Abstract
Purpose.
Investigate whether retinas of mice with impaired retinal cycles exposed to light or kept in the dark tolerate prolonged high-dose administration of QLT091001, which contains as an active ingredient, the 9-cis-retinal precursor, 9-cis-retinyl acetate.
Methods.
Four- to six-week-old Lrat−/− and Rpe65−/− mice (n = 126) as well as crossbred Gnat1−/− mice lacking rod phototransduction (n = 110) were gavaged weekly for 6 months with 50 mg/kg QLT091001, either after being kept in the dark or after light bleaching for 30 min/wk followed by maintenance in a 12-hour light ≤ 10 lux)/12-hour dark cycle. Retinal health was monitored by spectral-domain optical coherent tomography (SD-OCT) and scanning laser ophthalmoscopy (SLO) every other month and histological, biochemical, and visual functional analyses were performed at the end of the experiment. Two-photon microscopy (TPM) was used to observe retinoid-containing retinosome structures in the RPE.
Results.
Retinal thickness and morphology examined by SD-OCT were well maintained in all strains treated with QLT091001. No significant increases of fundus autofluorescence were detected by SLO imaging of any strain. Accumulation of all-trans-retinyl esters varied with genetic background, types of administered compounds and lighting conditions but retinal health was not compromised. TPM imaging clearly revealed maintenance of retinosomes in the RPE of all mouse strains tested.
Conclusions.
Retinas of Lrat−/−, Rpe65−/−, and crossbred Gnat1−/− mice tolerated prolonged high-dose QLT091001 treatment well.
This study provides evidence that retinas of 4 to 6-week-old Lrat−/− and Rpe65−/− mice gavaged with high doses of the experimental drug QLT091001 targeting a subset of LCA cases over a 6-month period tolerated the regimen well.
Introduction
Continuous regeneration of visual pigments by the visual chromophore 11-cis-retinal is essential to maintain vision and preserve retinal health.1–5 This process, which occurs in photoreceptor outer segments (ROS) and RPE, involves several enzymatic steps collectively named the retinoid or visual cycle.6–11 Disruption of this cycle due to gene mutations causes various inherited retinal degenerative diseases in humans5,6 due to inadequate or severely delayed 11-cis-retinal regeneration. Thus mutations in genes encoding LRAT and retinal pigment epithelium-specific 65 kDa protein (RPE65) account for 5% of all cases of Leber congenital amaurosis (LCA) and retinitis pigmentosa (RP).12–14 LRAT catalyzes the conversion of all-trans-retinol to all-trans-retinyl esters in the RPE.15,16 The resulting all-trans-retinyl esters, stored in RPE-specific organelles called retinosomes, serve as substrates for retinoid isomerization catalyzed by the RPE65.15–21 Notably, these retinosomes can be visualized by TPM under both ex vivo and in vivo conditions.20
Animal models have been extensively used to investigate the pathology and develop treatments for LCA such as gene transfer21–24 and pharmacological therapy21,25,26 because genetic, physiological, and biochemical attributes are broadly shared between the mouse, dog, and human retina. In Lrat−/− and Rpe65−/− mice, rod photoreceptor cells degenerate slowly whereas cone photoreceptors show an early onset of cell death.27,28 Spontaneous activity of opsin apoprotein reportedly can cause rod photoreceptor degeneration ameliorated by deletion of Gnat1, the gene encoding the rod specific G protein, transducin.29 The pathology of early cone cell death in Lrat−/− and Rpe65−/− mice could be caused by instability of cone pigments lacking visual chromophore and intracellular stress induced by anomalous cone opsin localization to different compartments of photoreceptors.30–34 Moreover, all-trans-retinyl esters accumulate massively in the RPE of Rpe65−/− mice.29 Whether such ester accumulation is pathological remains unclear, but it must be considered when pharmacological rescue of vision is employed in animal models and humans with defective 11-cis-retinal renewal.15,35,36
Pharmacological retinoid replacement therapy for 11-cis-retinal deficiency offers great promise for treatment of human retinal diseases with deficient chromophore biosynthesis.3,5,21,26,37,38 Administered 9-cis-retinoid can bypass defects in the visual cycle and regenerate visual pigment as iso-rhodopsin, thereby restoring visual function and ameliorating the progression of retinal degeneration in Lrat−/− and Rpe65−/− animals.21,25,37,39 In fact, 9-cis-retinoids are preferred over 11-cis-retinoids for replacement therapy due to their superior chemical stability and ease of synthesis.37,40,41 9-cis-Retinyl esters, including 9-cis-retinyl acetate (9-cis-R-Ac), are prodrugs used to generate 9-cis-retinal in vivo.21,25 QLT091001, which contains 9-cis-retinyl acetate as a 9-cis-retinoid—and is developed by QLT, Inc.—has been tested in preliminary human clinical trials (ClinicalTrials.gov number, NCT01014052; LCA/RP; Koenekoop RK, et al. IOVS 2012;53: ARVO E-Abstract 4642; and Cideciyan AV, et al. IOVS 2012;53:ARVO E-Abstract 6965).
Whether or not the retina and RPE can tolerate continuous exposure to high levels of QLT091001 and its metabolites has yet to be investigated. Possibly photo-isomerized 9-cis-retinal, the bioactive form of QLT091001 released from photoreceptors, could facilitate accumulation of all-trans-retinyl esters or retinoid byproducts in the RPE of patients with either LRAT or RPE65 deficiency. Such aberrant processes could eventually adversely affect both the retina and RPE. We designed this study to evaluate the effects of long-term, high-dose QLT091001 therapy on retinas of Lrat−/− and Rpe65−/− mice kept under lighting conditions that facilitate the release of 9-cis-retinal to the RPE and compared the results with those produced by identical regimens of control compounds (vehicle and all-trans-retinyl acetate). Gnat1−/− mice crossbred with either Lrat−/− or Rpe65−/− mice also were employed to evaluate unrelated QLT091001 side effects on the retina because GNAT1 deletion can ameliorate photoreceptor cell death caused by pathology induced by 11-cis-retinal deficiency itself.29 Noninvasive ophthalmic in vivo imaging systems such as spectral-domain optical coherent tomography (SD-OCT) and scanning laser ophthalmoscopy (SLO) were employed to monitor retinal health and TPM was used to document changes in RPE morphology and biochemistry.
Materials and Methods
Animals
All animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology. The generation and genotyping of Lrat−/−, Rpe65−/−, Gnat1−/−, Gnat1−/−Rpe65−/−, and Gnat1−/−Lrat−/− mice were previously described.16,39,42,43 Albino Gnat1−/−Rpe65−/− and Gnat1−/−Lrat−/− mice were generated by crossbreeding Gnat1−/− mice with C57BL/6 albino mice for ex vivo imaging by two-photon microscopy (TPM).44 C57BL/6J mice were purchased from Jackson laboratory (Bar Harbor, ME). Four- to 6-week-old animals of mixed gender were used to initiate all experiments (Table). Until each experiment was launched by the initial retinoid/vehicle gavage (see below), mice were maintained in a 12-hour light (≤ 10 lux)/12-hour dark (6 AM/6 PM) cycle at the Animal Resource Center, Case Western Reserve University. Then mice were either transferred to a darkroom just before their first retinoid/vehicle gavage where they remained or they were continuously kept in a 12-hour light (≤ 10 lux)/12-hour dark (6 AM/6 PM) cycle for the duration of the 6-month experiment.
