Abstract
Purpose.
To study the role of neuronal nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase–dependent reactive oxygen species (ROS) production in retinal ganglion cell (RGC) death after ischemia.
Methods.
Ischemic injury was induced by unilateral elevation of intraocular pressure via direct corneal cannulation. For in vitro experiments, RGCs isolated by immunopanning from retinas were exposed to oxygen and glucose deprivation (OGD). The expression levels of NAD(P)H oxidase subunits were evaluated by quantitative PCR, immunocytochemistry, and immunohistochemistry. The level of ROS generated was assayed by dihydroethidium. The NAD(P)H oxidase inhibitors were then tested to determine if inhibition of NAD(P)H oxidase altered the production of ROS within the RGCs and promoted cell survival.
Results.
It was reported that RGCs express catalytic Nox1, Nox2, Nox4, Duox1, as well as regulatory Ncf1/p47phox, Ncf2/p67phox, Cyba/p22phox, Noxo1, and Noxa1 subunits of NAD(P)H oxidases under normal conditions and after ischemia. However, whereas RGCs express only low levels of catalytic Nox2, Nox4, and Duox1, and regulatory Ncf1/p47, Ncf2/p67 subunits, they exhibit significantly higher levels of catalytic subunit Nox1 and the subunits required for optimal activity of Nox1. It was observed that the nonselective NAD(P)H oxidase inhibitors VAS-2870, AEBSF, and the Nox1 NAD(P)H oxidase–specific inhibitor ML-090 decreased the ROS burst stimulated by OGD, which was associated with a decreased level of RGC death.
Conclusions.
The findings suggest that NAD(P)H oxidase activity in RGCs renders them vulnerable to ischemic death. Importantly, high levels of Nox1 NAD(P)H oxidase subunits in RGCs suggest that this enzyme could be a major source of ROS in RGCs produced by NAD(P)H oxidases.
In this study, the authors provide evidence that RGCs express NAD(P)H oxidases, which produce ROS and mediate RGC death after ischemia.
Introduction
Retinal ischemia–reperfusion (IR) injury is a clinical condition that remains a common cause of visual impairment and blindness, which has a final pathway of retinal ganglion cell (RGC) death.1 Thus, the identification, detection, and neutralization of triggers of IR injury will lead to better management and improved clinical outcomes. Reactive oxygen species (ROS) constitute one of many factors influencing the survival of RGCs following IR injury.1 Mitochondria are considered to be the main source of ROS after ischemia.2–5 However, increasing evidence suggests that nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase complexes are also an important source of cellular ROS after IR injury.6–11
Several homologs (Nox1, Nox3, Nox4, Nox5, Duox1, and Duox2) of classical phagocyte NAD(P)H oxidase gp91phox (Cybb/Nox2) have been identified in various tissues and organ systems.6–11 To produce superoxide ions (O2−), Nox1 requires an interaction with p22phox (Cyba), the cytosolic subunits Noxo1 and Noxa1, and the small Rho GTP-binding protein Rac1.8 Nox2 needs to interact with p22phox, phosphorylated p47phox (Ncf1), p67phox (Ncf2), and Rac1, 2, or 3.8 Although not absolutely required, p40phox (Ncf4) also associates with this complex and may contribute to activation.8 Nox3 activation is similar to that of Nox1.8 Nox4 requires p22phox, but in reconstituted systems it is constitutively active without the requirement for other subunits.8 Nox5, Duox1, and Duox2 are activated by Ca2+ and do not appear to require other subunits.8 To date, a major concept in redox signaling is that NAD(P)H oxidase–derived ROS are necessary for normal cellular function.8,12,13 However, excessive ROS production can contribute to pathologic disease.8,12,13 This is true in the central nervous system (CNS), where normal function of NAD(P)H oxidase complexes appears to be required for processes such as neuronal signaling and memory, but overproduction of ROS contributes to neurotoxicity and neurodegeneration.7–10,12–15
Until relatively recently, it was thought that the primary source of NAD(P)H oxidase–derived ROS in the CNS were microglia, immigrant macrophages, and astrocytes.7–9,16,17 In neurons, the expression of ROS-generating NAD(P)H oxidase subunits was considered unlikely because of their high susceptibility to oxidative damage. However, this paradigm has changed, because it has been shown that neurons express NAD(P)H oxidase complexes.18–21 The neuronal expression level of the Nox4 catalytic subunit of NAD(P)H oxidase was upregulated during stroke and the Nox1 catalytic subunit was upregulated in response to nerve growth factor (NGF).18,19 In addition, Nox2 contributes directly to oxidative stress and apoptosis in NGF-deprived sympathetic neurons.20 In the experiments described in the following text, we demonstrate that ischemia-induced activation of neuronal NAD(P)H oxidases increases ROS production in RGCs, which mediates neuronal death.
