Abstract
Background & Aims
Multidrug resistance–associated protein 2 (MRP2) excretes conjugated organic anions including bilirubin and bile acids. Malfunction of MRP2 leads to jaundice in patients. Studies in rodents indicate that Radixin plays a critical role in determining Mrp2 canalicular membrane expression. However, it is not known how human hepatic MRP2 expression is regulated in cholestasis.
Methods
We assessed liver MRP2 expression in patients with obstructive cholestasis caused by gallstone blockage of bile ducts, and investigated the regulatory mechanism in HepG2 cells.
Results
Western blot detected that liver MRP2 protein expression in obstructive cholestatic patients (n=30) was significantly reduced to 25% of the non-cholestatic controls (n=23). Immunoprecipitation identified Ezrin but not Radixin associating with MRP2 in human livers, and the increased amount of phospho-Ezrin Thr567 was positively correlated with the amount of co-precipitated MRP2 in cholestatic livers, whereas Ezrin and Radixin total protein levels were unchanged in cholestasis. Further detailed studies indicate that Ezrin Thr567 phosphorylation plays an important role in MRP2 internalization in HepG2 cells. Since increased expression of PKCα, δ and ε were detected in these cholestatic livers, we further confirmed that these PKCs stimulated Ezrin phosphorylation and reduced MRP2 membrane expression in HepG2 cells. Finally, we identified GP78 as the key ubiquitin ligase E3 involved in MRP2 proteasome degradation.
Conclusions
Activation of liver PKCs during cholestasis leads to Ezrin Thr567 phosphorylation resulting in MRP2 internalization and degradation where ubiquitin ligase E3 GP78 is involved. This process provides a mechanistic explanation for jaundice seen in patients with obstructive cholestasis.
Keywords: Multidrug resistance–associated protein 2, Ezrin phosphorylation, Protein kinase C, Ubiquitin ligase E3 GP78, Protease degradation
Introduction
Multidrug resistance–associated protein 2 (MRP2; ABCC2) is a member of the adenosine triphosphate (ATP)-binding cassette (ABC) transporter superfamily [1]. It is widely expressed in many human and rodents tissues, such as liver, kidney, intestine, brain, and breast in humans and rodents [2–4]. MRP2/Mrp2 is localized on the apical membrane of epithelial cells in these tissues [1–3]. Specifically, it is expressed at the canalicular membrane of hepatocytes, the proximal tubule epithelial cells in kidney, and the brush-border membrane in small intestine for excreting conjugated organic anions, including bilirubin, bile acid divalent conjugates and drugs [1, 2]. In addition, MRP2/Mrp2 secretes glutathione into bile and is the major determinant of bile acid-independent bile flow [2]. Dysfunction of MRP2/Mrp2 leads to jaundice and hyperbilirubinemia in patients with Dubin-Johnson syndrome (DJS) and in GY/TR- EHBR rats [3, 4]. Jaundice is also seen in patients with obstructive cholestasis. Hepatic MRP2 mRNA expression was not changed in these patients [5, 6], but how its protein expression is regulated remains uncertain.
Previous studies in cholestatic rodent models have found reduced hepatic Mrp2 protein expression without changes in mRNA expression [7–9], indicating that post-transcriptional regulation most likely plays an important role in regulating Mrp2 function. Indeed, loss of Mrp2 from bile canalicular membranes without altering its mRNA expression in Radixin knockout mice and Radixin knockdown rat hepatocytes emphasized the importance of Radixin in the expression and function of rodent Mrp2 at post-transcriptional levels [10, 11]. Radixin is a member of the ERM protein family that consists of two more closely related proteins, i.e. Ezrin and Moesin [12]. ERM proteins are tether proteins, they connect certain plasma membrane proteins with actin filaments in cells [12]. The expression of individual ERM proteins varies in different tissues. Specifically, Radixin was abundantly expressed in rat hepatocytes, where Ezrin was undetectable by immunofluorescent labeling [13]. Instead, Ezrin was detected in biliary epithelia in the rat. Claperon et al. confirmed that Ezrin was mainly detected in bile duct epithelial cells in young children [14]. Interestingly, they also detected Ezrin at the canalicular membrane of hepatocytes in the livers of patients with biliary atresia and neonatal sclerosing cholangitis. Furthermore, both Ezrin and Radixin were detected in human hepatoma HepG2 and Huh7 cells [15, 16]. However, it is not known if Ezrin plays any role in MRP2 protein expression in human hepatocytes.
Studies from Caco2 cells and rat intestine tissue indicate that Ezrin regulates MRP2/Mrp2 membrane expression and function [17–19]. When the intestine was treated with the PKCα specific activator Thymeleatoxin, Ezrin Thr567 phosphorylation, Mrp2 brush-border membrane expression and efflux activity were reduced although the mechanism remained unexplained [18]. In contrast, activation of PKCα stimulated Ezrin phosphorylation in human breast carcinoma cells (MCF-7 cells) [20]. Elevated PKC activity, including PKCα, δ, and ε, was also reported in cholestatic rodent liver [7, 21]. But it remains to be determined if PKCs are involved in Ezrin phosphorylation in human cholestatic livers, and whether this process alters MRP2 expression and function.
In this report, we investigated the molecular mechanism of MRP2 expression regulation in the livers of patients with gallstone obstructive cholestasis. We found that hepatic MRP2 protein expression was significantly reduced in these patients. Immunoprecipitation identified Ezrin but not Radixin as the ERM protein that interacted with MRP2 in human livers. Phosphorylation of Ezrin positively correlated with its ability to associate with MRP2 protein but negatively correlated with the total amount of MRP2 protein expression. Further studies in HepG2 cells indicated that phosphorylation of Thr567 in Ezrin by PKCs reduced MRP2 membrane expression, whereas ubiquitin E3 GP78 controlled the degradation of internalized MRP2 in human hepatic cells. Our findings provide a novel mechanistic explanation for the pathogenesis of jaundice seen in patients with obstructive cholestasis and possibly other forms of cholestasis. Understanding the regulation of human MRP2 protein expression regulation may lead to new strategies for treating patients with various forms of hyperbilirubinemia.
Materials and Methods
Patients and liver samples collection
This research has been carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association, and has been approved by the Southwest Hospital Institutional Ethics Review Board (Chongqing, China). The corresponding written informed consent was obtained from all patients. Cholestatic liver samples (n=30) were surgically resected from patients with obstruction by biliary stones originating from the intrahepatic bile duct and/or common bile duct within several days of admission because of severe symptoms of biliary obstruction, such as jaundice. Neither ursodeoxycholic acid nor other preoperative therapies were administered. Control liver samples were acquired by liver biopsy and analyzed for exclusion of liver disease or staging of hematologic malignancy (n=7); in addition, control liver tissue was also obtained from patients undergoing resection of liver metastases without cholestasis (n=16; 6 colorectal, 7 colonic, and 3 rectal metastases). Liver samples were immediately cut into small pieces and fixed in 4% paraformaldehyde or stored in liquid nitrogen. The biochemical characteristics of patients were described in Table 1.