QLT091001 and All-trans-Retinyl Acetate (all-trans-RAc) Preparation
Stock solutions of QLT091001 and all-trans-RAc at a concentration of 20 mg/mL were provided by QLT Inc. (Vancouver, BC, Canada). Solutions were stored in the dark at 4°C for less than 1 month before experimental use. Retinoid concentrations in these stock solutions were measured with a spectrophotometer (Agilent 8453, UV-Visible, Chemstation ver. 8.02.01). For this procedure, 2 μL of stock solution were added to 1.0 mL of 100% ethanol and retinoids were quantified by maximum absorption at λmax 322 nm for QLT091001 and 325 nm for all-trans-RAc with molar extinction coefficients of ε = 42,300 and ε = 51,180, respectively. Less than 200 μL of each solution was administered to each animal through a gavage needle (20 G straight, No. 7902; Popper & Sons, Inc., New Hyde Park, NY) at a dose of 50 mg/kg body weight in a volume of 6.25 mL/kg. Each compound was given to 4- to 6-week-old mice every 7 days for 6 months. Dilution of dosing solution was with vehicle provided by QLT Inc.
Bleaching Endogenous Retinoids in Mice and QLT091001 and All-trans-RAc Administration
One group of mice was continuously maintained in darkness for the 6-month experiment starting just prior to their initial retinoid/vehicle gavage, whereas the second group of mice was kept in a regular 12-hour dark/12-hour light (≤ 10 lux) cycle with weekly bleaching and retinoid/vehicle administration as specified in the Table. Eyes of mice in the cyclic lighting group were first treated with Midorin-P solution to dilate the pupils (Santen, Osaka, Japan) and then exposed to fluorescent light in an animal fume hood (average luminance 1000 lux) for 30 minutes to bleach and convert remaining visual pigments prior to either QLT091001, all-trans-RAc or vehicle administration. Body weight changes and general health of both groups of mice were monitored over the total experimental period.
ERG
All ERG experimental procedures were performed under dim red light transmitted through a safelight filter (Kodak No. 1; Eastman Kodak Company, Rochester, NY; transmittance >560 nm) as previously described.45,46 Briefly, mice first were dark-adapted overnight prior to recording. Then they were anesthetized under a safety light by intraperitoneal injection of 20 μL/g body weight of 6 mg/mL ketamine and 0.44 mg/mL xylazine diluted with 10 mM sodium phosphate, pH 7.2, containing 100 mM NaCl. Pupils were dilated with Midorin-P ophthalmic solution (Santen Pharmaceutical, Osaka, Japan). A contact lens electrode was placed on the eye and a reference electrode and ground electrode were positioned on the ear and tail, respectively. ERGs were recorded by the universal testing and electrophysiological system (UTAS) with BigShot Ganzfeld (LKC Technologies, Gaithersburg, MD).
Single-Flash Recording.
White light flash stimuli were employed with a range of intensities (from −3.7 to 1.6 log cd·s·m−2) and flash durations were adjusted according to intensity (from 20 μs to 1 ms). Two to five recordings were made at sufficient intervals between flash stimuli (from 10 seconds to 1 minute) to allow mice time to recover.
SD-OCT and SLO Imaging
SD-OCT (Envisu R2200; Bioptigen, Durham, NC) and SLO (HRA2; Heidelberg Engineering, Carlsbad, CA) were used for in vivo imaging of mouse retinas as previously described.33,47,48 SLO was carried out for whole fundus imaging of mouse retinas.49 Mice were anesthetized by intraperitoneal injection of the anesthetic cocktail indicated above, followed by pupil dilation with an ophthalmic solution (Midorin-P; Santen Pharmaceutical, Osaka, Japan) prior to SLO imaging under the autofluorescence (AF) mode. AF levels were quantified by using “mean gray scale values” obtained by data analysis software (Heidelberg Eye Explorer, version 1.5.9.0; Heidelberg Engineering). These gray values were obtained by digitizing images in a gray scale from black: 0; white: 100. Baseline data were acquired from each mouse strain at 4 to 6 weeks of age. SD-OCT images were obtained in a liner B-scan mode at two orientations: 90 and 180 degrees. Outer nuclear layer (ONL) thickness was measured at the region 500 μm away from the optic nerve in both inferior and superior retinal SD-OCT images acquired at 90 degrees and measured similarly in temporal and nasal SD-OCT images at 180 degrees.
Cryo and Plastic Retina Cross-Sectioning
Mouse eye cups and retinal cross-sections were prepared according to a published procedure.45,50
Retinoid Analyses
All experimental procedures related to extraction, derivatization, and separation of retinoids from whole mouse eyes were carried out under dim red light employing a safelight filter (Eastman Kodak Company; transmittance >560 nm). Polar and nonpolar retinoids were extracted from mouse eyes and analyzed by HPLC as previously reported.25,45
TPM Imaging
TPM was carried out under ex vivo conditions as described previously.44 Imaging was done with a confocal microscope (Leica TCS SP2; Leica Microsystems, Wetzlar, Germany) equipped with a tunable femto-second laser (Chameleon XR; Coherent, Santa Clara, CA) that delivered 90 MHz, 150 fs laser light pulses. Laser light was premodulated with automated dispersion compensation (Chameleon Pre-Comp; Coherent) and then focused on the sample by an objective lens with a 1.25 numerical aperture (Planapochromat; Leica Microsystems). Fluorescence was collected by the same objective lens and directed in a non-descanned manner to a photomultiplier tube (Hamamatsu R6357; Hamamatsu City, Japan). Emission spectra were obtained with a confocal microscope (Leica Microsystems), spectrally sensitive detector in the descanned configuration. Laser power in the sample plane, measured with a calibrated laser power meter (FieldMax-TO; Coherent) and a sensor (PM10; Coherent) for each mouse strain, was maintained constant for all types of treatment. Signal intensities, reflected by mean pixel values derived from raw images and RPE areas, were analyzed off-line by using confocal software (Leica LCS Lite 2.6; Leica Microsystems) and data analysis and graphing software (Sigma Plot 11.0; Systat Software, Inc., San Jose, CA). Areas covered by fluorescent deposits were calculated with Java-based image processing software (ImageJ; National Institutes of Health, Bethesda, MD).
Statistical Analyses
Statistical analyses were carried out with ANOVA. Differences with P values < 0.05 were considered statistically significant.
Results
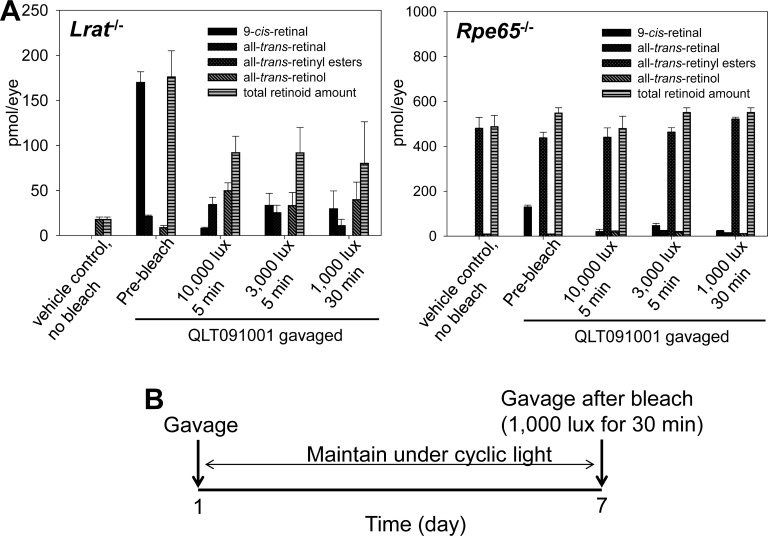
The primary purpose of this study was to evaluate the effects of prolonged high-dose QLT091001 treatment on the retinal health of mice with an impaired visual cycle. To address this issue, light bleaching conditions were optimized to release residual 9-cis-retinal from QLT091001 treatment in the form of all-trans-retinal derived from photoreceptor iso-rhodopsin to the RPE in form of all-trans-retinol. In a preliminary experiment, Lrat−/− and Rpe65−/− mice gavaged with a single 50 mg/kg body weight (bw) dose of QLT091001 were maintained under a regular 12-hour light (≤10 lux)/12-hour dark cycle for a week and then their retinas were bleached for various periods under different intensities of light. This experiment revealed that a 30-minute exposure under fluorescent light (1000 lux) photoisomerized >70% of the residual 9-cis-retinal in both Lrat−/− and Rpe65−/− mice (Fig. 1A). Based on this result, a weekly light exposure and retinoid administration protocol was designed (Fig. 1B) and repeated for 6 months. After being maintained on the same daily light/dark cycle, control groups of mice were placed in a darkroom just prior to their first retinoid/vehicle gavage and then kept in the dark for the remainder of the experiment. Otherwise these mice were treated identically to the light plus bleach group to test the effect of high-dose QLT091001, other retinoids and control vehicle on the health of the dark-exposed retina (Table).