Methods
Animals
All experiments and postsurgical care were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Association for Research in Vision and Ophthalmology statement for use of animals in ophthalmic and vision research, and according to the University of Miami Institutional Animal Care and Use Committee approved protocols. C57BL/6J (stock number 000664) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were housed under standard conditions of temperature and humidity, with a 12-hour light/dark cycle and free access to food and water. All animals used in our experiments were 3-month-old male mice or 10- to 14-day-old pups.
Transient Retinal Ischemia
Retinal ischemia was induced for 60 minutes by introducing into the anterior chamber of the eye a 33-gauge needle attached to a normal (0.9% NaCl) saline-filled reservoir raised above the animal to increase intraocular pressure (IOP, increased to 120 mm Hg). The contralateral eye was cannulated and maintained at normal IOP to serve as a normotensive control. Body temperature was maintained at 37 ± 0.5°C. Complete retinal ischemia, evidenced by a whitening of the anterior segment of the eye and blanching of the retinal arteries, was verified by microscopic examination.
Isolation of Retinal Ganglion Cells
Retinal ganglion cells (RGCs) were isolated according to the two-step immunopanning method.22,23 Briefly, the whole retinas were incubated in papain solution (16.5 U/mL) for 30 minutes. In the next step, macrophage and endothelial cells were removed from the cell suspension by panning with antimacrophage antiserum (Accurate Chemical, Westbury, NY). RGCs were specifically bound to the panning plates containing anti-Thy1.2 antibody and released by trypsin incubation. RGCs were grown in commercial media (Neurobasal/B27 media; Invitrogen, Carlsbad, CA).
Oxygen and Glucose Deprivation (OGD) Model
RGCs in Neurobasal/B27 (Invitrogen, Carlsbad, CA) were plated on 12-mm glass coverslips and allowed to adhere for at least 18 hours. Normal media was then replaced with OGD media containing (in mM): 1.8 CaCl2, 0.814 MgCl2, 5.33 KCl, 26.19 NaHCO3, 68.97 NaCl, 0.906 NaH2PO4–H2O, and 10 sucrose (pH 7.4). OGD media was deoxygenated before use by bubbling for 1 hour with 95% N2/5% CO2. RGCs were deprived of oxygen using an anaerobic chamber (0% O2, 5% CO2, and 95% N2) for 4 hours at 37°C. After the oxygen and glucose deprivation, OGD media was replaced with “sham media,” which had the same composition, except that sucrose was replaced with 10 mM d-glucose, and cultures were returned to a normoxic environment. Parallel cultures were exposed to oxygenated “sham media” in a normoxic incubator (37°C; atmosphere 5% CO2) to serve as controls.
Real-Time PCR Analysis
Real-time PCR analysis was performed as described previously,9,24 using gene-specific primers (Table 1). Specifically, total RNA was extracted from retinas using a commercial expression profiling solution (Absolutely RNA Nanoprep Kit; Stratagene, Santa Clara, CA) and reverse transcribed with commercial kit (Reverse Transcription System; Promega, Madison, WI) to synthesize cDNA. Real-time PCR was then performed (Bio-Rad iCycler; Hercules, CA) using a commercial mix (SYBR GREEN PCR MasterMix; Qiagen, Valencia, CA). Relative expression was calculated by comparison with a standard curve following normalization to expression of the housekeeping gene β-actin, chosen as control.
Table.