Table 1.
Clinical Features of Patients
| Clinical Features | Control patients | Obstructive Cholestatic patients |
|---|---|---|
| Total samples (Male/Female) | 23 (11/12) | 30 (17/13) |
| Age (years) | 48±14 | 49±13 |
| ALT (IU/L) | 37.1±27.5 | 197±192* |
| AST (IU/L) | 39.3±23.2 | 234±228* |
| ALP (IU/L) | 120±77 | 410±416† |
| GGT (IU/L) | 68.9±78.4 | 513±550* |
| TBA (μmol/L) | 4.8±5.2 | 59.9±91.8† |
| TBIL (μmol/L) | 13.1±4.7 | 180±142* |
| DBIL (μmol/L) | 3.9±3.4 | 89.6±74.8* |
| IBIL (μmol/L) | 9.2±2.8 | 91.6±86.7* |
Values are means ± SD.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; TBA, total bile salts; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin.
P < 0.001.
P < 0.01 versus controls.
Plasmids construction, transfection, and stably transfected cell lines
Plasmids eGFP-Ezrin WT (Wild type), eGFP-Ezrin T576A (non phosphorylatable mutation by changing Thr567 to Ala), eGFP-Ezrin T567D (simulating a constantly phosphorylated status by changing Thr567 to Asp), pHACE-PKCα, PKCδ and PKCε WT, pHACE-PKCα, PKCδ, and PKCε DN (dominant negative mutation), pHACE-PKCα CAT (constitutively active mutation), GFP-UbWT and GFP-UbK0 (all seven lysine residues that are potentially involved in polyubiquitination are replaced by arginines, but allow mono-ubiquitination) were provided by Addgene (Cambridge, MA). Human MRP2 plasmid was kindly provided by Dr. Dietrich Keppler (Division of Tumor Biochemistry, Deutsches Krebsforschungszentrum, Heidelberg, Germany) [3]. Plasmids pRNAT-GP78 shRNA#1, shRNA#2 and shRNA#3 targeting knockdown of human GP78 were designed and generated by GENCHEM (Shanghai, China). The sequences of these shRNAs are listed in Table S1. GP78 shRNA#3 construct demonstrated the best knockdown efficiency in HepG2 cells, and therefore were used throughout this report. G418 (750μg/ml) or puromycin (2μg/ml) were used to select stably transfected HepG2 cells.
RNA extraction, reverse transcription and real-time quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from tissues or cultured cells using Trizol reagent (Invitrogen; San Diego, CA). The cDNA was prepared and analyzed using real-time qPCR as described previously [6]. The primers used in this study are listed in Table S2 or as described in a previous report [6].
Western blot analysis
Total cell lysate and enriched membrane protein were prepared as previously described [6, 22]. Cell surface protein biotinylation were performed according to the manufacturer’s instruction (Thermo Scientific, MA). Protein samples were resolved using SDS-PAGE and transferred to PVDF membrane. The sources of primary antibodies and their dilution are listed in Table S3. In particular, to ensure Ezrin antibody’s specificity, we used an antibody from Sigma-Aldrich that was raised against a region where it does not share any identity to Radixin (Fig. S1).
Co-immunoprecipitation assay
The liver tissues were homogenized in RIPA buffer (Sigma-Aldrich) containing Complete EDTA-free Protease and PhosSTOP Phosphatase inhibitors (Roche, Palo Alto, CA). The homogenates were centrifuged at 8,000 × g for 10 min, and the resulting supernatant (1 mg protein) was then incubated with Protein A-Sepharose CL-4Ba or Protein G on Sepharose 4B fast flow (Sigma-Aldrich) for 1h at 4°C to remove nonspecific binding protein to the beads. Meanwhile, the primary antibodies (MRP2, Ezrin, or Radixin) were diluted with PBS and incubated with protein A or G agarose beads in a new tube for 1h at room temperature. Normal mouse or rabbit IgG was also used as negative control. After primary antibodies bound to the beads, the bead pre-absorbed homogenate was added to the antibody-bound beads and slowly rotated overnight at 4°C. The beads were spun down at 3,000 × g for 5 min and washed four times with cold PBS containing protease and phosphatase inhibitors (Roche) at 4°C. Finally, 2 x loading buffer was added to strip the protein from agarose beads, and then centrifuged at 10,000 × g for 1 min. The supernatant was subjected to SDS-PAGE and followed by Western blot analysis.
Alkaline phosphatase treatment
The cholestatic liver tissue whole lysate containing protease and phosphatase inhibitors or only containing protease inhibitor were treated with or without alkaline phosphatase (10μg total protein/5U alkaline phosphatase) (Beyotime, Suzhou, China) at 37°C for 1 h, and used for immunoprecipitation as described above.
Immunofluorescence analysis
Immunofluorescence (IF) and Immunohistochemistry (IHC) were performed as previously described [6] with the dilution of the primary antibodies as described in Table S3.
Statistical analysis
All data were analyzed using the independent-samples Student’s t-test (two-tailed) and are expressed as means ± standard deviation (SD), using SPSS software (PASW Statistics 18, IBM; SPSS, Inc., Chicago, IL). A value of p<0.05 was considered to be statistically significant.
Results
MRP2 protein expression and canalicular membrane localization were reduced in the livers of patients with obstructive cholestasis
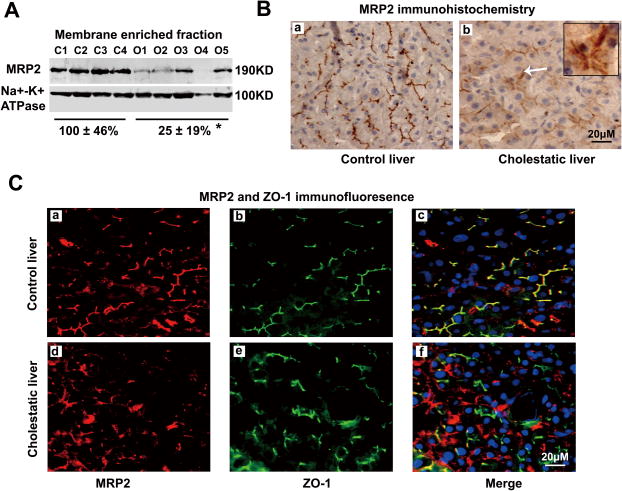
Western blot analysis demonstrated that MRP2 protein expression in the membrane enriched fraction and whole liver lysate from obstructive cholestatic livers were significantly lower (only 25±19% and 32±27% respectively by setting control livers as 100%, p<0.01) than in the control livers (Fig. 1A and Fig. S2), despite the lack of change in MRP2 mRNA as previously reported ([6] and data not shown). Both immunohistochemistry (IHC) and immunofluorescent (IF) labeling further confirmed that MRP2 canalicular membrane expression in cholestatic livers was markedly lower when compared with control livers, whereas intracellular MRP2 expression was enhanced in the cytoplasm of hepatocytes of these cholestatic livers (Fig. 1B and 1C). In contrast, the amounts of MDR1 (ABCB1) and BSEP (ABCB11) protein expression were not changed in these cholestatic livers ([6] and data not shown). Furthermore, in situ cell death analysis did not show substantially more apoptotic cells in the cholestatic livers than in the control livers (Fig. S3). Together, these results demonstrated that MRP2 protein expression and canalicular membrane localization were reduced in obstructive cholestatic human livers.
