperfusion injury (16). By contrast, PLA2 activity is important in the phospholipids of lung surfactant metabolism (17), and is required for optimal nicotinamide adenine dinucleotide phosphate-oxidase activity (18). A recent study highlighted that PLA2 activity has a substantial role in the protection of cells against oxidative stress (19). Prdx6 is regulated via a number of signaling pathways by nuclear factor-κB (NF-κB) (20), nuclear factor erythroid 2-related factor 2 (21) and Jun N-terminal kinase (22). However, to the best of our knowledge, the function and mechanism of Prdx6 in liver damage after brain death has not been reported in any previous studies.
Therefore, we propose that Prdx6 may attenuate ischemia and hypoxia-induced liver damage of DBD. In the present study, the effects of Prdx6 on liver damage were investigated in vivo and in vitro, and the underlying mechanisms were explored. The present study determined that the expression of Prdx6 and inhibitor of κB-α (IκB-α) proteins are decreased in DBD liver tissue. When normal liver cells (L02 cells) were exposed to ischemia and hypoxia to mimic the conditions of brain death, cell damage increased with exposure time. In addition, overexpression of Prdx6 partially attenuated cell damage and the expression of Prdx6 appeared to be regulated by the NF-κB signaling pathway. Furthermore, Prdx6 PLA2 activity has been revealed to be involved in protecting cells against oxidative stress induced by ischemia and hypoxia. Therefore, in agreement with our hypothesis, it is considered that Prdx6 can attenuate liver damage after brain death. These results provide valuable information to improve the quality of donor livers and thus increase transplant success from DBD livers.
Materials and methods
Cell culture and treatment
Normal human liver L02 cells were obtained from Kunming Institute of Zoology (Chinese Academy of Sciences, Kunming, China). The cells were grown to 70–80% confluence in 6-well culture plates in HyClone™ Dulbecco's modified Eagle medium (DMEM; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Beyotime Institute of Biotechnology, Nantong, China).
Liver damage following brain death was mimicked in L02 cells by means of serum deprivation and hypoxia for 6, 12, 18 and 24 h. In order to simulate the ischemic and hypoxic environment, L02 cells were cultured in HyClone serum-free DMEM/low glucose medium (GE Healthcare Life Sciences), and exposed to a gaseous mixture of 95% N2 and 5% CO2 (0% O2) in a 37°C humidified incubator (Thermo Fisher Scientific Inc.) for different time periods (0, 6, 12, 18, 24 h), as previously described (23).
For the transfection experiment, L02 cells were divided into three groups: A WT group (L02 cells cultured without transfection), a vector group [L02 cells transfected with pIRES2-ZsGreen1-vector plasmids (Wuhan Sanying Biotechnology, Inc., Wuhan, China)], and a Prdx6(+) group (L02 cells transfected with pIRES2-ZsGreen1-Prdx6 plasmids). L02 cells were transfected with pIRES2-ZsGreen1-Prdx6 plasmids using Lipofectamine 2000, according to manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc.), to over-express Prdx6. The cells were subjected to serum deprivation and hypoxia for 12 h, then harvested for further biochemical analyses 48 h after transfection. Transfection efficiency was evaluated by green fluorescence levels as determined by fluorescence microscopy, as well as protein expression determined western blotting.
The activity of NF-κB was inhibited by pretreating the cells with BAY11-7082 (5 µM; Santa Cruz Biotechnology Inc., Dallas, TX, USA) for 1 h, prior to being exposed to the ischemic-hypoxic conditions. The cells were then lysed using lysis buffer (1 M Tris-HCl, pH 8.0, 5 M NaCl, and 1% NP-40) and harvested for western blotting and CCK-8 assay.
To determine the protective effect of Prdx6 PLA2 against ischemia- and hypoxia-induced oxidative stress, L02 cells (1×104) were treated with 0, 10, 20, 30 and 50 µM MJ33, a PLA2 inhibitor. One group of cells was cultured with MJ33 for 0.5 h, then MJ33 was removed and the cells were cultured in normal or ischemic-hypoxic conditions for 12 h (MJ33+0.5 h group). The other group of cells was directly cultured with MJ33 in normal or ischemic-hypoxic conditions for 12 h (MJ33+12 h group). Determination of hepatocellular injury marker concentrations and cell viability were then performed.
Clinical DBD samples, treatment and ethical considerations
Liver tissue samples were collected from ten patients with DBD and six patients that had accepted liver hemangioma surgery, between February 2013 and December 2013 at the Zhongnan Hospital of Wuhan University, Wuhan, China. The present study was approved by the Ethics Committee of the Zhongnan Hospital of Wuhan University and informed consent was obtained from all patients prior to commencement of the study. Although the tissue used for the control group was harvested from patients with liver hemangioma it did not contain any cancerous tissue.
