Abstract
Introduction
The present study was conducted to examine the effect of conjugated docosahexaenoic acid (CDHA) on cell growth, cell cycle progression, mode of cell death, and expression of cell cycle regulatory and/or apoptosis-related proteins in KPL-1 human breast cancer cell line. This effect of CDHA was compared with that of docosahexaenoic acid (DHA).
Methods
KPL-1 cell growth was assessed by colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay; cell cycle progression and mode of cell death were examined by flow cytometry; and levels of expression of p53, p21Cip1/Waf1, cyclin D1, Bax, and Bcl-2 proteins were examined by Western blotting analysis. In vivo tumor growth was examined by injecting KPL-1 cells subcutaneously into the area of the right thoracic mammary fat pad of female athymic mice fed a CDHA diet.
Results
CDHA inhibited KPL-1 cells more effectively than did DHA (50% inhibitory concentration for 72 hours: 97 μmol/l and 270 μmol/l, respectively). With both CDHA and DHA growth inhibition was due to apoptosis, as indicated by the appearance of a sub-G1 fraction. The apoptosis cascade involved downregulation of Bcl-2 protein; Bax expression was unchanged. Cell cycle progression was due to G0/G1 arrest, which involved increased expression of p53 and p21Cip1/Waf1, and decreased expression of cyclin D1. CDHA modulated cell cycle regulatory proteins and apoptosis-related proteins in a manner similar to that of parent DHA. In the athymic mouse system 1.0% dietary CDHA, but not 0.2%, significantly suppressed growth of KPL-1 tumor cells; CDHA tended to decrease regional lymph node metastasis in a dose dependent manner.
Conclusion
CDHA inhibited growth of KPL-1 human breast cancer cells in vitro more effectively than did DHA. The mechanisms of action involved modulation of apoptosis cascade and cell cycle progression. Dietary CDHA at 1.0% suppressed KPL-1 cell growth in the athymic mouse system.
Keywords: apoptosis, breast cancer, conjugated docosahexaenoic acid, docosahexaenoic acid, human
Introduction
The etiology of human breast cancer is complex and remains poorly understood. At least one-third of all human cancers may be associated with dietary factors [1]. In particular, it has been hypothesized that dietary fat intake plays a role in the development and progression of breast cancer. Evidence from very large prospective studies strongly suggests that there is no association between overall dietary fat intake and breast cancer in humans [2]. However, such findings do not necessarily mean that fat has no effect on breast cancer, because other findings indicate that the type of dietary fat consumed is of particular importance in breast carcinogenesis [3]. Epidemiologic data in Alaskan and Greenland Eskimos [4,5] and in non-Eskimo populations [6] indicate that consumption of fish oil correlates with reduced incidence of breast cancer. In experimental studies using human breast cancer cells [7,8] eicosapentaenoic acid and docosahexaenoic acid (DHA) – n-3 polyunsaturated fatty acids (PUFAs) that are abundant in fish oil – have exhibited protective effects. Perilla oil, which contains a high level of α-linolenic acid (an n-3 PUFA), similarly inhibits mammary carcinogenesis in rats [9,10]. Thus, n-3 PUFAs appear to be of particular importance in suppression of breast carcinogenesis.
Conjugated fatty acids are positional and geometrical isomers with conjugated double bonds. Conjugated linoleic acid (CLA) is found in meat from ruminants and in dairy products [11], and has been shown to have anticarcinogenic effects [12-14]. It has been reported that CLA reduces mammary cancer risk in rats [15-17] and inhibits growth of human breast cancer cells in culture [18]. Linoleic acid, an n-6 PUFA, accelerates growth of human breast cancer cells [8]. Thus, the finding that CLA has the opposite effect is of particular interest. Given that n-3 PUFAs have been shown to have anticarcinogenic activity in vitro and in vivo, some conjugated fatty acids converted from n-3 PUFAs may have greater tumor suppressing activity than CLA or n-3 PUFAs themselves. We recently showed that DHA, an n-3 PUFA, suppresses mammary cancer in rats more effectively than does eicosapentaenoic acid [19]. Conjugated eicosapentaenoic acid is naturally found in seaweeds [20]. Conjugated DHA (CDHA) is not found naturally and is artificially prepared by alkaline isomerization of DHA [21]. In the present study, the tumor-suppressing effect of CDHA was examined in vitro and in vivo, and its anticarcinogenic activity was compared with that of DHA. Also, the mechanisms by which CDHA suppresses cancer cell growth were investigated.
Methods
Human breast cancer cell line and culture conditions
KPL-1 is a human breast carcinoma cell line that was established from the malignant effusion of a breast cancer patient [22]. This cell line is estrogen receptor positive, grows rapidly in female athymic mice, and often causes regional lymph node metastasis when inoculated into the mammary fat pad. KPL-1 cells were maintained in Dulbecco's modified Eagle's minimum essential medium (DMEM 0/5921; Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY, USA), and were grown at 37°C in 5% carbon dioxide/95% humidified air.