Figure 1.
A bleaching protocol tests the effect of QLT091001 on retinas of Lrat−/− and Rpe65−/− mice. (A) Optimization of bleaching conditions to eliminate residual 9-cis-retinal from retinas of Lrat−/− or Rpe65−/− mice after a single gavage with 50 mg/kg of QLT091001. Four-week-old Lrat−/− or Rpe65−/− mice were used for this experiment. Three days after retinoid/vehicle administration, mice were exposed to three different lighting conditions to photoisomerize residual iso-rhodopsin. After exposure to 1000 lux light for 30 minute that bleached more than 70% of the retina, 9-cis-retinal was released from photoreceptor cells in both mouse strains. (B) Schematic protocol for weekly light exposure with retinoid/vehicle gavage. Based on the results shown in (A), the experimental weekly light exposure protocol included a 30 minute bleach with 1000 lux light followed by retinoid/vehicle gavage in addition to maintenance of mice in a 12-hour light (≤10 lux)/12-hour dark environment for 6 months. Dark control groups of mice were gavaged weekly while maintained in the dark. Error bars indicate SDs, n ≥ 3.
Table.
Mouse Strains, Treatment, and Assessment/Examination for the QLT091001 Study
|
Strain |
Number (n) |
Treatment |
Lighting |
Assessment/Examination |
| C57BL/6J, pigmented (n = 24) | 8 | Vehicle | Light cycle plus bleach | Health condition and body weight measurement: every second week. SD-OCT and SLO: every 2 months. Histology, retinoid analyses: at the final endpoint. TPM imaging: albino mice only at the final endpoint. |
| 8 | QLT091001 | |||
| 8 | all-trans-RAc | |||
| *Rpe65−/−, pigmented (n = 50) | 8 | Vehicle | Light cycle plus bleach | |
| 9 | QLT091001 | |||
| 8 | all-trans-RAc | |||
| 8 | Vehicle | Dark | ||
| 9 | QLT091001 | |||
| 8 | all-trans-RAc | |||
| *Lrat−/−, pigmented (n = 52) | 8 | Vehicle | Light cycle plus bleach | |
| 10 | QLT091001 | |||
| 8 | all-trans-RAc | |||
| 8 | Vehicle | Dark | ||
| 10 | QLT091001 | |||
| 8 | all-trans-RAc | |||
| *Gnat1−/− (n = 30; pigmented, 15; albino, 15) | 10 per group (5 pigmented and 5 albino) | Vehicle | Light cycle plus bleach | |
| QLT091001 | ||||
| all-trans-RAc | ||||
| Gnat1−/−Rpe65−/− (n = 40; pigmented, 20; albino, 20) | 10 per group (5 pigmented and 5 albino) | Vehicle | Light cycle plus bleach | |
| QLT091001 | ||||
| all-trans-RAc | ||||
| QLT091001 | Dark | |||
| Gnat1−/−Lrat−/− (n = 40; pigmented, 20; albino, 20) | 10 per group (5 pigmented and 5 albino) | Vehicle | Light cycle plus bleach | |
| QLT091001 | ||||
| all-trans-RAc | ||||
| QLT091001 | Dark |
Loci at mouse chromosome: Rpe65, 3 H4; Lrat, 3 E3; Gnat1, 9 F1.
High Doses of QLT091001 Are Tolerated after Oral Administration by Gastric Gavage.
A total of 236 mice were used in this study (Table). The dose of 50 mg/kg was selected for all retinoids tested based on the efficacy of 9-cis-R-Ac for visual restoration in our previous study.25 Body weights of mice obtained before starting compound administration and every second week thereafter showed no differences between experimental and control groups. No apparent physical distress or toxic effects were observed during administration of all tested compounds over the 6 month course of this study. Over the duration of these experiments, 16 mice died out of 236 with an overall mortality rate of 6.8%. The breakdown was as follows: 6 pigmented Lrat−/−; 3 pigmented Rpe65−/−; 1 albino Gnat1−/−; 2 albino Gnat1−/−Lrat−/−; 1 pigmented Gnat1−/−Lrat−/−; 3 pigmented Gnat1−/−Rpe65−/−; and no WT mice. The Gnat1−/− pigmented and Gnat1−/−Rpe65−/− albino mice most likely died from anesthesia administered during in vivo examinations. This percentage of fatal events is within the range of fatalities exhibited by untreated mice over more than 10 years of our work with these animals. In summary, the present observations suggest that this experimental population of mice tolerated QLT091001 treatment well.
Monitoring Retinal Health with In Vivo Imaging Systems.
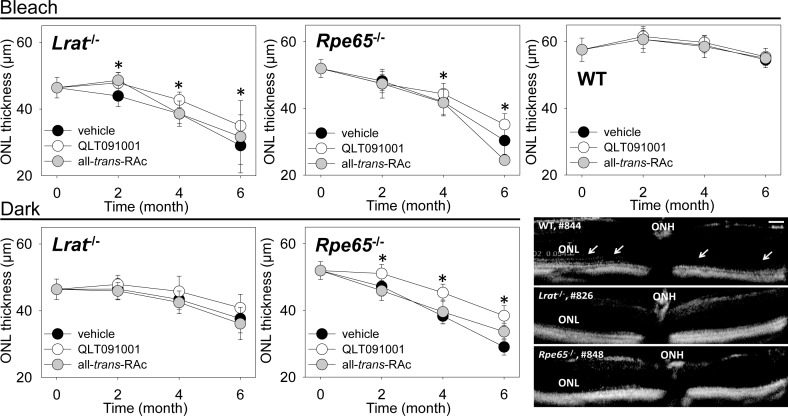
Retinal morphology and fundus autofluorescence levels were monitored with SD-OCT and SLO every 2 months during compound/vehicle administration. SD-OCT imaging was done to measure ONL thickness at regions 500 μm away from optic nerve head in the superior, inferior, temporal and nasal retina (Fig. 2). In Lrat−/− and Rpe65−/− mice, ONL thicknesses were measured at 5 weeks of age and at 2 and 4 months after starting drug administration. However, the external limiting membrane (ELM) could not be measured accurately due to its weak signal in major populations of vehicle- and all-trans-RAc-treated mice after 6 months (Fig. 2, right-bottom images): (Lrat−/− mice: 3 out of 5 treated with all-trans-RAc in the dark, 3 out of 5 treated with vehicle under a regular light cycle plus bleach, 3 out of 5 treated with all-trans-RAc under a regular light cycle plus bleach; Rpe65−/− mice: 5 out of 5 treated with vehicle and 5 out of 5 treated with all-trans-RAc under dark conditions, 3 out of 5 mice treated with vehicle and 6 out of 6 treated with all-trans-RAc under a regular light cycle plus bleach). Only measurable ONL thicknesses from 4 different retinal regions were plotted for these animals. Importantly, ONL thickness measurements were readily obtained from mice treated with QLT091001 because the ELM was clearly visible due to a well-preserved ONL/IS interface under both lighting conditions, that is a regular cycle plus bleach and darkness. The ELM was also detectable in some Rpe65−/− mouse retinas.
