Abstract
Background
We developed a hemorrhagic shock animal model to replicate an urban prehospital setting where resuscitation fluids are limited to assess the effect of saline versus plasma in coagulopathic patients. An in vitro model of whole blood dilution with saline exacerbated tPA mediated fibrinolysis, while plasma dilution did not change fibrinolysis. We hypothesize that shock induced hyperfibrinolysis can be attenuated by resuscitation with plasma while exacerbated by saline.
Methods
Sprague Dawley rats were hemorrhaged to a mean arterial pressure (MAP) of 25 mmHg and maintained in shock for 30 minutes. Animals were resuscitated with either normal saline (NS) or platelet free plasma (PFP) with a 10% total blood volume bolus, followed by an additional 5 minutes of resuscitation with NS to increase blood pressure to a MAP of 30 mmHg. Animals were observed for 15 minutes for assessment of hemodynamic response and survival. Blood samples were analyzed with thrombelastography (TEG) paired with protein analysis.
Results
The median percent of total blood volume shed per group were similar (NS 52.5% vs PFP 55.7 p=0.065). Survival was 50% in NS compared to 100% in PFP. The change in LY30 and tPA levels from baseline to shock was similar between groups (LY30 PFP10 IQR 4.3 to 11.2, NS 4.5 IQR 4.1 to 14.2 p=1.00; tPA PFP 16.6 ng/ml IQR 13.7–27.8, NS 22.4 IQR 20.1 to 25.5 p=0.240). After resuscitation, the median change in LY30 was greater in the NS group (13.5 IQR 3.5 to 19.9) compared to PFP (−4.9 % IQR −9.22 to 0.25 p=0.004) but tPA levels did not significantly change (NS 1.4 IQR-6.2 to 7.1 vs PFP 1.7 IQR-5.2 to 6.8 p=0.699).
Conclusions
Systemic hyperfibrinolysis is driven by hypoperfusion and associated with increased levels of tPA. Plasma is a superior resuscitation fluid to normal saline in a prehospital model of severe hemorrhagic shock as it attenuates hyperfibrinolysis and improves systemic perfusion.
Keywords: Hyperfibrinolysis, Plasma first resuscitation, Hemorrhagic shock
Background
Hyperfibrinolysis is the most lethal phenotype of trauma induced coagulopathy (TIC) (1). Depending on the degree of fibrinolysis, mortality ranges from 50–100% (1–4). High levels of tissue plasminogen activator (tPA) combined with a reduction in fibrinolysis inhibitors appear to drive this process (5). Profound shock is the most consistent clinical predictor of hyperfibrinolysis in retrospective trauma studies (6–8). This is further supported by non-trauma literature in which hyperfibrinolysis is prevalent in patients with prehospital cardiac arrest (9). The observation that inadequate perfusion promotes fibrinolysis is not new. A review by Sherry et al. in 1959 described multiple studies in which occlusion of vascular in flow or out flow increased fibrinolytic activity (10). This is further supported by an animal model in which profound shock increased systemic tPA levels and resulted in clots more susceptible to fibrinolysis (Moore et al. Surgery 2015 in press). Therefore, prompt and adequate resuscitation of patients in shock has the potential to attenuate hyperfibrinolysis.
Prehospital volume restoration to correct shock in severely injury patients remains a challenge. Crystalloid administration has been associated with the development of hyperfibrinolysis (2). Prior in vitro work has shown that whole blood dilution with saline increases susceptibility to tissue plasminogen activator (tPA) mediated fibrinolysis (5). However, equivalent dilution of whole blood with plasma does not increase fibrinolytic activity (Moore et al 2015 JACS in press). Plasma contains high concentrations of protease inhibitors that can regulate fibrinolysis (11, 12), and contains fibrinogen and other factors that promote a fibrinolytic resistant clot (13–15).
Plasma first resuscitation in hyperfibrinolytic patients may be life saving as it provides substrates for controlled hemostasis in addition to effective volume resuscitation with enhanced metabolic restoration. The initial resuscitation fluid may be critical to improve survival in trauma patients in profound shock as they are likely releasing large amounts of tPA, that can become unregulated by dilution of circulating plasma inhibitors with crystalloid resuscitation. We are currently conducting the COMBAT (Control of Major Bleeding After Trauma) study, a randomized clinical trial designed to evaluate the effects of receiving plasma versus normal saline in the field. To begin to understand shock induced coagulopathy and the impact of field resuscitation fluids, we created an animal model representing the COMBAT study conditions, i.e., an urban prehospital setting where resuscitation fluids are limited and normalization of blood pressure is not always obtainable. The objective of this study was to elucidate the molecular mechanisms mediating shock induced hyperfibrinolysis and the effects of early administration of plasma versus saline in this phenotype of coagulopathy. This manuscript describes the animal model and tests the hypothesis that severe shock increases systemic levels of tPA resulting in hyperfibrinolysis, which can be attenuated by resuscitation with plasma and exacerbated by saline.
Methods
Subjects
The University of Colorado Institutional Animal Care and Use Committee approved this animal study under protocol #90814. Male Sprague Dawley rats (age 14–16 weeks) with a weight between 300 and 400 grams were used for the experiment.