DHA and preparation of CDHA by alkaline treatment
DHA ethyl ester (purity 97%) was obtained from Bizen Chemical (Okayama, Japan). CDHA was prepared by alkaline treatment [21]. Briefly, a solution of potassium hydroxide in ethylene glycol was prepared at a concentration of 21% (weight:weight), and this solution was bubbled with nitrogen gas. Then, 100 g DHA ethyl ester was added to 1000 ml of the 21% potassium hydroxide/ethylene glycol solution. The mixture was bubbled with nitrogen gas and allowed to stand for 10 min at 180°C. The reaction mixture was cooled and 1000 ml methanol was added. The mixture was acidified with 2000 ml of 6 N HCl. After dilution with 2000 ml distilled water, the conjugated fatty acid was extracted with 5000 ml n-hexane. The hexane extract was then washed with 3000 ml of 30% methanol/distilled water, washed with 3000 ml distilled water, and finally evaporated under the nitrogen gas stream to produce CDHA. DHA isomerized with 21% potassium hydroxide for 10 min preferentially forms conjugated pentaene and conjugated hexaene [21]. The purity of CDHA used in the following experiments was approximately 65% (35% was unreacted and remained as DHA). DHA and CDHA were stored at -80°C in the dark.
MTT assay
Viable cells exposed to DHA or CDHA were quantified using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Stock solutions of DHA or CDHA in ethanol were prepared at a concentration of 500 mmol/l. For each experiment test compound was prepared from the stock solution and diluted to a final concentration of 1 μmol/l to 5 × 102 μmol/l with culture medium. Initially, 5 × 103 KPL-1 cells were seeded in 96-well culture plates and cultured for 24 hours to allow them to adhere to the plates. After preincubation, the culture medium was changed to the experimental medium supplemented with DHA or CDHA. We used four concentrations of each test compound, covering a 3-log range (1 μmol/l to 5 × 102 μmol/l) that was chosen to span the 50% inhibitory concentration (IC50) determined in preliminary assays. The final concentration of ethanol never exceeded 0.2% (vol:vol). Control cells were exposed to test medium supplemented with 0.2% ethanol. After incubation with the test compound for 24, 48 or 72 hours, MTT (Sigma) was added and the plate samples were then read in eight wells at an optical density value of 540 nm. Untreated controls were incubated with culture medium alone. The percentages of surviving cells, as compared with untreated controls, were calculated.
Flow cytometry
To evaluate the mode of cell death, asynchronous KPL-1 cells were exposed to DHA or CDHA at IC50 for 72 hours, in DMEM supplemented with 10% FBS. To evaluate cell cycle progression, after serum starvation (incubated with FBS-free medium) for 24 hours synchronous KPL-1 cells were exposed to each test compound at the same concentration in DMEM supplemented with 10% FBS for 0, 3, 6, or 24 hours. Both asynchronous and synchronous KPL-1 cells were treated as follows. The adhered and floating cells were mixed and washed twice in phosphate-buffered saline (PBS)(-) followed by centrifugation, and fixed in 70% ethanol. Then, the cells were treated with 1 mg/ml RNase, diluted with PBS(-), and stained with 50 μg/ml propidium iodide in PBS(-). Cells were analyzed by FACScan (Becton Dickson, Mountain View, CA, USA) using Cell Quest software. Cell cycle distribution was quantified with Modfit LT software (Verity Software House, Topsham, ME, USA), using a doublet discrimination module to eliminate the possibility of confusing multiples of G1 cells with ordinary G2 cells.
Western blotting analysis
KPL-1 cells were examined for expression of p53, p21Cip1/Waf1, cyclin D1, Bax, and Bcl-2 proteins. KPL-1 cells were exposed to DHA or CDHA for 3, 6 and 24 hours, or for 24, 48 and 72 hours (at the IC50 dose for 72 hours) and compared with unexposed cells. Immunoblotting was performed as previously described [23]. Briefly, cell pellets were homogenized using lysis buffer (50 mmol/l Tris-HCl [pH 6.8], 2% SDS, 5 mmol/l β-mercaptoethanol, 10% glycerol). Cell lysates were clarified by centrifugation at 13,500 g for 40 min. Protein concentrations were measured by Bio-Rad assay (Bio-Rad, Richmond, CA, USA), and 70 μg protein from each sample was mixed with loading buffer, electrophoresed on 15% SDS polyacrylamide gels, and blotted onto nitrocellulose membrane (Hybond-P; Amersham Pharmacia Biotech, Buckinghamshire, UK). The membranes were blocked with 5% dry milk in Tris-buffered saline–Tween for 1 hour at room temperature and probed with anti-p53 (clone DO-7; Dako, Glostrup, Denmark), anti-p21Cip1/Waf1 (clone SX-118; BD Biosciences, San Jose, CA, USA), and anti-cyclin D1 (clone P2D11F11; Novocastra Laboratories, Newcastle upon Tyne, UK) antibodies, and with anti-Bax (BD Biosciences) or anti-Bcl-2 (BD Biosciences) antiserum. Next, the membrane was treated with peroxidase-conjugated goat antimouse or antirabbit IgG antiserum (Envision + system HRP; Dako). Immobilized antigen was detected using ECL plus Western-blotting detection reagents and hyperfilm (Amersham), according to the manufacturer's instructions. Intensity of bands was quantified with the NIH Image1.47 Processing and Analysis program for use with Macintosh computers http://rsb.info.nih.gov/nih-image/.
