Abstract
Nanotechnologies are emerging as highly promising technologies in many sectors in the society. However, the increasing use of engineered nanomaterials also raises concerns about inadvertent exposure to these materials and the potential for adverse effects on human health and the environment. Despite several years of intensive investigations, a common paradigm for the understanding of nanoparticle-induced toxicity remains to be firmly established. Here, the so-called oxidative stress paradigm is scrutinized. Does oxidative stress represent a secondary event resulting inevitably from disruption of biochemical processes and the demise of the cell, or a specific, non-random event that plays a role in the induction of cellular damage e.g. apoptosis? The answer to this question will have important ramifications for the development of strategies for mitigation of adverse effects of nanoparticles. Recent examples of global lipidomics studies of nanoparticle-induced tissue damage are discussed along with proteomics and transcriptomics approaches to achieve a comprehensive understanding of the complex and interrelated molecular changes in cells and tissues exposed to nanoparticles. We also discuss instances of non-oxidative stress-mediated cellular damage resulting from direct physical interference of nanomaterials with cellular structures.
Keywords: Carbon nanotubes, Oxidative stress, Oxidative lipidomics, Proteomics, Transcriptomics, Apoptosis
Introduction
The trouble with the world is not that people know too little, but that they know so many things that ain’t so
Mark Twain
Developments in nanotechnology have already yielded breakthrough discoveries in nanomedical devices, biosensors, drug delivery protocols and theranostics (Cabezon et al., 2011; Fazil et al., 2012; Leucuta, 2010; Motwani et al., 2011). A large diversity of nanomaterials have found their way into many consumer items, such as clothing and cosmetics, thus increasing the likelihood of human contact and exposure to nanomaterials. In addition, occupational, environmental and indoor exposures to nanoparticles remain a public concern and a potentially serious health hazard (Maynard et al., 2011). Extrapolations from the field of toxicology of particulate matter (PM), especially from extensive work on PM with less than 10 μm in diameter (PM10), laid the background for the suspicions that nanoparticles are not innocuous but may yield a range of harmful effects (Oberdörster, 2012). Indeed, some animal studies of pulmonary toxicity of nanoparticles, particularly carbon nanotubes (CNTs), revealed robust, pro-inflammatory responses accompanied by oxidative stress (Ravichandran et al., 2011; Shvedova et al., 2005).
In 2009, the marked demand for single-walled CNTs (SWCNTs) was 90 million US$ versus 120 million US$ for multi-walled CNTs (MWCNTs). However, the projected market growth predicts that in year 2014 the demand for SWCNTs and MWCNTs will be 600 and 470 million US$, respectively (http://nextbigfuture.com/2008/06/carbon-nanotube-production.html). Thus potential adverse health effects of both SWCNTs and MWCNTs, particularly in occupational settings, should be seriously considered along with the toxicity mechanisms of these types of CNTs.
There is evidence that ENMs, including CNTs, may be released in the work environment: many activities have been investigated such as workplace cleaning, packaging/bag filling, reactor cleanout, mixing, cutting composite, and in almost all cases evidence for some release of ENMs in the workplace has been detected (Bello et al., 2009; Han et al., 2008; Kuhlbusch and Fissan, 2006; Maynard et al., 2004; Peters et al., 2009; Tsai et al., 2009; Vosburgh et al., 2011). Some reports describing serious adverse effects of occupational exposure to ENMs have been published: These include several cases of rescue workers present at the World Trade Center in New York City after the tragic events of 9/11 (Aldrich et al., 2010; Gupta, 2011; NIOSH, 2011; Romano, 2011; Zeig-Owens et al., 2011), reports of occupational exposures to mixtures containing nanoparticles in factories in China and India (Jaakkola et al., 2011; Song et al., 2009), a case-report regarding a worker who inhaled an estimated gram of nickel nanoparticles over an approximate 90-minute period, and who died from adult respiratory distress syndrome (Phillips et al., 2010), the development of unusual pulmonary disease in workers exposed to silica nanoparticles (Song et al., 2011), and the development of toxic epidermal necrolysis-like dermatitis in a chemist exposed to high levels of intermediate or final products of dendrimers while performing dendrimer synthesis (Toyama et al., 2009). Finally, a recent occupational survey showed an association with mortality due to chronic obstructive pulmonary disease and to ischemic heart disease in a cohort of workers exposed to metalworking fluids containing a substantial amount of incidental nanoparticles (Eisen et al., 2011).
Is oxidative stress a uniform and sensible paradigm for nanoparticle-induced cytotoxicity?
The intrinsic toxicity of nanoparticles has been attributed to their unique physico-chemical characteristics — i.e. their smallness and the remarkably large surface area per unit mass and high surface reactivity (Auffan et al., 2009). This physico-chemical reactivity is frequently correlated with the ability of nanoparticles, especially metal-based nanoparticles, to trigger the formation of free radicals (i.e. reactive oxygen species (ROS) such as superoxide radical anions and hydroxyl radicals) directly or via activation of oxidative enzymatic pathways leading to oxidative stress. It is believed that ROS generation by nanoparticles is realized through several mechanisms frequently including redox-cycling pathways. These may include the oxidant-generating properties of particles themselves as well as their ability to stimulate generation of ROS as a part of cellular response to nanoparticles (Knaapen et al., 2004). Among the former, the major sources of oxidative stress are (i) transition metal-based nanoparticles or transition metal contaminants used as catalysts during the production of non-metal nanoparticles, including SWCNT (ii) relatively stable free radical intermediates (detectable by electron paramagnetic resonance spectroscopy) present on “reactive” surfaces of particles such as quartz, carbonaceous particles, and (iii) redox-active groups resulting from functionalization of nanoparticles (e.g., quinones capable of generating ROS) (Knaapen et al., 2004; Kovacic and Somanathan, 2010; Li et al., 2010). In addition to direct prooxidant effects of nanoparticles, they may activate the redox-machinery of the cell especially in the lungs where inflammatory cells – neutrophils and macrophages – can act as potent generators of ROS. Mitochondria are the major source of ROS in the cell; indeed, the production of superoxide is an inevitable “side-effect” of respiration and, hence, of life itself, and cells balance this continuous production of ROS through expression of a plethora of anti-oxidant enzymes. However, in phagocytic cells of the immune system, NADPH oxidase-dependent generation of ROS is also a critical source of ROS in response to ingestion of offending agents e.g. bacteria, which are killed through a combination of oxidative and proteolytic activities within the phagocytic cell (more recent studies suggest that neutrophils may also capture extracellular bacteria through the generation of so-called neutrophil extracellular traps or NETs; however, this formation of NETs is also NADPH oxidase-dependent) (Fadeel, 2009; Fadeel and Kagan, 2003). Recent studies have shown that ROS-producing mitochondria can trigger inflammasome activation in phagocytic cells (Zhou et al., 2011), thus providing a link between mitochondria and inflammation, with ROS acting as the signal. In addition, the activation of lysosomes, cellular “suicide bags” with high content of digestive enzyme, may in turn lead to the triggering of mitochondrial apoptosis, with production of ROS (Torres Andon and Fadeel, in press). It is our firm belief that we may gain a much better appreciation for the biological/toxicological effects of nanoparticles if we consider the highly specialized (organelle-specific) pathways of ROS production and the ways in which nanoparticles may impinge upon these pathways. Nel and co-workers provided evidence for the differential induction of cytotoxicity by different nanoparticles depending on cell type-specific uptake mechanisms (Xia et al., 2008). In a recent study, we provided an example of nanoparticle-specific, ROS-dependent cytotoxicity insofar as overexpression of the anti-oxidant enzyme, microsomal glutathione transferase-1 (MGST1) protected cells from silica nanoparticle-induced cell death but not from ZnO nanoparticle-induced cell death; the protective effect was likely exerted through the prevention of nanoparticle-induced lipid peroxidation in critical cellular membranes. Both nanoparticles, however, triggered the production of cellular ROS (Shi et al., 2012). Moreover, active cellular internalization of nanoparticles or fibers through phagocytic pathways may, at least in professional phagocytic cells, result in activation of the NADPH oxidase with resultant massive generation of ROS (the so-called “oxidative burst”). Indeed, studies in mice lacking expression of a functional NADPH oxidase have shown that NADPH oxidase-derived ROS are critical in determining the course of pulmonary response to SWCNT (Shvedova et al., 2008a). The localization of nanoparticles to lysosomes may lead to activation of the lysosomal–mitochondrial axis with attendant cell death. Using rapid multicolor 3D live cell confocal fluorescence microscopy, combined with transient overexpression of small GTPases marking various endocytic membranes, Dawson and co-workers were able to reveal the kinetics of nanoparticle trafficking through early endosomes to late endosomes and lysosomes (Sandin et al., 2012).
CNT represents an illustrative case. Hence, several groups have shown that the presence of iron in SWCNT may be important in determining redox-dependent responses of macrophages. Hence, in zymosan-stimulated RAW 264.7 macrophages, non-purified iron-rich SWCNT were more effective in generating hydroxyl radicals (documented by EPR spin-trapping with 5,5-dimethyl-1-pyrroline-N-oxide, DMPO) than purified (iron-stripped) SWCNT (Kagan et al., 2006). Similarly, Pulskamp et al. (2007) reported a dose- and time-dependent increase of intracellular ROS and a decrease of the mitochondrial membrane potential with non-purified CNT in rat macrophages and human lung cells whereas incubation with the purified (acid-treated) SWCNT had no effect. Furthermore, others have suggested that, contrary to traditional toxicants, such as asbestos, CNT may quench rather than generate oxygen free radicals. This scavenging activity was suggested to be related to the presence of structural defects (Fenoglio et al., 2008). Notwithstanding, upon interaction with phagocytic cells of the immune system, CNT may induce ROS production through NADPH oxidase-dependent pathways, as we shall discuss in a following section. Notably, we have found that NADPH oxidase-deficient mice respond to SWCNT exposure with a marked accumulation of neutrophils and production of pro-inflammatory cytokines, decreased production of the anti-inflammatory and pro-fibrotic cytokine, TGF-β, and significantly lower levels of collagen deposition, as compared to wild-type control mice, thus supporting a prominent role for NADPH oxidase-ROS in determining course of pulmonary responses to SWCNT.
This emphasis and wide acceptance of “oxidative stress” pathways as one of the fundamental paradigms for toxicity necessitates a very careful analysis of specific mechanisms elicited in cells and tissues by nanoparticles and assessment of possible role and significance of oxidative reactions in responses elicited by exposures to nanoparticles.