Figure 1. Reduced MRP2 protein expression in the liver of patients with obstructive cholestasis.
(A) A representative Western blot of MRP2 protein in membrane enriched fraction, and the quantitative analysis (% of control group, n=23 for control group, n=30 for obstructive cholestatic group). (B) Immunohistochemistry labeling of MRP2 in the livers of a patient with obstructive cholestasis (b) and a control patient (a). (C) Immunofluorescent labeling of MRP2 protein (Red) and ZO-1 (Green) in a control liver (a–c) and a cholestatic liver (d–f). Nuclei were stained with DAPI (blue). C1-C4, controls; O1-O5, obstructive cholestasis liver samples. *p<0.01 versus controls. DAPI, 40,6-diamidino-2-phenylindole.
MRP2 protein associates with Ezrin but not Radixin in human liver
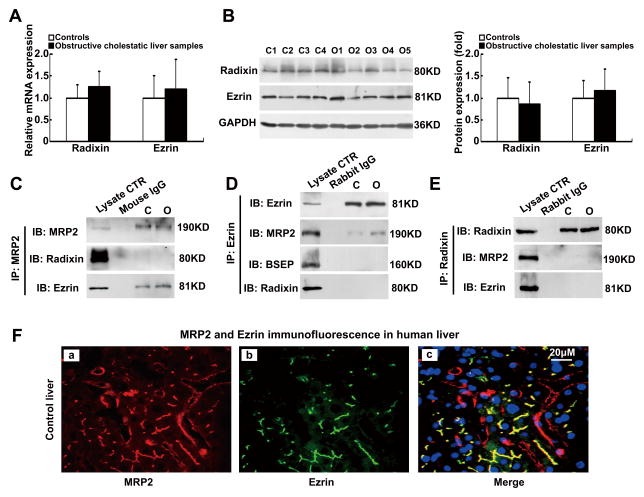
To understand the molecular mechanism that leads to the reduction of MRP2 protein expression in human cholestatic livers, we examined whether the membrane-cytoskeleton linker proteins Radixin and Ezrin were involved since they were detected in the hepatocytes of young children and in human hepatoma cells [14–16]. As shown in Figure 2A and 2B, we detected the expression of both Radixin and Ezrin mRNA and protein in human livers, but there were no differences in their levels of expression between the control and cholestatic livers. To test whether these two proteins are associated with MRP2 in human liver, we performed protein immunoprecipitation using liver homogenates from both control and cholestatic patients. Interestingly, when MRP2 protein was pulled down, we were able to detect Ezrin, but not Radixin (Fig. 2C). To confirm this observation, we then pulled down Ezrin and Radixin using their specific antibodies respectively. Again, MRP2 was detected in Ezrin pull-down complexes (Fig. 2D). In contrast, neither MRP2 nor Ezrin were detected in Radixin pull-down complexes (Fig. 2E). These results indicate that MRP2 protein is associated with Ezrin but not Radixin in human liver. To check if Ezrin is also associated with other canalicular transporters, we tested for BSEP and MDR1. However, neither protein was detected in the complex (Fig. 2D and data not shown). Furthermore, immunofluorescent labeling revealed that both MRP2 and Ezrin were colocalized at the apical region in human hepatocytes, where Radixin was also detected (Fig. 2F and Fig. S4). Together, these findings indicate that Ezrin is specifically associated with MRP2 in human hepatocytes, suggesting that it may be involved in regulating MRP2 canalicular membrane expression.
Figure 2. MRP2 protein is associated with Ezrin but not Radixin protein in human liver.
(A) Real-time qPCR detected unchanged mRNA expression of Radixin and Ezrin in the livers of obstructive cholestatic patients (n=30) and control patients (n=23) (fold of control group). (B) Representative Western blot of Radixin and Ezrin proteins expression and their corresponding densitometry in patient livers (fold of control group, n=23 for control group, n=30 for obstructive cholestatic group). (C–E), Western blots of MRP2, BSEP, Ezrin, and Radixin after immunoprecipitation as indicated. (F) Immunofluorescent labeling of MRP2 protein (Red) and Ezrin (Green) in a control liver (a–c). Nuclei of hepatocytes from patients’ liver samples were stained with DAPI (blue). C1-C4, controls; O1-O5, obstructive cholestasis liver samples. DAPI, 40,6-diamidino-2- phenylindole.
MRP2/Ezrin proteins association in human liver requires EzrinThr567 phosphorylation
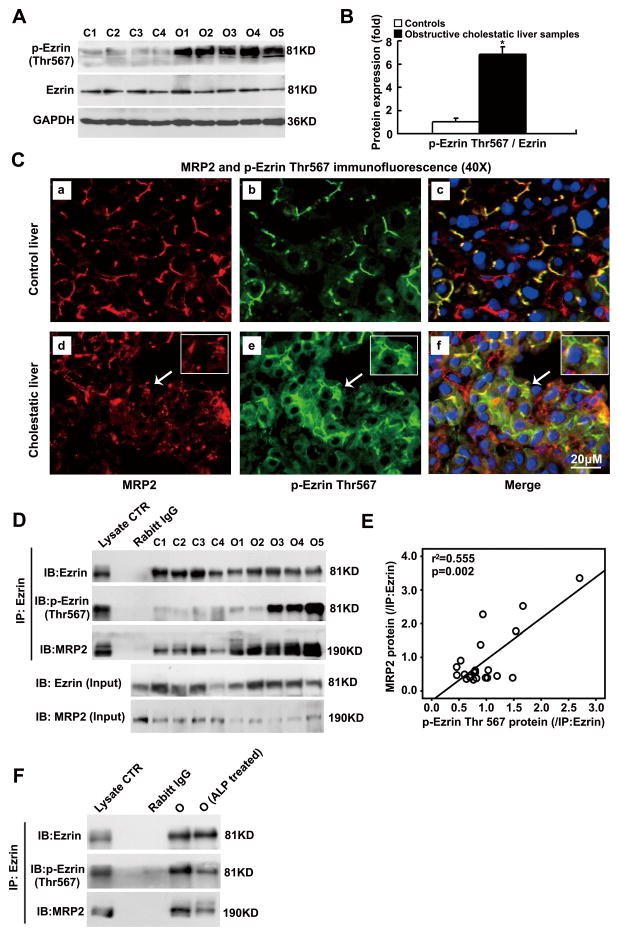
To gain insights into how Ezrin might regulate MRP2 membrane expression, we examined whether Ezrin Thr567 phosphorylation occurred in human livers since a previous study suggested that phospho-Thr567 in Ezrin was required for its interaction with Mrp2 in rat intestine [18]. As demonstrated in Figure 3A and 3B, Ezrin Thr567 phosphorylation was markedly higher in the cholestatic livers than in the controls (6.5-fold, p<0.01) despite total Ezrin protein remaining unchanged in these samples. Immunofluorescent labeling using anti-phospho-Ezrin Thr567 antibody further confirmed increased Ezrin Thr567 phosphorylation in these cholestatic livers (Fig. 3C panel b vs e), with most labeling localized in the intracellular region of the hepatocytes, where decreased canalicular membrane labeling of MRP2 was also seen (Fig. 3C panel a vs d). To test if Ezrin Thr567 phosphorylation plays a role in interacting with MRP2 protein in human livers, we immunoprecipitated Ezrin from both cholestatic and control livers. As shown in Figure 3D and 3E, the increased amount of phospho-Ezrin Thr567 in cholestatic livers positively correlated with the amount of co-precipitated MRP2 protein in the Ezrin pull-down complex (r2=0.555, p=0.002). In contrast, the amount of total precipitated Ezrin protein from these samples did not correlate with the amount of co-precipitated MRP2 protein (Figure S5A). When we immunoprecipitated MRP2, we detected more phosphor-Ezrin and total Ezrin in the complex (Fig. S5B). In addition, less MRP2 protein was pulled down with Ezrin protein when Ezrin phosphorylation was reduced by alkaline phosphatase (ALP) treatment (Fig. 3F). Together, these findings indicate that it is the phosphorylated Ezrin that interacts with MRP2 protein in human livers.