List of PCR Primers and the Primary Antibodies
|
Gene |
Oligonucleotides |
Primary Antibodies |
|
| Nox1 | Forward | GGAATTGCAGATGAGGAAGC | GTX103888 (GeneTex, Irvine, CA) |
| Reverse | CCCAACCAGTACAGCCACTT | ||
| Nox2 | Forward | GACTGCGGAGAGTTTGGAAG | SC-5827 (Santa Cruz Biotechnology, Santa Cruz, CA) |
| Reverse | ACTGTCCCACCTCCATCTTG | ||
| Nox3 | Forward | ATCTTCATCCAGTGCCCATC | |
| Reverse | AGTGACTCCAATTCCCGTTG | ||
| Nox4 | Forward | TCTGGAAAACCTTCCTGCTG | GTX121929 (GeneTex, Irvine, CA) |
| Reverse | GTTGAGGGCATTCACCAAGT | ||
| Duox1 | Forward | TGGGGTTCCACTTAGCTCTG | |
| Reverse | GTACGGTTCCGAAGAGATGG | ||
| Duox2 | Forward | CTAGTTCTCCTGGGCGCTCT | |
| Reverse | AAGAAGACCCCCAGCACTGT | ||
| Noxo1 | Forward | ACTTAAACGCCTGTGCCATC | GTX108043 (GeneTex, Irvine, CA) |
| Reverse | CCCCAACACTGCCCTAAGTA | ||
| Noxa1 | Forward | TACAACATGGCATCAGCACA | |
| Reverse | CGTTGTGGTCATCAGGAATG | ||
| Ncf1 | Forward | CGAGAAGAGTTCGGGAACAG | SC-14,015 (Santa Cruz Biotechnology, Santa Cruz, CA) |
| Reverse | AGCCATCCAGGAGCTTATGA | ||
| Ncf2 | Forward | CTACCTGGAGCCAGTTGAGC | |
| Reverse | AGCGCCAGCTTCTTAGACAC | ||
| Ncf4 | Forward | TCAGAGAGCGACTTTGAGCA | |
| Reverse | GTTTTGCGCCATGTAGACT | ||
| Cyba | Forward | AAAGAGGAAAAAGGGGTCCA | |
| Reverse | CTCCTCTTCACCCTCACTCG | ||
| Rac1 | Forward | CTGAAGTGCGACACCACTGT | |
| Reverse | CAGCAGGCATTTTCTCTTCC | ||
| Rac2 | Forward | CATCAGCTACACCACCAACG | |
| Reverse | TTGGTACCCACCAGGATGAT | ||
| Rac3 | Forward | ACTGCTGGCCAGGAAGACTA | |
| Reverse | TCCAGGTACTTGACGGAACC | ||
| Thy1 | Forward | CAAGGATGAGGGCGACTAC | |
| Reverse | TCTTGGGGAGGGAGTCAGC | ||
| Gfap | Forward | AGAAAGGTTGAATCGCTGGA | C9205 (Sigma-Aldrich, St. Louis, MO) |
| Reverse | CGGCGATAGTCGTTAGCTTC | ||
| S100b | Forward | GGTGACAAGCACAAGCTGAA | |
| Reverse | CCCTCATGTCTGTTGCAGAA | ||
| Cd11b | Forward | ACAATGTGACCGTATGGGATC | RM2805 (Invitrogen, Grand Island, NY) |
| Reverse | GCAAACGCAGAGTCATTAAAC | ||
| Actb | Forward | CACCCTGTGCTGCTCACC | |
| Reverse | GCACGATTTCCCTCTCAG | ||
| NeuN | MAB377X (Millipore, Billerica, MA) | ||
| Tub β III | MMS-435P (Covance, Dedham, MA) | ||
Immunohistochemistry
Eyes were enucleated upon euthanasia by CO2 inhalation under anesthesia, incised at the ora serrata, and immersion-fixed in 4% paraformaldehyde (PF). After an hour, the fixed retinas were removed and sectioned to a thickness of 100 μm with a vibratome (Vibratome, St. Louis, MO) and immunostained using the protocol described previously.9 Sections were incubated with various primary antibodies (Table 1), followed by species-specific secondary fluorescent antibodies (AlexaFluor; Invitrogen, Carlsbad, CA). Negative controls were incubated with secondary antibody only. Imaging was performed with a confocal microscope (Leica TSL AOBS SP5; Leica Microsystems, Exton, PA).
Immunocytochemistry
Cultured cells were fixed in 4% PF and blocked with 5% normal donkey serum with 0.15% Tween-20 in PBS at pH 7.4. Cells were then incubated (β-tubulin III antibody, 1:500; Covance, Princeton, NJ) and various primary antibodies (Table 1) followed by species-specific secondary fluorescent antibodies (Invitrogen). Negative controls were incubated with secondary antibody only. Imaging was performed with a confocal microscope (Leica TSL AOBS SP5; Leica Microsystems).
Measurement of Superoxide Formation Using Dihydroethidium (DHE)
The cell-permeate DHE (D23107, Invitrogen) was used to assess formation of superoxide in RGCs. RGCs were plated on 12-mm glasses and allowed to adhere for at least 18 hours. RGCs were treated during and after OGD with Nox1 NAD(P)H oxidase–specific inhibitor ML-090 [5,12-dihydroquinoxalino[2,3-b]quinoxaline] (0.09 μM)25 (Sigma-Aldrich, St. Louis, MO) and with the nonselective NAD(P)H oxidase inhibitors (VAS-2870, 0.5 μM; and AEBSF, 0.5 μM; both from Enzo Life Sciences, Farmingdale, NY). DHE (3 μM) was added to the medium of untreated and treated RGCs, the incubation was continued for 15 minutes, and then images were sampled randomly to collect a total of 10 images with a confocal microscope using a ×20 objective lens (Leica TSL AOBS SP5). The averages of fluorescence intensity values (518-nm excitation, 605-nm emission) from at least 100 cells were calculated using commercial image software (Leica TSL AOBS SP5). The experiment was repeated at least three times.