Figure 2.
ONL thickness assessed by SD-OCT in Lrat−/−, Rpe65−/−, and WT mice after treatment with either vehicle, QLT091001, or all-trans-RAc. ONL thicknesses were measured at regions 500 μm away from the optic nerve head (ONH) in the superior, inferior, temporal, and nasal retina in SD-OCT images obtained at 0° and 90° every second month. ONL thicknesses from these 4 different regions of the retina were averaged and plotted. Protective effects of QLT091001 became statistically significant at 2 months in Lrat−/− mice maintained under light cycle and bleach and Rpe65−/− mice maintained in dark, and at 4 months in Rpe65−/− mice maintained in the dark. These positive effects were maintained after 6 months treatment. Effects were more prominent in Rpe65−/− mice maintained in dark, whereas only mild protective effects were observed in Lrat−/− mice maintained in the dark. There were no significant differences noted in WT mice. Representative SD-OCT images from WT, Lrat−/− and Rpe65−/− after 6 months treatment are shown in the right bottom corner. The ELM was clearly evident in SD-OCT images of WT retina (white arrows), but not those from Lrat−/− and Rpe65−/− mice. Error bars indicate SDs. Magnification bar indicates 50 μm, n ≥3 per group. *P < 0.05.
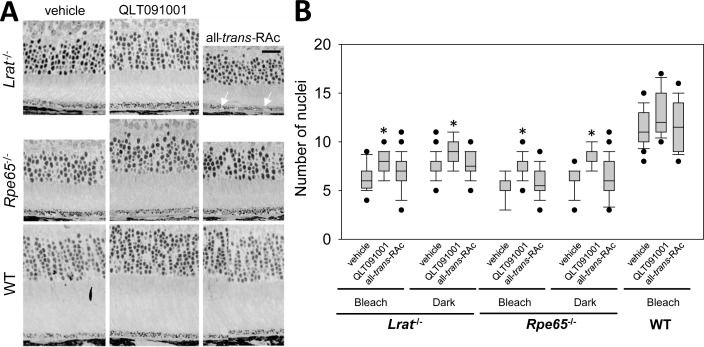
Protective effects of QLT091001 became statistically significant at 2 months in Lrat−/− mice maintained under light cycle and bleach (bleach) and at 4 months in Rpe65−/− mice maintained under the same conditions. Rpe65−/− mice maintained in the dark (dark) evidenced significant protection at 2 months which was retained at 6 months as contrasted with mice treated with vehicle. These protective effects were more prominent in Rpe65−/− mice maintained in dark whereas only mild protective effects were observed in Lrat−/− mice maintained in dark. No difficulties were encountered with ONL measurements in WT mice which displayed no significant differences in ONL thickness related to the type of administered compounds (Fig. 2, right-bottom images). To compensate for the deficiencies of ONL imaging by SD-OCT, we counted the numbers of nuclei attributable to the photoreceptor population in two types of retinal cross sections, plastic sections (Fig. 3A) and cryosections and the numbers of nuclei obtained from each mouse were plotted (Fig. 3B). By histological analysis, we found that the nuclei numbers were well maintained in QLT091001-treated Lrat−/− and Rpe65−/− mice maintained in the dark or under regular cyclic light plus bleach conditions.
Figure 3.
ONL thickness assessed by histology in Lrat−/−, Rpe65−/−, and WT mice after treatment with either vehicle, QLT091001, or all-trans-RAc. (A) Nuclei present in photoreceptor populations of representative retinal plastic cross sections from Lrat−/−, Rpe65−/−, and WT mice treated with either vehicle, QLT091001 or all-trans-retinyl esters (all-trans-RAc) are shown. Oil-droplet like structures were observed in the RPE of some Lrat−/− mice maintained under a regular light cycle plus bleach protocol (white arrows). (B) Numbers of photoreceptor nuclei counted at 4 different regions in the retina similar to those assessed for ONL thickness by SD-OCT in each mouse strain treated with retinoids/vehicle were averaged and plotted. Numbers of nuclei were significantly preserved in the eyes of mice treated with QLT091001 (P < 0.05) whereas numbers were comparable in vehicle- or all-trans-RAc-treated eyes regardless of mouse strain. Boxes denote interquartile range, lines within boxes denote medians, whiskers denote 10th and 90th percentiles, and symbols denote outliers (A). Magnification bar indicates 20 μm (B), n ≥3 per group at each time point. *P < 0.05.
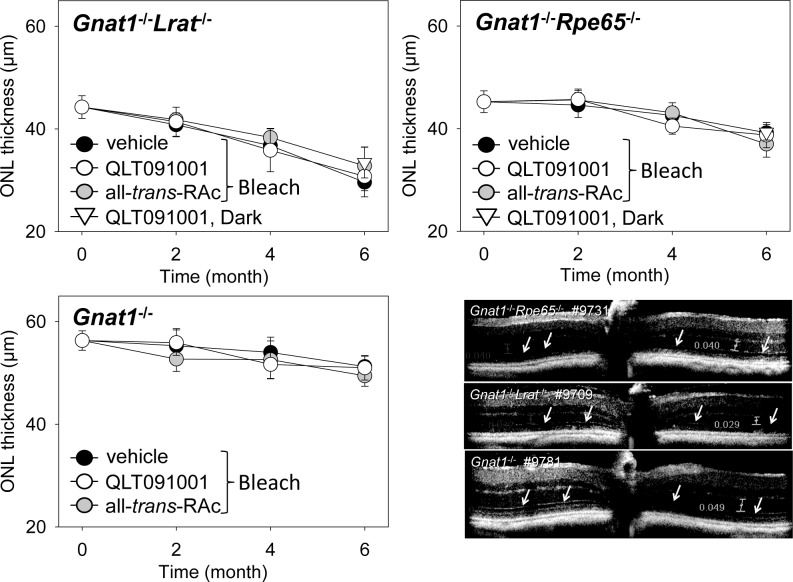
To examine if the observed degeneration is due to spontaneous activity of unliganded opsin and continuous activation of phototransduction or resulted instead from accumulation of retinoids in these mice (especially in Rpe65−/− mice), we bred additional mouse strains. Gnat1−/− mice crossbred with Lrat−/− and Rpe65−/− mice (Gnat1−/−Rpe65−/− and Gnat1−/−Lrat−/− mice, pigmented) were treated and examined by the same protocol used for single Rpe65−/− and Lrat−/− mouse strains. ONL measurements also were obtained from these mice at the same time points (Fig. 4). ELMs were well preserved and ONL thicknesses were measurable 6 months after starting drug treatment (Fig. 4). Notably, trends in ONL thickness losses were comparable (∼10% loss during 6 months of aging) in Gnat1−/−Rpe65−/− and Gnat1−/− mice; moreover, there were no apparent protective effects evident after Gnat1 deletion in Gnat1−/−Lrat−/− mice. Nearly 30% of ONL thickness was lost similar to that shown by Lrat−/− mice under the same treatment (Fig. 2). In addition, the ONL thicknesses in these Gnat1−/− mice crossbred with Lrat−/− and Rpe65−/− mice were not affected by the types of retinoid compounds used in our experimental protocol.
Figure 4.
ONL thickness assessed by SD-OCT in retinas of Gnat1−/−Lrat−/−, Gnat1−/−, and Gnat1−/−Rpe65−/− mice after 6 months of treatment with vehicle, QLT091001, or all-trans-RAc. Mice were 4-weeks-old at the beginning of treatment. Averaged thicknesses of the ONL measured from SD-OCT images obtained at 0 and 90 degrees from four different regions of the retina were not affected by the compounds administered to Gnat1−/−Lrat−/− or Gnat1−/−Rpe65−/− mice. Representative SD-OCT images after 6 months of treatment are shown (right-bottom panel). ELMs were discerned in SD-OCT images from all strains (white arrows). Error bars indicate SDs, n ≥3 per group at each time point. *P < 0.05.