Western blot analysis
Cell lysate and liver tissue (from DBD and control groups) samples were prepared in ice-cold radioimmunoprecipitation assay lysis buffer (Applygen Technologies Inc., Beijing, China), as previously described (24), then centrifuged at 10,000 × g for 15 min at 4°C. The protein concentration of the lysates was determined by a Bradford assay (Beyotime Institute of Biotechnology). Equal quantities of protein samples (30 µg) were loaded on a 15% sodium dodecyl sulfate gel (60 V) and blotted onto polyvinylidene difluoride membrane (0.2 µm; EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk at room temperature for 1 h. The membranes were subsequently incubated at 4°C overnight with the following specific primary antibodies: Rabbit polyclonal anti-human/mouse/rat Prdx6 (1:500; cat. no. 13585-1-AP; Wuhan Sanying Biotechnology, Inc.), mouse monoclonal anti-rat/human p-IκB-α (1:200; cat. no. sc-8404; Santa Cruz Biotechnology, Inc.), rabbit polyclonal anti-human/mouse/rat IκB-α (1:300; cat. no. 18220-1-AP; ProteinTech Group, Inc., Chicago, IL USA) and rabbit polyclonal anti-human/mouse/rat β-actin (1:3,000; cat. no. BS1002; Bioworld Technology Inc., St. Louis Park, MN, USA), then washed three times with Tris-buffered saline and Tween-20 (TBST). Subsequently, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:3,000; cat. no. BL003A; BioSharp, Hefei, China) or anti-mouse antibody (1:3,000; cat. no. BL001A; BioSharp), and washed three times with TBST. The immunoreactive proteins were visualized using a DAB Horseradish Peroxidase Color Development enhanced chemiluminescence method kit (cat. no. P0202; Beyotime Institute of Biotechnology).
Measurement of alanine transaminase (ALT), aspartate transaminase (AST) and lactate dehydrogenase (LDH) levels
ALT, AST and LDH were used as markers of hepatocellular injury. L02 cells were exposed to ischemia and hypoxia for different time periods (0, 6, 12, 18, 24 h). Subsequently, ALT, AST and LDH concentration levels were measured using an AU5400 Clinical Chemistry System (Beckman Coulter, Inc., Brea, CA, USA), which is an automatic biochemistry analyzer.
Measurement of intracellular ROS
The ROS in L02 cells were measured with a ROS assay kit (Genmed Scientific Inc., Shanghai, China) according to manufacturer's protocol. Subsequently, cells were cultured and subjected to ischemia and hypoxia for different time periods. The cells were maintained in the dark and incubated with working solution (obtained from the ROS assay kit) at 37°C for 20 min; resulting in the development of a green color proportional to the amount of ROS present. Fluorescence was monitored by excitation/emission of 490/530 nm using a fluorescence microscope (IX71-A12FL; Olympus Corporation, Tokyo, Japan), and fluorescence intensity of 10,000 cells was measured using an FC500 flow cytometer (Beckman Coulter, Inc.).
Cell Counting Kit-8 (CCK-8) viability assay
Cell viability was assessed using a CCK-8 detection kit (Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according to the manufacturer's protocol. Briefly, 1×105 cells/well were cultured in 96-well plates. Cells were treated with BAY11-7082 or MJ33, as described, and exposed to ischemic and hypoxic conditions for different periods of time. Subsequently, 100 µl 10% CCK-8 solution was added per well and then incubated at 37°C for 1 h. Absorbance was measured at 450 nm using a microplate reader (SpectraMax 190; Eppendorf, Hamburg, Germany).
Measurement of PLA2 enzymatic activity
PLA2 activity was measured using a PLA2 Assay kit (Invitrogen, Thermo Fisher Scientific Inc.), according to the manufacturer's instructions. Fluorescence was measured using a microplate reader (SpectraMax 190; Molecular Devices, LLC, Sunnyvale, CA, USA) with excitation and emission wavelengths of 485 and 520 nm, respectively. The effect of 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol (MJ33; Sigma-Aldrich, St. Louis, MO, USA) on PLA2 activity was measured. Three MJ33 treatments were applied to the L02 cells: i) Following a 0.5 h treatment with MJ33 PLA2 enzymatic activity in L02 cells was measured immediately; ii) Following a 0.5 h treatment with MJ33 PLA2 enzymatic activity in L02 cells was measured after 12 h; and iii) Following a 12 h treatment with MJ33, PLA2 enzymatic activity in L02 cells was measured immediately. The concentrations of MJ33 used were 10, 20, 30 and 50 µM.
Statistical analysis
The results are expressed as the mean ± standard deviation for three or more independent experiments. One-way analysis of variance and independent Student's t-test were performed using SPSS software (version 11.5; SPSS, Inc., Chicago, IL, USA). P<0.05 indicated a statistically significant difference.
Results
Prdx6 expression is decreased and the NF-κB signaling pathway is activated in DBD livers
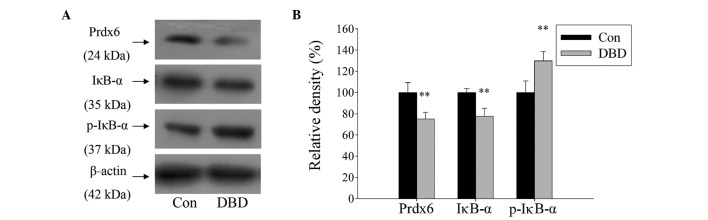
The role of Prdx6 and its regulation by NF-κB in DBD livers was explored. A total of ten DBD patients and six normal controls were examined. The present study identified that the expression of Prdx6 in liver tissue was significantly reduced in the DBD group compared with the control group (P<0.01). Simultaneously, the expression of IκB-α was significantly reduced and its phosphorylated form (p-IκB-α) was significantly increased in DBD (Fig. 1; P>0.01). The results suggest that Prdx6 may be involved in liver damage processes and that its expression is regulated by the NF-κB signaling pathway.