Effect of CDHA on KPL-1 tumor growth in female athymic mice
Female athymic BALB/c-nu/nu mice (age 4 weeks) were purchased from Charles River Japan (Atsugi, Japan). After arrival the mice were randomly assigned to three different dietary groups, with 10 mice in each group and two cages for each group. For basal diet without CDHA, an American Institute of Nutrition (AIN-76 A) standard reference diet [24] was used. This diet consists of 20% casein, 0.3% DL-methionine, 55% cornstarch, 10% sucrose, 5% cellulose, 5% corn oil, 3.5% AIN-76 mineral mix, 1.0% AIN-76 vitamin mix, and 0.2% choline bitartrate. For diets containing 0.2% or 1.0% CDHA, the appropriate amount of CDHA was added to the basal diet. The diets were prepared at Oriental Yeast (Chiba, Japan) and were stored in sealed plastic bags in the dark at 4°C. During the experimental period the animals were housed in plastic cages with sterilized white pine chips for bedding. The animal room was maintained under specific pathogen-free conditions and was controlled for temperature (22 ± 2°C), light (12-hour cycle), and humidity (60 ± 10%). After the animals had become acclimatization to their respective powdered diets over 2 weeks, the semiconfluent KPL-1 cells growing in DMEM supplemented with 10% FBS at 37°C were trypsinized, and 2 × 107 viable cells/0.25 ml of medium were injected subcutaneously into the area of the right thoracic mammary fat pad using a 26-gauge needle.
The local growing tumor was checked every day before it became visible, and was measured once a week after it became visible. Tumor volume was measured with calipers and calculated using a standard formula [25]: width2 × length × 0.5. All mice were killed when the tumors of the basal diet fed group began to exhibit central necrosis (41 days after tumor cell inoculation), and all animals underwent necropsy. At necropsy tumors were weighed and all organs were checked macroscopically. Abnormal organs, locally growing tumors, and axillary lymph nodes were fixed in 10% neutral buffered formalin and processed for hematoxylin–eosin staining. Axillary lymph nodes were evaluated microscopically for metastases.
Data analysis
All results are expressed as means ± standard error. In all in vitro experiments, significance of differences was determined using the two-tailed Mann–Whitney U-test for unpaired samples after assuring homogeneity of variance. In in vivo experiments, after assurance of homogeneity of variance, analysis was performed using the non-repeated-measures analysis of variance parametric test or the Kruskal–Wallis nonparametic test. If the P value of these pretests was below 0.05, then post hoc analysis was performed using Fisher's protected least significant difference test to compare body weight, tumor volume, tumor weight, and frequency of metastasis. P < 0.05 was considered statistically significant.
Results
Effects of DHA and CDHA in vitro
The number of viable KPL-1 cells decreased after treatment with DHA and CDHA (Fig. 1). Dose and time dependent cytotoxity (reduction in cell number to below the initial plating density) was observed. For 72 hours of treatment, the IC50s of DHA and CDHA were 270 and 97 μmol/l, respectively. CDHA was more cytotoxic than DHA toward KPL-1 cells.
Figure 1.
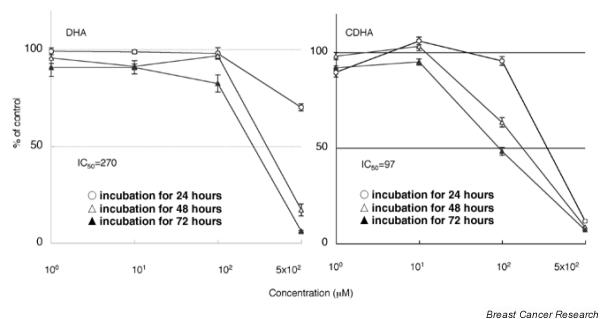
Growth inhibition curves of KPL-1 cells after treatment with docosahexaenoic acid (DHA) or conjugated DHA (CDHA) for 24, 48, and 72 hours. Shown are means ± standard error for three experiments. The 50% inhibitory concentration (IC50) values indicated are for the 72-hour treatment.
Mode of cell death
To determine whether the growth inhibitory activity of DHA and CDHA was related to induction of apoptosis, the effect of both reagents on the cell cycle was analyzed by flow cytometry. Representative DNA histograms of asynchronous KPL-1 cells are shown in Fig. 2. At the IC50 for 72 hours, both DHA and CDHA induced appearance of a sub-G1 fraction, which is characteristic of apoptosis.
Figure 2.