Remarkable advancements in chemistry of liquid-phase oxidation of organic compounds in the mid 1960–70s had a huge impact on several biomedical disciplines and designated the emergence of a new field of knowledge and practice — free radical biology and medicine (Emanuel, 1976). Initially applied to explain unusual features of radiation induced damage, these frontier chemical ideas propagated into different areas of biomedicine, particularly after the discoveries of oxygen radicals and their regulating enzymes in cells and tissues (Fridovich, 1995). Based on the premise of the essentiality of low molecular weight sacrificial antioxidants – small water- and lipid-soluble molecules such as vitamins C and E – capable of scavenging reactive radicals and slowing down the overall oxidation rates of essential biomolecules in biofluids and tissues (Packer, 1992), the concept of “oxidative stress” was put forward and quickly gained in popularity (Sies, 1985). According to this concept the disturbances in the balance between pro- and antioxidants disrupt the equilibrium in the redox “powers” resulting in accumulation of oxidation products and, potentially, cell damage and death. While the two corner-stone doctrines: i) oxidative stress and ROS are essential mechanisms of injury and diseases and ii) antioxidants can be used as preventive/protective treatments — have received much attention, they have ignored, at least for some time, possible important metabolic functions of oxidative reactions. The latter re-emerged and became central in the recent studies, particularly as simplistic understanding of “oxidative stress” as an exclusive mechanism of injury has been sobered by the outcome of clinical intervention trials that did not reveal significant beneficial effects of antioxidants (Abner et al., 2011; Azzi, 2007; Niki, 2010). There is a mounting consensus that the meaning of oxidatively modified biomolecules in biomedicine and toxicology may need a serious re-evaluation with regards to the major pathways involved and their significance in the pathogenesis of injury and disease. An illuminating example in this respect may be to assess the importance of oxidative reactions for the toxicity of nanoparticles.
Lipid peroxidation in nanoparticle-induced cell death: oxidative lipidomics approaches
Lipid peroxidation products are among the most common types of oxygenated molecules implicated in oxidative stress responses. Overall, lipid peroxidation reactions are sub-categorized into two types: enzymatic and non-enzymatic. The latter proceed as typical “free radical” reactions whereby the oxidation rates are defined mainly by the number of double bonds in their polyunsaturated fatty acid (PUFA) residues. Consequently, this random process should affect all different (phospho)lipid classes with six, five and four double bonds. The dependence of this type of reactions on the chemical nature of phospholipid polar heads is usually minimal (Kagan, 1988). Enzymatic oxygenations of free PUFA, on the other hand, have been known for a long time as the major source of numerous extra- and intracellular biological regulators that include a large diversity of eicosanoids, prostanoids, docosapentanoids and docosahexanoids (Praticò, 2008; Serhan, 2010). Their major metabolic sources are polyunsaturated phospholipids that undergo an enzymatic attack by selective phospholipases with subsequent specific enzymatic oxygenation of free PUFA. Until recently, enzymatic peroxidation of PUFA esterified into phospholipids has not been recognized as one of the major metabolic pathways. This was mostly due to the fact that detailed analysis of diversified peroxidation products in different classes of phospholipids and identification of their individual molecular species was difficult. Current advancements in different types of mass-spectrometry (MS) of small molecules gave a remarkable boost to MS of phospholipids (Blanksby and Mitchell, 2010; Hsu and Turk, 2009; Ivanova et al., 2009; Tyurin et al., 2009) and their oxidation products thus heralding the appearance of the research field of (global) oxidative lipidomics (Kagan and Quinn, 2004; Kagan et al., 2004). As we shall discuss below, the application of oxidative lipidomics protocols to the study of nanomaterial-induced cell and tissue damage may yield novel and important information.
It is a common belief that nanomaterials cause non-specific oxidative damage that underlies, to a large extent, their cytotoxicity (Li et al., 2008; Nel et al., 2006). This may be true for some specific cases e.g. for nanoparticles capable of acting as pro-oxidants due to the chemical nature of their surface (see above). Indeed, Fenoglio et al. (2008) have suggested that, contrary to traditional toxicants, such as asbestos, CNT may quench rather than generate oxygenated free radicals. This scavenging activity, related to the presence of structural defects, appeared to be associated with the genotoxic and inflammatory potential of CNT (Muller et al., 2008).
However, this view may be an oversimplification of the complex interactions between a robust inflammatory response, cytotoxicity and oxidative reactions accompanying these processes. Notably, no detailed lipidomics/oxidative lipidomics studies have been performed to characterize “oxidative stress” elicited by nanoparticles in vivo. These considerations prompted us to undertake an MS-based oxidative lipidomics analysis of all major pulmonary phospholipid classes in mice exposed to CNT through inhalation (5 mg/m3, whole body inhalation for 4 consecutive days, 5 h/day) and euthanized 1, 7, and 28 days thereafter. Because transition metals, particularly iron, are frequently used as catalysts in single-walled CNT (SWCNT) synthesis (Tessonnier, 2011), in these experiments, we intentionally utilized the preparations of CNT with a relatively high content of Fe (17.7% weight) as well as other transition metals capable of inducing non-enzymatic oxidative stress – Cu (0.16%), Cr (0.049%), and Ni (0.046%) (Valko et al., 2005). In spite of the presence of several potential transition metal catalysts of non-enzymatic lipid peroxidation, we found highly selective patterns of pulmonary phospholipid peroxidation after the inhalation exposure of mice to SWCNT. No oxidized molecular species were found in the two most abundant phospholipid classes: phosphatidylcholine (PC) and phosphatidylethanolamine (PE) – that were rich in peroxidizable PUFA residues. Instead, peroxidation products were detected and identified in three relatively minor classes of anionic phospholipids, cardiolipin (CL), phosphatidylserine (PS) and phosphatidylinositol (PI), whereby oxygenation of polyunsaturated fatty acid residues showed unusual substrate specificity. Moreover, we demonstrated that this non-random profile of SWCNT-induced lipid peroxidation in the lungs of exposed mice was associated with an accumulation of significant amounts of apoptotic cells in the lung. We believe that the pattern of lipid peroxidation reflects the specific cytotoxic (pro-apoptotic) effects of SWCNT and therefore argues against non-specific oxidative damage.
It is intriguing to uncover the catalytic nature of the selectivity of SWCNT-triggered phospholipid peroxidation in spite of the presence of high doses of “non-specific” transition metal catalysts commonly associated with induction of “oxidative stress”. Indeed, our studies indicated that phospholipid peroxidation was secondary to the cytotoxic effects of inhaled SWCNT that caused massive apoptotic cell death. We previously identified an intermembrane hemoprotein of mitochondria, cytochrome c (cyt c), as a catalyst of selective peroxidation of CL and PS in mitochondrial and extramitochondrial compartments of apoptotic cells, respectively (Ardail et al., 1990; Bayir et al., 2006; Jiang et al., 2003, 2008; Kagan et al., 2002, 2003, 2005; Schlame and Haldar, 1993; Tyurina et al., 2004a, 2004b). Notably, a similar selective phospholipid peroxidation profile was detected upon incubation of a mixture of total lung lipids with H2O2/cyt c known to catalyze CL and PS peroxidation in apoptotic cells (Fig. 1). Using similar oxidative lipidomics protocols, we have previously characterized the pattern of phospholipid peroxidation following several other insults, including hyperoxic acute lung injury, γ-radiation-induced lung injury, and traumatic brain injury (Bayir et al., 2007; Sparvero et al., 2010; Tyurin et al., 2010; Tyurina et al., 2008, 2009, 2010, 2011a). Strikingly, in all cases, a selective pattern of phospholipid peroxidation involving primarily CL and/or PS was observed (Tyurina et al., 2011a). The fact that inhalation exposure to SWCNT with high content of transition metals, particularly iron, known to effectively catalyze non-specific peroxidation of polyunsaturated phospholipids, also induces a similar, selective pattern of peroxidation of phospholipids (Fig. 1) suggests that common pathways of cellular and tissue damage are engaged. This is encouraging because it implies that common “anti-apoptotic” countermeasures against a diverse range of insults, including nanomaterials, may be envisioned. This is based on the essentiality of CL peroxidation for the execution of the apoptotic program (Tyurin et al., 2007); thus, suppression of this important function of cyt c/CL peroxidase complexes may be used as a target for the regulation of apoptosis (Hoye et al., 2008; Huang et al., 2008; Kagan et al., 2009a, 2009b). This protective/mitigative approach has very recently been proven successful in vitro (Belikova et al., 2009a; Jiang et al., 2009) and against total body irradiation-induced injury (Atkinson et al., 2011). On the other hand, treatment with antioxidant compounds should not be neglected. For example, in one recent study, the presence of vitamin E was reported to protect PC12 cells from the injury induced by SWCNT through the down-regulation of oxidative stress and prevention of mitochondria-mediated apoptosis (Wang et al., 2012). In line with these studies, lowered levels of antioxidants in vitamin E-deficient mice were associated with a higher sensitivity to SWCNT-induced acute inflammation and enhanced pro-fibrotic responses (elevation of TGF-β and collagen deposition) (Shvedova et al., 2007).
Fig. 1.
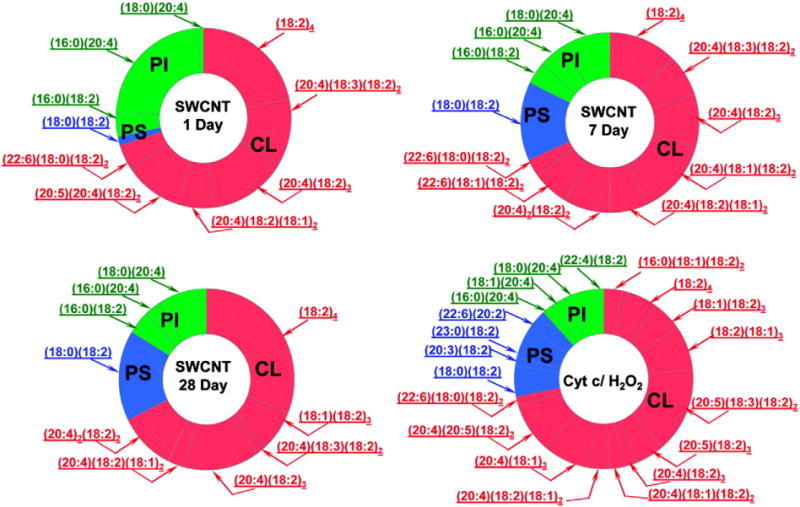
Oxidative lipidomics reveals non-random phospholipid oxidation in the lungs of CNT-exposed mice. Profiles of phospholipid oxidation in the lung induced by the exposure of mice to SWCNT and by incubation of phospholipids extracted from the lung in a model system containing cyt c and H2O2. Lipids from lungs of control mice and mice exposed to SWCNT were isolated and resolved by LC/MS. The data are presented as decreases in the amounts of oxidizable molecular species of phospholipids after exposure to SWCNT. CL = cardiolipin, PS = phosphatidylserine, PI = phosphatidylinositol.
Reprinted from Tyurina et al. (2011b) with permission from American Chemical Society.