Figure 1.
Western blot analysis demonstrating modulated expression of Prdx6 and IκB-α protein in liver tissue from DBD. (A) Protein expression in liver tissues, collected from DBD and patients who accepted liver hemangioma tumor excision surgery. (B) Histogram showing relative density (%) of protein bands. Data are expressed as mean ± standard deviation. **P<0.01 vs. Con. Prdx6, peroxiredoxin 6; p-IκB-α, phosphrylated-inhibitor of κB-α; Con, control; DBD, donors after brain death.
Prdx6 may attenuate ischemic and hypoxic damage in L02 cells
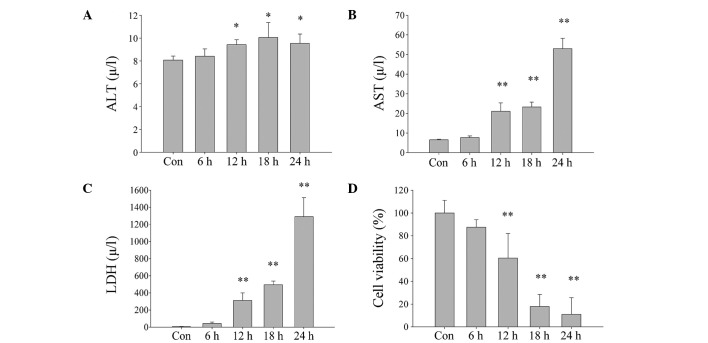
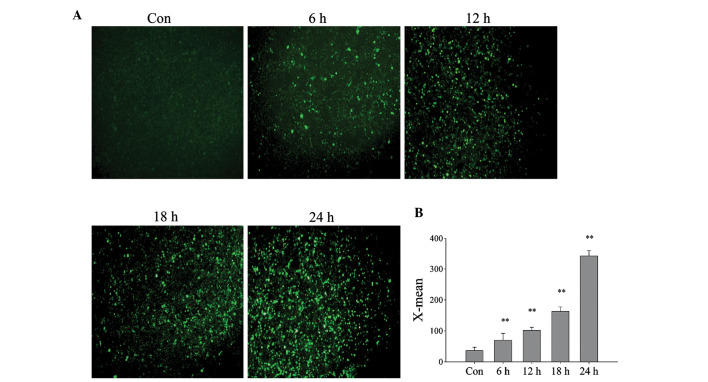
In order to determine the role of Prdx6 in DBD livers, L02 cells were cultured under ischemic-hypoxic conditions to mimic the state of brain death. AST and LDH concentration levels were demonstrated to significantly increase (Fig. 2A–C) and cell viability was significantly reduced as cell exposure time to DBD conditions was increased (Fig. 2D). However, the increase in levels of ALT were not as marked as those of AST, which suggests that ALT may be located in the cytoplasm, and AST in the mitochondria. ALT, AST and LDH concentration levels were identified to significantly increase (Fig. 2A–C) and cell viability was significantly reduced as cell exposure time to DBD conditions was increased (Fig. 2D). Using an ROS assay kit and a fluorescence microscope, it was detected that the intracellular ROS levels of L02 cells also increase with exposure time (Fig. 3A), and the relative fluorescence value significantly increased (Fig. 3B; P<0.01). This suggests that cell damage occurs in a time-dependent manner that may be caused by increased ROS.
Figure 2.
Damage of L02 cells exposed to ischemia and hypoxia increases with increased exposure times (0, 6, 12, 18 and 24 h). (A) ALT, (B) AST and (C) LDH concentration levels measured in cell culture following exposure to ischemia and hypoxia. (D) Survival rate of cells with increased exposure to hypoxia and ischemia, as determined by Cell Counting Kit-8 assay. Data are expressed as mean ± standard deviation *P<0.05, **P<0.01 vs. Con. ALT, alanine transaminase; Con, control; AST, aspartate transaminase; LDH, lactate dehydrogenase.
Figure 3.
Change of intracellular reactive oxygen species (ROS) during exposure of L02 cells to ischemia and hypoxia. L02 cells were cultured in ischemic and hypoxic conditions for 0, 6, 12, 18 and 24 h. (A) Intracellular ROS were displayed using fluorescence microscopy. Original magnification, ×100. (B) Histogram showing intracellular ROS in L02 cells, measured by flow cytometry. X-mean, fluorescence intensity. **P<0.01 vs. control. Con, control.