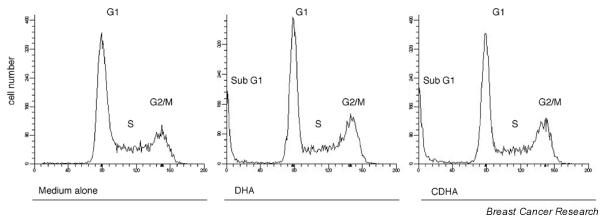
Cell cycle analysis of KPL-1 cells grown in medium alone or treated with 270 μmol/l docosahexaenoic acid (DHA) or 97 μmol/l conjugated DHA (CDHA) 50% inhibitory concentration [IC50] for 72 hours, respectively) for 72 hours. Appearance of sub-G1 fraction is seen after DHA and CDHA treatment.
Cell cycle progression
Cells in asynchronous cell populations are in different stages of the cell cycle at any given time, making it difficult to interpret changes in cell cycle progression precisely. To examine inhibition of cell growth by DHA or CDHA, synchronous KPL-1 cells were treated with 270 μmol/l DHA or 97 μmol/l CDHA (IC50 concentration for 72 hours). Cell cycle was synchronized by serum starvation for 24 hours followed by refeeding. Cell cycle fraction was recorded after 3, 6, and 24 hours of treatment with each test compound, and was compared with cells incubated in medium alone. Representative DNA histograms for synchronous cells and data from three independent experiments are shown in Fig. 3. Among synchronous cells incubated in medium alone, 71 ± 2% of cells were in G0/G1 phase and 19 ± 2% were in S phase. At 3 hours after refeeding, the percentage of cells in G0/G1 decreased to 37 ± 1% and the percentage in S phase increased to 43 ± 5%. At 6 hours the percentage of cells in G2/M phase increased to 22 ± 0%. In cells treated with DHA or CDHA, cell cycle progression was comparable with that in cells in medium alone until 6 hours after re-feeding. However, after 24 hours of incubation with DHA or CDHA, the percentage of cells in S phase was lower (15 ± 7% and 12 ± 0%, respectively) and the percentage of cells in G0/G1 was higher (53 ± 10% and 76 ± 4%, respectively), as compared with cells incubated in medium alone, suggesting G0/G1 arrest. After 24 hours of incubation, the percentage of cells in G2/M phase was high (32 ± 3%) among cells treated with DHA but not among cells treated with CDHA (17 ± 5%), as compared with cells incubated in medium alone (12 ± 1%). DHA may have induced cell arrest in the early stage of G0/G1 phase, leading to accumulation of cells in G2/M phase.
Figure 3.
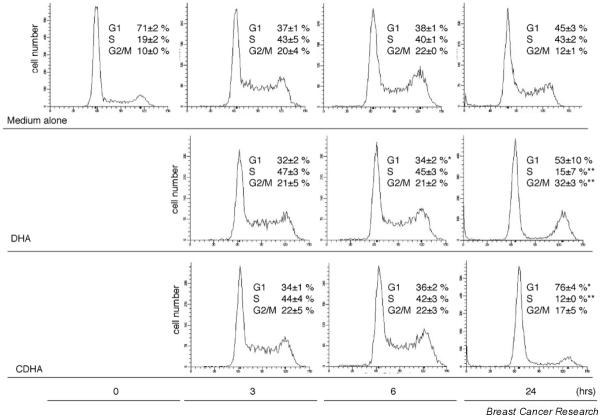
Cell cycle analysis of synchronous KPL-1 cells grown in medium alone or treated with docosahexaenoic acid (DHA) or conjugated DHA (CDHA) (50% inhibitory concentration [IC50] for 72 hours, respectively) for up to 24 hours. G0/G1 arrest is seen after DHA and CDHA treatment. All results were calculated from three independent experiments. *P < 0.05, **P < 0.01, versus controls at the same time point.
Changes in cell cycle regulators and apoptosis inducers
Cell cycle regulatory proteins, including p53, p21Cip1/Waf1, and cyclin D1, were examined. Representative Western blotting results are shown in Fig. 4a, and data from three independent experiments using individually prepared lysates are summarized in Fig. 5a. Density of immunoreactive bands in DHA-treated or CDHA-treated samples is expressed as percentage of the density of corresponding bands in control samples, which were designated 100%. In KPL-1 cells, both DHA and CDHA at IC50 for 72 hours increased levels of p53 and p21Cip1/Waf1, and decreased levels of cyclin D1 at 3, 6 and 24 hours, as compared with untreated control cells. Changes in the apoptosis accelerating protein Bax and the apoptosis suppressing protein Bcl-2 were examined 24, 48 and 72 hours after treatment. Although the level of Bax was not altered at these time points, a decrease in Bcl-2 level was observed (Figs 4b and 5b).
Figure 4.