Neuronal Death Assay
RGCs were plated on 12-mm glasses and allowed to adhere for at least 18 hours. After OGD, necrotic and apoptotic cells were determined using a commercial kit (Vybrant Apoptosis Assay Kit #2; Invitrogen). Cells were imaged using a confocal microscope (Leica TSL AOBS SP5; Leica Microsystems). Individual glasses were sampled randomly to collect a total of 10 images using a ×20 objective lens. The apoptotic RGCs (Annexin V-positive) and necrotic RGCs (Annexin V and propidium iodide [PI] positive) were counted semiautomatically using commercial software (MetaMorph; Molecular Devices, Sunnyvale, CA). The percentage of necrotic and apoptotic RGCs relative to the total number of cells was determined. The experiment was repeated at least three times.
Statistical Analysis
Data are presented as average ± SEM. Real-time PCR measurements, fluorescence quantification, and levels of live cells as well as necrotic and apoptotic cells were analyzed with Student's t-test. Values of P < 0.05 were designated as statistically significant.
Results
NAD(P)H Oxidase Subunits Are Present at the mRNA and Protein Levels in Normal and Ischemic RGCs
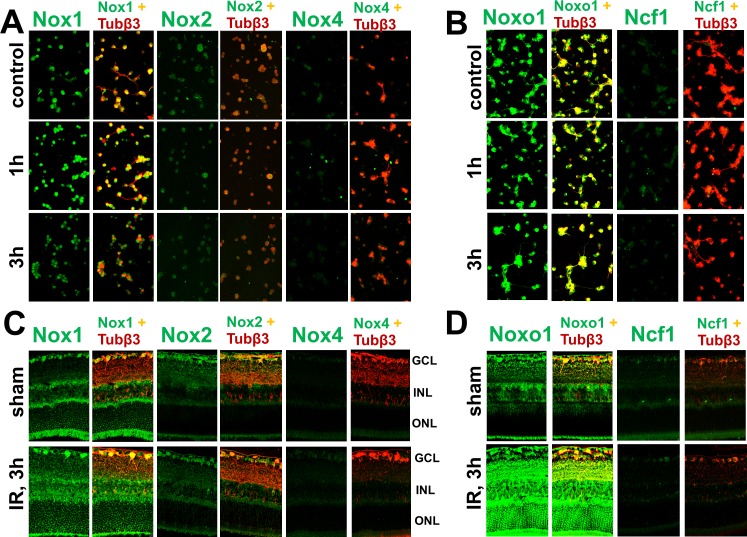
To determine whether NAD(P)H oxidase subunits are expressed in normal and ischemic RGCs, we analyzed the expression of catalytic (with the exception of the Nox5 gene, which is absent in the rodent genome8) and regulatory subunits in RGCs in response to oxygen and glucose deprivation (OGD) using real-time PCR analysis. Primary RGC cultures were deprived of oxygen and glucose for 4 hours. Cultures of RGCs were assessed for levels of NAD(P)H oxidase subunits 1 and 3 hours after OGD. We found that OGD-treated and untreated RGCs express catalytic Nox1, Nox2, Nox4, Duox1 (Fig. 1A, Supplement 1; http://www.iovs.org/content/53/6/2823/suppl/DC1), as well as regulatory Ncf1/p47phox, Ncf2/p67phox, Cyba/p22phox, Noxo1, Noxa1 subunits (Fig. 1B, Supplement 1; http://www.iovs.org/content/53/6/2823/suppl/DC1) and Rac1, Rac2, and Rac3 (Fig. 1C, Supplement 1; http://www.iovs.org/content/53/6/2823/suppl/DC1). The level of catalytic Nox3 and Duox2, and regulatory Ncf4/p40phox subunits were statistically insignificant in RGCs (Figs. 1A, 1B, Supplement 1; http://www.iovs.org/content/53/6/2823/suppl/DC1). In addition, RGCs express only low levels of catalytic Nox2, Nox4, and Duox1, and regulatory Ncf1/p47phox, Ncf2/p67phox subunits, but exhibit significantly higher levels of catalytic subunit Nox1 and subunits required for optimal activity of Nox1, including Cyba/p22phox, Noxo1, and Rac1 (Fig. 1, Supplement 1; http://www.iovs.org/content/53/6/2823/suppl/DC1). Importantly, the expression level of Nox1 was increased after OGD treatment, whereas the levels of Nox2 and Nox4 were not increased (Fig. 1A, Supplement 1; http://www.iovs.org/content/53/6/2823/suppl/DC1). To exclude the possibility of contamination of the primary RGC culture by other cells, which might contribute to the detected expression of NAD(P)H oxidase subunits, we evaluated the purity of the isolated cells with the use of specific markers for RGC (Thy1), astrocytes (Gfap and S100b), and microglia/macrophages (Cd11b). The calculated efficiency of RGC immunoaffinity purification was 95% to 99% (Fig. 1D). The gene expression profiles for Nox1, Nox2, Nox4, as well as regulatory subunits Noxo1 and Ncf1/p47phox in RGCs were consistent with the corresponding protein accumulation levels detected by immunocytochemistry: levels of Nox1 and Noxo1 in control and ischemic RGCs were significantly higher compared with Nox2, Nox4, and Ncf1/p47phox, respectively (Figs. 2A, 2B).