Animal Induction and Cannulation
Anesthesia was induced with pentobarbital (5mg/kg, intraperitoneal) followed by tracheostomy and femoral artery cannulation. The femoral artery cannula was used for invasive measurement of blood pressure, blood withdrawal for controlled hemorrhage sampling, and resuscitation. The animals were allowed to recover from these initial procedures for airway and vascular access and then randomized into resuscitation groups as described below. Due to the need for plasma, the first three animals were allocated to the normal saline (NS) group. Thereafter, animal resuscitation randomization was alternated between PFP and NS.
Hemorrhagic shock
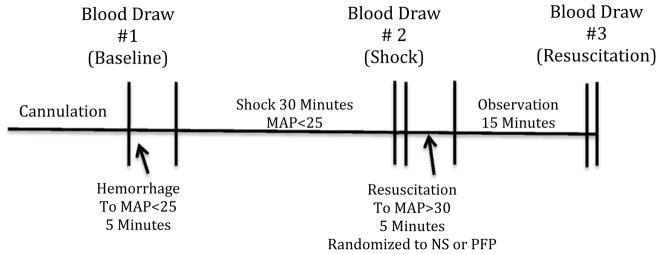
Hemorrhagic shock was performed through the femoral artery catheter with blood draws (volume 0.5 to 1.5 ml) every 30 seconds to achieve a mean arterial pressure (MAP) of 25 mmHg within 5 minutes. A MAP of 25 was selected as previous experiments with a MAP >30 were insufficient in producing coagulation changes (16); and rodents with a MAP <20 did not survive a second blood draw. Blood pressure was maintained at a MAP of 25 +/− 2 mmHG for 30 minutes performing additional blood draws as needed (Figure 1). The 30 minutes of shock was selected based on a previous model in which 30 minutes was adequate to increase systemic tPA levels (Moore et al. Surgery 2015 in press).
Figure 1. Schematic of profound shock model.
Animals after cannulation underwent hemorrhage form 5 minutes reduce blood pressure to a mean arterial pressure of less than 35 mmHg, 30 minutes of shock, 5 minutes of resuscitation, and 15 minutes of observation. During resuscitation animals were randomized to either plasma or saline with an initial 10% estimated blood volume bolus followed by an addition as needed saline resuscitation to obtain a mean arterial pressure greater than 30.
MAP= mean arterial pressure in mmHG
Replicating Prehospital Resuscitation
Fluid resuscitation prior to arrival at the hospital is limited on what can be delivered to critically injured patients. It is common for patients who have coagulation changes to be incompletely resuscitated before they arrive at the hospital. An estimated 10% total blood volume of the rat (0.06ml/gram*rat weight grams*0.10) was transfused over 2 minutes (plasma vs normal saline) through the femoral artery, with an additional 3 minutes of resuscitation (saline only) to obtain a MAP >30 mm Hg. This initial 10% volume for resuscitation was based on the estimate of what a trauma patient would receive prior to arrival to the hospital in an ongoing prehospital plasma trial (17). It was anticipated that plasma resuscitation would increase blood pressure more effectively than crystalloid due to oncotic forces of proteins. The additional 3 minutes of resuscitation allowed for both arms of the experiment to increase blood pressure to a MAP >30 mm Hg. Following 5 minutes of resuscitation, both arms of the experiment were observed for 15 minutes. Continued resuscitation was not performed during this time frame to reduce confounding of ongoing whole blood dilution and for assessment of effectiveness of resuscitation fluid on blood pressure and survival.
Blood samples
Baseline blood samples were obtained after cannulation, tracheostomy and rest period. A second blood draw occurred 30 minutes after achieving the targeted blood pressure of 35 mm Hg representing the shock time point. The third time point of blood samples were obtained after 15 minutes of observation following resuscitation. To keep blood sampling volume constant between different animal weights, 8% of estimated total blood volume was drawn at each time point. The 8% blood volume was established based off the minimal amount of blood from a 300 gram rodent required to conduct thrombelastography (TEG) and plasma assays. Blood samples obtained after the shock point dramatically reduced blood pressure to a point at which animals would have not survive without immediate resuscitation. Animals that did not survive the observation period or died during the final blood draw had an immediate laparotomy and sternotomy with blood draw through the supra-hepatic vena cava using 20 gauge needle and 1 ml syringe.
Blood Component Banking
Blood banking techniques in rodents were described by Gilson et al (18). A modified protocol was developed to minimize the number of animals used in the experiment by eliminating the need to sacrifice animals to obtain blood products. During hemorrhage, the first 3 ml of blood were collected in citrate phosphate dextrose adenine (CPDA-1) with a final concentration of 14%, as used by the Gilson group. These blood samples underwent serial centrifuge spins to remove red cells and platelets to reduce the contamination of cellular lysate, which has previously been shown to alter fibrinolysis (19). The remaining platelet free plasma (PFP) was flash frozen in liquid nitrogen and stored at −80 degrees Celsius. Prior to transfusion PFP from 3 animals was warmed in a 37 degree Celsius incubator for 15 minutes and pooled. Pooling allowed for less variability in animal plasma.