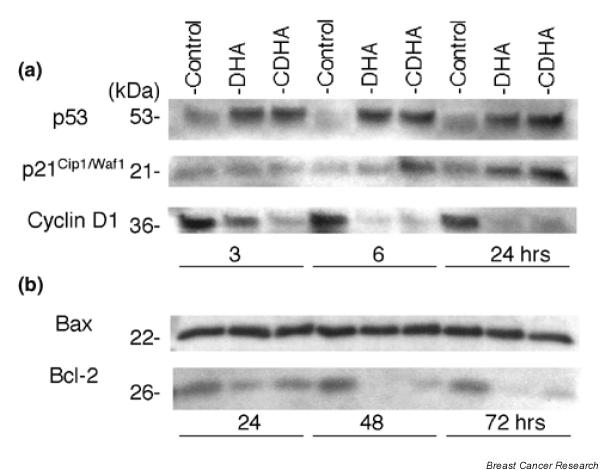
Expression of (a) p53, p21Cip1/Waf1 and cyclin D1, and (b) Bax and Bcl-2 in KPL-1 cells grown in medium alone (control) or treated with docosahexaenoic acid (DHA) or conjugated DHA (CDHA).
Figure 5.
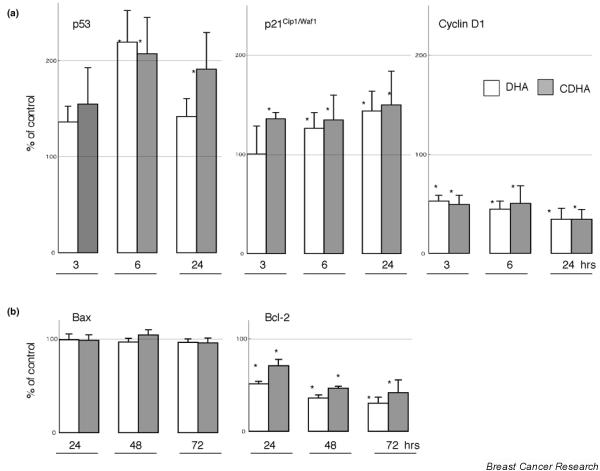
Quantification of (a) p53, p21Cip1/Waf1 and cyclin D1, and (b) Bax and Bcl-2 protein expression in KPL-1 cells treated with docosahexaenoic acid (DHA) or conjugated DHA (CDHA), compared with control (designated 100%). All results were calculated from three independent samples. *P < 0.05, versus controls.
Effects of CDHA in vivo
There was no evidence of gross toxicity resulting from the 0.2% or 1.0% CDHA diets. There were no significant differences in body weight change among groups (Fig. 6) and no deaths occurred. However, growth of KPL-1 cells, as evaluated by tumor volume, was always less in CDHA-fed mice than in CDHA-unfed controls (Fig. 7). At the end of the study tumor volume was significantly reduced in 1.0% CDHA-treated mice (855 ± 88 mm3) compared with CDHA-unfed controls (1314 ± 223 mm3; P < 0.05); the 0.2% CDHA diet (1193 ± 138 mm3) had no significant effect. Although a 26% reduction in tumor weight was observed in 1.0% CDHA treated mice compared with CDHA-unfed controls (1170 ± 158 and 1580 ± 261 mg, respectively), the difference was not significant; tumor weight was not reduced in 0.2% CDHA treated mice (1581 ± 157 mg). Axillary lymph node metastasis was observed in 60% (6/10) of control mice, 50% (5/10) of 0.2% CDHA-treated mice, and 40% (4/10) of 1.0% CDHA-treated mice (i.e. metastasis gradually decreased with increasing amount of ingested CDHA). In each dietary group metastasis tended to correlate with the size of the primary tumor (Fig. 8). Further experiments are needed to determine whether metastasis per se is affected by CDHA.
Figure 6.
Effect of dietary conjugated docosahexaenoic acid (CDHA) on body weight change in female BALB/c athymic mice before and after KPL-1 inoculation. Values are expressed as mean ± standard error for 10 mice.
Figure 7.
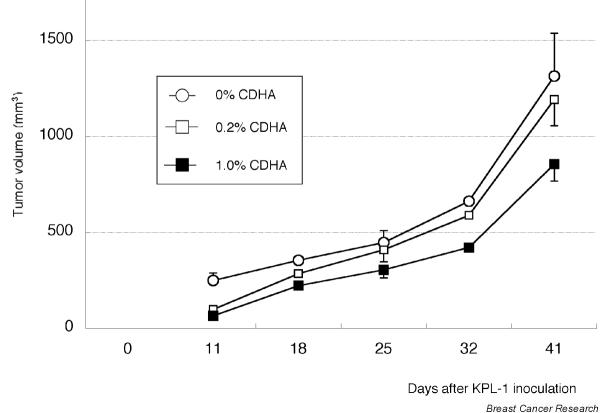
Growth of KPL-1 cells injected subcutaneously into the area of the right thoracic mammary fat pad of female BALB/c athymic mice at 6 weeks of age. Values are expressed as mean ± standard error for 10 tumors. CDHA, conjugated docosahexaenoic acid.
Figure 8.