Figure 1.
NAD(P)H oxidase subunits are present in RGCs at the mRNA level: RGC expression of (A) catalytic and (B, C) regulatory subunits of NAD(P)H oxidases after 4 hours OGD followed by 1 and 3 hours of reoxygenation, compared with control. (D) Isolated RGCs were tested for the presence of specific RGC and contaminating cell type markers using real-time PCR.
Figure 2.
NAD(P)H oxidase subunits are present in RGCs at the protein level: Immunocytochemistry for (A) catalytic Nox1, Nox2, and Nox4 and (B) regulatory Noxo1 and Ncf1/p47phox subunit accumulation in control and OGD-treated RGCs were consistent with increased levels of corresponding transcripts. Immunohistochemistry showed accumulation of (C) catalytic as well as (D) regulatory subunits in RGCs of sham-operated and ischemic (3 hours after reperfusion) retinas. Antibodies against tubulin β-III were used to identify RGCs. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
To evaluate whether our in vitro data were consistent in vivo, we induced unilateral retinal ischemia in mice and studied the corresponding protein levels and cellular localization of catalytic Nox1, Nox2, and Nox4, and regulatory Noxo1 and Ncf1/p47phox subunits by immunohistochemistry. Nox1 and regulatory subunit Noxo1 were easily detected in RGCs of ischemic and sham-operated retinas, whereas Nox2, Nox4, and regulatory Ncf1/p47phox subunit were detected at significantly lower levels (Figs. 2C, 2D). Thus, our in vivo and in vitro data indicate that Nox1 is the main NAD(P)H oxidase expressed in RGCs and could be a major source of ROS produced by NAD(P)H oxidases in ischemic RGCs.
Oxygen and Glucose Deprivation Induces the Activation of NAD(P)H Oxidases, Which Facilitates ROS Production in RGCs
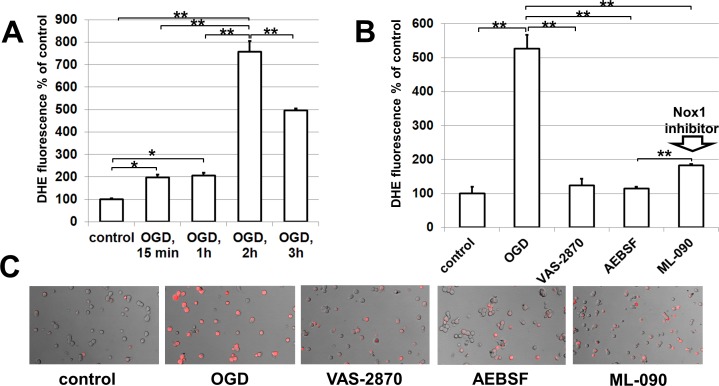
The NAD(P)H oxidase complex donates an electron from NAD(P)H to molecular oxygen (O2) to produce superoxide (O2−), which initiates the respiratory burst.8 Thus, to test whether neuronal NAD(P)H oxidases can regulate ROS production in RGCs after ischemia, we exposed primary RGC cultures to OGD and then measured dihydroethidium (DHE) oxidation as an indicator of NAD(P)H oxidase–dependent superoxide production in a time-dependent manner26 (Fig. 3A). Our results indicate that initial ROS production was rapid, with 2-fold increases in DHE oxidation within 15 minutes after OGD (Fig. 3A). OGD-induced ROS formation remained stable up to 1 hour, and then increased significantly after 2 hours (Fig. 3A). Although ROS formation decreased between 2 and 3 hours post-OGD treatment, these levels remained 2.5-fold higher than ROS formation at 15 minutes and 1 hour post-OGD treatment and 5-fold higher than the control. The NAD(P)H oxidase nonselective inhibitors VAS-2870,27 AEBSF,8 and the Nox1 NAD(P)H oxidase–specific inhibitor ML-09025 were then tested to verify the involvement of neuronal NAD(P)H oxidase in ROS production in RGCs after ischemia. To this end, control and OGD-treated primary RGC cultures were incubated without or in the presence of the NAD(P)H oxidase inhibitors. Inhibitors were present in the RGC culture medium during and after OGD. Two hours after reoxygenation, the levels of ROS generated were assayed by DHE. We detected that treatment of RGCs during and after OGD with the NAD(P)H oxidase inhibitors significantly abolished OGD-induced enhancement of DHE oxidation (Figs. 3B, 3C, Supplement 2; http://www.iovs.org/content/53/6/2823/suppl/DC1). It should be noted that we found no statistically significant differences in ROS levels after OGD between the RGC cultures treated with the nonselective inhibitor VAS-2870 and the Nox1 NAD(P)H oxidase–specific inhibitor ML-090 (P value = 0.099; refer to Supplement 2, http://www.iovs.org/content/53/6/2823/suppl/DC1). These data suggest that neuronal NAD(P)H oxidases can regulate ROS production in RGCs. Nox1 NAD(P)H oxidase could be a major source of ROS produced by NAD(P)H oxidases in RGCs.