Blood Samples and Rodent Thrombelastography
Whole blood was collected in 3.2 % sodium citrate tubes at a 1:10 ratio, based on a standardized model of performing TEG in rodents (20). Individual microcentrifuge tubes were prefilled with citrate and marked to an appropriate fill level to ensure reproducible ratios of whole blood to citrate. Citrated native TEG assays were re-calcified and run according to the manufacturer’s instructions on a TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics, Niles IL). The LY30 parameter was used to quantify fibrinolysis. Blood not used for TEG was spun to yield plasma for protein analysis. Whole blood was centrifuged at 6,000 g for 10 minutes at 4 °C. Plasma was removed and then spun at 12,500 g for 10 minutes at the same temperature to remove contaminating platelets and a cellular debris. The remaining plasma was flash frozen in liquid nitrogen and stored at −80 °C until analyzed.
ELISA
Assays for detection of total plasma tissue levels of tPA were purchased from Molecular Innovations (Novi, MI product # RTPAKT-TOT). Plasma and tissue homogenate supernatants were used in a 1:1 dilution and were run according to the manufacture’s recommendations. Samples were run in duplicate and the average of two samples was used for quantification. Using the “ladder” of standards, standard curves were constructed, and plasma levels were quantified in ng/ml after adjusting for dilution.
Mass Spectrometric Analysis
The albumin was removed from the plasma samples Capture Select™ Multi Species Albumin Depletion affinity resin according to manufacturer’s protocol. Protein preparation and analysis was conducted using a similar methodology previously used to analyze human lymph and plasma samples (21)
Statistical Analysis
SPSS software version 22 (IBM, Armonk, NY) was used for statistical analysis. All experimental results were represented as the group median and variability was represented by interquartile range (IQR), as several variables were not normally distributed (e.g., LY30, tPA levels). Correlations were explored using the Spearman test. Experimental groups were contrasted with the Mann-Whitney-U test and the Kaplan-Meyer survival curves evaluated survival with differences between strata tested via the log rank test. Group sample sizes of 6 and 6 achieve 80% power to detect a minimum difference of 10 percent points in the LY30 change from baseline to resuscitation between experimental groups assuming a change of 20% (resuscitation/baseline) in the NS group (with known group standard deviations of 8% and 4%) and significance level (alpha) of 0.05.
Proteomic analysis was a descriptive assessment of pooled samples because of limited volumes at each time point. To visualize the relative changes from baseline protein spectral counts to counts during shock and resuscitation, the experimental groups were contrasted using a conditional formatting in Excel version 14.7.4 (Microsoft, Redmond, WA). The color red represented the 95th percentile change in protein detection, white represented 50th percentile (no change) and blue represented 5th percentile change (decreased protein count). Due to a large number of identified proteins, we selected only proteins containing baseline spectral counts greater than 10 and had a similar counts between experimental groups at baseline and shock.
Results
Hyperfibrinolysis in Rodent Near Lethal Hemorrhage
A total of 23 rodents were used for this experiment. Seven rodents died before resuscitation (three in PFP, four in NS). An additional four rodents (one PFP, three NS) did not develop hyperfibrinolysis after shock (LY30 > 3%). These 11 animals were not included in the analysis. The remaining twelve animals were equally distributed to each experimental arm. The percentage of total estimated blood volume shed between groups was similar NS 52.5% (IQR 51.2–55.3) vs PFP 55.7 (IQR 54.3–59.2) p=0.065. Blood pressure (MAP) prior to resuscitation was similar between groups (Figure 2) with a similar nadir reach prior to resuscitation (PFP 16.5 mm Hg IQR 15.8–18.3 versus NS 16.0 mm Hg IQR 14.8–19.0 p=0.684).
Figure 2. Saline resuscitation improves blood pressure.
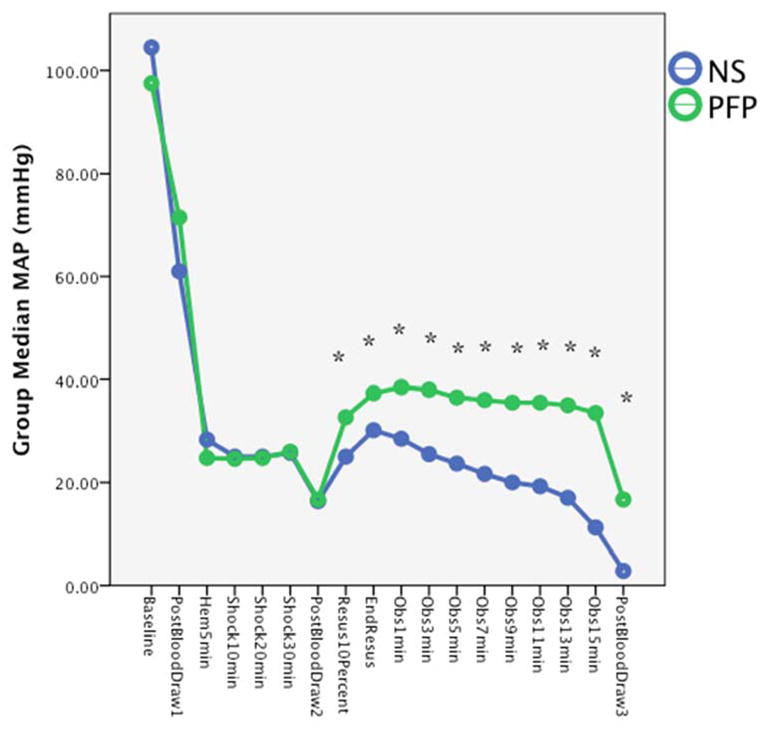
The median mean arterial blood pressure is represented on the Y axis. The X axis represents time points at which blood pressure were measured. Animals in both experimental arms had similar blood pressure during the hemorrhage and shock period. After initial bolus of resuscitation plasma increased blood pressure greater than saline, and continued to have a higher blood pressure during the observation period.