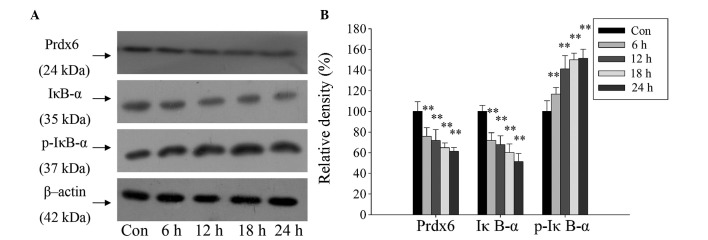
Furthermore, a western blot assay revealed the same expression pattern for Prdx6, IκB-α and p-IκB-α as in the DBD liver samples. The expression of Prdx6 and IκB-α proteins in L02 cells were significantly reduced, and the expression of p-IκB-α protein significantly increased over time (Fig. 4; P<0.01). The changes in concentration of IκB-α and p-IκB-α indicate that the NF-κB signaling pathway was activated. This demonstrates the potential stress-preventive role of Prdx6 in L02 cells under ischemia and hypoxia exposure, and indicates that Prdx6 may be regulated by NF-κB.
Figure 4.
Western blot analysis revealing modulated expression of Prdx6 and IκB-α protein in L02 cells following exposure to ischemia and hypoxia over time. (A) Protein expression in L02 cells after culture in ischemic and hypoxic conditions for 0, 6, 12, 18 and 24 h. (B) Histogram showing relative density of the protein bands. **P<0.01 vs. control. Prdx6, peroxiredoxin 6; p-IκB-α, phosphorylated-inhibitor of κB-α; Con, control.
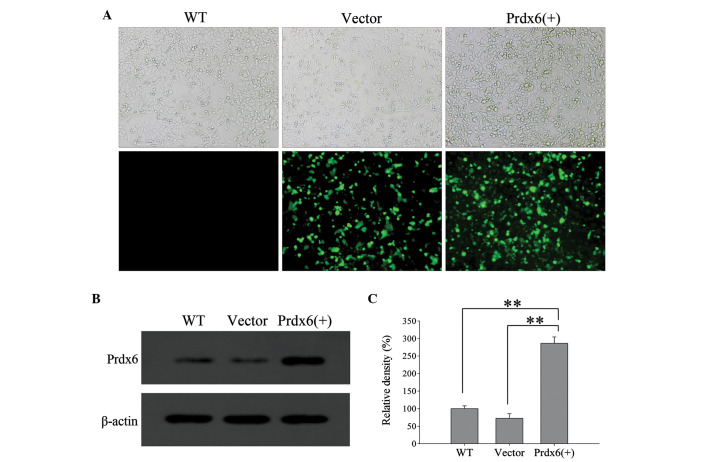
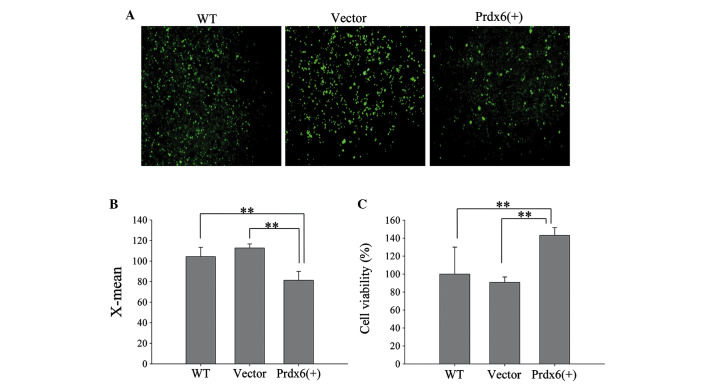
The efficiency of L 02 transfection wit h pIRES2-2sGreen1-Prdx6 was evaluated using green fluorescence (Fig. 5A) and western blotting (Fig. 5B). L02 cells significantly overexpressed Prdx6 compared with the controls (Fig. 5C; P<0.01). Furthermore, L02 cells overexpressing Prdx6 exhibited significantly reduced levels of intracellular ROS when exposed to ischemia and hypoxia (Fig. 6A and B; P<0.01), and cell viability significantly increased in these cells versus the control cells (Fig. 6C; P<0.01). The results confirm that Prdx6 has a protective function against cell death caused by ischemia and hypoxia-induced oxidative stress by optimizing ROS levels.
Figure 5.
Efficiency of transfection of Prdx6 into L02 cells. L02 cells were transfected with Vector or Prdx6(+), and compared with WT cells. (A) Transfection was evaluated by green fluorescence. Upper row, phase contrast microscope images; lower row, green fluorescence from transfected cells. Original magnification, ×200. (B) Western blot analysis of protein expression following transfection. (C) Histogram of the relative density of the protein bands **P<0.01. WT, wild-type; Vector, pIRES2-ZsGreen1; Prdx6(+), pIRES2-ZsGreen1-peroxiredoxin 6.
Figure 6.
Protective role of Prdx6 in L02 cells during ischemic and hypoxic conditions. Three groups of L02 cells, WT, Vector, and Prdx6(+), were exposed to ischemia and hypoxia for 12 h. (A) Intracellular reactive oxygen species (ROS) were determined using a fluorescence microscope. Original magnification, ×100. (B) Histogram showing intracellular ROS in L02 cells, measured by flow cytometry. (C) Survival rate of cell groups following exposure to ischemia and hypoxia for 12 h. **P<0.01. WT, wild-type; Vector, pIRES2-ZsGreen1; Prdx6(+), pIRES2-ZsGreen1-peroxiredoxin 6.