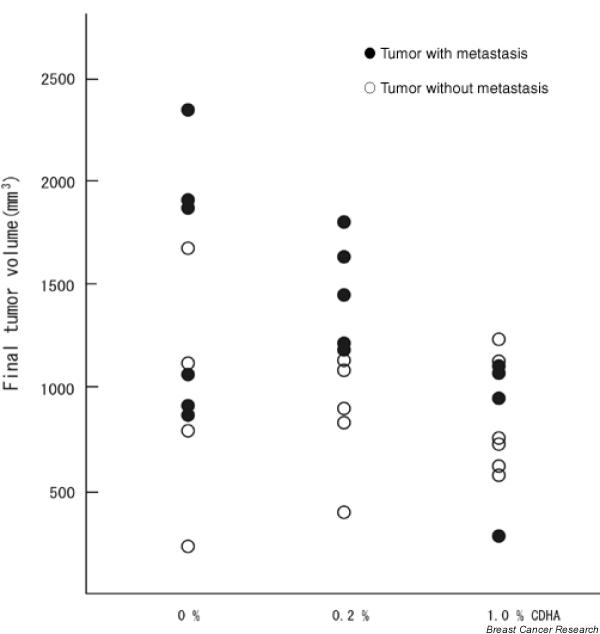
Scattergram showing KPL-1 primary tumor volume and lymph node metastasis for diets containing 0%, 0.2%, or 1.0% conjugated docosahexaenoic acid (CDHA).
Discussion
DHA inhibited the growth of KPL-1 human breast cancer cells in vitro, with an IC50 of 270 μmol/l. In a previous study serum concentration of DHA in rats was 97.3 μg/ml (297 μmol/l) after 4 weeks of DHA supplementation [26]. Thus, the present IC50 for DHA may be relevant to tumor progression in vivo. CDHA inhibited KPL-1 cell growth more effectively than did DHA in vitro. CDHA isomerized by alkaline treatment exhibited cytotoxity, with an IC50 of 97 μmol/l, which is 2.8-fold more potent than the parent DHA. The conjugated fatty acid most widely studied for its anticarcinogenic effects is CLA, a conjugated dienoic acid. In addition, conjugated trienoic fatty acids exhibit cytotoxic effects in cultured human tumor cell lines [27], and conjugated tetraenes exert cytotoxicity on human leukemia cells [28]. In the present study CDHA rich in conjugated pentaene and hexaene was shown to be cytotoxic against KPL-1 human breast cancer cells.
Garden balsam seed oil, which contains conjugated tetraenes, and tung, bitter gourd, pomgranata, catalpa and pot marigolg seed oil, which contain high amounts of conjugated trienes, are cytotoxic to tumor cells of mouse and human origin [28,29]. These naturally occurring seed oils each contain one major isomer [30]. In contrast, like CLA formed by partial bacterial hydrogenation of the parent linoleic acid in the rumen of ruminant animals, conjugated fatty acids prepared by alkaline isomerization contain a mixture of complex positional (location of double bonds) and geometric (cis/trans combinations) isomers. Determining which isomer(s) exhibits potent anticarcinogenic action requires further study.
DHA-induced reduction of tumor cell growth is generally attributed to induction of apoptotic cell death [31] and its effects on cell cycle progression [32]. DHA reduces cyclin D1, E and A associated kinase activity in HT-29 human colon cancer cells. Thus, DHA may exert its negative effect on growth of tumor cells by inhibiting activation and expression of G1-associated cell cycle regulatory proteins [33]. In the present study, like its parent DHA, CDHA induced apoptosis and affected cell cycle regulatory proteins. Cell cycle analysis exhibited G1 arrest, which involves increases in p53 and p21Cip1/Waf1 and a decrease in G1 cyclin (cyclin D1). CDHA induces apoptosis in human colorectal adenocarcinoma cells [21]. In the present study apoptosis was seen in KPL-1 cells; the apoptosis cascade involved downregulation of Bcl-2, whereas Bax expression was unchanged. Thus, the growth inhibitory mechanisms of CDHA involve positive regulation of apoptosis and negative regulation of cell cycle progression.
In the present study, CDHA suppressed growth of human breast cancer cells transplanted into athymic mice. Previous studies indicate that CLA is a powerful anticancer agent in the rat mammary cancer model, with significant effects of CLA obtained at dietary doses of 1.0% and less [34]. Suppression of mammary carcinogenesis in rats can be achieved with a diet containing less than 1.0% conjugated α-linolenic acid (derived from perilla oil) [35]. The dietary doses at which conjugated fatty acids have tumor-suppressing effects is strikingly low compared with n-3 PUFAs, which require a dietary dose of 5–10% to achieve comparable effects [8,19,36]. In the present study the dose of CDHA (0.2–1.0% in the AIN-76A diet) was determined in accordance with findings of previous studies of conjugated fatty acid. A dietary dose of 1.0% CDHA suppressed KPL-1 cell growth in athymic mice.
Conclusion
CDHA exhibited an inhibitory effect on KPL-1 human breast carcinoma cells in culture, and modulated apoptosis cascades and cell cycle progression. Also, CDHA suppressed KPL-1 cell growth in athymic mice with no side effects. CDHA, which has relatively low toxicity, has potential as a dietary chemopreventive/chemotherapeutic agent against human breast cancer, and possibly in management of other forms of cancer. Determining which positional and geometric isomers are responsible for this anticarcinogenic effect will require further study.
Competing interests
None declared.