It will be important in the future to evaluate the extent to which the apoptosis-associated selectivity of phospholipid peroxidation detected after SWCNT exposure is characteristic of exposures to other engineered nanomaterials in the lung or in other tissues. Other forms of cell death including accidental necrosis, regulated necrosis (necroptosis), and cell death resulting from excessive autophagy may also be associated with the exposure to nanoparticles (Torres Andon and Fadeel, in press). It will be of considerable interest to establish whether these various cell death modalities are associated with specific patterns of phospholipid peroxidation catalyzed by specific enzymatic mechanisms.
Oxidative stress as a mechanism of biodegradation of carbon-based nanomaterials
The fact that specific rather than random oxidative reactions were elicited by exposure to nanomaterials raises a possibility that other responses, different from cell death mechanisms, may be critical as determinants of their fate and effects, including distribution and clearance from the tissues and body, degradation, etc. Indeed, the dimensions of nanoparticles are comparable to those of viruses or bacteria for which our immune system has elaborated sophisticated oxidative responses effectuated by cells of the innate immune system (Shvedova et al., 2010). Engulfment of bacteria provokes the “oxidative burst” in phagocytes with massive generation of ROS. Given that the oxidizing potentials of these reactive species are sufficient for breakage of C–H and C–C bonds it is likely that oxidative modification of nanoparticles will occur, if they are engulfed. Myeloperoxidase (MPO) – an abundant enzyme of neutrophils – is one of the most potent enzymatic sources of oxidants involved in host defense responses. We reported that, in simple biochemical model systems, MPO turned out to be effective in oxidative biodegradation of SWCNT via a plethora of intermediate aliphatic and aromatic products to ultimately yield CO2 and H2O by a combination of two pathways associated with: i) hypochlorite generated by the enzyme in the presence of chloride, and ii) reactive intermediates of the enzyme (Kagan et al., 2010). Molecular modeling showed that the carboxyls on the carbon nanotubes – through the interaction with the basic amino acids-rich domain of the enzyme – position the SWCNT in the proximity of the catalytic site. It is likely that oxidative biodegradation of pristine SWCNT is initiated by the hypochlorite-driven carboxylation that primes them for binding with the enzyme and subsequent degradation that also involves the reactive intermediates of the enzyme. Notably, the biodegradation products were ineffective – in contrast to SWCNT – at inducing pulmonary inflammatory responses in mice upon pharyngeal aspiration (Kagan et al., 2010). Importantly, this propensity of MPO to oxidatively biodegrade SWCNT was also realized in activated human neutrophils in vitro — suggesting that MPO-driven reactions may represent an important biodegradation pathway.
Indeed, our recent studies using MPO-deficient mice provided direct evidence for the effectiveness of this mechanism in vivo (Shvedova et al., 2012). We found that clearance of SWCNT from the lung of MPO knockout mice was markedly compromised compared to wild-type animals. It is likely that two major pathways – hypochlorite generated by the enzyme and MPO reactive intermediates – are both involved in the oxidative biodegradation process (Bianco et al., 2011; Shvedova et al., 2012; Vlasova et al., 2011). Overall, these experiments identified a previously unknown role of MPO in the body to oxidatively biodegrade SWCNT. It has been demonstrated that other carbonaceous nanoparticles — multi-walled carbon nanotubes, fullerenes, and graphene may also undergo MPO-catalyzed modifications and degradation (Bianco et al., 2011). Given that fate and distribution of CNT in the body are defined, to a large extent, by their high aspect ratios (Donaldson et al., 2011), oxidative shortening of CNT should inevitably affect their toxicity. This warrants further studies of specific interactions of different types of oxidative reactions involved in oxidative stress and oxidative biodegradation of nanoparticles by inflammatory responses of immune cells.
Unlike NADPH oxidase-deficient individuals who suffer from life-threatening infections, persons with MPO deficiency are relatively unaffected apart from an increased susceptibility to Candida albicans, a common fungal pathogen (Hampton et al., 1998) suggesting an auxiliary role for MPO in immunity against infections. One may speculate that the catalytic competence of MPO towards CNT is a reflection of a very ancient role of this enzyme towards carbonaceous particles generated during food cooking, high temperature processing of metals, forest fires, volcanic eruptions, etc. to which animals and humans have been exposed from primordial times.
Several other peroxidase – lactoperoxidase, eosinophil peroxidase, thyroid peroxidase, and prostaglandin synthase – are activated by H2O2 to form intermediates with the oxidation capacities high enough to degrade carbonaceous nanomaterials. In addition, several other hemo-proteins in animal and plant tissues (e.g., hemoglobin and myoglobin, cytochromes P450, horseradish peroxidase) are also known to generate highly oxidizing reactive intermediates. Indeed, several of these enzymes have been shown to effectively degrade CNT (Allen et al., 2008, 2009; Vlasova et al., 2011). The initiation and specific mechanisms of these oxidation reactions may differ in different oxidizing systems. For example, pristine SWCNT demonstrates no degradation with horseradish peroxidase (HRP) but displays significant degradation by either hemin or FeCl3. This suggests that a heterolytic mechanism of cleavage of H2O2 (Belikova et al., 2009b) by HRP is not effective towards pristine nanotubes, whereas Fenton catalysis results in the homolytic cleavage of H2O2 producing free radicals that oxidize pristine SWCNTs.
In a recent study, Donaldson and co-workers reported on the durability of four types of CNTs in simulated biological fluid (so-called Gambles solution) and their subsequent pathogenicity in vivo using a mouse model sensitive to inflammogenic effects of fibers (Osmond-McLeod et al., 2011). The results supported the view that CNTs are generally durable but may be subject to bio-modification in a sample-specific manner. This study also indicated that durable carbon nanotubes that are either short or form tightly bundled aggregates with no isolated long fibers are less inflammogenic in fiber-specific assays. In a related study, Liu et al. (2010) reported that SWCNTs with carboxylated surfaces are unique in their ability to undergo 90-day degradation in vitro in an artificial phagolysosomal simulant fluid leading to length reduction and accumulation of ultrafine solid carbonaceous debris. Unmodified, ozone-treated, and aryl-sulfonated tubes did not degrade under these conditions. The authors attributed the difference to the specific chemistry of acid carboxylation, which not only introduces COOH surface groups, but also causes collateral damage to the tubular graphenic backbone in the form of neighboring active sites that provide points of attack for further oxidative degradation.
Finally, not only SWCNTs but also other types of carbonaceous nanomaterials such as multi-walled CNT are sensitive to oxidants produced by peroxidases (Russier et al., 2011; Zhao et al., 2011).
A recent study demonstrated that two-dimensional graphitic carbon – a new material with many emerging applications – also undergoes enzymatic oxidation by HRP (Kotchey et al., 2011). Overall, the discovery of enzymatic biodegradation of carbonaceous nanomaterials by peroxidases emphasizes the general thesis of the current review that “oxidative stress” is not a uniform platform that is indiscriminatively responsible for toxic effects of nanoparticles but should be viewed as an array of regulatable oxidative reactions. A better understanding of the nature and significance of such oxidative (signaling) responses will offer new opportunities to control the distribution, degradation and clearance of nanoparticles as well as their toxicity.
Oxidative stress as a signaling component in phagocytic cells: inflammasome activation
The dual role of ROS is particularly evident when considering the function of phagocytic cells of the innate immune system. Hence, direct oxidative damage is involved in the killing of microorganisms and chronic granulomatous disease (CGD) patients lacking a functional NADPH oxidase, the major source of ROS in phagocytic cells, are at risk of life-threatening bacterial and fungal infections (van den Berg et al., 2009). However, phagocyte-derived ROS are also known to injure human tissues and to contribute to inflammation. Therefore, if one focuses on non-specific “oxidative stress” as a main culprit underlying toxicity of nanoparticles or other exogenous agents one may overlook other, vital signaling functions of reactive oxygen and nitrogen species, including the protective role of the so-called “oxidative burst” in phagocytic cells engaged in the engulfment and degradation of microorganisms. Of note, when mice were exposed to SWCNT and then challenged with Listeria monocytogenes this sequential exposure amplified lung inflammation (Shvedova et al., 2008b). However, despite the robust inflammatory response, SWCNT pre-exposure was associated with decreased phagocytosis of bacteria by macrophages in the lung and a decrease in nitric oxide production by these phagocytes. It is intriguing to speculate that the preoccupation with SWCNT leads to an inability of alveolar macrophages to handle subsequent exposure to microorganisms. The study suggests that exposure to CNT may exacerbate infections in exposed individuals.
One-half of the Nobel Prize in Physiology or Medicine in 2011 was awarded to Beutler and Hoffmann for their work on toll-like receptors (TLRs), cell surface proteins that provide a broad, first-line defense against microbial pathogens. Activation of the so-called inflammasome complex in the cytoplasm of phagocytic cells occurs via engagement of TLRs leading to subsequent assembly of the NLRP3 (NLR-related protein 3)-containing inflammasome complex and activation of caspase-1 with processing and secretion of the pro-inflammatory cytokine, interleukin (IL)-1β (Dagenais et al., 2012). ROS production is thought to be an essential requirement for inflammasome activation. NLRP3 is also activated in response to host-derived particulate matter precipitates such as uric acid and cholesterol crystals and studies in recent years have shown that exogenous structures including asbestos fibers and crystalline silica also activate the inflammasome (Cassel et al., 2008; Dostert et al., 2008). Furthermore, very recent studies have shown that long and needle-like MWCNT also can activate the NLRP3 inflammasome, at least in vitro (Palomäki et al., 2011). How do CNT activate the inflammasome? One possibility is that phagocytic cells attempt to engulf the fiber-like structures leading to activation of the NADPH oxidase and production of ROS with or without internalization of the CNT. However, the role of NADPH oxidase-derived ROS for inflammasome activation has been called into question (van de Veerdonk et al., 2010) and an alternative hypothesis is therefore that nanotubes are recognized by phagocytes not as single fibers but as entangled particles leading to their internalization via endocytosis with subsequent fusion with lysosomes within the cell. The ensuing disruption of lysosomes may lead to the release of cathepsins and activation of the inflammasome. Notably, Zhou and colleagues reported recently that mitophagy/autophagy blockade leads to the accumulation of damaged, ROS-generating mitochondria, and this in turn activates the NLRP3 inflammasome (Zhou et al., 2011). One may thus speculate that autophagy blockade by nanoparticles (Ma et al., 2011) may provide an alternative pathway for inflammasome activation. The contribution of mitochondria-generated versus NADPH oxidase-generated ROS for nanomaterial-induced activation of the inflammasome merits further investigation.