NF-κB signaling pathway negatively regulates Prdx6 expression
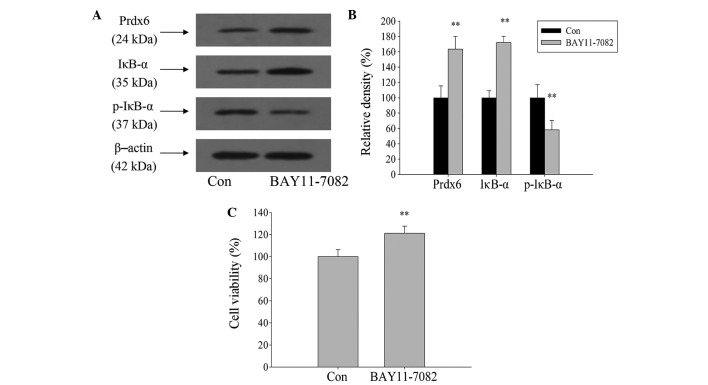
NF-κB was activated during ischemia and hypoxia. To clarify whether NF-κB activation is involved in the regulation of Prdx6 expression in L02 cells during ischemia and hypoxia, BAY11-7082 was used to inhibit the activation of NF-κB. Western blot analysis showed that expression levels of IκB-α and Prdx6 significantly increased, and p-IκB-α expression significantly decreased after 1 h treatment of L02 cells with BAY11-7082, when compared with the control (Fig. 7A and B; P<0.01). Following a 12-h exposure to ischemia and hypoxia, cell viability significantly increased compared with the control group (Fig. 7C; P<0.01). Therefore, this indicates that Prdx6 expression is negatively regulated by NF-κB during ischemia and hypoxia.
Figure 7.
Effects of nuclear factor-κB signaling pathway on regulating Prdx6 expression. (A) L02 cells were treated with BAY11-7082 (5 µM) for 1 h, then harvested for western blot analysis. (B) Histogram revealing relative density of protein bands. (C) After pretreatment with BAY11-7082 (5 µM) for 1 h, L02 cells were exposed with ischemia and hypoxia for 12 h, then cell viability was measured by Cell Counting Kit-8 assay. **P<0.01 vs. Con. p-IκB-α, phosphrylated-inhibitor of κB-α; Con, control.
Inhibition of PLA2 activity by MJ33 exacerbates ischemia and hypoxia-induced cell injury in L02 cells
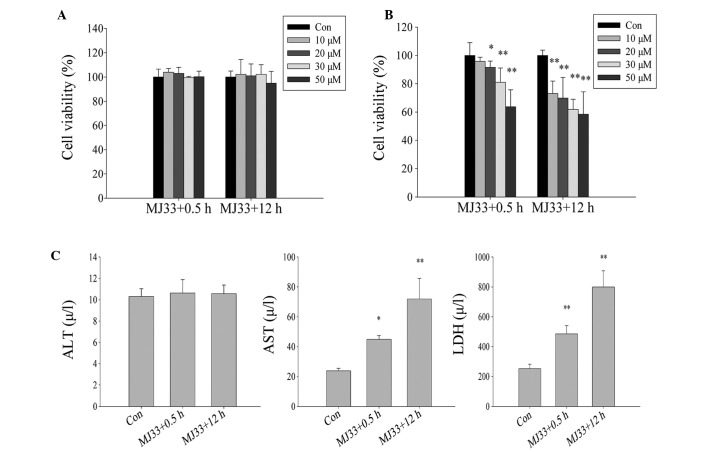
The role of Prdx6 PLA2 in oxidative stress induced by ischemia and hypoxia was explored. Initially, L02 cells were treated with different concentrations of MJ33 for two different time periods (MJ33+0.5 h and MJ33+12 h), without exposure to ischemia-hypoxia. There was no significant difference in cell viability between the two MJ33 treatment groups (Fig. 8A), thus cytotoxicity of MJ33 at concentrations of <50 µM was not evident. However, when the cells were pretreated with MJ33 and then exposed to ischemic and hypoxic conditions (MJ33+0.5 h) or concurrently treated with MJ33 and ischemia-hypoxia (MJ33+12 h), a significant difference in cell viability was observed. The cell viability in each group declined as MJ33 concentration increased. Notably, the cell viability of the MJ33+0.5 h group was marginally higher than that of the MJ33+12 h group cells (Fig. 8B). The ALT concentration level in cells exposed to culture medium containing 0 µM (control) and 20 µM MJ33 exhibited no significant difference, while AST and LDH levels were significantly increased in both MJ33 treatment groups. However, those in the MJ33+0.5 h group exhibited lower concentrations than MJ33+12 h group cells, which coincides with the CCK-8 data (Fig. 8C).
Figure 8.
Effect of inhibition of PLA2 activity by MJ33 (0, 10, 20, 30 and 50 µM) on L02 cells damage when subjected to ischemia and hypoxia. Viability of cells following (A) treatment with MJ33 and following (B) treatment with MJ33 and exposure to ischemic and hypoxic conditions. (C) The damage L02 cells suffered following exposure to ischemia and hypoxia was measured by ALT, AST and LDH concentration levels. MJ33+0.5 h group, cultured with MJ33 for 0.5 h, then MJ33 was removed and the cells were cultured in normal or ischemic-hypoxic conditions for 12 h; MJ33+12 h group, directly cultured with MJ33 in normal or ischemic-hypoxic conditions for 12 h. *P<0.05, **P<0.01 vs. control. ALT, alanine transaminase; Con, control; AST, aspartate transaminase; LDH, lactate dehydrogenase.