Abbreviations
CDHA = conjugated docosahexaenoic acid; CLA = conjugated linoleic acid; DHA = docosahexaenoic acid; DMEM = Dulbecco's modifed Eagle's minimum essential medium; FBS = fetal bovine serum; IC50 = 50% inhibitory concentration; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PBS = phosphate-buffered saline; PUFA = polyunsaturated fatty acid.
Acknowledgments
Acknowledgements
The authors thank Ms T Akamatsu for her excellent technical assistance in tissue preparation, and Ms Y Yoshida for preparing the manuscript. This study was supported in part by a Health and Labor Science Research Grant for Research on Food and Chemical Safety, from the Ministry of Health, Labor and Welfare, Japan.
Contributor Information
Miki Tsujita-Kyutoku, Email: tsujita@takii.kmu.ac.jp.
Takashi Yuri, Email: yurit@takii.kmu.ac.jp.
Naoyuki Danbara, Email: danbaran@takii.kmu.ac.jp.
Hideto Senzaki, Email: senzaki@takii.kmu.ac.jp.
Yasuhiko Kiyozuka, Email: kiyozuka@takii.kmu.ac.jp.
Norihisa Uehara, Email: ueharan@takii.kmu.ac.jp.
Hideho Takada, Email: takadahi@kouri.kmu.ac.jp.
Takahiko Hada, Email: hada@bizen-c.com.jp.
Teruo Miyazawa, Email: miyazawa@biochem.tohoku.ac.jp.
Yutaka Ogawa, Email: ogaway@takii.kmu.ac.jp.
Airo Tsubura, Email: tsubura@takii.kmu.ac.jp.
References
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- Hunter DJ, Spiegelman D, Adami HO, Beeson L, van den Brandt PA, Folsom AR, Fraser GE, Goldbohm RA, Graham S, Howe GR, Kushi LH, Marshall JR, McDermott A, Miller AB, Speizer FE, Wolk A, Yaun S-S, Willett W. Cohort studies of fat intake and the risk of breast cancer: a pooled analysis. N Engl J Med. 1996;334:356–361. doi: 10.1056/NEJM199602083340603. [DOI] [PubMed] [Google Scholar]
- Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- Bang HO, Dyerberg J, Hjoorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- Nobmann ED, Byers T, Lanier AP, Hankin JH, Jackson MY. The diet of Alaska Native adults: 1987–1988. Am J Clin Nutr. 1992;55:1024–1032. doi: 10.1093/ajcn/55.5.1024. [DOI] [PubMed] [Google Scholar]
- Kaizer L, Boyd NF, Kriukov V, Tritcher D. Fish consumption and breast cancer risk: an ecological study. Nutr Cancer. 1989;12:61–68. doi: 10.1080/01635588909514002. [DOI] [PubMed] [Google Scholar]
- Rose DP, Connolly JM, Coleman M. Effect of omega-3 fatty acids on the progression of metastasis after the surgical excision of human breast cancer cell solid tumor growing in nude mice. Clin Cancer Res. 1996;2:1751–1756. [PubMed] [Google Scholar]
- Senzaki H, Iwamoto S, Ogura E, Kiyozuka Y, Arita S, Kurebayashi J, Takada H, Hioki K, Tsubura A. Dietary effects of fatty acids on growth and metastasis of KPL-1 human breast cancer cells in vivo and in vitro. Anticancer Res. 1998;18:1621–1628. [PubMed] [Google Scholar]
- Hirose M, Masuda A, Ito N, Kamano K, Okuyama H. Effect of dietary perilla oil, soybean oil and safflower oil on 7,12-dimethylbenzanthracene (DMBA) and 1,2-dimethylhydrazine (DMH)-induced mammary gland and colon carcinogenesis in female SD rats. Carcinogenesis. 1990;11:731–735. doi: 10.1093/carcin/11.5.731. [DOI] [PubMed] [Google Scholar]
- Hirose M, Nishikawa A, Shibutani M, Imai T, Shirai T. Chemoprevention of heterocyclic amine-induced mammary carcinogenesis in rats. Environ Mol Mutagen. 2002;39:271–278. doi: 10.1002/em.10066. [DOI] [PubMed] [Google Scholar]
- Ha YL, Grimm NK, Pariza MW. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis. 1987;8:1881–1887. doi: 10.1093/carcin/8.12.1881. [DOI] [PubMed] [Google Scholar]
- MacDonald HB. Conjugated linoleic acid and disease prevention: a review of current knowledge. J Am Coll Nutr. 2000;Suppl:111S–118S. doi: 10.1080/07315724.2000.10718082. [DOI] [PubMed] [Google Scholar]
- Kelly GS. Conjugated linoleic acid: a review. Altern Med Rev. 2001;6:367–382. [PubMed] [Google Scholar]
- Pariza MW, Park Y, Cook ME. The biologically active isomers of conjugated linoleic acid. Prog Lipid Res. 2001;40:283–298. doi: 10.1016/S0163-7827(01)00008-X. [DOI] [PubMed] [Google Scholar]
- Ip C, Chin SF, Scimeca JA, Pariza MW. Mammary cancer prevention by conjugated dienoic derivative of linoleic acid. Cancer Res. 1991;51:6118–6124. [PubMed] [Google Scholar]