The work cited above on the interaction of needle-like CNT with immune cells highlights a non-trivial point: all “carbon nanotubes” are not alike. Hence, these materials may be long or short, thin or wide, aggregated or well-dispersed, purified or raw (containing other contaminants), pristine or functionalized (Shvedova et al., 2009). Moreover, it remains possible that different properties of CNTs could underlie different toxicities, e.g. the length (high aspect ratio) may be an important determinant for frustrated phagocytosis/inflammation (Poland et al., 2008) whereas the diameter/rigidity may be critical for mesothelial cell damage and mesothelioma induction (Nagai et al., 2011). In the former case, toxicity may conceivably result from phagocytosis (or an attempt by macrophages to engulf the foreign material) leading to ROS production and inflammation. In the latter case, physical interference with cells i.e. the needle-like piercing of the plasma membrane may serve as the main driving mechanism of toxicity. In a very recent study, Fenoglio et al. (2012) provided evidence that the thickness of MWCNT plays a role in lung toxicity following intratracheal instillation in rats. They found that thin MWCNT appeared significantly more toxic than the thicker ones, both in vitro and in vivo, when compared on a mass-dose basis. Both forms of CNT were highly purified.
Other examples of non-oxidative stress mediated toxicities of nanomaterials are discussed below.
Non-oxidative stress-related mechanisms of nanoparticle-induced cellular damage
The data on selective phospholipid peroxidation induced upon exposure to CNT (Tyurina et al., 2011b) strongly suggest that oxidative damage is associated to induction of the apoptotic process. However, substantial cellular damage in the absence of any sign of oxidative stress or apoptosis has also been reported (Tabet et al., 2009). Interestingly, in the latter study, no evidence of cellular internalization of the CNT was presented. Research aimed at the elucidation of alternative, non-oxidative stress mediated pathways of cellular damage is therefore warranted. Some data are available and may be of help in orienting future studies. One potentially relevant mechanism of damage is represented by the physical interference of CNT with cellular and extracellular constituents, which may cause alterations of vital cellular processes, leading to various degrees of cellular injury, and in some cases even to cell death. Sargent et al. reported that the physical similarity of SWCNT to cellular microtubules forming the mitotic spindle was the cause of significant cytotoxicity in human airway epithelial cells exposed to this nanomaterial (Sargent et al., 2009). SWCNTs were in fact incorporated into the centrosome structure and induced DNA damage. Notably, these findings were observed at doses of SWCNT compatible with those expected in an occupational setting (Sargent et al., 2011). Another example of physical interference of carbon nanotubes with intracellular structures has been reported by Holt et al. who found that the intracellular presence of SWCNT caused the assembly of actin filaments into bundles with strong impairment in proliferative activity (Holt et al., 2010). This is not surprising, because the actin cytoskeleton is essential in maintaining cellular shape and in performing cellular functions (Heng and Koh, 2010). These findings are in keeping with previous experiments, in which profound alterations of the actin cytoskeleton of mesothelioma cells were seen after 5 day exposure to 15 μg/mL SWCNT, whereas milder changes were observed in epithelial cells (Kaiser et al., 2008). The outcome of these experiments was dependent on CNT quality (i.e. whether the materials were purified or not).
The cell may be viewed essentially as a collection of “nanomachines” as many enzymes possess nano-scale dimensions (van den Heuvel and Dekker, 2007). It is therefore perhaps not surprising that engineered nanomaterials may interfere directly with cellular functions. Indeed, Park et al. (2003) reported that SWCNTs of certain dimensions act as ion channel blockers. The authors speculated that nanotubes dampened receptor function by fitting into the pore and thus either hindering ion movement or alternatively preventing further conformational changes. Using large-scale molecular dynamics simulations, other investigators have shown that SWCNT can plug into the hydrophobic core of proteins to form stable complexes, leading to the disruption of ligand binding and loss of protein function (Zuo et al., 2010). Similarly, using a docking algorithm, Calvaresi and Zerbetto (2010) provided evidence for the interaction of C60 fullerenes with a range of proteins. The authors proposed that the findings could be exploited for future biomedical applications of fullerenes, but the information may also shed light on the potential toxicity of nanomaterials. Overall, the above mentioned studies suggest that nanomaterials may exert biological or toxicological effects through direct physical interaction.
SWCNT may also cause the appearance of unusual ultrastructural modifications in cells. Mangum et al. have shown in an in vivo study the presence of “carbon bridges” between alveolar macrophages in rats intratracheally instilled with SWCNT, in the absence of any sign of inflammation (Mangum et al., 2006). These peculiar structures were not observed after exposure with other carbonaceous material. It has been hypothesized that carbon bridges could affect the phagocytic function of macrophages or inhibit their ability to release or respond to cytokines. However, the process underlying this intercellular rearrangement remains elusive. Our studies have shown that primary human macrophages exposed to SWCNT exhibit a decreased ability to engulf apoptotic cells in the absence of overt cytotoxicity (Witasp et al., 2009). This could potentially be explained by effects of the nanomaterials on cellular architecture especially on the actin cytoskeleton.
Adsorption of protein constituents of cellular plasma membrane onto the CNT surface, with subsequent loss of membrane integrity and cellular damage, is suggested by a recent study of mouse macrophages exposed to MWCNT (Hirano et al., 2008). The relevance of this mechanism is questionable, however, since serum proteins are likely adsorbed onto nanomaterials in vivo so that cells are not directly exposed to the surface of nanoparticles. In fact, using both experimental and theoretical approaches Ge et al. show that binding of blood proteins to CNT strongly reduces their toxicity approaches (Ge et al., 2011). Further analysis of the protein “corona” on various nanoparticles, including MWCNT, was reported recently by Sund et al. (2011) who found that adsorbed proteins derived from plasma are involved in cellular internalization and seem to cover the nanomaterials independently of their surface properties while the binding efficiency to cell lysate proteins (derived from the cell) appears to depend on the characteristics of the nanomaterial surface. Overall, these studies elucidate the importance of understanding how cells “see” nanomaterials (Walczyk et al., 2010).
Other investigators have recently reported that pulmonary phospholipid coating may alter the plasma protein coating of functionalized MWCNT. This change in the pattern of adsorbed proteins is hypothesized to influence significantly the interaction and subsequent effects of the MWCNT on biological systems (Gasser et al., 2010; Schleh et al., 2011).
Notably, the corona of proteins and/or lipids may vary depending on the biological compartment, e.g. the blood stream versus lungs or the GI-tract.
Interactions of nanoparticles with cells of the innate immune system and the role of oxidants as signaling molecules were discussed in previous sections. Can nanomaterials also impact on cells of the adaptive immune system? Recent studies on SWCNT and MWCNT suggest that this is the case. Dendritic cells are professional antigen-presenting cells that play a key role in orchestrating immune responses and one-half of the Nobel Prize in Physiology or Medicine in 2011 was awarded to Steinman for his discovery of the dendritic cell (DC) and its role in adaptive immunity. Our recent work has shown that pulmonary exposure of mice to SWCNT leads to systemic immunosuppression as evidenced by decreased proliferative T cell responses in the spleen (Tkach et al., 2011). Notably, these effects were apparently mediated by a direct effect of SWCNT on DCs that had infiltrated into the lung upon exposure to the nanomaterials. In contrast, other investigators have previously reported that exposure of mice to MWCNT via inhalation involved activation of cyclooxygenase enzymes in the spleen in response to a signal from the lungs, likely transforming growth factor (TGF)-β1 (Mitchell et al., 2009). Spleen cells from exposed animals partially recovered their immune function when treated with ibuprofen, a drug that blocks the formation of cyclooxygenase enzymes. Hence, it appears that CNT may adversely affect systemic immune responses either directly (Tkach et al., 2011) or indirectly (Mitchell et al., 2009).
Omics approaches to understand nanoparticle-induced toxicity: the response of the Sybil
Ibis redibis non morieris in bello was the response (in its Latin translation) of the Sybil to a Roman soldier asking her about his fate in the war. However, the interpretation of the response depends on how it is written: if the comma is inserted before the word “non” it means: you will come back and will not die in war, but if the comma is after “non”, the meaning is the opposite: you will not come back and will die in war. Incidentally, the soldier was killed in war. In a similar manner, according to the proteomic response, two opposite outcomes may be predicted when challenging cells with CNT: apoptosis (i.e. death) or resistance to apoptosis (i.e. survival). In this case, it is an adjective and not a comma that determines the fate, as we shall explain below. However, before making explicit the adjective, it should be emphasized that the information provided by proteomic analysis of cells and tissues is far from being sybilline or cryptic; on the contrary, given the high level of complexity of the network of apoptosis regulation, the proteomic approach serves as an important aid in understanding the mechanisms behind the induction of susceptibility or resistance to cell death in response to exogeneous substances. Global proteomic analysis allows the mapping of protein expression data into relevant molecular interaction networks and may thus provide additional insights into nanomaterial effects on apoptosis signaling and other cellular pathways.
The most extensively studied protein families in the evaluation of the interaction of CNT with mammalian cells are the caspase family and p53. Caspases are aspartate-specific proteases that play a critical role in apoptosis and inflammation (Fadeel et al., 2000). p53 is a pro-apoptotic protein and a tumor suppressor and is considered as the “guardian of the genome” (Rufini and Melino, 2011). Most studies of cellular protein profiles performed to date show over-expression of the p53 dependent and/or the caspase-dependent pro-apoptotic pathway after exposure to CNT (Cui et al., 2005; Pacurari et al., 2008; Ravichandran et al., 2010; Teeguarden et al., 2011; Yuan et al., 2011). This is generally associated with evidence of cellular oxidative stress and also with upregulation of genes involved in oxidative stress or with upregulation of oxidative stress sensitive proteins (Pacurari et al., 2008; Ravichandran et al., 2010; Teeguarden et al., 2011; Yuan et al., 2011). Thus, these proteomics data support the concept that apoptosis and oxidative stress are tightly interconnected events in response to CNT exposure (Fig. 2). It should be noted, however, that the experimental studies above refer to models of acute exposure, whereas in human populations, such as workers and consumers, a chronic low-dose pattern of exposure is expected. This gap is filled by a recent study in which an in vitro model of chronic exposure to SWCNT was applied (Wang et al., 2011). Interestingly, the findings are in sharp contrast to the acute exposure studies, showing not only that chronically exposed cells (i.e. 24 weeks of exposure, at a subcytotoxic dose of 0.02 μg/cm2) do not develop apoptosis, but also that these cells become apoptosis resistant. Protein array analysis of human lung epithelial cells BEAS-2B, showed a distinctive protein profile of apoptosis proteins, and in particular a striking decrease in the expression of phospho-p53 (Ser 15) protein, which is critical for p53-associated apoptosis (She et al., 2000). Testing SWCNT-exposed cells for sensitivity to known apoptotic inducers, such as etoposide and TNF-α, confirmed the acquired resistance to apoptosis. Thus, the critical adjective determining the cell’s fate after exposure to CNT has perhaps been found: acute exposure causes increased apoptosis, chronic exposure apoptosis resistance. Of note, the aberrant survival of cells that have sustained DNA damage may produce adverse effects (cancer).