NS= normal saline; PFP = platelet free plasma; ng = nanogram
Plasma is a Superior Resuscitation Fluid to Normal Saline
Blood pressure was significantly higher in the PFP group compared to NS group after an initial 10% of estimated blood volume resuscitation (PFP 32.5 mm Hg IQR 31.3–35.0 versus NS 25.0 mm Hg IQR 23.5–29.0 p=0.01). After the initial 10% bolus of resuscitation fluids, animals in the PFP group did not require on going resuscitation, while all animals in the NS group required ongoing crystalloid resuscitation to increase the MAP above 30 mm Hg. Therefore, each group represented a pure resuscitation of plasma or crystalloid. Total fluid resuscitation was significantly higher in the NS group (20% IQR 16.8–23.1 vs 10.0% IQR 9.9–10.1 p<0.001). During the observation period, all blood pressures remained higher in the PFP group (Figure 2). Survival was 50% in NS compared to 100% in PFP during observation. Survival time trended towards significance (log rank p=0.055 Figure 3). All animals in the PFP group survived the final blood draw, while only one of the three animals in the NS group that survived the observations period survived the final blood draw.
Figure 3. Kaplan Meyer curve.
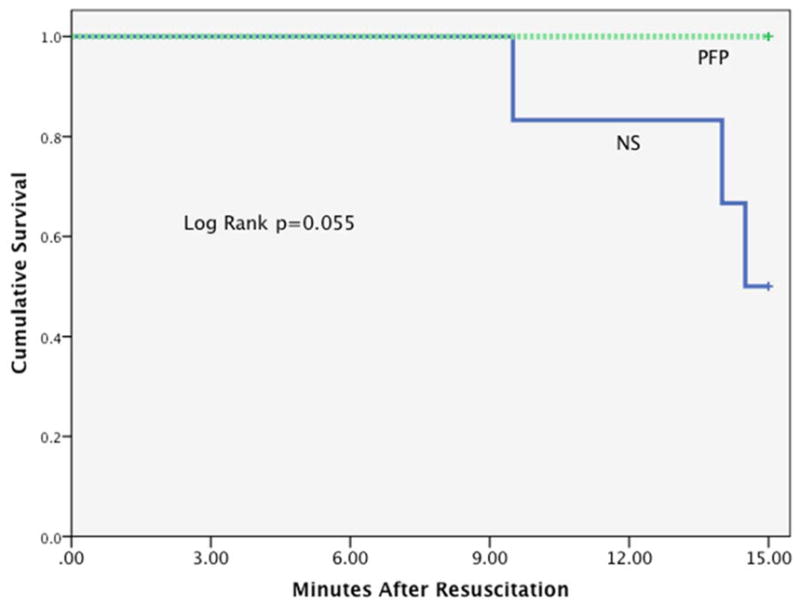
Y axis represents cumulative survival, and X axis represents observation times in minutes.
NS= normal saline; PFP= platelet free plasma; ng= nanogram
LY30 Increases with Shock and is Exacerbated by Saline Resuscitation Despite tPA level Stabilization After Resuscitation
Baseline LY30, and tPA levels were similar between experimental arms (LY30 PFP 1.2% IQR 0.5–5.0, NS 0.8% IQR 0.2–2.7 p=0.349; tPA PFP 10.5 ng/ml IQR 9.2–11.5 vs NS 11.8 IQR 8.8–13.7 p=0.560). Shock increased both LY30 and tPA levels in both experimental arms (LY30 PFP 10% IQR 4.3–12.0, NS 4.5% IQR 4.1–14.0; tPA PFP 16.6 ng/ml IQR 13.7–27.8, NS 22.4 IQR 20.8–25.5). The change in LY30 and tPA levels from baseline to shock were not statistically different between groups (LY30 PFP10 IQR 4.3 to 11.2, NS 4.5 IQR 4.1 to 14.2 p=1.00 Figure 4A; tPA PFP 16.6 ng/ml IQR 13.7–27.8, NS 22.4 IQR 20.1 to 25.5 p=0.240 Figure 4B). Although both experiment arms had an increase in LY30 and tPA for all animals, the magnitude of change did not directly correlate (Spearman’s Rho 0.315 p= 0.319). After resuscitation, LY30 was greater in the NS group (22.5 IQR 7.8–33.7) compared to PFP (2.6 IQR 2.2–5.4) but, tPA levels remained in a similar range (NS 22.9 IQR 15.7–30.5 vs PFP 14.1 IQR 13.5–27.4). The median change from shock to resuscitation in LY30 was significant in the NS group (13.5 IQR 3.5 to 19.9) compared to the PFP group (−4.9 % IQR −9.22 to 0.25 p=0.004 Figure 5A) but changes in tPA levels did not reach statistical significance (NS 1.4 IQR-6.2 to 7.1 vs PFP 1.7 IQR-5.2 to 6.8 p=0.699 Figure 5B).