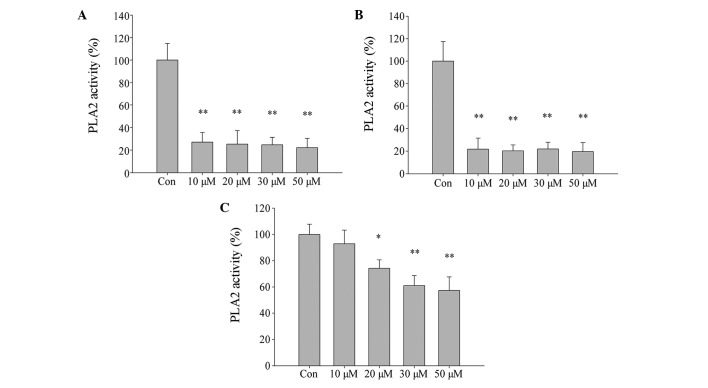
The effect of MJ33 on cell PLA2 activity was evaluated using an assay kit. PLA2 activity was significantly inhibited by treatment of L02 cells with increasing concentrations of MJ33 for 0.5 h and 12 h, then immediately harvested (Fig. 9A and B, respectively; P<0.01). However, in cells that were pretreated with MJ33 for 0.5 h and then cultured for 12 h after MJ33 removal, PLA2 activity did not decrease as significantly compared with the other two groups (Fig. 9C). This may explain why cell viability was higher in the equivalent MJ33+0.5 h group of cells. Thus, the current results indicate that the Prdx6 PLA2 activity is involved in cell protection against oxidative stress caused by ischemia and hypoxia.
Figure 9.
Effect of MJ33 on inhibition of PLA2 activity. L02 cells were treated with different concentration MJ33 for varied time periods. (A) PLA2 activity decreases rapidly with increased MJ33 concentrations (0.5 h treatment and immediate harvesting). (B) PLA2 activity decreases rapidly with increased MJ33 concentrations (12 h treatment and immediate harvesting). (C) PLA2 activity decreases with increased MJ33 concentrations (0.5 h treatment and 12 h culture). *P<0.05, **P<0.01 vs. Con. PLA2, phospholipase A2; Con, control.
Discussion
The present study identified a reduction in the expression of Prdx6 in DBD livers. Liver cells from DBD are considered to be subjected to ischemic-hypoxic damage, and Prdx6 is an antioxidant protein that can protect cells from oxidative stress (15). Thus, Prdx6 is possibly involved in the prevention of ischemia- and hypoxia-induced liver damage.
L02 cells were subjected to ischemia and hypoxia to mimic DBD liver cells. The present study determined that the expression of Prdx6 was reduced in L02 cells. Furthermore, ALT, AST and LDH concentration levels were increased, in addition to intracellular ROS levels, resulting in reduced cell viability. In a previous study, Prdx6 expression was downregulated upon serum deprivation in mouse liver cells (25). In addition, it has been reported that retinal ganglion cells exposed to hypoxia demonstrate reduced expression of Prdx6 with higher ROS levels and increased cell death (26). The current findings are consistent with these studies, as it was observed that over-expression of Prdx6 could reduce the levels of intracellular ROS and improve cell viability. ROS can activate apoptosis in hepatocytes (27), therefore, the increased cell damage possibly results from higher levels of intracellular ROS, which are in turn caused by the reduced expression of Prdx6, indicating that Prdx6 has a protective role when liver cells are exposed to ischemia and hypoxia.
In the present study, the expression of IκB-α was reduced and p-IκB-α was increased in DBD liver tissue and ischemic-hypoxic L02 cells, indicating that NF-κB activity was increased. However, there remain inconsistencies in the association between Prdx6 and NF-κB. For example, previous studies have reported that upregulation of Prdx6 may inhibit the activation of NF-κB (26,28,29). Other studies state that NF-κB may negatively regulate Prdx6 expression in oxidative stress, as observed in mice livers treated with ethanol (30) and mouse hippocampal cells subjected to hypoxia (20). BAY11-7082, a specific IκB kinase/NF-κB inhibitor, was used to inhibit NF-κB transcriptional activity in L02 cells in the present study, resulting in increased expression of Prdx6 protein and cell viability when cells were exposed to ischemia and hypoxia. Previous studies revealed that ROS could activate NF-κB in cell-type specific molecular mechanisms (31,32). Therefore, it is possible that NF-κB is an important signaling pathway that mediates Prdx6 expression in DBD ischemic-hypoxic liver cells, leading to elevated ROS activating NF-κB and resulting in the inhibition of Prdx6 expression. This, in turn, leads to ROS elevation, triggering a positive-feedback mechanism and causing more liver injury.