Fig. 2.
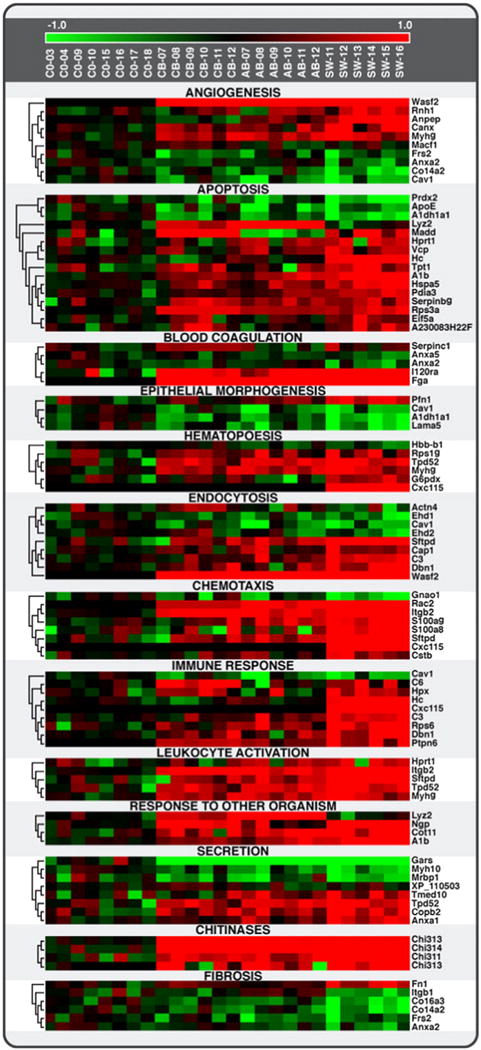
Comparative proteomics of instilled single-walled carbon nanotubes, crocidolite asbestos, and ultrafine carbon black in mice. Heat map showing protein levels for 13 cellular processes (GO functional categories) related to inflammation/immune response, fibrosis, and tissue remodeling in response to SWCNT, AB, and UFCB. SWCNT appeared to have the strongest effect on protein levels for most biological processes. Among the 109 proteins uniquely affected by SWCNT exposure were some related to the GO functional category “cell redox homeostasis”, suggesting a high level of oxidative stress, either directly from the SWCNT or indirectly from the resulting inflammation.
Reprinted from Teeguarden et al. (2011) with permission from Oxford University Press.
Transcriptomic analysis of mRNA expression has also been applied to understand cellular and tissue responses to nanomaterials. For instance, in an attempt to obtain a molecular characterization of the cytotoxic mechanism of MWCNT versus so-called multi-walled carbon nano-onions (MWCNO), Ding et al. (2005) performed whole genome expression analysis and high-content image analysis-based phenotypic measurements on exposed human fibroblasts. The micorarray analysis indicated that multiple cellular pathways are perturbed after exposure to the nanomaterials, with material-specific toxigenomic profiles observed. Promoter analysis of the microarray results demonstrated that interferon and p38/ERK–MAPK cascades are critical pathway components contributing to the more adverse effects observed upon exposure to MWCNT as compared to MWCNO. Similarly, Zhang et al. (2006) performed genome-wide expression analysis of human fibroblasts exposed to PEG-coated silanized quantum dots and found that the quantum dots, unlike CNT, do not activate genes indicative of a strong immune and inflammatory response or heavy-metal-related toxicity. In a more recent study, the concentration-dependent cytotoxicity of SWCNT and SWCNT functionalized with polyethylene glycol (SWCNT-PEG) was compared in neuronal PC12 cells (Zhang et al., 2011). SWCNT elicited cytotoxicity in a concentration-dependent manner while SWCNT-PEG was less cytotoxic. ROS were generated after exposure to both nanomaterials. Gene expression analysis using a real-time PCR array showed that the genes involved in oxidoreductases and antioxidant activity, nucleic acid or lipid metabolism, and mitochondria dysfunction were highly represented (Fig. 3). The alterations of genes were surface coating-dependent. The findings suggest that surface functionalization of SWCNT decreases the oxidative stress-associated response. We have found that the exposure of primary human immune-competent cells to cytotoxic ZnO nanoparticles results in upregulation of genes encoding metallothioneins, low-molecular weight proteins with metal-binding capacity and antioxidant properties (unpublished observations). The results are not unexpected in light of the fact that ZnO nanoparticles undergo dissolution when immersed in cell culture.
Fig. 3.
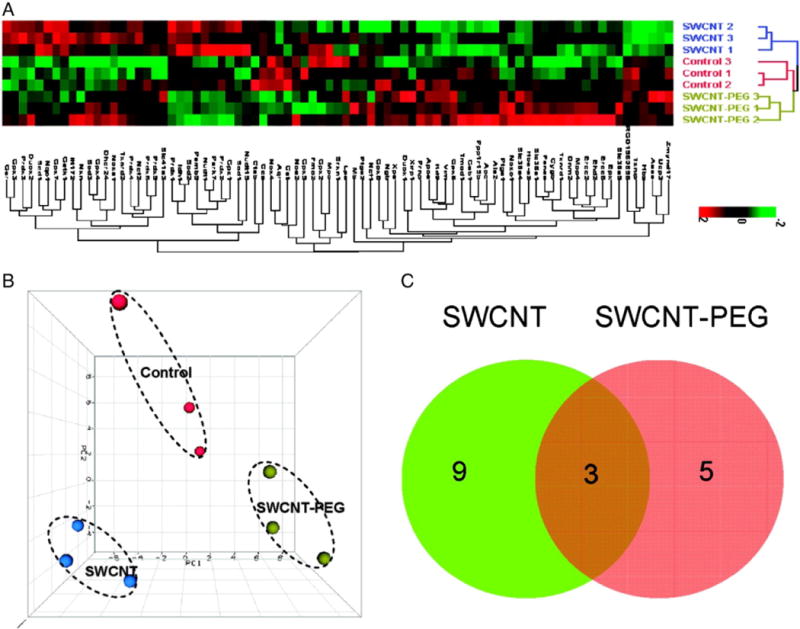
Transcriptomic assessment of cytotoxic effects of SWCNT. The effect of surface modification of SWCNT on oxidative stress genes was determined using real-time PCR array to evaluate 84 genes related to oxidative stress. (A) Hierarchical cluster analysis (HCA) and (B) principal component analysis (PCA) of nine array data from neuronal PC12 cells treated with SWCNT (blue dots), SWCNT-PEG (green dots), and vehicle (red dots). (C) Venn diagram of the genes significantly changed by SWCNT or SWCNT-PEG treatment.
Reprinted from Zhang et al. (2011) with permission from American Chemical Society.
Conclusions
Nanoparticle toxicity may be understood in the context of the oxidative stress paradigm. However, this statement is only true insofar as we understand the meaning of “oxidative stress”. In the present review, we have emphasized that oxidative stress does not necessarily mean non-discriminative production of oxygen radicals with ensuing nonspecific damage to cells and tissues. Instead, the induction of oxidative stress and related signaling pathways in response to nanoparticle exposure may also be viewed as a non-random event resulting in specific (programmed) cellular fates, e.g. apoptosis. Understanding the pathways leading to oxidative stress and the consequences thereof may yield novel approaches to mitigate the toxicity of engineered nanoparticles. To this end, it is important not only to emphasize the need for careful physico-chemical characterization of the nanomaterials, but also to consider cell type-specific differences in responses to nanomaterial exposure. We have also discussed examples of non-oxidative stress-mediated cellular damage resulting, for instance, from direct physical interference with the nano-scale machineries of cells. Fig. 4 illustrates oxidative stress dependent pathways and alternative, non-oxidative stress mediated pathways of nanoparticle toxicity.
Fig. 4.
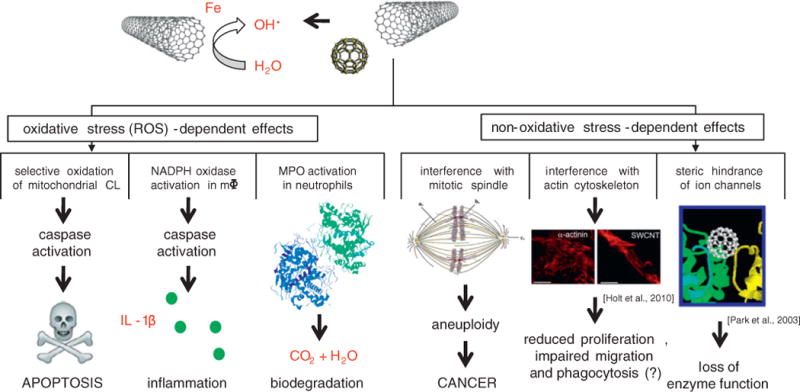
Schematic figure depicting reactive oxygen species (ROS)-dependent and ROS-independent pathways of cellular toxicity induced by nanoparticles. Consult text for details.
We have also presented recent evidence for the enzymatic biodegradation of engineered nanoparticles and attempted to elucidate the importance of considering interactions of nanoparticles with the immune system, our principal defense system against foreign intrusion. Cells of the innate system are equipped with oxidative degradative machinery to dismantle microorganisms and several studies have shown that similar mechanisms may also be harnessed for the degradation of engineered nanomaterials.
Finally, we have highlighted the potential of global omics approaches (e.g. lipidomics, proteomics, transcriptomics) for the understanding of biological interactions of nanomaterials. The latter approaches may not only lead to the generation of new hypotheses regarding bio-nano-interactions but could also yield biomarkers of human exposure to engineered nanomaterials.
Acknowledgments
The authors are supported, in part, by grants from the NIOSH OH008282, the NORA 0HELD015, the NIEHS R01ES019304, the Swedish Research Council for Environment, the Agricultural Sciences and Spatial Planning (FORMAS), and the European Commission (FP7-MARINA, grant agreement no. 263215).
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Conflict of interest
None.