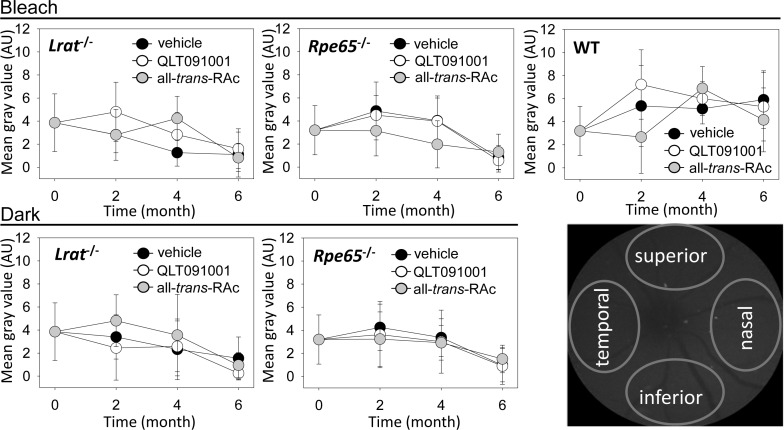
Fundus AF levels were measured as mean gray values to evaluate accumulation of potentially toxic retinoid byproducts every second month during retinoid/vehicle administration. These values obtained from each of 4 different regions of the fundus—superior, inferior, temporal, and nasal—then were averaged and plotted (Fig. 5). AF levels in Lrat−/− and Rpe65−/− mice were comparable with or less than those in WT mice under both lighting conditions. AF levels in WT mice showed a mild increase in an age-dependent manner whereas significant increases due to QLT091001 or all-trans-RAc treatment were not observed in either Lrat−/− or Rpe65−/− mice at 6 months of treatment (Fig. 5). Similar results were obtained with Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice (data not shown). These observations indicate that there was no obvious accumulation of potential toxic retinoid byproducts or pathological changes in the fundus after administration of high-doses of QLT091001 or all-trans-RAc in all tested mouse strains.
Figure 5.
AF measurements by SLO during 6 months of vehicle, QLT091001 or all-trans-RAc treatment of Lrat−/−, Rpe65−/−, or WT mice. AF levels in SLO fundus images were quantified every other month during weekly retinoid/vehicle administration to Lrat−/−, Rpe65−/− and WT mice that were either maintained under a regular light cycle plus bleach protocol or kept in the dark. Mean gray values obtained from four different regions of the fundus (right-bottom panel) were averaged and plotted. No significant differences were noted between vehicle-, QLT091001- or all-trans-RAc-treated Lrat−/−, RPE65−/−, and WT mice maintained in either a normal light/dark cycle with bleach or in a dark environment. Error bars indicate SDs, n = 3 to 5 per group at each time point.
Retinoid Levels in the Eyes of Treated Mice.
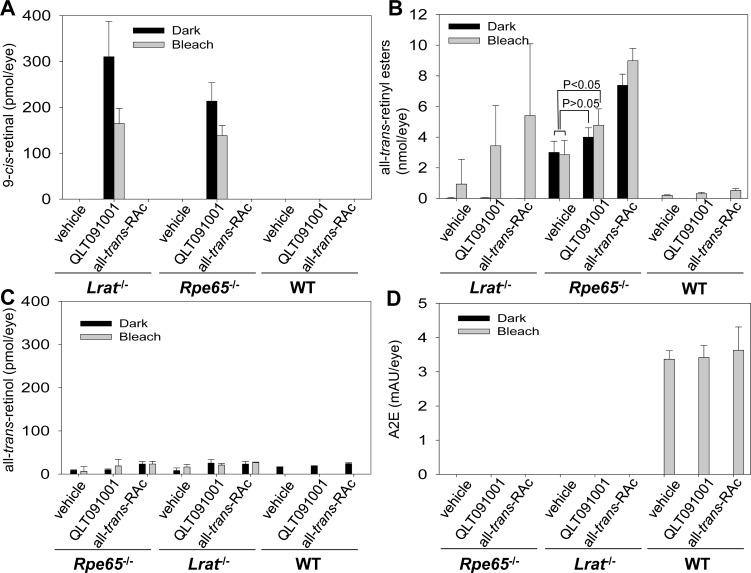
Changes of retinoid content in the eye after QLT091001 or all-trans-RAc treatment were evaluated by normal phase HPLC after 6 months of retinoid/vehicle administration. Retinoid contents determined in mouse eyes were averaged and amounts of each retinoid derivative were plotted (Fig. 6). 9-cis-Retinal was readily detected in QLT091001-treated Lrat−/− and RPE65−/− mice, but not in WT mice (Fig. 6A). Moreover, the amount of 9-cis-retinal was nearly 1.5-fold higher in mice maintained in the dark compared to levels in mice undergoing the weekly bleach protocol. Unexpectedly, all-trans-retinyl esters were detected in pigmented Lrat−/− mice maintained under the light cycle plus bleach protocol irrespective of the compounds administered whereas accumulated levels were quite variable and highest in mice treated with all-trans-RAc (Fig. 6B). Unfortunately, only noninvasive 2PO is suitable for imaging retinosomes in non-pigmented mice; this method is not yet suitable for imaging retinosomes in pigmented mice. Only trace levels of all-trans-retinyl esters were detected in Lrat−/− mice maintained in the dark and treated with either QLT091001 or all-trans-RAc (Fig. 6B). Lighting conditions did not significantly change high levels of all-trans-retinyl esters in Rpe65−/− mice treated with QLT091001 or all-trans-RAc (Fig. 6B). Moreover, there was no more than a 1.5-fold increase of all-trans-retinyl esters levels in Rpe65−/− mice treated with QLT091001 maintained under the light cycle plus bleaching protocol whereas ester levels were not significantly different between vehicle and QLT091001 in the dark-maintained controls (Fig. 6B). Ester levels were 3-fold higher in the all-trans-RAc treated group as compared to vehicle treated mice (Fig. 6B). All-trans-retinyl esters were modestly increased in eyes of WT mice treated by retinoids as well (Fig. 6B). The low levels of all-trans-retinol found were not affected by the different genetic backgrounds of studied mice; however, these tended to be higher in the eyes of mice treated with either QLT091001 or all-trans-RAc (Fig. 6C). 11-cis-Retinal was detected only in the eyes of WT mice and its level was not affected by the administered retinoids (data not shown). Retinoid analyses also were carried out in selected numbers of Gnat1−/− mice crossbred with Lrat−/− and Rpe65−/− mice. Accumulation of all-trans-retinyl esters was not detected in Gnat1−/−Lrat−/− mice. Otherwise, levels of each retinoid in the eyes were similarly affected in Gnat1−/− mice crossbred with Lrat−/− and Rpe65−/− mice, irrespective of the types of retinoid/vehicle treatment (data not shown). Accumulation of the age-related retinoid byproduct N-retinyl-N-retinylidene-ethanolamine (A2E) was not detected by normal phase HPLC in the eyes of Lrat−/− and Rpe65−/− mice irrespective of the retinoid regimen employed (Fig. 6D). However, physiological accumulation of A2E,48 independent of the type of treatment, was observed in WT mice 6 months after initiation of drug treatment. These data suggest that an active visual cycle is essential for the accumulation of the retinal condensation products. Together the above observations indicate that the QLT091001 and all-trans-RAc gavage protocols used in this study induced all-trans-retinyl ester accumulation at different levels, whereas even the large accumulation of esters in Rpe65−/− mice treated with all-trans-RAc or the aberrant accumulation of esters in Lrat−/− mice produced no significant negative effects on photoreceptor populations of LCA mouse models, all suggesting an apparently negligible toxicity of ester accumulation in the RPE. Importantly, the retinoid administration protocol used in this study did not cause an excess accumulation of the toxic retinoid byproduct, A2E, in RPE cells of tested strains regardless of whether the visual cycle was normal or impaired. The last had been anticipated as one of the adverse effects of prolonged administration of high doses of QLT091001.