Figure 3. Phospho-Ezrin (p-Ezrin) Thr567 interacted with MRP2 in human obstructive cholestatic livers.
Western blot detection of Ezrin Thr567 phosphorylation (A) and their corresponding densitometry analysis (B) (fold of control group, n=23 for control group, n=30 for obstructive cholestatic group, *p< 0.01 to the control group). (C) Enhanced intracellular labeling of MRP2 (Red) and Ezrin Thr567 phosphorylation (Green) were detected in the cholestatic hepatocytes. a–c, the control liver; d–f, the obstructive cholestatic liver. Nuclei were stained using DAPI (blue). (D) Western blot analysis of Ezrin Thr567 phosphorylation and its interaction with MRP2 using immunoprecipitation. (E) The amount of co-immunoprecipitated MRP2 protein was positively correlated to the amount of Ezrin Thr567 phosphorylation in the obstructive cholestatic livers (r2=0.555, p=0.002, n=21). (F) Alkaline phosphatase (ALP) treatment reduced of Ezrin Thr567 phosphorylation and its association with MRP2 protein in cholestatic liver homogenates. C1-C4, controls; O1-O5, obstructive cholestasis liver samples. DAPI, 40, 6-diamidino-2-phenylindole.
Ezrin Thr567 phosphorylation reduces MRP2 membrane expression in stably transfected HepG2 cells
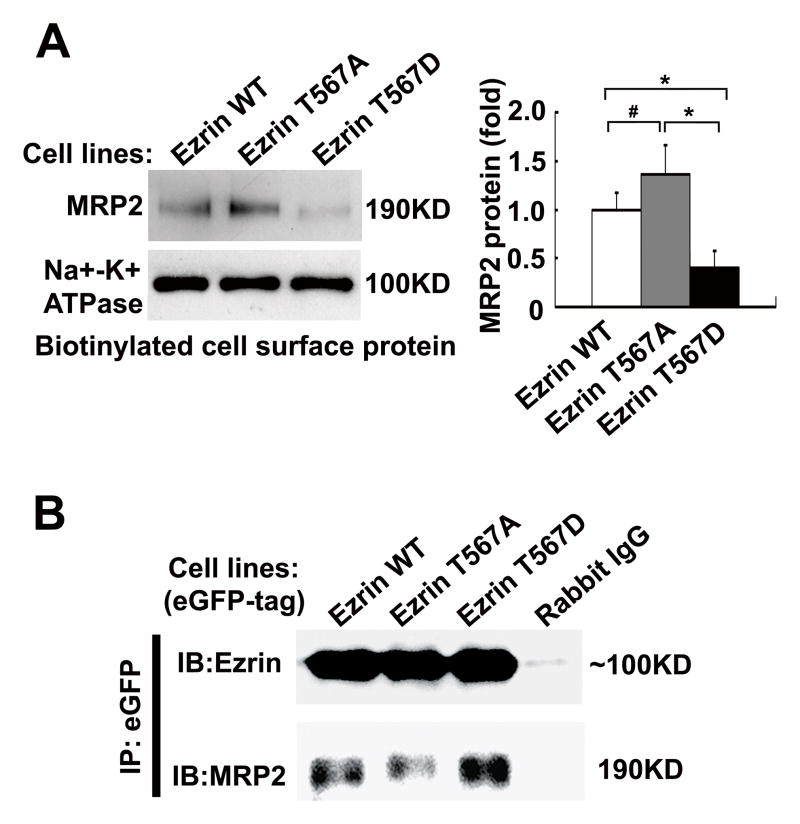
Since the amount of phospho-Thr567 Ezrin appears to be negatively correlated with the amount of MRP2 protein expression (Fig 1A and 3D), we speculated that Ezrin phosphorylation may stimulate MRP2 internalization in human cholestatic liver. To test this hypothesis, we selectively mutated Thr567 in human Ezrin and generated three lines of HepG2 cells that were stably transfected with different Ezrin expression constructs, i.e. WT, T567, and T567D. Biotinylation analysis of cell surface protein expression detected slightly but significantly more MRP2 protein in the T567A mutant (1.4-fold of WT control, p<0.05, n=4) whereas less MRP2 protein (64% of WT control, p<0.01, n=4) was expressed in the T567D mutant transfected cells (Fig. 4A). In contrast, we detected more MRP2 protein in the intracellular fraction in the T567D mutant cells (Fig. S6A). The corresponding enhanced or diminished MRP2 plasma membrane expression in Ezrin T567A and T567D mutants were further confirmed by immunofluorescent labeling (Fig. S6B). To further determine the role of Ezrin p-T567 in the association of Ezrin with MRP2, we performed pull-down assay using the lysate from these stably transfected HepG2 cells. As shown in Fig. 4B, the amount of MRP2 in the eGFP-Ezrin complex was increased from the Ezrin T567D mutant while decreased in Ezrin T567A mutant when compared with the WT. Together, these findings indicate that Ezrin Thr567 phosphorylation plays an important role in regulating MRP2 membrane expression, most likely by stimulating its internalization.
Figure 4. Ezrin Thr567 phosphorylation reduces MRP2 membrane expression in stably transfected HepG2 cells.