Figure 4.
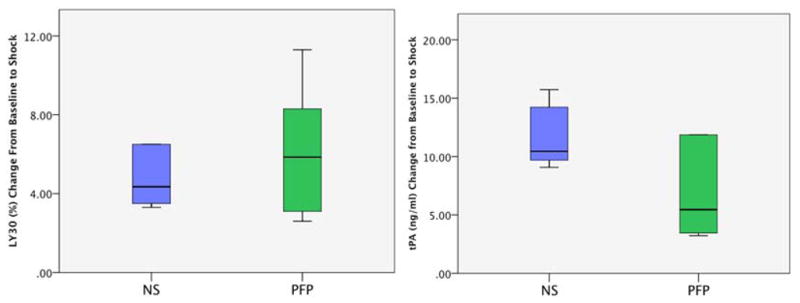
A, LY30 increased with shock: Y Axis represents the change in LY30 from baseline to shock. Both experimental groups had an increase in LY30 following 30 minutes of hemorrhagic shock.
B, tPA levels increased with shock: Y axis represents the change in tPA from baseline to shock. Both experimental groups had an increase in tPA following shock.
NS= normal saline; PFP = platelet free plasma; MAP = mean arterial pressure; Hem = hemorrhage; Obs = Observation; *= P<0.05
Figure 5.
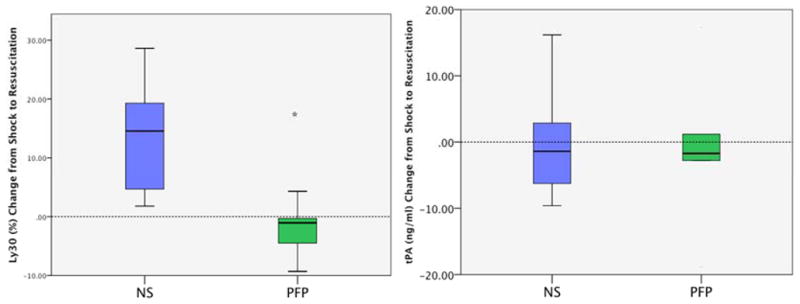
A, LY30 after NS resuscitation and was attenuated with plasma. Y Axis represents the change in LY30 from shock to partial resuscitation and 15 minutes of observation. NS resuscitation increased LY30 while plasma resuscitation tended to decrease LY30.
B, tPA levels similar regardless of resuscitation. Y axis represents the change in tPA from shock to partial resuscitation and 15 minutes of observation. Both experimental groups showed a similar distribution of change in tPA.
PFP= platelet free plasma; NS= normal saline
Proteomics
Pooled animal plasma at each time point identified over 400 proteins. Table 1 represents 16 proteins with similar baseline spectral counts. These proteins are ordered in ranked according to changes after shock, ordered from the largest increase of protein count from baseline to resuscitation with NS to the largest decrease in baseline to resuscitation with NS. Notably, fibrinogen, histidine rich glycoprotein (HRG), and Von Willebrand Factor (vWF) were reduced in the NS group. These proteins have been implicated in regulation of fibrinolysis.
Table 1.
Table 1 represents proteomic analysis of rodents at baseline, post shock, and after resuscitation. The first column represents proteins of interest followed by baseline spectral counts. The following columns are reflective of the groups change from baseline after shock or resuscitation.
| Baseline NS |
Baseline PFP |
Shock NS |
Shock PFP |
Resus NS |
Resus PFP |
|
|---|---|---|---|---|---|---|
| Zero beta-1 globin | 14 | 19 | 35% | 47% | 128% | 5% |
| Beta-2-microglobulin | 20 | 20 | 15% | 15% | 80% | 5% |
| Carbonic anhydrase 2 | 13 | 15 | 7% | 0% | 76% | 0% |
| complement factor properdin | 11 | 13 | 27% | 15% | 63% | 7% |
| Ficolin-1 | 12 | 11 | 8% | −9% | 50% | −9% |
| Complement C1q subcomponent | 13 | 14 | 7% | 7% | 30% | −7% |
| Murinoglobulin 1 homolog | 110 | 114 | −2% | 9% | 28% | 3% |
| Ceruloplasmin | 293 | 327 | −2% | 0% | 24% | −1% |
| Serine peptidase inhibitor | 70 | 79 | 2% | 7% | 18% | −7% |
| Serum amyloid A4 | 51 | 49 | −3% | −6% | 17% | −22% |
| Complement factor I | 74 | 68 | 14% | 5% | 17% | −11% |
| Isoform HMW of Kininogen-1 | 154 | 157 | 9% | −5% | −22% | −2% |
| Fibrinogen gamma chain | 642 | 617 | 5% | 20% | −45% | −15% |
| Histidine-rich glycoprotein | 174 | 198 | −23% | −38% | −47% | −15% |
| Coagulation factor X | 36 | 34 | −19% | −9% | −47% | −11% |
| Von Willebrand factor | 11 | 15 | −72% | −73% | −72% | −13% |
Discussion
In this rodent model of prehospital resuscitation in hyperfibrinolytic animals, plasma was a superior resuscitation fluid to saline. Plasma per volume was more effective at increasing mean arterial blood pressure, and the effects were sustained over 15 minutes of observation. This was accompanied by an overall improved survival rate. In addition, these animals all tolerated an additional blood draw suggesting increased physiologic reserve. Shock increased tPA in all animals, but the type of resuscitation did not change tPA concentrations. Proteomics identified several proteins that may account for these changes. Collectively, the beneficial effect of plasma resuscitation appears to be restoring unbalanced plasma proteins closer to their baseline level.