Figure 6.
Eye retinoid content in Lrat−/−, Rpe65−/−, or WT mice treated with either vehicle, QLT091001 or all-trans-RAc for 6-months. RPE65−/−, Lrat−/−, and WT mice were 4 to 6 weeks old at the beginning of treatment. Eye retinoids were extracted and quantified after separation by normal phase HPLC. 9-cis-Retinal was detected in eyes of both Lrat−/− and RPE65−/− mice treated with QLT091001 under both normal lighting plus bleach conditions and maintenance in the dark ([A], left and center). Several Lrat−/− light-exposed mice showed an unexpected all-trans-retinyl ester accumulation enhanced by treatment with either QLT091001 or all-trans-RAc ([B], left). High levels of all-trans-retinyl esters in Rpe65−/− mice were not affected by lighting conditions ([B], center). The ester levels in this strain maintained in the dark and treated with QLT091001 were comparable to those of the vehicle control (P > 0.05) whereas ester levels of mice maintained in the dark treated with all-trans-RAc and mice maintained under light cycle plus bleach treated with QLT091001 and all-trans-RAc were significantly increased compared to ester levels of those treated with vehicle ([B], center, P < 0.05). Levels of all-trans-retinyl esters were mildly increased in eyes of WT mice treated by QLT091001 and all-trans-RAc (B). The levels of all-trans-retinol varied but were higher in the eyes of all mice treated with either QLT091001 or all-trans-RAc (C). Age-dependent accumulation of the retinoid byproduct, A2E was observed only in WT mice maintained under normal light with bleach and this was not altered by exposure to either QLT091001 or all-trans-RA (D). Error bars indicate SDs, n ≥3 in each group.
Visual Function Tested with ERG Recordings.
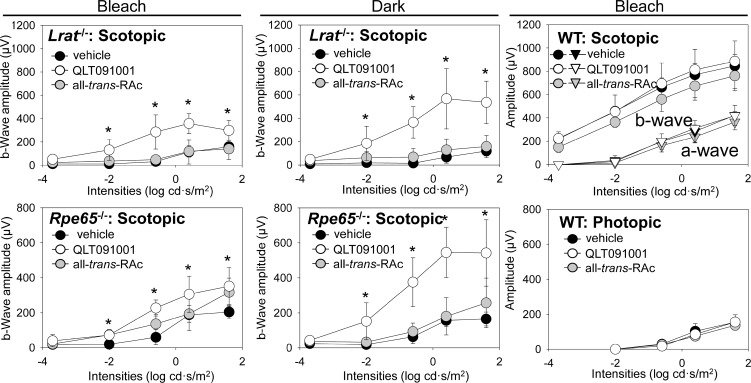
ERG recordings were employed to evaluate the effects of QLT091001 and all-trans-RAc administration on the visual function of mutant mice. Scotopic ERG responses were obtained from Lrat−/− and Rpe65−/− mice and both scotopic and photopic responses were recorded in WT mice 6 months after starting the retinoid/vehicle regimens. Response amplitudes from examined mice were averaged and plotted (Fig. 7). Overall, significant improvement of visual function was observed in both Lrat−/− and Rpe65−/− mice gavaged with QLT091001 whereas administration of all-trans-RAc produced no significant difference from vehicle-treated controls. In WT mice, the slight improvement observed in the QLT091001-treated group failed to achieve statistical significance. QLT091001 provided effective therapy for visual dysfunction in Lrat−/− and Rpe65−/− mice without any adverse effects under the two contrasting experimental lighting conditions.
Figure 7.
ERG recordings of visual function in Lrat−/−, Rpe65−/−, and WT mice after 6 months of treatment with either vehicle, QLT091001 or all-trans-RAc. Animals were 4 weeks old at the beginning of treatment. ERG recordings were obtained from Lrat−/− and Rpe65−/− mice under scotopic conditions and from WT mice under both scotopic and photopic conditions. Plots of b-wave amplitudes from Lrat−/− and Rpe65−/− mice and scotopic a- and b-wave and photopic b-wave amplitudes from WT mice are shown. ERG amplitudes of QLT091001-treated groups were significantly higher than those of all-trans-RAc- or vehicle-treated Lrat−/− and Rpe65−/− mice. ERG responses of WT mice were comparable between the different regimens. Error bars indicate SDs, n >3 in each group. *P < 0.05.
Ex Vivo RPE Observations with TPM.
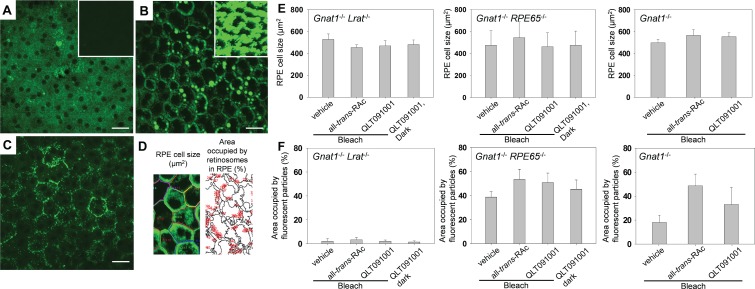
Albino Gnat1−/− mice crossbred with Lrat−/− and Rpe65−/− mice were used to evaluate RPE health at the termination of the QLT091001 regimen. To discern subcellular details of the RPE, TPM conditions were optimized separately and then kept constant for mice from each genetic background (Figs. 8A–8C). Two parameters, namely RPE cell size and areas occupied by retinosomes in the RPE, were evaluated (Fig. 8D). There was no significant difference in the size of the RPE cells of these mice due to the administered retinoids (Fig. 8E). Moreover, the area occupied by retinosomes in Gnat1−/−Rpe65−/− mice treated with QLT091001 was not significantly increased compared to that in mice treated with all-trans-RAc or vehicle under either lighting condition. In Gnat1−/− mice, the area occupied by retinosomes was significantly greater in all-trans-RAc-treated animals whereas a lesser increase was observed in QLT091001-treated mice (Fig. 8F). The randomly distributed fluorescent deposits in Gnat1−/−Lrat−/− mice were visible with both 730 and 850 nm excitation light (data not shown), indicating that they were not retinosomes, an observation consistent with the retinoid analyses and previous findings.44 These data indicate that long-term high dose QLT091001 administration had no obvious damaging effects on the RPE of Lrat−/− and Rpe65−/− mice.
Figure 8.
Characterization of the RPE from Lrat−/−, Rpe65−/− or WT mice by TPM after 6 months of treatment with vehicle, all-trans-RAc or QLT091001. Animals were 4 weeks-old at the beginning of treatment. Representative TPM images are shown for albino Gnat1−/−Lrat−/− (A), albino Gnat1−/−Rpe65−/− (B), and albino Gnat1−/− (C) mice. Imaging conditions were optimized to visualize the subcellular structure of RPE cells. Insets in (A) and (B) show images obtained under the same conditions as those used in (C). Areas of individual RPE cells and areas occupied by fluorescent deposits in the RPE were estimated as shown in (D). There was no significant difference in the size of RPE cells between strains and treatments in these mice (E). As shown in (F), the fraction of an area occupied by fluorescent deposits was consistently greatest in mice treated with all-trans-RAc. Error bars in (E) and (F) show SDs, n ≥3 in each group. *P < 0.05.