(A) Western blot detected altered cell surface expression of MRP2 protein in Ezrin mutants stably transfected HepG2 cells and their corresponding densitometry analysis (fold of WT control, n=4, *p< 0.01; #p< 0.05 to controls). WT (Wild type), T567A (non-phosphorylatable mutation), and T567D (simulates a constitutively phosphorylated mutation). (B) Ezrin phosphorylation enhanced its association with MRP2 in these stably transfected HepG2 cells. Antibody against GFP was used to pull-down Ezrin WT and its mutants from the lysates of these stably transfected HepG2 cells. Normal rabbit IgG was used as the negative control.
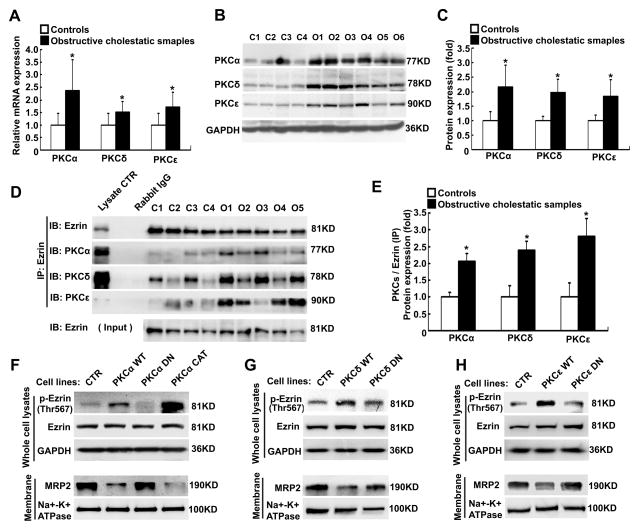
Up-regulation of PKCs expression increased Ezrin Thr567 phosphorylation in human cholestatic livers and HepG2 cells
Previous reports indicate that PKCs are responsible for Ezrin Thr567 phosphorylation in human breast carcinoma cells (MCF-7 cells) and canine osteosarcoma cells [20, 23]. However, it is not known which PKC isoform can phosphorylate Ezrin Thr567 in human hepatocytes. To address this question, we assessed the expression of PKCα, δ and ε in liver samples from both cholestatic and control patients. As shown in Figure 5A, the expression of all three PKC mRNAs were significantly increased in human obstructive cholestatic liver as compared to controls (2.4-fold, 1.5-fold, and 1.7-fold, respectively, p<0.01). Western-blots also detected increased protein expression of these PKCs in the cholestatic livers (2.2-fold, 2.0-fold, and 1.8-fold of controls, respectively, p<0.01, Fig. 5B and 5C). To test if these PKCs interacted with Ezrin in human livers, we again performed immunoprecipitation using an Ezrin specific antibody. As shown in Figure 5D–E, increased amounts of PKCα, δ and ε were co-precipitated with Ezrin from the cholestatic livers (2.1-fold, 2.4-fold, and 2.8-fold over the controls, respectively, p<0.01), suggesting these PKCs may be responsible for Ezrin phosphorylation in human liver. To test this hypothesis, we manipulated PKCα expression and activity in stably transfected HepG2 cells. Overexpression of PKCα WT and its mutants did not alter Ezrin protein expression in HepG2 cells (Fig. 5F). However, both the WT and its constitutive active mutant (CAT) markedly increased Ezrin Thr567 phosphorylation, whereas its dominant negative (DN) mutant did not alter Ezrin phosphorylation when compared with untransfected HepG2 control cells. In contrast, MRP2 protein expression was substantially lower in the WT transfected cells and nearly eliminated in CAT mutant transfected cells (Fig. 5F). Similar results were obtained when HepG2 cells were stably transfected with PKCδ and PKCε WT forms and their DN mutants (Fig. 5G and H). Together, these findings indicate that increased expression and activity of PKCs in human cholestatic livers may lead to Ezrin Thr567 phosphorylation, and result in MRP2 internalization from the canalicular membrane.
Figure 5. PKCα, δ and ε stimulate Ezrin Thr567 phosphorylation in human obstructive cholestatic livers and HepG2 cells.
(A) PKCα, δ, and ε mRNA expression in the livers of obstructive cholestatic patients (n=30) and control patients (n=23) (fold of control group). (B) A representative Western blot of PKCα, δ, and ε protein expression, and (C) their densitometry (fold of control group, n=23 for control group, n=30 for obstructive cholestatic group). (D) The increased PKCα, PKCδ, and PKCε proteins were detected in the Ezrin pull-down complex, and (E) their corresponding densitometry analysis (fold of control group, n=15 for control group, n=21 for obstructive cholestatic group). (F)–(G) Modulating PKCα, δ, and ε activities affected Ezrin phosphorylation and MRP2 protein expression in stably transfected HepG2 cells. Cells were transfected with PKC wild-type (WT), dominant negative mutant (DN) and constitutively active mutant (CAT) constructs, or not transfected control (CTR). C1-C4, controls; O1-O5, obstructive cholestasis liver samples. *p< 0.01 versus controls.
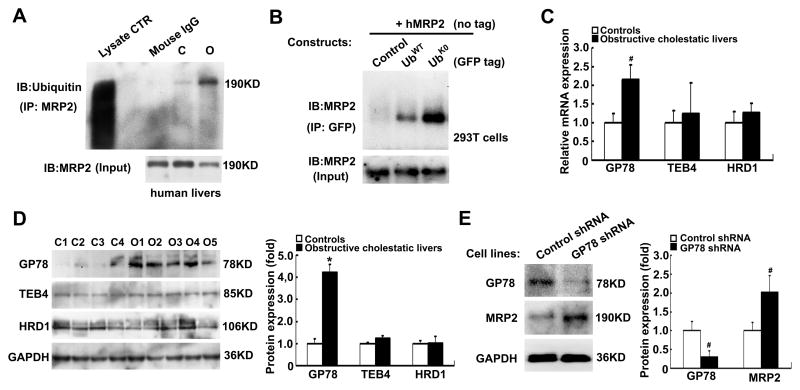
Ubiquitin ligase (E3) GP78 plays an important role in MRP2 protein degradation
Because MRP2 protein expression was reduced in these obstructive cholestatic livers, we hypothesized that the phospho-Ezrin mediated internalization of MRP2 protein was subject to proteasome degradation. To examine this possibility, we first determined whether MRP2 is ubiquitinated in human liver. As demonstrated in Figure 6A, MRP2 ubiquitination was markedly increased in the cholestatic liver compared to the control liver when we pulled down MRP2 from liver homogenates and blotted with ubiquitin antibody. To further confirm that MRP2 can be ubiquitinated in cells, we transiently transfected Hek293T cells with human MRP2 expression construct along with GFP vector, GFP-UbWT, or GFP-UbK0 expression constructs. Of note, GFP-UbK0 only allows mono-ubiquitination so that the target protein will not be subjected to polyubiquitination and subsequent degradation [24]. As shown in Figure 6B, when we immunoprecipitated GFP, we were able to detect ubiquitin conjugated MRP2. Interestingly, substantially more ubiquitin conjugated MRP2 was detected in cells cotransfected with GFP-UbK0 than with GFP-UbWT, supporting that polyubiquitination of MRP2 leads to its degradation [25]. As ubiquitin conjugation is the rate limiting step in target protein degradation [26, 27], we examined which ubiquitin ligase E3s was responsible for MRP2 ubiquitination in human cholestatic livers, i.e., GP78, TEB4 and HRD1. As shown in Figure 6C and D, only GP78 expression was significantly increased at both mRNA and protein levels (2.1-flod, p<0.05 and 4.2-fold, p<0.01 of control livers, respectively), whereas hepatic expression of TEB4 and HRD1 did not change. To test if GP78 modulates MRP2 protein expression, we used a plasmid construct to knock down GP78 expression. As shown in Figure 6E, knockdown of GP78 resulted in significant increase in MRP2 protein expression (2.0-fold of control, p<0.05) in stably transfected HepG2 cells, confirming that GP78 plays an important role in regulating the expression of MRP2 protein.