References
- Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4:158–170. doi: 10.2174/1874609811104020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich TK, Gustave J, Hall CB, Cohen HW, Webber MP, Zeig-Owens R, Cosenza K, Christodoulou V, Glass L, Al-Othman F, Weiden MD, Kelly KJ, Prezant DJ. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362:1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BL, Kichambare PD, Gou P, Vlasova II, Kapralov AA, Konduru N, Kagan VE, Star A. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8:3899–3903. doi: 10.1021/nl802315h. [DOI] [PubMed] [Google Scholar]
- Allen BL, Kotchey GP, Chen Y, Yanamala NV, Klein-Seetharaman J, Kagan VE, Star A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J Am Chem Soc. 2009;131:17194–17205. doi: 10.1021/ja9083623. [DOI] [PubMed] [Google Scholar]
- Ardail D, Privat JP, Egret-Charlier M, Levrat C, Lerme F, Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J Biol Chem. 1990;265:18797–18802. [PubMed] [Google Scholar]
- Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, Pearce L, Peterson J, Huang Z, Jiang J, Samhan-Arias AK, Maeda A, Feng W, Wasserloos K, Belikova NA, Tyurin VA, Wang H, Fletcher J, Wang Y, Vlasova II, Klein-Seetharaman J, Stoyanovsky DA, Bayir H, Pitt BR, Epperly MW, Greenberger JS, Kagan VE. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nat Commun. 2011;2:497–505. doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- Azzi A. Oxidative stress: a dead end or a laboratory hypothesis? Biochem Biophys Res Commun. 2007;362:230–232. doi: 10.1016/j.bbrc.2007.07.124. [DOI] [PubMed] [Google Scholar]
- Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek PM, DeKosky ST, Greenberger JS, Shvedova AA, Kagan VE. Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria. Biochim Biophys Acta. 2006;1757:648–659. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, Amoscato AA, Zhao Q, Zhang XJ, Janesko-Feldman KL, Alexander H, Basova LV, Clark RS, Kochanek PM, Kagan VE. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann Neurol. 2007;62:154–169. doi: 10.1002/ana.21168. [DOI] [PubMed] [Google Scholar]
- Belikova NA, Jiang J, Stoyanovsky DA, Glumac A, Bayir H, Greenberger JS, Kagan VE. Mitochondria-targeted (2-hydroxyamino-vinyl)-triphenyl-phosphonium releases NO(.) and protects mouse embryonic cells against irradiation-induced apoptosis. FEBS Lett. 2009a;583:1945–1950. doi: 10.1016/j.febslet.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belikova NA, Tyurina YY, Borisenko G, Tyurin V, Samhan, Arias AK, Yanamala N, Furtmuller PG, Klein-Seetharaman J, Obinger C, Kagan VE. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: antioxidant function in mitochondria. J Am Chem Soc. 2009b;131:11288–11289. doi: 10.1021/ja904343c. [DOI] [PubMed] [Google Scholar]
- Bello D, Wardie BL, Yamamoto N, deVilloria RG, Garcia EJ, Hart AJ, Ahn K, Ellenbecker MJ, Hallock M. Exposure to nanoscale particles and fibres during machining of hybrid advanced composites containing carbon nanotubes. J Nanopart Res. 2009;11:231–249. [Google Scholar]
- Bianco A, Kostarelos K, Prato M. Making carbon nanotubes biocompatible and biodegradable. Chem Commun (Camb) 2011;47:10182–10188. doi: 10.1039/c1cc13011k. [DOI] [PubMed] [Google Scholar]
- Blanksby SJ, Mitchell TW. Advances in mass spectrometry for lipidomics. Annu Rev Anal Chem (Palo Alto Calif) 2010;3:433–465. doi: 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- Cabezon E, Lanza VF, Arechaga I. Membrane-associated nanomotors for macromolecular transport. Curr Opin Biotechnol. 2011 Dec 19; doi: 10.1016/j.copbio.2011.11.031. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Calvaresi M, Zerbetto F. Baiting proteins with C60. ACS Nano. 2010;4:2283–2299. doi: 10.1021/nn901809b. [DOI] [PubMed] [Google Scholar]
- Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Tian F, Kong Y, Titushikin I, Gao H. Effects of single-walled carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Dagenais M, Skeldon A, Saleh M. The inflammasome: in memory of Dr. Jürg Tschopp. Cell Death Differ. 2012;19:5–12. doi: 10.1038/cdd.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Stilwell J, Zhang T, Elboudwarej O, Jiang H, Selegue JP, Cooke PA, Gray JW, Chen FF. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005;5:2448–2464. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety-by-design. Nanomedicine (Lond) 2011;6:143–156. doi: 10.2217/nnm.10.139. [DOI] [PubMed] [Google Scholar]
- Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen EA, Costello S, Chevrier J, Picciotto S. Epidemiologic challenges for studies of occupational exposure to engineered nanoparticles: a commentary. J Occup Environ Med. 2011;53(Suppl):S57–S61. doi: 10.1097/JOM.0b013e31821bde98. [DOI] [PubMed] [Google Scholar]
- Emanuel NM. Free radicals and the action of inhibitors of radical processes under pathological states and ageing in living organisms and in man. Q Rev Biophys. 1976;9:283–308. doi: 10.1017/s0033583500002420. [DOI] [PubMed] [Google Scholar]
- Fadeel B. Babies born without safety NET. Blood. 2009;113:6270–6271. doi: 10.1182/blood-2009-03-210328. [DOI] [PubMed] [Google Scholar]
- Fadeel B, Kagan VE. Apoptosis and macrophage clearance of neutrophils: regulation by reactive oxygen species. Redox Rep. 2003;8:143–150. doi: 10.1179/135100003225001511. [DOI] [PubMed] [Google Scholar]
- Fadeel B, Orrenius S, Zhivotovsky B. The most unkindest cut of all: on the multiple roles of mammalian caspases. Leukemia. 2000;14:1514–1525. doi: 10.1038/sj.leu.2401871. [DOI] [PubMed] [Google Scholar]
- Fazil M, Shadab, Baboota S, Sahni JK, Ali J. Nanotherapeutics for Alzheimer’s disease (AD): past, present and future. J Drug Target. 2012;20:97–113. doi: 10.3109/1061186X.2011.607499. [DOI] [PubMed] [Google Scholar]
- Fenoglio I, Aldieri E, Gazzano E, Cesano F, Colonna M, Scarano D, Mazzucco G, Attanasio A, Yakoub Y, Lison D, Fubini B. Thickness of multiwalled carbon nanotubes affects their lung toxicity. Chem Res Toxicol. 2012;25:74–82. doi: 10.1021/tx200255h. [DOI] [PubMed] [Google Scholar]
- Fenoglio I, Greco G, Tomatis M, Muller J, Raymundo-Pinero E, Beguin F, Fonseca A, Nagy JB, Lison D, Fubini B. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: toxicological aspects. Chem Res Toxicol. 2008;21:1690–1697. doi: 10.1021/tx800100s. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Gasser M, Rothen-Rutishauser B, Krug HF, Gehr P, Nelle M, Yan B, Wick P. The adsorption of biomolecules to multi-walled carbon nanotubes is influenced by both pulmonary surfactant lipids and surface chemistry. J Nanobiotechnol. 2010;8:31. doi: 10.1186/1477-3155-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Du J, Zhao L, Wang L, Liu Y, Lia D, Yang Y, Zhou R, Zhao Y, Chai Z, Chen C. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci USA. 2011;108:16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Terror in the dustSept 7. 2011 http://cnnpressroom.blogs.cnn.com/2011/09/072011.
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Han JH, Lee EJ, Lee JH, So KP, Lee YH, Bae GN, Lee SB, Ji JH, Cho MH, Yu IJ. Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal Toxicol. 2008;20:741–749. doi: 10.1080/08958370801942238. [DOI] [PubMed] [Google Scholar]
- Heng YW, Koh CG. Actin cytoskeleton dynamics and the cell division cycle. Int J Biochem Cell Biol. 2010;42:1622–1633. doi: 10.1016/j.biocel.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Hirano S, Kanno S, Furuyama A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharmacol. 2008;232:244–251. doi: 10.1016/j.taap.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Holt BD, Short PA, Rapet AD, Wang YL, Islam MF, Dahl KN. Carbon nanotubes reorganize actin structures in cells and ex vivo. ACS Nano. 2010;4:4872–4878. doi: 10.1021/nn101151x. [DOI] [PubMed] [Google Scholar]
- Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- Hsu FF, Turk J. Electrospray ionization with low-energy collisionally activated dissociation tandem mass spectrometry of glycerophospholipids: mechanisms of fragmentation and structural characterization. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2673–2695. doi: 10.1016/j.jchromb.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Jiang J, Tyurin VA, Zhao Q, Mnuskin A, Ren J, Belikova NA, Feng W, Kurnikov IV, Kagan VE. Cardiolipin deficiency leads to decreased cardiolipin peroxidation and increased resistance of cells to apoptosis. Free Radic Biol Med. 2008;44:1935–1944. doi: 10.1016/j.freeradbiomed.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova PT, Milne SB, Myers DS, Brown HA. Lipidomics: a mass spectrometry based systems level analysis of cellular lipids. Curr Opin Chem Biol. 2009;13:526–531. doi: 10.1016/j.cbpa.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola MS, Sripaiboonkij P, Jaakkola JJ. Effects of occupational exposures and smoking on lung function in tile factory workers. Int Arch Occup Environ Health. 2011;84:151–158. doi: 10.1007/s00420-010-0603-6. [DOI] [PubMed] [Google Scholar]
- Jiang J, Serinkan BF, Tyurina YY, Borisenko GG, Mi Z, Robbins PD, Schroit AJ, Kagan VE. Peroxidation and externalization of phosphatidylserine associated with release of cytochrome c from mitochondria. Free Radic Biol Med. 2003;35m:814–825. doi: 10.1016/s0891-5849(03)00429-5. [DOI] [PubMed] [Google Scholar]