Figure 3.
Neuronal NAD(P)H oxidase leads to the generation of ROS in RGCs after ischemia: (A) OGD increases ROS production in RGCs via NAD(P)H oxidase activity in a time-dependent manner. (B) NAD(P)H oxidase inhibitors VAS-2870 (0.5 μM), AEBSF (0.5 μM), and ML-090 (0.09 μM) reduce ROS production after OGD. RGCs were deprived of oxygen and glucose for 4 hours followed by 2 hours of reoxygenation in either the presence or the absence of NAD(P)H inhibitors. The average fluorescence intensity values were calculated, **P < 0.01. (C) Representative results of DHE are shown.
Neuronal NAD(P)H Oxidases Mediate RGC Death
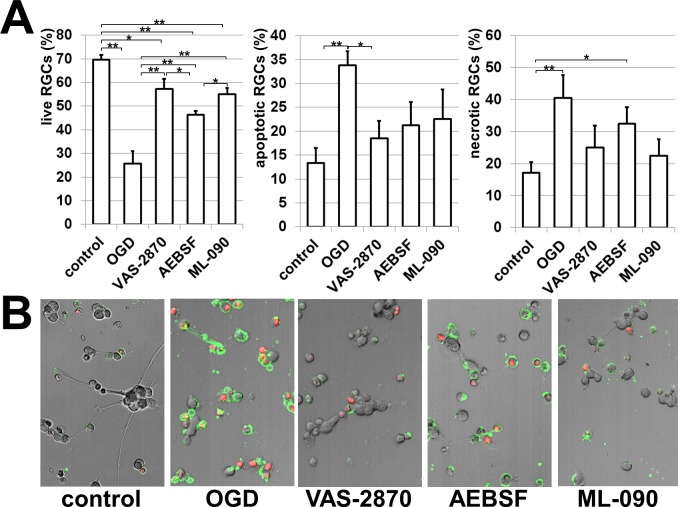
To evaluate the role of NAD(P)H oxidases in RGC death, we analyzed whether the NAD(P)H oxidase inhibitors VAS-2870, AEBSF, and ML-090 protected these neurons from death after OGD. RGCs were deprived of oxygen and glucose for 4 hours in an anaerobic chamber with or without inhibitors and the level of RGC survival was detected 24 hours after reoxygenation. Cultures of primary RGCs were assessed for levels of live cells as well as necrotic and apoptotic cells using a commercial marker of apoptotic cells (Annexin V; BD Pharmingen) and to identify necrotic cells (Annexin V/PI) (Fig. 4). Our results indicate that RGC death was largely blocked by VAS-2870, AEBSF, and ML-090 treatment (Fig. 4, Supplement 3, http://www.iovs.org/content/53/6/2823/suppl/DC1). Importantly, we observed that the Nox1-specific inhibitor ML-090 demonstrated a similar neuroprotective effect as the nonselective NAD(P)H oxidase inhibitors VAS-2870 and AEBSF. This indicates that Nox1 NAD(P)H oxidase could be more responsible for RGC death, compared with other NAD(P)H oxidases. In summary, our results suggest that reduced activity of neuronal NAD(P)H oxidases protect RGCs from OGD-induced death.
Figure 4.
NAD(P)H oxidases contribute to RGC death: (A) The NAD(P)H oxidase inhibitors VAS-2870 (0.5 μM), AEBSF (0.5 μM), and ML-090 (0.09 μM) reduced OGD-induced RGC death. RGCs were deprived of oxygen and glucose for 4 hours followed by 24 hours of reoxygenation in either the presence or the absence of the NAD(P)H oxidase inhibitors. The percentage of live RGCs as well as necrotic and apoptotic cells relative to the total number of cells was determined (*P < 0.05, **P < 0.01). (B) Necrotic and apoptotic cells were identified using Annexin V (green) as a marker of apoptotic cells and Annexin V/propidium iodide (PI, red) to recognize necrotic cells.