Figure 6. MRP2 protein ubiquitination and the role of ubiquitin ligase E3 GP78 in obstructive cholestasis livers and HepG2 cells.
(A) MRP2 was ubiquitinated in the livers of a cholestatic patient. Normal mouse IgG was used as the negative control. (B) GFP tagged ubiquitin was conjugated to MRP2 in Hek293T cells. Cells were transiently transfected with human MRP2 and GFP-control, GFP-UbWT, or GFP-UbK0 expression constructs, and collected 48 hour after transfection. (C) Ubiquitin ligase E3s GP78, TEB4 and HDR1 mRNA expression in the livers of obstructive cholestatic patients (n=30) and the controls (n=23). (D) Representative Western blot of GP78, TEB4 and HDR1 protein expression, and their densitometry analyses (fold of control group, n=23 for control group, n=30 for obstructive cholestatic group). (E) Knockdown of GP78 expression increased MRP2 protein expression in HepG2 cells stably transfected with GP78 shRNA construct, and their corresponding densitometry analysis (% of control group, n=3). C1-C4, controls; O1-O5, obstructive cholestasis liver samples.*p< 0.01; #p< 0.01 to controls.
Discussion
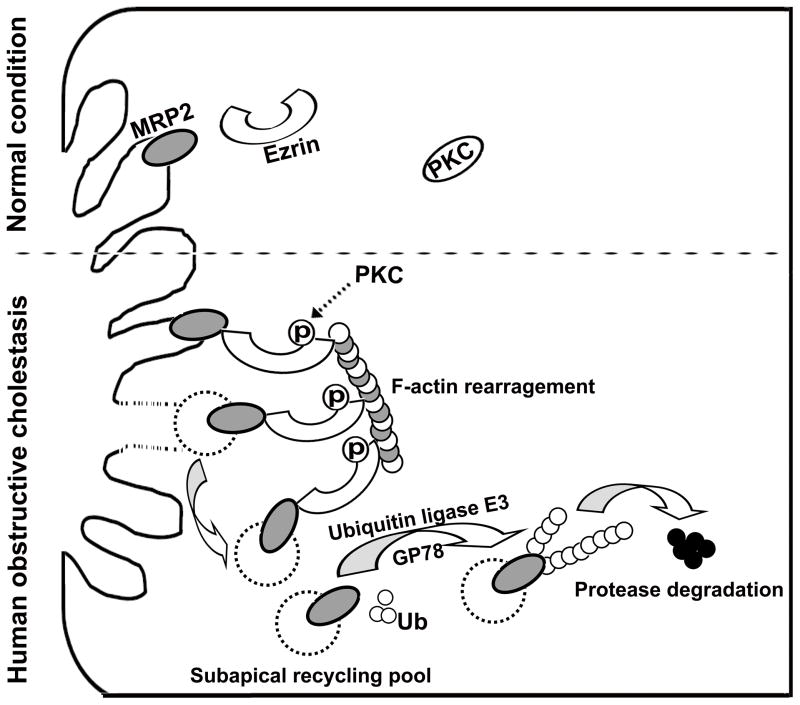
When jaundice develops in cholestatic patients, impaired function of MRP2 is thought to be the cause. Previous studies in rodent models of cholestasis have demonstrated translocation of Mrp2 from the canalicular membrane to the cytosol [28, 29]. Since hepatic MRP2 mRNA expression was not changed in patients with obstructive cholestasis [5, 6], posttranscriptional mechanisms are likely involved. As described in this report, MRP2 protein expression was significantly reduced in livers of obstructive cholestatic patients when compared to control patients. Further biochemical analysis revealed that phospho-Ezrin binds to MRP2 in cholestatic human livers, where elevated levels of Ezrin phosphorylation and PKCα, δ and ε are also detected. The role for PKCs in Ezrin phosphorylation and MRP2 membrane expression was further verified in vitro in stably transfected HepG2 cells. Because Ezrin phosphorylation was negatively correlated with MRP2 membrane expression, we propose that during cholestasis, PKCs activation phosphorylates Ezrin resulting in MRP2 internalization from the canalicular membrane. The internalized MRP2 protein is then subject to ubiquitination by GP78 and undergoes proteasome degradation (Fig. 7). This process provides a novel mechanism for the regulation of MRP2 protein expression and function in human cholestatic livers as well as explaining why jaundice develops in these and possibly other patients with cholestasis.
Figure 7. A proposed model for MRP2 canalicular membrane expression regulation in human obstructive cholestasis.
Increased PKC expression and activity phosphorylate Ezrin Thr567. The phosphorylated Ezrin enhances its binding to canalicular membrane MRP2 and retrieves MRP2 from canalicular membrane into cytosol. The internalized MRP2 is polyubiquitinated by ubiquitin ligase E3 GP78, and then is degraded.
The mechanism by which PKCs are activated in these patients with obstructive jaundice remains unclear. There are a number of possibilities. First, elevated levels of bile acids in the liver during cholestasis may activate PKCs as indicated in previous reports [30–32]. Second, TNFα signaling can also activate PKCs [33]. During cholestasis, elevated levels of TNFα have been observed in both humans and rodents [6, 34, 35]. Activated PKCs can phosphorylate Ezrin Thr567 and perhaps also MRP2 as previously reported [21]. This phosphorylation enables Ezrin to interact with MRP2 presumably through PDZ domains, and causes MRP2 internalization from the canalicular membrane. This speculation may also explain why MDR1 and BSEP protein levels were not changed in the liver of these obstructive cholestatic patients as these two proteins do not have binding motifs that interact with PDZ domains. The internalized MRP2 may then be subject to proteasome degradation if it is ubiquitinated by GP78. Alternatively, the internalized MRP2 may also recycle back to the canalicular membrane if phosphorylated Ezrin is dephosphorylated before its polyubiquitin proteasome degradation. These sequential events would provide a mechanistic explanation for reduced MRP2 protein expression and function in these cholestatic livers.