- Jiang J, Huang Z, Zhao Q, Feng W, Belikova NA, Kagan VE. Interplay between bax, reactive oxygen species production, and cardiolipin oxidation during apoptosis. Biochem Biophys Res Commun. 2008;368:145–150. doi: 10.1016/j.bbrc.2008.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, Kapralova V, Huang Z, Mintz AH, Greenberger JS, Kagan VE. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172:706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE. Lipid Peroxidation in Biomembranes. CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- Kagan VE, Quinn PJ. Toward oxidative lipidomics of cell signaling. Antioxid Redox Signal. 2004;6:199–202. doi: 10.1089/152308604322899260. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Gleiss B, Tyurina YY, Tyurin VA, Elenstrom-Magnusson C, Liu SX, Serinkan FB, Arroyo A, Chandra J, Orrenius S, Fadeel B. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002;169:487–499. doi: 10.4049/jimmunol.169.1.487. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Borisenko GG, Serinkan BF, Tyurina YY, Tyurin VA, Jiang J, Liu SX, Shvedova AA, Fabisiak JP, Uthaisang W, Fadeel B. Appetizing rancidity of apoptotic cells for macrophages: oxidation, externalization, and recognition of phosphatidylserine. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1–L17. doi: 10.1152/ajplung.00365.2002. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, Kisin ER, Schwegler-Berry D, Mercer R, Castranova V, Shvedova AA. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett. 2006;165:88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Bayir A, Bayir H, Stoyanovsky D, Borisenko GG, Tyurina YY, Wipf P, Atkinson J, Greenberger JS, Chapkin RS, Belikova NA. Mitochondria-targeted disruptors and inhibitors of cytochrome c/cardiolipin peroxidase complexes: a new strategy in anti-apoptotic drug discovery. Mol Nutr Food Res. 2009a;53:104–114. doi: 10.1002/mnfr.200700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Wipf P, Stoyanovsky D, Greenberger JS, Borisenko G, Belikova NA, Yanamala N, Samhan, Arias AK, Tungekar MA, Jiang J, Tyurina YY, Ji J, Klein-Seetharaman J, Pitt BR, Shvedova AA, Bayir H. Mitochondrial targeting of electron scavenging antioxidants: regulation of selective oxidation vs random chain reactions. Adv Drug Deliv Rev. 2009b;61:1375–1385. doi: 10.1016/j.addr.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi J, Kisin ER, Murray AR, Franks J, Stolz D, Gou P, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser JP, Wick P, Manser P, Spohn P, Bruinink A. Single walled carbon nanotubes (SWCNT) affect cell physiology and cell architecture. J Mater Sci Mater Med. 2008;19:1523–1527. doi: 10.1007/s10856-007-3296-y. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, Borm PJA, Albrecht C, Schins RPF. Inhaled particles and lung cancer. Part A: mechanisms Int J Cancer. 2004;109:799–809. doi: 10.1002/ijc.11708. [DOI] [PubMed] [Google Scholar]
- Kotchey GP, Allen BL, Vedala H, Yanamala N, Kapralov AA, Tyurina YY, Klein-Seetharaman J, Kagan VE, Star A. The enzymatic oxidation of graphene oxide. ACS Nano. 2011;5:2098–20108. doi: 10.1021/nn103265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic P, Somanathan R. Biomechanisms of nanoparticles (toxicants, antioxidants and therapeutics): electron transfer and reactive oxygen species. J Nanosci Nanotechnol. 2010;10:7919–7930. doi: 10.1166/jnn.2010.3028. [DOI] [PubMed] [Google Scholar]
- Kuhlbusch TA, Fissan H. Particle characteristics in the reactor and pelletizing areas of carbon black production. J Occup Environ Hyg. 2006;3:558–567. doi: 10.1080/15459620600912280. [DOI] [PubMed] [Google Scholar]
- Leucuta SE. Nanotechnology for delivery of drugs and biomedical applications. Curr Clin Pharmacol. 2010;5:257–280. doi: 10.2174/157488410793352003. [DOI] [PubMed] [Google Scholar]
- Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Muralikrishnan S, Ng CT, Yung LY, Bay BH. Nanoparticle-induced pulmonary toxicity. Exp Biol Med (Maywood) 2010;235:1025–1033. doi: 10.1258/ebm.2010.010021. [DOI] [PubMed] [Google Scholar]
- Liu X, Hurt RH, Kane AB. Biodurability of single-walled carbon nanotubes depends on surface functionalization. Carbon N Y. 2010;48:1961–1969. doi: 10.1016/j.carbon.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y, Yu L, Liang XJ. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano. 2011;5:8629–8639. doi: 10.1021/nn202155y. [DOI] [PubMed] [Google Scholar]
- Mangum JB, Turpin EA, Anta-Menezes A, Cesta MF, Bermudez E, Bonner JC. Single-walled carbon nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part Fibre Toxicol. 2006;3:15. doi: 10.1186/1743-8977-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Warheit DB, Philbert MA. The new toxicology of sophisticated materials: nanotoxicology and beyond. Toxicol Sci. 2011;120(Suppl. 1):S109–S129. doi: 10.1093/toxsci/kfq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani MS, Rafiei Y, Tzifa A, Seifalian AM. In situ endothelialization of intravascular stents from progenitor stem cells coated with nanocomposite and functionalized biomolecules. Biotechnol Appl Biochem. 2011;58:2–13. doi: 10.1002/bab.10. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Fonseca A, Nagy JB, Moreau N, Delos M, Raymundo-Pinero E, Beguin F, Kirsch-Volder M, Fenoglio I, Fubini B, Lison D. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: physicochemical aspects. Chem Res Toxicol. 2008;21:1698–1705. doi: 10.1021/tx800101p. [DOI] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H, Jiang L, Ohara H, Takahashi T, Ichihara G, Kostarelos K, Miyata Y, Shinohara H, Toyokuni S. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci USA. 2011;108:E1330–E1338. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Niki E. Assessment of antioxidant capacity of natural products. Curr Pharm Biotechnol. 2010;11:801–809. doi: 10.2174/138920110793262097. [DOI] [PubMed] [Google Scholar]
- NIOSH Publication Number 2011–197. First periodic review of cancer for the WTC health program. 2011 http://www.cdc.gov/niosh/topics/wtc/prc/prc-1.html2011.
- Oberdörster G. Nanotoxicology: in vitro–in vivo dosimetry. Environ Health Perspect. 2012;120:a13. doi: 10.1289/ehp.1104320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond-McLeod MJ, Poland CA, Murphy F, Waddington L, Morris H, Hawkins SC, Clark S, Aitken R, McCall MJ, Donaldson K. Durability and inflammogenic impact of carbon nanotubes compared with asbestos fibres. Part Fibre Toxicol. 2011;8:15. doi: 10.1186/1743-8977-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L. Interactions among antioxidants in health and disease: vitamin E and its redox cycle. Proc Soc Exp Biol Med. 1992;200:271–276. doi: 10.3181/00379727-200-43433. [DOI] [PubMed] [Google Scholar]
- Pacurari M, Yin XJ, Zhao J, Ding M, Steve SS, Schwegler-Berry D, Ducatman BS, Sbarra D, Hoover MD, Castranova V, Vallyathan V. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-κB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116:1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomäki J, Välimäki E, Sund J, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, Alenius H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Park KH, Chhowalla M, Iqbal Z, Sesti F. Single-walled carbon nanotubes are a new class of ion channel blockers. J Biol Chem. 2003;278:50212–50216. doi: 10.1074/jbc.M310216200. [DOI] [PubMed] [Google Scholar]
- Peters TM, Elzey S, Johnson R, Park H, Grassian VH, Maher T, O’Shaughnessy P. Airborne monitoring to distinguish engineered nanomaterials from incidental particles for environmental health and safety. J Occup Environ Hyg. 2009;6:73–81. doi: 10.1080/15459620802590058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JI, Green FY, Davies JC, Murray J. Pulmonary and systemic toxicity following exposure to nickel nanoparticles. Am J Ind Med. 2010;53:763–767. doi: 10.1002/ajim.20855. [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Praticò D. Prostanoid and isoprostanoid pathways in atherogenesis. Atherosclerosis. 2008;201:8–16. doi: 10.1016/j.atherosclerosis.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Pulskamp K, Diabaté S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168:58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ravichandran P, Baluchamy S, Sadanandan B, Gopikrishnan R, Biradar S, Ramesh V, Hall JC, Ramesh GT. Multiwalled carbon nanotubes activate NF-jB and AP-1 signaling pathways to induce apoptosis in rat lung epithelial cells. Apoptosis. 2010;15:1507–1516. doi: 10.1007/s10495-010-0532-6. [DOI] [PubMed] [Google Scholar]
- Ravichandran P, Baluchamy S, Gopikrishnan R, Biradar S, Ramesh V, Goornavar V, Thomas R, Wilson BL, Jeffers R, Hall JC, Ramesh GT. Pulmonary biocompatibility assessment of inhaled single-wall and multiwall carbon nano-tubes in BALB/c mice. J Biol Chem. 2011;286:29725–29733. doi: 10.1074/jbc.M111.251884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E. World Trade Center dust and 9/11 first responders with cancer. 2011 http://workerslawwatch.com/2011/09/study2011.
- Rufini A, Melino G. Cell death pathology: the war against cancer. Biochem Biophys Res Commun. 2011;414:445–450. doi: 10.1016/j.bbrc.2011.09.110. [DOI] [PubMed] [Google Scholar]
- Russier J, Ménard-Moyon C, Venturelli E, Gravel E, Marcolongo G, Meneghetti M, Doris E, Bianco A. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale. 2011;3:893–896. doi: 10.1039/c0nr00779j. [DOI] [PubMed] [Google Scholar]
- Sandin P, Fitzpatrick LW, Simpson JC, Dawson KA. High-speed imaging of rab family small GTPases reveals rare events in nanoparticle trafficking in living cells. ACS Nano. 2012;6:1513–1521. doi: 10.1021/nn204448x. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Shvedova AA, Hubbs AF, Salisbury JL, Benkovic SA, Kashon ML, Lowry DT, Murray AR, Kisin ER, Friend S, McKinstry KT, Battelli L, Reynolds SH. Induction of aneuploidy by single-walled carbon nanotubes. Environ Mol Mutagen. 2009;50:708–717. doi: 10.1002/em.20529. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Hubbs AF, Young SH, Kashon ML, Dinu CZ, Salisbury JL, Benkovic SA, Lowry DT, Murray AR, Kisin ER, Siegrist KJ, Battelli L, Mastovich J, Sturgeon JL, Bunker KL, Shvedova AA, Reynolds SH. Single-walled carbon nanotube-induced mitotic disruption. Mutat Res. 2011 Dec 11; doi: 10.1016/j.mrgentox.2011.11.017. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J Biol Chem. 1993;268:74–79. [PubMed] [Google Scholar]
- Schleh C, Rothen-Rutishauser B, Kreyling WG. The influence of pulmonary surfactant on nanoparticulate drug delivery systems. Eur J Pharm Biopharm. 2011;77:350e2. doi: 10.1016/j.ejpb.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She QB, Chen N, Dong ZJ. ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chem. 2000;275:20444–20449. doi: 10.1074/jbc.M001020200. [DOI] [PubMed] [Google Scholar]