Discussion
A major concept in redox signaling is that NAD(P)H oxidase–derived ROS are necessary for normal cellular function.8 This certainly is true in the central nervous system (CNS), where normal NAD(P)H oxidase function appears to be required for processes including neuronal signaling and memory, but overproduction of ROS contributes to neurotoxicity and neurodegeneration.8 Retinal neurons, RGCs, were found to express NAD(P)H oxidase subunits under physiologic conditions and after ischemia. In addition, this report provides evidence that neuronal NAD(P)H oxidases contribute to ROS production and RGC death after ischemic injury.
Recent studies confirm the expression of ROS-generating NAD(P)H oxidases in neurons. Indeed, all elements of phagocyte Nox2 NADPH oxidase were found in sympathetic20 and hippocampal neurons,28 whereas Nox4 immunoreactivity has been demonstrated in Purkinje cells,18 and Nox1 expression was detected in the PC12 neuronal cell line.19 Our detailed analysis of the NAD(P)H oxidase subunits in RGCs has revealed that the Nox1, Nox2, and Nox4 catalytic subunits as well as their regulatory subunits are present in normal and ischemic RGCs at the mRNA and protein levels. However, RGCs exhibit a significantly higher level of catalytic subunit Nox1 as well as the subunits required for optimal activity of Nox1. Importantly, oxygen and glucose deprivation increased Nox1 expression, whereas expressions of Nox2 and Nox4 were slightly reduced. The next question that was important to answer was whether neuronal NAD(P)H oxidase subunits form active ROS-producing enzymes. We found that the NAD(P)H oxidase–specific inhibitors significantly abolished OGD-induced ROS production in RGC cultures. These data suggest that neuronal NAD(P)H oxidases can regulate ROS production in RGCs. Importantly, we found no statistically significant differences in ROS levels after OGD between the RGC cultures treated with the nonselective inhibitor VAS-2870 and the Nox1 NAD(P)H oxidase–specific inhibitor ML-090. This suggests that NAD(P)H oxidase–dependent ROS production after ischemia can be mediated predominantly via Nox1 activity. However, it is also known that the rate of ROS production from mitochondria is increased in neurons after ischemia or axonal injury.2–5 These results do not contradict our findings. It was found that mitochondria and Nox1 NAD(P)H oxidase do not act independently but rather function in a cooperative manner to extend the production of ROS.29–32 Lee et al.32 have shown a signaling link between the mitochondria and Nox1 NAD(P)H oxidase, which is crucial for the sustained accumulation of ROS and cell death. This mechanism should be explored in future studies.
It has been shown that NAD(P)H-oxidase–related production of ROS plays a role in normal neuronal function (for review, see Bedard and Krause8). However, an excessive and/or sustained increase in NAD(P)H-oxidase–derived ROS production after ischemia has been implicated in neuronal death. Walder et al.14 have demonstrated that mice lacking the classical phagocyte-type Nox2 are protected against ischemic injury and concluded that a putative neuronal Nox is involved in the development of neuronal damage following brain ischemia.14 It has been shown that the deletion of Nox2 can reduce IR-induced cell death and preserve retinal neurons after IR injury.11 This report provides evidence that neuronal NAD(P)H oxidases contribute to RGC death after ischemia. However, our data suggest that Nox1 NAD(P)H oxidase is more responsible for RGC death compared to Nox2 since the Nox1 specific inhibitor ML-090 demonstrated a similar neuroprotective effect as the nonselective NAD(P)H oxidase inhibitors VAS-2870 and AEBSF. In this regard, it is important to note that Nox1 NAD(P)H oxidase contributes to ischemic injury in experimental stroke in mice.6
In conclusion, our findings suggest that NAD(P)H oxidase activity in RGCs renders them vulnerable to ischemic death. The critical role of NAD(P)H oxidase Nox1 was proposed. Importantly, the expression of subunits of NAD(P)H oxidase complexes in RGCs under physiologic conditions suggests that NAD(P)H-oxidase–related production of ROS can play a role in normal RGC function, but also that excessive NAD(P)H oxidase–derived ROS production after ischemia can mediate RGC death. Thus, therapies based on agents that inhibit NAD(P)H oxidase activity can prove to be effective in reducing neuronal injury after ischemia. The effectiveness of the NAD(P)H oxidase inhibitors VAS-2870, AEBSF, and ML-090 has already been demonstrated in vitro.
Supplementary Material
Acknowledgments
The authors thank Gabriel Gaidosh for his expert technical assistance.
Footnotes
Supported in part by National Eye Institute/National Institutes of Health (NIH) Grants R21 EY020613 and R01 EY022348 (DI), unrestricted grant from the Research to Prevent Blindness, NIH Center Core Grant P30EY014801, and Department of Defense (DOD) Grant W81XWH-09-1-0675 to the University of Miami Department of Ophthalmology.