Prior hemorrhagic shock models in rodents have observed the beneficial effects of plasma over saline resuscitation. Kozar et al. (22) have previously shown the restorative effects of plasma over crystalloid resuscitation on the endothelial glyocalyx and reduction in lung injury. In this non-lethal pressure control model of hemorrhagic shock to a MAP of 30 mmHg for 90 minutes, the plasma-resuscitated rats required less than half the volume to recover from shock required for animals resuscitated with crystalloid. In a different rat hemorrhage volume control model (40% blood volume shed) over 30 minutes with 30 minutes of shock, plasma resuscitation also improved the endothelium in addition to preventing a decrease in clot strength compared to rodents resuscitated with crystalloid or colloids (23). The survival benefit of plasma resuscitation was also observed in a polytrauma swine model with severe hemorrhagic shock (24). Alam et al. demonstrated that plasma alone was an effective resuscitation fluid that corrected coagulopathy and improved survival compared to colloid and was equivalent to transfusion of whole blood with 60% bloodshed. The limitation of these prior animal models was the failure to generate a coagulopathy prior to resuscitation. Our model was designed to produce coagulation changes within 30 minutes, which has been documented in a number of clinical studies (25, 26) and was first suggested by Hardaway in 1970 (27). We demonstrated the beneficial effects of plasma on coagulation, even with incomplete resuscitation using plasma in rodents with hyperfibrinolysis, which is representative of trauma patients at the highest risk of death that arrive at the emergency department.
Based on previous work, we anticipated that circulating tPA levels would be elevated after 30 minutes of non-survivable shock in rats, and their clots would become more susceptible to clot degradation when challenges with a profibrinolytic (Moore et al Surgery 2015 manuscript submitted). Due to the fact these animals would not survive an 8% blood volume draw in this pressure control model at a MAP of 20 mmHG, the depth of shock in our current animal model was decreased to 25 mm Hg. This allowed rodents to survive hemorrhage and develop a profound shock state that was lethal unless resuscitated promptly. Bringing these animals to a near lethal state, but not into circulatory arrest, produced the hyperfibinolytic phenotype. The observation that 7 animals died during the post shock blood draw supports the hypothesis that fibrinolysis appears to be a pre-mortal event.
The tPA levels that increased with shock in this animal model are within the range of tPA levels measure by Brohi et al. proposing the mechanism of trauma coagulopathy in human patients driven by hypoperfusion (28). These authors point out the importance of unbalance of tPA regulation as a mechanism for increases in fibrinolysis. This is consistent with changes in rat tPA from baseline that did not correlate with changes in the magnitude of LY30 after shock, as individual animals resistance to fibrinolysis likely reflects variability of plasma proteins at baseline, which was evident in our proteomic analysis. High local levels of tPA can be produced by degranulation of small vessel endothelial cells (29). This regionalized fibrinolysis keeps the microvascular patent but circulating plasma under physiologic conditions prevents systemic hyperfibrinolysis. As previously mentioned, systemic fibrinolysis is not only impaired by PAI-1, but by a number of additional proteins in human plasma (30) and inhibited in the presence of platelet degranulation (31). Our proteomic analysis suggests depletion of fibrinogen, HRG and vWF after saline resuscitation when compared to plasma. Low levels of fibrinogen (14), HRG (32) and vWF (33) have been associated with increased susceptibility to fibrinolysis. Additionally, the proteins with the greatest change (Zero beta-1 globin, Beta-2-microglobulin) have been implicated in non-trauma related cellular damage (34, 35). Finally, there are a number of proteins associated with complement activation (Properiden, Ficolin) that have been implicated in up regulation of fibrinolysis after shock (36).
The high variability of LY30 and tPA in this study are representative of the complexity of the fibrinolytic system. There does not appear to be a finite threshold for development for hyperfibrinolysis as we observed that not all rodents developed the hyperfibrinolytic phenotype. This variability of susceptibility to fibrinolysis is also observed in the clinical setting. Due to restrictions of blood sampling in these animals, we were not able to perform concurrent platelet function assays. The abundance of antifibrinolytic stored in platelets granules makes them a probable suspect for fibrinolysis regulation. Recently, plasma resuscitation compared to saline has been shown to improve platelet function after hemorrhagic shock in a swine model (37). As previously discussed, there are numerous potential benefits of plasma resuscitation compared to crystalloid, many of which remain to be elucidated. The ongoing randomized prehospital trials (NCT01838863, NCT02303964, NCT01818427) are essential to evaluate the risk/benefit of treating trauma patients in the field with plasma, as it has been reported that plasma transfusion is associated with organ failure (38). Regardless, there is compelling evidence that plasma first resuscitation in the right patient at the right time has the potential to be lifesaving.