To the best of our knowledge, the present study observed for the first time, that Prdx6 PLA2 activity has a protective role in liver cells. ROS can peroxidase unsaturated fatty acids in the phospholipids of cellular membranes, thus causing impaired membrane function (33). PLA2 can hydrolyze an acyl or alkyl linkage at the sn-2 position of phospholipids to produce free fatty acids (34). Therefore, PLA2 contributes to membrane repair by releasing free fatty acids and forming 2-lysophospho-lipid acceptors that may be reacylated by acyltransferases (35). Although Prdx6 PLA2 activity is high at pH 4 and low at pH 7, as Prdx6 binds to oxidize phospholipids at pH 7, PLA2 activity increases (36). PLA2 activity is not as efficient in the reduction of peroxidized phospholipids as Prdx6 peroxidase activity, however it is still an important alternative pathway in the event of a decline in peroxidase activity due to reduced Prdx6 expression.
In conclusion, the current study observed that the Prdx6 expression was reduced in DBD liver tissue, and liver cells subjected to ischemia and hypoxia. This was associated with liver injury. Conversely, overexpression of Prdx6 was found to partially reverse the liver cell damage. In addition, expression of Prdx6 appeared to be regulated by the NF-κB signaling pathway, and Prdx6 PLA2 activity contributed to cell protection during ischemia and hypoxia. Thus, the present study provides a novel perspective on the protection mechanism against liver damage after brain death, and provides valuable information for the development of a novel strategy for the improvement of donor liver quality.
References
- 1.Telles-Correia D, Mega I. Candidates for liver transplantation with alcoholic liver disease: Psychosocial aspects. World J Gastroenterol. 2015;21:11027–11033. doi: 10.3748/wjg.v21.i39.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heimbach JK, Hirose R, Stock PG, Schladt DP, Xiong H, Liu J, Olthoff KM, Harper A, Snyder JJ, Israni AK, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61:1643–1650. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SJ, Lim YS, Hwang S, Heo NY, Lee HC, Suh DJ, Yu E, Lee SG. Emergency adult-to-adult living-donor liver transplantation for acute liver failure in a hepatitis B virus endemic area. Hepatology. 2010;51:903–911. doi: 10.1002/hep.23369. [DOI] [PubMed] [Google Scholar]
- 4.Huang JF, Wang HB, Zheng SS, Liu YF, Shi BY, Shen ZY, Hu SS, Ye QF, Xue WJ, He XS. Advances in China's organ transplantation achieved with the guidance of law. Chin Med J (Engl) 2015;128:143–146. doi: 10.4103/0366-6999.149183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss S, Kotsch K, Francuski M, Reutzel-Selke A, Mantouvalou L, Klemz R, Kuecuek O, Jonas S, Wesslau C, Ulrich F, et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7:1584–1593. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 6.Barklin A, Larsson A, Vestergaard C, Koefoed-Nielsen J, Bach A, Nyboe R, Wogensen L, Tønnesen E. Does brain death induce a pro-inflammatory response at the organ level in a porcine model? Acta Anaesthesiol Scand. 2008;52:621–627. doi: 10.1111/j.1399-6576.2008.01607.x. [DOI] [PubMed] [Google Scholar]
- 7.Kusaka M, Pratschke J, Wilhelm MJ, Ziai F, Zandi-Nejad K, Mackenzie HS, Hancock WW, Tilney NL. Activation of inflammatory mediators in rat renal isografts by donor brain death. Transplantation. 2000;69:405–410. doi: 10.1097/00007890-200002150-00017. [DOI] [PubMed] [Google Scholar]
- 8.Takada M, Nadeau KC, Hancock WW, Mackenzie HS, Shaw GD, Waaga AM, Chandraker A, Sayegh MH, Tilney NL. Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation. 1998;5:1533–1542. doi: 10.1097/00007890-199806270-00001. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Hoeven JA, Moshage H, Schuurs T, Nijboer M, Van Schilfgaarde R, Ploeg RJ. Brain death induces apoptosis in donor liver of the rat. Transplantation. 2003;76:1150–1154. doi: 10.1097/01.TP.0000080983.14161.95. [DOI] [PubMed] [Google Scholar]
- 10.Adrie C, Monchi M, Fulgencio JP, Cottias P, Haouache H, Alvarez-Gonzalvez A, Guerrini P, Cavaillon JM, Adib-Conquy M. Immune status and apoptosis activation during brain death. Shock. 2010;33:353–362. doi: 10.1097/SHK.0b013e3181b65b99. [DOI] [PubMed] [Google Scholar]
- 11.Golling M, Mehrabi A, Blum K, Jahnke C, Kellner H, Bud O, Hashemi B, Breitkreutz R, Becker-Brandenbutg K, Schemmer P, et al. Effects of hemodynamic instability on brain death-induced prepreservation liver damage. Transplantation. 2003;75:1154–1159. doi: 10.1097/01.TP.0000062868.34247.8F. [DOI] [PubMed] [Google Scholar]
- 12.Dutkiewicz G, Domanski L, Binczak-Kuleta A, Pawlik A, Safranow K, Dziedziejko V, Wisniewska M, Ciechanowicz A, Ciechanowski K. Lack of association of polymorphisms 239+34A/C in the SOD1 gene and 47C/T in the SOD2 gene with delayed graft function and acute and chronic rejection of kidney allografts. Transplant Proc. 2009;41:3701–3703. doi: 10.1016/j.transproceed.2009.06.221. [DOI] [PubMed] [Google Scholar]