Discussion
Pharmacological replacement therapy with 9-cis-retinal for 11-cis-retinal deficiency in Rpe65−/− mice was initiated by our group in 2000 37 and many of its pharmacological aspects were investigated over the past decade.21,25,26,39,51–54 This proof of concept study initiated the pharmaceutical application of 9-cis-retinoids to remedy retinal dysfunction caused by delayed or deficient regeneration of 11-cis-retinal.21,25,26,39,51–53 In subsequent studies, the 9-cis-retinyl esters 9-cis-R-Ac and 9-cis-retinyl succinate were developed to avoid the high chemical reactivity of the 9-cis-retinal aldehyde. These 9-cis retinoids possess improved chemical stability and bioavailability compared with 9-cis-retinal.21 Further pharmacokinetic, systemic toxicity and efficacy studies of 9-cis-R-Ac then were done in WT and Rpe65−/− mice with various dosing regimens.25
The present study focused specifically on the impact of prolonged high-dose QLT091001 administration on the retinas of mice serving as models for LCA. Key features of this study design were the weekly regimen of either bleaching live mouse retinas with 1000-lux light followed by high dose (50 mg/kg) retinoid administration and maintenance of mice in a 12-hour light (≤10 lux)/dark cycle for the 6-month experiment or the identical retinoid/vehicle protocol except that mice were continuously maintained in the dark starting just prior to their first gavage with the experimental compounds (Fig. 1B). Bleaching prior to gavage with QLT091001, all-trans-RAc or vehicle was employed to enhance release (>70%) of residual 9-cis-retinal from photoreceptor cells to the RPE. This weekly regimen, initiated in 4-6-week-old-mice, was repeated for 6 months. The dose of 50 mg/kg employed corresponds to more than 50-fold the dose used for initial human clinical trials. QLT091001 caused improved maintenance of ONL thickness attributable to a higher density of photoreceptors (Figs. 2, 3) without a significant increase of fundus autofluorescence indicative of retinoid byproduct accumulation (e.g., A2E in the RPE; Fig. 5). Negligible A2E accumulation was further confirmed by direct HPLC analyses of eyes from Lrat−/− and Rpe65−/− mice and no A2E increase was found in WT retina after QLT091001 treatment as well (Fig. 6D). The same treatment regimen was tested in Gnat1−/− mice crossbred with Lrat−/− and Rpe65−/− mice because it had been reported that GNAT1 deletion can decrease photoreceptor cell death in Rpe65−/− mice29 by preventing spontaneous activation of phototransduction in mice lacking rhodopsin but having high concentrations of activated opsin instead. Therefore, Gnat1−/− crossbred LCA mouse models could theoretically demonstrate direct effects of drug treatment on the retina which are masked by photoreceptor cell death due to 11-cis-retinoid deficiency. Importantly, there were no adverse effects of QLT091001 treatment on either ONL thickness or the increase of fundus autofluorescence in either Gnat1−/−Rpe65−/− or Gnat1−/−Lrat−/− mice (Fig. 4). However, this treatment did not stop the natural age-related degeneration observed in mice. Daily phagocytosis of rod outer segments (∼10% each day)55 and other metabolic processes deplete the pool of retinoids available to the retina. It should also be noted that the thickness of the retina decreases even in WT mice under ordinary lighting conditions by about 20% in 6 months (duration of this experiment). But, most important is that the retina did not deteriorate more rapidly after treatment.
Amounts of all-trans-retinyl esters accumulated by Lrat−/− mice were sporadic and quite variable, especially when animals were treated with all-trans-RAc. This was surprising because Lrat−/− mice have an impaired ability to generate retinyl esters.16 But these esters could be formed by other enzymes, such as acetyl-coenzyme A acyltransferase (ARAT)15,56 or diacylglycerol acyltransferase 1 (DGAT1).56 The influence of ARAT or DGAT1 could be minor in WT and Rpe65−/− mice because LRAT is the dominant enzyme involved in esterification of all-trans-retinol whereas the influence of ARAT and DGAT1 is controversial when the RPE is exposed to high levels of all-trans-retinol in mice with LRAT deficiency.57 All-trans-retinyl ester accumulation could also result from impaired elimination of retinoids in mice that do not continuously produce chromophore and thus visual pigments. Consistent with this idea is the fact that ester accumulation was found only in light-exposed mice and not in dark-reared control animals.
The massive accumulation of all-trans-retinyl esters exhibited by Rpe65−/− mice was not anticipated to cause retinal degeneration.13,35,42 Two possible scientific explanations have been advanced for this result.26,29 Woodruff et al. reported that GNAT1 deletion can ameliorate photoreceptor cell death caused by RPE65 deficiency, and speculated that all-trans-retinyl ester accumulation does not cause photoreceptor cell death in Rpe65−/− mice.29 Moreover, Van Hooser et al. showed that administration of 9-cis-retinal can decelerate accumulation of all-trans-retinyl esters in Rpe65−/− mice.26 Importantly, all-trans-retinyl ester levels in Rpe65−/− mice treated with QLT091001 under the weekly-based light plus bleach regimen showed only a mild increase in all-trans-retinyl esters compared with mice in the vehicle treated group whereas the all-trans-RAc-treated group exhibited more than a 3-fold increase (Fig. 6B). Of note, even the latter group's high levels of retinyl ester accumulation failed to accelerate progression of photoreceptor cell death and retinal dysfunction in Rpe65−/− mice (Figs. 2–4, 7). How 9-cis-retinoid slows retinyl ester accumulation most likely suggests that trapping retinoids from the circulation by the RPE is impaired when iso-rhodopsin is formed from this artificial chromophore. Higher all-trans-retinoid concentrations in the circulation would cause even more accumulation of retinyl esters in the all-trans-RAc-treated group because excretion of all-trans-retinol is decreased in Rpe65−/− as compared with WT mice and this could indirectly enhance accumulation of retinyl esters in the RPE.58 We observed no adverse retinal effects of QLT091001 in either Gnat1−/−Rpe65−/− or Gnat1−/−Lrat−/− mice (Fig. 4).
The potential effects of QLT091001 on the RPE were evaluated specifically by in vivo SLO and ex vivo TPM. SLO is widely used to monitor accumulation of retinoid byproducts such as A2E, both in clinical and basic research.59 Because fundus AF levels related to accumulation of A2E can be quantified by SLO,49 we used this technique to assess A2E accumulation in Lrat−/− and Rpe65−/− mice during QLT091001 administration. No significant increase of AF levels was observed in these mice (Fig. 5), consistent with HPLC quantification of A2E in their eyes (Fig. 6D). The advanced imaging system, TPM, can picture individual RPE cells due to signals from natural fluorophores such as retinosomes in the RPE.20 In TPM images, the size and morphology of the RPE were well maintained in both Lrat−/− and Rpe65−/− mice, irrespective of the type of administered retinoid (Fig. 8). Occupation ratios of retinosomes within the RPE also showed trends similar to the results obtained by retinoid analyses (Figs. 6B, 8F).
In summary, this study provides evidence that retinas of 4 to 6-week-old Lrat−/− and Rpe65−/− mice gavaged with high doses of QLT091001 over a 6-month period tolerated the regimen well.
Acknowledgments
We thank Leslie T. Webster Jr (Case Western Reserve University) for comments on the manuscript; Satsumi Roos and Midori Hitomi (Case Western Reserve University) for block preparation and plastic sectioning; and Lindsay Perusek, Hiroko Matsuyama and Masami Matsuyama (Case Western Reserve University) for retinoid analyses. We also thank Jenis Lem (Tufs University) for Gnat1 knockout mice and Michael Redmond (National Institutes of Health) for RPE knockout mice.
Footnotes
Supported in part by QLT Inc, Vancouver, Canada.