Acknowledgments
This study was supported in part by National Institute of Health grants: T32-GM008315 (NIGMS), P50-GM0492221 (NIGMS), UM 1HL120877 (NHLBI) and CCTSI supported in part by Colorado CTSA Grant UL1 TR001082 from NCATS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional research support was provided by Haemonetics (inc).
Footnotes
This study was presented at the 45th annual meeting of the Western Trauma Association, March 1–6, 2015, in Telluride, Colorado.
Author Contribution
H.M., E.M., A.B., and C.S. developed study design. H.M., A.M., E.G., and M.F. completed animal experiments and data collection. M.D., K.H., and M.C. completed protein assays. H.M., A.M., E.G., and M.C. completed literature review. H.M. and A.S. completed statistical analyses. H.M., A.M., E.G., M.D., and M.F. drafted the manuscript. H.M., E.M., K.H., A.B., A.S., and C.S. completed critical revisions.
Contributor Information
Hunter B. Moore, Email: hunter.moore@ucdenver.edu.
Alexander P. Morton, Email: alexander.morton@ucdenver.edu.
Eduardo Gonzalez, Email: eduardo.gonzalez@ucdenver.edu.
Miguel Fragoso, Email: miguel.fragoso@ucdenver.edu.
Michael P. Chapman, Email: michael.chapman@ucdenver.edu.
Monika Dzieciatkowska, Email: monika.dzieciatkowska@ucdenver.edu.
Kirk C. Hansen, Email: kirk.hansen@ucdenver.edu.
Anirban Banerjee, Email: anirban.banerjee@ucdenver.edu.
Angela Sauaia, Email: angela.sauaia@ucdenver.edu.
Christopher C. Silliman, Email: christopher.silliman@ucdenver.edu.
References
- 1.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. The journal of trauma and acute care surgery. 2014;77(6):811–7. doi: 10.1097/TA.0000000000000341. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schochl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. The journal of trauma and acute care surgery. 2012;73(2):365–70. doi: 10.1097/TA.0b013e31825c1234. discussion 70. [DOI] [PubMed] [Google Scholar]
- 3.Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. The Journal of trauma. 2009;67(1):125–31. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 4.Ives C, Inaba K, Branco BC, Okoye O, Schochl H, Talving P, Lam L, Shulman I, Nelson J, Demetriades D. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. Journal of the American College of Surgeons. 2012;215(4):496–502. doi: 10.1016/j.jamcollsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. doi: 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 6.Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Redick BJ, Nelson MF, Cohen MJ. Criteria for empiric treatment of hyperfibrinolysis after trauma. The journal of trauma and acute care surgery. 2012;73(1):87–93. doi: 10.1097/TA.0b013e3182598c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, Biffl WL, Burlew CC, Johnson JL, Sauaia A. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Annals of surgery. 2010;252(3):434–42. doi: 10.1097/SLA.0b013e3181f09191. discussion 43–4. [DOI] [PubMed] [Google Scholar]
- 8.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De’Ath HD, Allard S, Hart DP, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. Journal of thrombosis and haemostasis: JTH. 2013;11(2):307–14. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 9.Schochl H, Cadamuro J, Seidl S, Franz A, Solomon C, Schlimp CJ, Ziegler B. Hyperfibrinolysis is common in out-of-hospital cardiac arrest: results from a prospective observational thromboelastometry study. Resuscitation. 2013;84(4):454–9. doi: 10.1016/j.resuscitation.2012.08.318. [DOI] [PubMed] [Google Scholar]
- 10.Sherry S, Fletcher AP, Alkjaersig N. Fibrinolysis and fibrinolytic activity in man. Physiological reviews. 1959;39(2):343–82. doi: 10.1152/physrev.1959.39.2.343. [DOI] [PubMed] [Google Scholar]
- 11.Booth NA, Walker E, Maughan R, Bennett B. Plasminogen activator in normal subjects after exercise and venous occlusion: t-PA circulates as complexes with C1-inhibitor and PAI-1. Blood. 1987;69(6):1600–4. [PubMed] [Google Scholar]
- 12.Schaller J, Gerber SS. The plasmin-antiplasmin system: structural and functional aspects. Cellular and molecular life sciences: CMLS. 2011;68(5):785–801. doi: 10.1007/s00018-010-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramanathan A, Karuri N. Fibronectin alters the rate of formation and structure of the fibrin matrix. Biochemical and biophysical research communications. 2014;443(2):395–9. doi: 10.1016/j.bbrc.2013.11.090. [DOI] [PubMed] [Google Scholar]
- 14.He S, Johnsson H, Zabczyk M, Hultenby K, Wallen H, Blomback M. Fibrinogen depletion after plasma-dilution: impairment of proteolytic resistance and reversal via clotting factor concentrates. Thrombosis and haemostasis. 2014;111(3):417–28. doi: 10.1160/TH13-06-0497. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe B. Fibrinogen and factor XIII at the intersection of coagulation, fibrinolysis and inflammation. Thrombosis and haemostasis. 2014;112(4):649–58. doi: 10.1160/TH14-01-0085. [DOI] [PubMed] [Google Scholar]