- Shi J, Karlsson HL, Johansson K, Gogvadze V, Xiao L, Li J, Burks T, Garcia-Bennett A, Uheida A, Muhammed M, Mathur S, Morgenstern R, Kagan VE, Fadeel B. Microsomal glutathione transferase 1 protects against toxicity induced by silica nanoparticles but not by zinc oxide nanoparticles. ACS Nano. 2012 Feb 13; doi: 10.1021/nn2021056. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Murray AR, Gorelik O, Arepalli S, Castranova V, Young SH, Gao F, Tyurina YY, Ouri TD, Kagan VE. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol. 2007;221:339–348. doi: 10.1016/j.taap.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Murray AR, Kommineni C, Castranova V, Fadeel B, Kagan VE. Increased accumulation of neutrophils and decreased fibrosis in the lung of NADPH oxidase-deficient C57BL/6 mice exposed to carbon nanotubes. Toxicol Appl Pharmacol. 2008a;231:235–240. doi: 10.1016/j.taap.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Fabisiak JP, Kisin ER, Murray AR, Roberts JR, Tyurina YY, Antonini JM, Feng WH, Kommineni C, Reynolds J, Barchowsky A, Castranova V, Kagan VE. Sequential exposure to carbon nanotubes and bacteria enhances pulmonary inflammation and infectivity. Am J Respir Cell Mol Biol. 2008b;38:579–590. doi: 10.1165/rcmb.2007-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Porter D, Schulte P, Kagan VE, Fadeel B, Castranova V. Mechanisms of pulmonary toxicity and medical applications of carbon nanotubes: two faces of Janus? Pharmacol Ther. 2009;121:192–204. doi: 10.1016/j.pharmthera.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kagan VE, Fadeel B. Close encounters of the small kind: adverse effects of man-made materials interfacing with the nano-cosmos of biological systems. Annu Rev Pharmacol Toxicol. 2010;50:63–88. doi: 10.1146/annurev.pharmtox.010909.105819. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kapralov O, Feng W, Kisin ER, Murray A, Mercer R, St Croix C, Lang M, Watkins S, Konduru N, Allen B, Conroy J, Mohamed BM, Meade AD, Volkov Y, Star A, Fadeel B, Kagan VE. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS ONE. 2012;7(3):e30923. doi: 10.1371/journal.pone.0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: introductory remarks. In: Sies H, editor. Oxidative Stress. Academic Press; London: 1985. pp. 1–7. [Google Scholar]
- Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34:559–567. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- Song Y, Li X, Wang L, Rojanasakul Y, Castranova V, Li H, Ma J. Nanomaterials in humans: identification, characteristics, and potential damage. Toxicol Pathol. 2011;2011:841–849. doi: 10.1177/0192623311413787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparvero LJ, Amoscato AA, Kochanek PM, Pitt BR, Kagan VE, Bayir H. Mass-spectrometry based oxidative lipidomics and lipid imaging: applications in traumatic brain injury. J Neurochem. 2010;115:1322–1336. doi: 10.1111/j.1471-4159.2010.07055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund J, Alenius H, Vippola M, Savolainen K, Puustinen A. Proteomic characterization of engineered nanomaterial–protein interactions in relation to surface reactivity. ACS Nano. 2011;5:4300–4309. doi: 10.1021/nn101492k. [DOI] [PubMed] [Google Scholar]
- Tabet L, Bussy C, Amara N, Setyan A, Grodet A, Rossi MJ, Pairon JC, Boczkowski J, Lanone S. Adverse effects of industrial multiwalled carbon nanotubes on human pulmonary cells. J Toxicol Environ Health A. 2009;72:60–73. doi: 10.1080/15287390802476991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Webb-Robertson BJ, Waters K, Murray A, Kisin E, Varnum SM, Jacobs J, Pounds JG, Zanger R, Shvedova A. Comparative proteomics and pulmonary toxicity of instilled single walled carbon nanotubes, crocidolite asbestos and ultrafine carbon black in mice. Toxicol Sci. 2011;120:123–135. doi: 10.1093/toxsci/kfq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessonnier JP. Recent progress on the growth mechanism of carbon nanotubes: a review. ChemSusChem. 2011;4:824–847. doi: 10.1002/cssc.201100175. [DOI] [PubMed] [Google Scholar]
- Tkach AV, Shurin GV, Shurin MR, Kisin ER, Murray AR, Young SH, Star A, Fadeel B, Kagan VE, Shvedova AA. Direct effects of carbon nanotubes on dendritic cells induce immune suppression upon pulmonary exposure. ACS Nano. 2011;5:5755–5762. doi: 10.1021/nn2014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres Andon F, Fadeel B. Programmed cell death: molecular mechanisms and implications for safety assessment of nanomaterials. Acc Chem Res. doi: 10.1021/ar300020b. in press. [DOI] [PubMed] [Google Scholar]
- Toyama T, Matsuda H, Ishida I, Tani M, Kitaba S, Sano S, Katayama I. A case of toxic epidermal necrolysis-like dermatitis evolving from contact dermatitis of the hands associated with exposure to dendrimers. Contact Dermatitis. 2009;59:122–127. doi: 10.1111/j.1600-0536.2008.01340.x. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hofmann M, Hallock M, Ada E, Kong J, Ellenbecker M. Characterization and evaluation of nanoparticlerelease during the synthesis of single-walled and multiwalled carbon nanotubes by chemical vapour deposition. Environ Sci Technol. 2009;43:6017–6023. doi: 10.1021/es900486y. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Osipov AN, Belikova NA, Basova LV, Kapralov AA, Bayir H, Kagan VE. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Differ. 2007;14:872–875. doi: 10.1038/sj.cdd.4402068. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Jung MY, Tungekar MA, Wasserloos KJ, Bayir H, Greenberger JS, Kochanek PM, Shvedova AA, Pitt B, Kagan VE. Mass-spectrometric analysis of hydroperoxy- and hydroxy-derivatives of cardiolipin and phosphatidylserine in cells and tissues induced by pro-apoptotic and pro-inflammatory stimuli. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2863–2872. doi: 10.1016/j.jchromb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Ritov VB, Lysytsya A, Amoscato AA, Kochanek PM, Hamilton R, Dekosky ST, Greenberger JS, Bayir H, Kagan VE. Oxidative lipidomics of apoptosis: quantitative assessment of phospholipid hydroperoxides in cells and tissues. Methods Mol Biol. 2010;610:353–374. doi: 10.1007/978-1-60327-029-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Kawai K, Tyurin VA, Liu SX, Kagan VE, Fabisiak JP. The plasma membrane is the site of selective phosphatidylserine oxidation during apoptosis: role of cytochrome C. Antioxid Redox Signal. 2004a;6:209–225. doi: 10.1089/152308604322899288. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Tyurin VA, Zhao Q, Djukic M, Quinn PJ, Pitt BR, Kagan VE. Oxidation of phosphatidylserine: a mechanism for plasma membrane phospholipid scrambling during apoptosis? Biochem Biophys Res Commun. 2004b;324:1059–1064. doi: 10.1016/j.bbrc.2004.09.102. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med. 2008;44:299–314. doi: 10.1016/j.freeradbiomed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Tyurin VA, Kapralova VI, Amoscato AA, Epperly MW, Greenberger JS, Kagan VE. Mass-spectrometric characterization of phospholipids and their hydroperoxide derivatives in vivo: effects of total body irradiation. Methods Mol Biol. 2009;580:153–183. doi: 10.1007/978-1-60761-325-1_9. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Tyurin VA, Kaynar AM, Kapralova VI, Wasserloos K, Li J, Mosher M, Wright L, Wipf P, Watkins S, Pitt BR, Kagan VE. Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L73–L85. doi: 10.1152/ajplung.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Tyurin VA, Kapralova VI, Wasserloos K, Mosher M, Epperly MW, Greenberger JS, Pitt BR, Kagan VE. Oxidative lipidomics of gamma-radiation-induced lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiat Res. 2011a;175:610–621. doi: 10.1667/RR2297.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Kisin ER, Murray A, Tyurin VA, Kapralova VI, Sparvero LJ, Amoscato AA, Samhan-Arias AK, Swedin L, Lahesmaa R, Fadeel B, Shvedova AA, Kagan VE. Global phospholipidomics analysis reveals selective pulmonary peroxidation profiles upon inhalation of single-walled carbon nanotubes. ACS Nano. 2011b;5:7342–7353. doi: 10.1021/nn202201j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, Netea MG. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci USA. 2010;107:3030–3033. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Español T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia MJ, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MG, Dekker C. Motor proteins at work for nanotechnology. Science. 2007;317:333–336. doi: 10.1126/science.1139570. [DOI] [PubMed] [Google Scholar]
- Vlasova II, Sokolov AV, Chekanov AV, Kostevich VA, Vasil’ev VB. Myeloperoxidase-induced biodegradation of single-walled carbon nanotubes is mediated by hypochlorite. Bioorg Khim. 2011;37:510–521. doi: 10.1134/s1068162011040157. [DOI] [PubMed] [Google Scholar]
- Vosburgh DJH, Boysen DA, Oleson JJ, Peters TM. Airborne nanoparticle concentration in the manufacturing of polytetrafluoroethylene (PTFE) apparel. J Occup Environ Health. 2011;8:139–146. doi: 10.1080/15459624.2011.554317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. J Am Chem Soc. 2010;132:5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- Wang L, Luanpitong S, Castranova V, Tse W, Lu Y, Pongrakhananon V, Rojanasakul Y. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011;11:2796–2803. doi: 10.1021/nl2011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun P, Bao Y, Dou B, Song D, Li Y. Vitamin E renders protection to PC12 cells against oxidative damage and apoptosis induced by single-walled carbon nanotubes. Toxicol In Vitro. 2012;26:32–41. doi: 10.1016/j.tiv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Witasp E, Shvedova AA, Kagan VE, Fadeel B. Single-walled carbon nanotubes impair human macrophage engulfment of apoptotic cell corpses. Inhal Toxicol. 2009;21(Suppl. 1):131–136. doi: 10.1080/08958370902942574. [DOI] [PubMed] [Google Scholar]
- Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- Yuan J, Gao H, Ching CB. Comparative protein profile of human hepatoma HepG2 cells treated with graphene and single-walled carbon nanotubes: an iTRAQ-coupled 2D LC–MS/MS proteome analysis. Toxicol Lett. 2011;207:213–221. doi: 10.1016/j.toxlet.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Zeig-Owens R, Webber MP, Hall CB, Schwartz T, Jaber N, Weakley J, Rohan TE, Cohen HW, Derman O, Aldrich TK, Kelly K, Prezant DJ. Early assessment of cancer outcomes in New York City firefighters after the 9/11 attacks: an observational cohort study. Lancet. 2011;378:898–905. doi: 10.1016/S0140-6736(11)60989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Stilwell JL, Gerion D, Ding L, Elboudwarej O, Cooke PA, Gray JW, Alivisatos AP, Chen FF. Cellular effect of high doses of silica-coated quantum dot profiled with high throughput gene expression analysis and high content cellomics measurements. Nano Lett. 2006;6:800–808. doi: 10.1021/nl0603350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu Y, Li Z, Chen T, Lantz SM, Howard PC, Paule MG, Slikker W, Jr, Watanabe F, Mustafa T, Biris AS, Ali SF. Mechanistic toxicity evaluation of uncoated and PEGylated single-walled carbon nanotubes in neuronal PC12 cells. ACS Nano. 2011;5:7020–7033. doi: 10.1021/nn2016259. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Allen BL, Star A. Enzymatic degradation of multiwalled carbon nanotubes. J Phys Chem A. 2011;115:9536–9544. doi: 10.1021/jp112324d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- Zuo G, Huang Q, Wie G, Zhou R, Fang H. Plugging into proteins: poisoning protein function by a hydrophobic nanoparticle. ACS Nano. 2010;4:7508–7514. doi: 10.1021/nn101762b. [DOI] [PubMed] [Google Scholar]