Previous studies in rodent cholestatic models and hepatocytes established Radixin’s critical role in Mrp2 protein expression as Ezrin was not detected in these cells [11, 13]. However, the expression of ERM proteins appear to be tissue specific and also species specific. Indeed Ezrin was first detected at the apical region of hepatocytes in children with cholestasis [14]. In this report, we were able to detect both Radixin and Ezrin at the apical region of hepatocytes from both control and cholestatic patient livers. While Claperon’s studies did not detect Ezrin in normal human liver, in contrast to our findings, their detection of Ezrin at the canalicular region in pediatric cholestatic liver disorders is consistent with our findings in adult patients with bile duct obstruction. Ezrin has also been detected in human hepatoma cells [15, 16]. Most importantly, we found that it is phospho-Ezrin that associated with MRP2 and causes its internalization and leads to subsequent degradation in human hepatocytes. Yang et al. has reported that both Ezrin and Radixin can interact with MRP2 in Caco2 cells [17], although they did not examine whether phosphorylation affects their binding abilities to MRP2 or not. In rat intestine, Nakanoet al. observed that a PKCα agonist reduced Mrp2 membrane expression and function [18], in agreement with what we saw in human livers and HepG2 cells. However, in that report, the authors detected reduced Ezrin Thr567 phosphorylation but did not provide an explanation for why PKCα activation leads to reduced phosphorylation of Ezrin in rat intestine cells. When PKC is activated, one would expect an increase of phosphorylation of Ezrin as we have seen in human livers and HepG2 cells, and as described in human breast carcinoma cells [20]. However, it is also possible that this discrepancy may be due to ERM proteins functioning differently in a tissue and species specific manner.
Our current study did not address any functional role of Radixin in MRP2 membrane expression in human hepatocytes as co-immunoprecipitation did not detect interaction between Radixin and MRP2. It is possible that Radixin may only facilitate nascent MRP2 protein membrane trafficking in human hepatocytes as described in the Radixin knockout mouse [11]. Because MRP2 protein has a relatively long half-life in hepatocytes (~ 30 hours) [36], human hepatocytes may only synthesize very small amount of nascent MRP2 protein. Therefore, we may miss the interaction of MRP2 with Radixin in human liver by co-immunoprecipitating these two proteins.
In summary, we detected reduced MRP2 protein expression in human cholestatic livers, and found that Ezrin Thr567 phosphorylation was associated with MRP2 internalization and subsequent degradation where PKCs and GP78 play important roles in this process. These findings provide a novel explanation for the development of jaundice in some patients with cholestasis. Understanding the molecular regulation of MRP2 expression may lead to new strategies for therapeutic interventions.
Supplementary Material
Figure S1. Sequence alignment of human Ezrin and Radixin at the region where Sigma-Aldrich Ezrin antibody (Cat#E1284) was raised (black box).
C1-C4, controls; O1-O5, obstructive cholestasis liver samples.*p< 0.01 to controls.
There was no substantially more apoptotic hepatocytes (arrows) in livers from obstructive cholestatic patients (b, n=3) when compared to control livers (a, n=2). The hepatocytes cell death was determined by In Situ Cell Death Detection Kit (POD) (Roche Diagnosis).
Immunofluorescent labeling of MRP2 (a, Red) and Radixin (b, Green) proteins in a control liver (a–c). Nuclei were stained with DAPI (blue).
(A) Correlation analysis of co-immunoprecipitated Ezrin and MRP2 proteins from obstructive cholestatic livers (n=21). (B) Western blot analysis of Ezrin and its Thr567 phosphorylation in immunoprecipitated MRP2 complex.
(A) Western blot detection of MRP2 protein in intracellular fractions after cell surface protein biotinylation and strepavidin pull down. (B) Immunofluorescent labeling of MRP2 protein (arrows) in these WT (a) and mutated Ezrin (b and c) stably transfected HepG2 cells. Intracellular MRP2 labeling was mostly detected in the Ezrin T567D mutant transfected cells. Nuclei were stained with DAPI (blue).
Acknowledgments
This work was supported by National Natural Science Foundation of China (81170430, 81070320, 81100280, and 81470880), Scholarship Foundation of China Scholarship Council (CSC No.201307610015) and Third Military Medical University (2013). S-Y.C and J.L.B were supported by NIH grants DK25636 and DK34989 (Yale Liver Center). We also thank Qiaobing Huang (Department of Pathophysiology, Key Lab for Shock and Microcirculation Research, Southern Medical University, Guangzhou, P. R. China) for Moesin constructs.
Abbreviations
- DJS
Dubin-Johnson syndrome
- ERAD
endoplasmic reticulum (ER)-associated degradation
- ERM
Ezrin/Radixin/Moesin
- GAPDH
glyceroldehyde 3-phosphate dehydrogenase
- ALP
alkaline phosphatase
- IF
immunofluorescence
- IHC
immunohistochemistry
- MRP2
multidrug resistance-associated protein 2
- PKCα
protein kinase C alpha
- PKCδ
protein kinase delta
- PKCε
protein kinase epsilon
Footnotes
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461(2):377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 2.Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009;51(3):565–580. doi: 10.1016/j.jhep.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto K, Uchiumi T, Konno T, Ebihara T, Nakamura T, Wada M, et al. Trafficking and functional defects by mutations of the ATP-binding domains in MRP2 in patients with Dubin-Johnson syndrome. Hepatology. 2002;36(5):1236–1245. doi: 10.1053/jhep.2002.36368. [DOI] [PubMed] [Google Scholar]
- 4.Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, et al. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271(5252):1126–1128. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- 5.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL, et al. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49(4):1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 6.Chai J, He Y, Cai SY, Jiang Z, Wang H, Li Q, et al. Elevated hepatic multidrug resistance-associated protein 3/ATP-binding cassette subfamily C 3 expression in human obstructive cholestasis is mediated through tumor necrosis factor alpha and c-Jun NH2-terminal kinase/stress-activated protein kinase-signaling pathway. Hepatology. 2012;55(5):1485–1494. doi: 10.1002/hep.24801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crocenzi FA, Sanchez Pozzi EJ, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD, et al. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology. 2008;48(6):1885–1895. doi: 10.1002/hep.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, et al. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113(1):255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 9.Paulusma CC, Kothe MJ, Bakker CT, Bosma PJ, van Bokhoven I, van Marle J, et al. Zonal down-regulation and redistribution of the multidrug resistance protein 2 during bile duct ligation in rat liver. Hepatology. 2000;31(3):684–693. doi: 10.1002/hep.510310319. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131(3):878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet. 2002;31(3):320–325. doi: 10.1038/ng905. [DOI] [PubMed] [Google Scholar]