- 16.Wohlauer MV, Moore EE, Droz NM, Harr J, Gonzalez E, Fragoso M, Silliman CC. Hemodilution is not critical in the pathogenesis of the acute coagulopathy of trauma. The Journal of surgical research. 2012;173(1):26–30. doi: 10.1016/j.jss.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore EE, Chin TL, Chapman MC, Gonzalez E, Moore HB, Silliman CC, Hansen KC, Sauaia A, Banerjee A. Plasma first in the field for postinjury hemorrhagic shock. Shock. 2014;41 (Suppl 1):35–8. doi: 10.1097/SHK.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49(8):1546–53. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore HB, Moore EE, Gonzalez E, Hansen KC, Dzieciatkowska M, Chapman MP, Sauaia A, West B, Banerjee A, Silliman CC. Hemolysis Exacerbates Hyperfibrinolysis While Platelolysis Shuts Down Fibrinolysis: Evolving Concepts of the Spectrum of Fibrinolysis in Response to Severe Injury. Shock. 2014 doi: 10.1097/SHK.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wohlauer MV, Moore EE, Harr J, Gonzalez E, Fragoso M, Silliman CC. A standardized technique for performing thromboelastography in rodents. Shock. 2011;36(5):524–6. doi: 10.1097/SHK.0b013e31822dc518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzieciatkowska M, Wohlauer MV, Moore EE, Damle S, Peltz E, Campsen J, Kelher M, Silliman C, Banerjee A, Hansen KC. Proteomic analysis of human mesenteric lymph. Shock. 2011;35(4):331–8. doi: 10.1097/SHK.0b013e318206f654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesthesia and analgesia. 2011;112(6):1289–95. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres LN, Sondeen JL, Ji L, Dubick MA, Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. The journal of trauma and acute care surgery. 2013;75(5):759–66. doi: 10.1097/TA.0b013e3182a92514. [DOI] [PubMed] [Google Scholar]
- 24.Alam HB, Bice LM, Butt MU, Cho SD, Dubick MA, Duggan M, Englehart MS, Holcomb JB, Morris MS, Prince MD, et al. Testing of blood products in a polytrauma model: results of a multi-institutional randomized preclinical trial. The Journal of trauma. 2009;67(4):856–64. doi: 10.1097/TA.0b013e3181b5ae75. [DOI] [PubMed] [Google Scholar]
- 25.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. The Journal of trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 26.Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, Kozar RA, Holcomb JB. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. The Journal of trauma. 2011;71(2):407–14. doi: 10.1097/TA.0b013e31821e1bf0. discussion 14–7. [DOI] [PubMed] [Google Scholar]
- 27.Hardaway RM. The significance of coagulative and thrombotic changes after haemorrhage and injury. Journal of clinical pathology Supplement. 1970;4:110–20. doi: 10.1136/jcp.s3-4.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Annals of surgery. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin EG, del Zoppo GJ. Localization of tissue plasminogen activator in the endothelium of a limited number of vessels. The American journal of pathology. 1994;144(5):855–61. [PMC free article] [PubMed] [Google Scholar]
- 30.Booth NA. Fibrinolysis and thrombosis. Bailliere’s best practice & research Clinical haematology. 1999;12(3):423–33. doi: 10.1053/beha.1999.0034. [DOI] [PubMed] [Google Scholar]
- 31.Brass LF, Stalker TJ. Minding the gaps--and the junctions, too. Circulation. 2012;125(20):2414–6. doi: 10.1161/CIRCULATIONAHA.112.106377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuchida-Straeten N, Ensslen S, Schafer C, Woltje M, Denecke B, Moser M, Graber S, Wakabayashi S, Koide T, Jahnen-Dechent W. Enhanced blood coagulation and fibrinolysis in mice lacking histidine-rich glycoprotein (HRG) Journal of thrombosis and haemostasis: JTH. 2005;3(5):865–72. doi: 10.1111/j.1538-7836.2005.01238.x. [DOI] [PubMed] [Google Scholar]
- 33.Tanka-Salamon A, Kolev K, Machovich R, Komorowicz E. Proteolytic resistance conferred to fibrinogen by von Willebrand factor. Thrombosis and haemostasis. 2010;103(2):291–8. doi: 10.1160/TH09-07-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaves DF, Carvalho PC, Lima DB, Nicastro H, Lorenzeti FM, Siqueira-Filho M, Hirabara SM, Alves PH, Moresco JJ, Yates JR, 3rd, et al. Comparative proteomic analysis of the aging soleus and extensor digitorum longus rat muscles using TMT labeling and mass spectrometry. J Proteome Res. 2013;12(10):4532–46. doi: 10.1021/pr400644x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo C, Yoon DH, Suh C. Serum beta-2 microglobulin in malignant lymphomas: an old but powerful prognostic factor. Blood research. 2014;49(3):148–53. doi: 10.5045/br.2014.49.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Barreno P, Balibrea JL, Aparicio P. Blood coagulation changes in shock. Surgery, gynecology & obstetrics. 1978;147(1):6–12. [PubMed] [Google Scholar]
- 37.Sillesen M, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam A, Hwabejire JO, Deperalta D, Duggan M, DeMoya M, et al. Fresh frozen plasma resuscitation attenuates platelet dysfunction compared with normal saline in a large animal model of multisystem trauma. The journal of trauma and acute care surgery. 2014;76(4):998–1007. doi: 10.1097/TA.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 38.Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, Sauaia A. Effect of blood products transfusion on the development of postinjury multiple organ failure. Archives of surgery. 2010;145(10):973–7. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]