Disclosure: G. Dvoriantchikova, None; J. Grant, None; A.R.C. Santos, None; E. Hernandez, None; D. Ivanov, None
References
- 1. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. [DOI] [PubMed] [Google Scholar]
- 2. Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–332. [DOI] [PubMed] [Google Scholar]
- 3. Lieven CJ, Hoegger MJ, Schlieve CR, Levin LA. Retinal ganglion cell axotomy induces an increase in intracellular superoxide anion. Invest Ophthalmol Vis Sci. 2006;47:1477–1485. [DOI] [PubMed] [Google Scholar]
- 4. Christophe M, Nicolas S. Mitochondria: a target for neuroprotective interventions in cerebral ischemia-reperfusion. Curr Pharm Des. 2006;12:739–757. [DOI] [PubMed] [Google Scholar]
- 5. Osborne NN. Mitochondria: their role in ganglion cell death and survival in primary open angle glaucoma. Exp Eye Res. 2010;90:750–757. [DOI] [PubMed] [Google Scholar]
- 6. Kahles T, Kohnen A, Heumueller S, et al. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis. 2010;40:185–192. [DOI] [PubMed] [Google Scholar]
- 7. Barakat DJ, Dvoriantchikova G, Ivanov D, Shestopalov VI. Astroglial NF-kappaB mediates oxidative stress by regulation of NADPH oxidase in a model of retinal ischemia reperfusion injury. J Neurochem. 2012;120:586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. [DOI] [PubMed] [Google Scholar]
- 9. Dvoriantchikova G, Barakat D, Brambilla R, et al. Inactivation of astroglial NF-kappaB promotes survival of retinal neurons following ischemic injury. Eur J Neurosci. 2009;30:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxidants Redox Signaling. 2009;11:2481–2504. [DOI] [PubMed] [Google Scholar]
- 11. Yokota H, Narayanan SP, Zhang W, et al. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci. 2011;52:8123–8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. [DOI] [PubMed] [Google Scholar]
- 13. Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxidants Redox Signaling. 2006;8:1583–1596. [DOI] [PubMed] [Google Scholar]
- 14. Walder CE, Green SP, Darbonne WC, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. [DOI] [PubMed] [Google Scholar]
- 15. Yokota H, Narayanan SP, Zhang W, et al. BNeuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci. 2011;52:8123–8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hur J, Lee P, Kim MJ, Kim Y, Cho YW. Ischemia-activated microglia induces neuronal injury via activation of gp91phox NADPH oxidase. Biochem Biophys Res Commun. 2010;391:1526–1530. [DOI] [PubMed] [Google Scholar]
- 18. Vallet P, Charnay Y, Steger K, et al. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. [DOI] [PubMed] [Google Scholar]
- 19. Ibi M, Katsuyama M, Fan C, et al. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free Radic Biol Med. 2006;40:1785–1795. [DOI] [PubMed] [Google Scholar]
- 20. Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000; 20:RC53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. [DOI] [PubMed] [Google Scholar]
- 22. Dvoriantchikova G, Hernandez E, Grant J, Santos AR, Yang H, Ivanov D. The high-mobility group box-1 nuclear factor mediates retinal injury after ischemia reperfusion. Invest Ophthalmol Vis Sci. 2011;52:7187–7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Liposome-delivered ATP effectively protects the retina against ischemia-reperfusion injury. Mol Vis. 2010;16:2882–2890. [PMC free article] [PubMed] [Google Scholar]
- 24. Dvoriantchikova G, Agudelo C, Hernandez E, Shestopalov VI, Ivanov D. Phosphatidylserine-containing liposomes promote maximal survival of retinal neurons after ischemic injury. J Cereb Blood Flow Metab. 2009;29:1755–1759. [DOI] [PubMed] [Google Scholar]
- 25. Brown SJ, Gianni D, Bokoch G, Mercer BA, Hodder P, Rosen HR. Probe Report for NOX1 Inhibitors. Probe Reports from the NIH Molecular Libraries Program [ Internet] Bethesda, MD: National Center for Biotechnology Information; 2010. [PubMed] [Google Scholar]
- 26. Robinson KM, Janes MS, Pehar M, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA. 2006;103:15038–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ten Freyhaus H, Huntgeburth M, Wingler K, et al. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–341. [DOI] [PubMed] [Google Scholar]
- 28. Tejada-Simon MV, Serrano F, Villasana LE, et al. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897–906. [DOI] [PubMed] [Google Scholar]
- 30. Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther. 2005;4:1367–1373. [DOI] [PubMed] [Google Scholar]
- 32. Lee SB, Bae IH, Bae YS, Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem. 2006;281:36228–36235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.