- 12.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11(4):276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouassier L, Duan CY, Feranchak AP, Yun CH, Sutherland E, Simon F, et al. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology. 2001;33(1):166–176. doi: 10.1053/jhep.2001.21143. [DOI] [PubMed] [Google Scholar]
- 14.Claperon A, Debray D, Redon MJ, Mergey M, Ho-Bouldoires TH, Housset C, et al. Immunohistochemical profile of ezrin and radixin in human liver epithelia during fetal development and pediatric cholestatic diseases. Clin Res Hepatol Gastroenterol. 2013;37(2):142–151. doi: 10.1016/j.clinre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhao R, Wang TZ, Kong D, Zhang L, Meng HX, Jiang Y, et al. Hepatoma cell line HepG2.2.15 demonstrates distinct biological features compared with parental HepG2. World J Gastroenterol. 2011;17(9):1152–1159. doi: 10.3748/wjg.v17.i9.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukong TN, Kodys K, Szabo G, et al. Human ezrin-moesin-radixin proteins modulate hepatitis C virus infection. Hepatology. 2013;58(5):1569–1579. doi: 10.1002/hep.26500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q, Onuki R, Nakai C, Sugiyama Y. Ezrin and radixin both regulate the apical membrane localization of ABCC2 (MRP2) in human intestinal epithelial Caco-2 cells. Exp Cell Res. 2007;313(16):3517–3525. doi: 10.1016/j.yexcr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Sekine S, Ito K, Horie T. Correlation between apical localization of Abcc2/Mrp2 and phosphorylation status of ezrin in rat intestine. Drug Metab Dispos. 2009;37(7):1521–1527. doi: 10.1124/dmd.108.024836. [DOI] [PubMed] [Google Scholar]
- 19.Nakano T, Sekine S, Ito K, Horie T. Ezrin regulates the expression of Mrp2/Abcc2 and Mdr1/Abcb1 along the rat small intestinal tract. Am J Physiol Gastrointest Liver Physiol. 2013;305(11):G807–817. doi: 10.1152/ajpgi.00187.2013. [DOI] [PubMed] [Google Scholar]
- 20.Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, et al. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001;20(11):2723–2741. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anwer MS. Role of protein kinase C isoforms in bile formation and cholestasis. Hepatology. 2014;60(3):1090–1097. doi: 10.1002/hep.27088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol. 2004;40(4):585–91. doi: 10.1016/j.jhep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Hong SH, Osborne T, Ren L, Briggs J, Mazcko C, Burkett SS, et al. Protein kinase C regulates ezrin-radixin-moesin phosphorylation in canine osteosarcoma cells. Vet Comp Oncol. 2011;9(3):207–218. doi: 10.1111/j.1476-5829.2010.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergink S, Salomons FA, Hoogstraten D, Groothuis TA, de Waard H, Wu J, et al. DNA damage triggers nucleotide excision repair-dependent monoubiquitylation of histone H2A. Genes Dev. 2006;20(10):1343–1352. doi: 10.1101/gad.373706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai YC, Mendoza A, Mariano JM, Zhou M, Kostova Z, Chen B, et al. The ubiquitin ligase Gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat Med. 2007;13(12):1504–1509. doi: 10.1038/nm1686. [DOI] [PubMed] [Google Scholar]
- 27.Jiang W, Song BL. Ubiquitin ligases in cholesterol metabolism. Diabetes Metab J. 2014;38(3):171–180. doi: 10.4093/dmj.2014.38.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rost D, Kartenbeck J, Keppler D. Changes in the localization of the rat canalicular conjugate export pump Mrp2 in phalloidin-induced cholestasis. Hepatology. 1999;29(3):814–21. doi: 10.1002/hep.510290319. [DOI] [PubMed] [Google Scholar]
- 29.Kubitz R, Wettstein M, Warskulat U, Häussinger D. Regulation of the multidrug resistance protein 2 in the rat liver by lipopolysaccharide dexamethasone. Gastroenterology. 1999;116(2):401–10. doi: 10.1016/s0016-5085(99)70138-1. [DOI] [PubMed] [Google Scholar]
- 30.Schonhoff CM, Webster CR, Anwer MS. Taurolithocholate-induced MRP2 retrieval involves MARCKS phosphorylation by protein kinase C in HUH-NTCP Cells. Hepatology. 2013;58(1):284–292. doi: 10.1002/hep.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beuers U, Throckmorton DC, Anderson MS, Isales CM, Thasler W, Kullak-Ublick GA, et al. Tauroursodeoxycholic acid activates protein kinase C in isolated rat hepatocytes. Gastroenterology. 1996;110(5):1553–1563. doi: 10.1053/gast.1996.v110.pm8613063. [DOI] [PubMed] [Google Scholar]
- 32.Beuers U, Probst I, Soroka C, Boyer JL, Kullak-Ublick GA, Paumgartner G. Modulation of protein kinase C by taurolithocholic acid in isolated rat hepatocytes. Hepatology. 1999;29(2):477–482. doi: 10.1002/hep.510290227. [DOI] [PubMed] [Google Scholar]
- 33.Koss M, Pfeiffer GR, 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, et al. Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol. 2006;176(2):1218–27. doi: 10.4049/jimmunol.176.2.1218. [DOI] [PubMed] [Google Scholar]
- 34.Dulundu E, Ozel Y, Topaloglu U, Toklu H, Ercan F, Gedik N, et al. Grape seed extract reduces oxidative stress and fibrosis in experimental biliary obstruction. J Gastroenterol Hepatol. 2007;22(6):885–92. doi: 10.1111/j.1440-1746.2007.04875.x. [DOI] [PubMed] [Google Scholar]
- 35.Bohan A, Chen WS, Denson LA, Held MA, Boyer JL. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003;278(38):36688–98. doi: 10.1074/jbc.M304011200. [DOI] [PubMed] [Google Scholar]
- 36.Jones BR, Li W, Cao J, Hoffman TA, Gerk PM, Vore M. The role of protein synthesis and degradation in the post-transcriptional regulation of rat multidrug resistance-associated protein 2 (Mrp2, Abcc2) Mol Pharmacol. 2005;68(3):701–710. doi: 10.1124/mol.105.013144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sequence alignment of human Ezrin and Radixin at the region where Sigma-Aldrich Ezrin antibody (Cat#E1284) was raised (black box).
C1-C4, controls; O1-O5, obstructive cholestasis liver samples.*p< 0.01 to controls.
There was no substantially more apoptotic hepatocytes (arrows) in livers from obstructive cholestatic patients (b, n=3) when compared to control livers (a, n=2). The hepatocytes cell death was determined by In Situ Cell Death Detection Kit (POD) (Roche Diagnosis).
Immunofluorescent labeling of MRP2 (a, Red) and Radixin (b, Green) proteins in a control liver (a–c). Nuclei were stained with DAPI (blue).
(A) Correlation analysis of co-immunoprecipitated Ezrin and MRP2 proteins from obstructive cholestatic livers (n=21). (B) Western blot analysis of Ezrin and its Thr567 phosphorylation in immunoprecipitated MRP2 complex.
(A) Western blot detection of MRP2 protein in intracellular fractions after cell surface protein biotinylation and strepavidin pull down. (B) Immunofluorescent labeling of MRP2 protein (arrows) in these WT (a) and mutated Ezrin (b and c) stably transfected HepG2 cells. Intracellular MRP2 labeling was mostly detected in the Ezrin T567D mutant transfected cells. Nuclei were stained with DAPI (blue).