Disclosure: T. Maeda, QLT, Inc. (F, C), Polgenix, Inc. (C), P; Z. Dong, QLT, Inc. (F); H. Jin, QLT, Inc. (F); O. Sawada, QLT, Inc. (F); S. Gao, QLT, Inc. (F); D. Utkhede, QLT, Inc. (F, E); W. Monk, QLT, Inc. (F, E); G. Palczewska, QLT, Inc. (F); K. Palczewski, QLT, Inc. (C), Polgenix, Inc. (F, C), P
References
- 1. Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010; 29: 398–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997; 17: 194–197. [DOI] [PubMed] [Google Scholar]
- 3. Palczewski K. Blind dogs that can see: pharmacological treatment of Leber congenital amaurosis caused by a defective visual cycle. Arch Ophthalmol. 2010; 128: 1483–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006; 75: 743–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci. 2010; 31: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Ann Rev Pharmacol Toxicol. 2007; 47: 469–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001; 20: 469–529. [DOI] [PubMed] [Google Scholar]
- 8. von Lintig J, Kiser PD, Golczak M, Palczewski K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci. 2010; 35: 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiser PD, Golczak M, Maeda A, Palczewski K. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim Biophys Acta. 2012; 1821: 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parker RO, Crouch RK. Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Research. 2010; 91: 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004; 23: 307–380. [DOI] [PubMed] [Google Scholar]
- 12. Thompson DA, Li Y, McHenry CL, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet. 2001; 28: 123–124. [DOI] [PubMed] [Google Scholar]
- 13. den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008; 27: 391–419. [DOI] [PubMed] [Google Scholar]
- 14. Bereta G, Kiser PD, Golczak M, et al. Impact of retinal disease-associated RPE65 mutations on retinoid isomerization. Biochemistry. 2008; 47: 9856–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saari JC, Bredberg DL, Farrell DF. Retinol esterification in bovine retinal pigment epithelium: reversibility of lecithin: retinol acyltransferase. Biochem J. 1993; 291 (pt 3); 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Batten ML, Imanishi Y, Maeda T, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004; 279: 10422–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005; 122: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacDonald PN, Ong DE. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J Biol Chem. 1988; 263: 12478–12482. [PubMed] [Google Scholar]
- 19. Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem. 1999; 274: 3834–3841. [DOI] [PubMed] [Google Scholar]
- 20. Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004; 164: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Batten ML, Imanishi Y, Tu DC, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005; 2: e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008; 16: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bemelmans AP, Kostic C, Cachafeiro M, et al. Lentiviral gene transfer-mediated cone vision restoration in RPE65 knockout mice. Adv Exp Med Biol. 2008; 613: 89–95. [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2006; 47: 1177–1184. [DOI] [PubMed] [Google Scholar]
- 25. Maeda T, Maeda A, Casadesus G, Palczewski K, Margaron P. Evaluation of 9-cis-retinyl acetate therapy in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2009; 50: 4368–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Hooser JP, Liang Y, Maeda T, et al. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem. 2002; 277: 19173–19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. Rpe65−/− and Lrat−/− mice: comparable models of leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2008; 49: 2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci. 2005; 46: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 29. Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003; 35: 158–164. [DOI] [PubMed] [Google Scholar]
- 30. Zhang T, Zhang N, Baehr W, Fu Y. Cone opsin determines the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc Natl Acad Sci U S A. 2011; 108: 8879–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sato K, Nakazawa M, Takeuchi K, Mizukoshi S, Ishiguro S. S-opsin protein is incompletely modified during N-glycan processing in Rpe65(−/−) mice. Exp Eye Res. 2010; 91: 54–62. [DOI] [PubMed] [Google Scholar]
- 32. Sato K, Ozaki T, Ishiguro S, Nakazawa M. M-opsin protein degradation is inhibited by MG-132 in Rpe65(−/−) retinal explant culture. Mol Vis. 2012; 18: 1516–1525. [PMC free article] [PubMed] [Google Scholar]
- 33. Maeda A, Okano K, Park PS, et al. Palmitoylation stabilizes unliganded rod opsin. Proc Natl Acad Sci U S A. 2010; 107: 8428–8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang T, Baehr W, Fu Y. Chemical chaperone TUDCA preserves cone photoreceptors in a mouse model of Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2012; 53: 3349–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001; 28: 92–95. [DOI] [PubMed] [Google Scholar]
- 36. Aguirre GD, Baldwin V, Pearce-Kelling S, Narfstrom K, Ray K, Acland GM. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998; 4: 23. [PubMed] [Google Scholar]
- 37. Van Hooser JP, Aleman TS, He YG, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci U S A. 2000; 97: 8623–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuksa V, Bartl F, Maeda T, et al. Biochemical and physiological properties of rhodopsin regenerated with 11-cis-6-ring- and 7-ring-retinals. J Biol Chem. 2002; 277: 42315–42324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maeda T, Cideciyan AV, Maeda A, et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet. 2009; 18: 2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wald G, Brown PK, Hubbard R, Oroshnik W. Hindered Cis isomers of vitamin A and retinene: the structure of the neo-B isomer. Proc Natl Acad Sci U S A. 1955; 41: 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DeGrip WJ, Liu RS, Ramamurthy V, Asato A. Rhodopsin analogues from highly hindered 7-cis isomers of retinal. Nature. 1976; 262: 416–418. [DOI] [PubMed] [Google Scholar]
- 42. Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998; 20: 344–351. [DOI] [PubMed] [Google Scholar]
- 43. Calvert PD, Krasnoperova NV, Lyubarsky AL, et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc Natl Acad Sci U S A. 2000; 97: 13913–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palczewska G, Maeda T, Imanishi Y, et al. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat Med. 2010; 16: 1444–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maeda A, Maeda T, Imanishi Y, et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005; 280: 18822–18832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maeda T, Lem J, Palczewski K, Haeseleer F. A critical role of CaBP4 in the cone synapse. Invest Ophthalmol Vis Sci. 2005; 46: 4320–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shiose S, Chen Y, Okano K, et al. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J Biol Chem. 2011; 286: 15543–15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Y, Okano K, Maeda T, et al. Mechanism of all-trans-retinal toxicity with implications for Stargardt disease and age-related macular degeneration. J Biol Chem. 2012; 287: 5059–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huber G, Beck SC, Grimm C, et al. Spectral domain optical coherence tomography in mouse models of retinal degeneration. Invest Ophthalmol Vis Sci. 2009; 50: 5888–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008; 283: 26684–26693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maeda A, Maeda T, Palczewski K. Improvement in rod and cone function in mouse model of Fundus albipunctatus after pharmacologic treatment with 9-cis-retinal. Invest Ophthalmol Vis Sci. 2006; 47: 4540–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maeda T, Maeda A, Leahy P, Saperstein DA, Palczewski K. Effects of long-term administration of 9-cis-retinyl acetate on visual function in mice. Invest Ophthalmol Vis Sci. 2009; 50: 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maeda T, Maeda A, Matosky M, et al. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Invest Ophthalmol Vis Sci. 2009; 50: 4917–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maeda T, Perusek L, Amengual J, Babino D, Palczewski K, von Lintig J. Dietary 9-cis-beta, beta-carotene fails to rescue vision in mouse models of leber congenital amaurosis. Mol Pharmacol. 2011; 80: 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology. 2010; 25: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wongsiriroj N, Piantedosi R, Palczewski K, et al. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem. 2008; 283: 13510–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaschula CH, Jin MH, Desmond-Smith NS, Travis GH. Acyl CoA: retinol acyltransferase (ARAT) activity is present in bovine retinal pigment epithelium. Exp Eye Res. 2006; 82: 111–121. [DOI] [PubMed] [Google Scholar]
- 58. Qtaishat NM, Redmond TM, Pepperberg DR. Acute radiolabeling of retinoids in eye tissues of normal and rpe65-deficient mice. Invest Ophthalmol Vis Sci. 2003; 44: 1435–1446. [DOI] [PubMed] [Google Scholar]
- 59. Maeda T, Golczak M, Maeda A. Retinal photodamage mediated by all-trans-retinal. Photochem Photobiol. 2012; 88: 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]