- 13.Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 14.Fisher AB. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid Redox Signal. 2011;15:831–844. doi: 10.1089/ars.2010.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simeone M, Phelan SA. Transcripts associated with Prdx6 (peroxiredoxin 6) and related genes in mouse. Mamm Genome. 2005;16:103–111. doi: 10.1007/s00335-004-2429-6. [DOI] [PubMed] [Google Scholar]
- 16.Eismann T, Huber N Shin T, Kuboki S, Galloway E, Wyder M, Edwards MJ, Greis KD, Shertzer HG, Fisher AB, Lentsch AB. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G266–G274. doi: 10.1152/ajpgi.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher AB, Dodia C, Feinstein SI, Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J Lipid Res. 2005;46:1248–1256. doi: 10.1194/jlr.M400499-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Ambruso DR, Ellison MA, Thurman GW, Leto TL. Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochim Biophys Acta. 18232012:306–315. doi: 10.1016/j.bbamcr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Lien YC, Feinstein SI, Dodia C, Fisher AB. The roles of peroxidase and phospholipase A2 activities of peroxiredoxin 6 in protecting pulmonary microvascular endothelial cells against peroxidative stress. Antioxid Redox Signal. 2012;16:440–451. doi: 10.1089/ars.2011.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhunchha B, Fatma N, Kubo E, Rai P, Singh SP, Singh DP. Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-κB regulation. Am J Physiol Cell Physiol. 2013;304:C636–C655. doi: 10.1152/ajpcell.00345.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdhury I, Fisher AB, Christofidou-Solomidou M, Gao L, Tao JQ, Sorokina EM, Lien YC, Bates SR, Feinstein SI. Keratinocyte growth factor and glucocorticoid induction of human peroxiredoxin 6 gene expression occur by independent mechanisms that are synergistic. Antioxid Redox Signal. 2014;20:391–402. doi: 10.1089/ars.2012.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paula FM, Ferreira SM, Boschero AC, Souza KL. Modulation of the peroxiredoxin system by cytokines in insulin-producing RINm5F cells: Down-regulation of PRDX6 increases susceptibility of beta cells to oxidative stress. Mol Cell Endocrinol. 2013;374:56–64. doi: 10.1016/j.mce.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Li YH, Xu SQ, Hu SH, Zhang L. Protective effects of peroxisome proliferator-activated receptor-α agonist, Wy14643, on hypoxia/reoxygenation injury in primary rat hepatocytes. PPAR Res. 2012;2012:547980. doi: 10.1155/2012/547980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhunchha B, Fatma N, Bhargavan B, Kubo E, Kumar A, Singh DP. Specificity protein, Sp1-mediated increased expression of Prdx6 as a curcumin-induced antioxidant defense in lens epithelial cells against oxidative stress. Cell Death Dis. 2011;2:e234. doi: 10.1038/cddis.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher BM, Phelan SA. Investigating transcriptional regulation of Prdx6 in mouse liver cells. Free Radic Biol Med. 2007;42:1270–1277. doi: 10.1016/j.freeradbiomed.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Tulsawani R, Kelly LS, Fatma N, Chhunchha B, Kubo E, Kumar A, Singh DP. Neuroprotective effect of peroxiredoxin 6 against hypoxia-induced retinal ganglion cell damage. BMC Neurosci. 2010;11:125. doi: 10.1186/1471-2202-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Xu Y, Wang H, Xue P, Li X, Li B, Zheng Q, Sun G. Arsenic induces mitochondria-dependent apoptosis by reactive oxygen species generation rather than glutathione depletion in Chang human hepatocytes. Arch Toxicol. 2009;83:899–908. doi: 10.1007/s00204-009-0451-x. [DOI] [PubMed] [Google Scholar]
- 28.Fatma N, Kubo E, Sen M, Agarwal N, Thoreson WB, Camras CB, Singh DP. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res. 2008;1233:63–78. doi: 10.1016/j.brainres.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun XG, Fu XQ, Cai HB, Liu Q, Li CH, Liu YW, Li YJ, Liu ZF, Song YH, Lv ZP. Proteomic analysis of protective effects of polysaccharides from Salvia miltiorrhiza against immunological liver injury in mice. Phytother Res. 2011;25:1087–1094. doi: 10.1002/ptr.3487. [DOI] [PubMed] [Google Scholar]
- 30.Roede JR, Stewart BJ, Petersen DR. Decreased expression of peroxiredoxin 6 in a mouse model of ethanol consumption. Free Radic Biol Med. 2008;45:1551–1558. doi: 10.1016/j.freeradbiomed.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Wang ZW, Wu HB, Li Z, Li LC, Hu XP, Ren ZL, Li BJ, Hu ZP. Transforming growth factor-β1 induces matrix metal-loproteinase-9 expression in rat vascular smooth muscle cells via ROS-dependent ERK-NF-κB pathways. Mol Cell Biochem. 2013;375:11–21. doi: 10.1007/s11010-012-1512-7. [DOI] [PubMed] [Google Scholar]
- 33.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 34.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A(2): Structure and function. Biochim Biophys Acta. 2000;1488:28–39. doi: 10.1016/S1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 36.Manevich Y, Shuvaeva T, Dodia C, Kazi A, Feinstein SI, Fisher AB. Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A(2) activities. Arch Biochem Biophys. 2009;485:139–149. doi: 10.1016/j.abb.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]