- Thompson H, Zhu Z, Banni S, Darcy K, Loftus T, Ip C. Morphological and biochemical status of mammary gland as influenced by conjugated linoleic acid: implication for a reduction in mammary cancer risk. Cancer Res. 1997;57:5067–5072. [PubMed] [Google Scholar]
- Ip C, Banni S, Angioni E, Carta G, McGinly J, Thompson HJ, Barbano D, Bauman D. Conjugated linoleic acid-enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J Nutr. 1999;129:2135–2142. doi: 10.1093/jn/129.12.2135. [DOI] [PubMed] [Google Scholar]
- O'Shea M, Devery R, Lawless F, Murphy J, Stanton C. Milk fat conjugated linoleic acid (CLA) inhibits growth of human mammary MCF-7 cancer cells. Anticancer Res. 2000;20:3591–3601. [PubMed] [Google Scholar]
- Yuri T, Danbara N, Tsujita-Kyutoku M, Fukunaga K, Takada H, Inoue Y, Hada T, Tsubura A. Dietary docosahexaenoic acid suppresses N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats more effectively than eicosapentaenoic acid. Nutr Cancer. 2003;45:211–217. doi: 10.1207/S15327914NC4502_11. [DOI] [PubMed] [Google Scholar]
- Lopez A, Gerwick WH. Two new icosapentaenoic acids from the temperate red seaweed Ptilota filicina J. Agardh. Lipids. 1987;22:190–194. doi: 10.1007/BF02537301. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Miyazawa T. Do conjugated eicosapentaenoic acid and conjugated docosahexaenoic acid induce apoptosis via lipid peroxidation in cultured human tumor cells? Biochem Biophys Res Commun. 2000;270:649–656. doi: 10.1006/bbrc.2000.2484. [DOI] [PubMed] [Google Scholar]
- Kurebayashi J, Kurosumi M, Sonoo H. A new human breast cancer cell line, KPL-1 secretes tumor-associated antigens and grows rapidly in female athymic nude mice. Br J Cancer. 1995;71:845–853. doi: 10.1038/bjc.1995.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, Tsubura A. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001;22:891–897. doi: 10.1093/carcin/22.6.891. [DOI] [PubMed] [Google Scholar]
- Bieri JG, Stoewsand GS, Briggs GM, Phillips RW, Woodard JC, Knapka JJ. Report of the American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- Geran RI, Greenberg NH, McDonald MM, Schumacher AM, Abbott BJ. Protocol for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep. 1972;3:1–103. [Google Scholar]
- Mizota A, Sato E, Taniai M, Adachi-Usami E, Nishikawa M. Protective effects of dietary docosahexaenoic acid against kainate-induced retinal degeneration in rats. Invest Ophthalmol Vis Sci. 2001;42:216–221. [PubMed] [Google Scholar]
- Igarashi M, Miyazawa T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett. 2000;148:173–179. doi: 10.1016/S0304-3835(99)00332-8. [DOI] [PubMed] [Google Scholar]
- Cornelius AS, Yerram NR, Kratz DA, Spector AA. Cytotoxic effect of cis-parinaric acid in cultured malignant cells. Cancer Res. 1991;51:6025–6030. [PubMed] [Google Scholar]
- Suzuki R, Noguchi R, Ota T, Abe M, Miyashita K, Kawada T. Cytotoxic effect of conjugated trienoic fatty acids on mouse tumor and human monocytic leukemia cells. Lipids. 2001;36:477–482. doi: 10.1007/s11745-001-0746-0. [DOI] [PubMed] [Google Scholar]
- Takagi T, Itabashi Y. Occurrence of mixtures of geometrical isomers of conjugated octadecatrienoic acids in some seed oils: analysis by open-tubular gas liquid chromatography and high performance liquid chromatography. Lipids. 1981;16:546–551. [Google Scholar]
- Connolly JM, Gilhooly EM, Rose DP. Effects of reduced dietary linoleic acid intake, alone or combined with an algal source of docosahexaenoic acid, on MDA-MB-231 breast cancer cell growth and apoptosis in nude mice. Nutr Cancer. 1999;35:44–49. doi: 10.1207/S1532791444-49. [DOI] [PubMed] [Google Scholar]
- Istfan NW, Wan J, Chen ZY. Fish oil and cell proliferation kinetics in a mammary carcinoma tumor model. Adv Exp Med Biol. 1995;375:149–156. doi: 10.1007/978-1-4899-0949-7_13. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Istfan NW. Docosahexaenoic acid, a major constituent of fish oil diets, prevents activation of cyclin-dependent kinases and S-phase entry by serum stimulation in HT-29 cells. Prostaglandins Leukot Essent Fatty Acids. 2001;64:67–73. doi: 10.1054/plef.2000.0239. [DOI] [PubMed] [Google Scholar]
- Ip C, Scimeca JA, Thompson HJ. Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources. Cancer. 1994;74:1050–1054. doi: 10.1002/1097-0142(19940801)74:3+<1050::aid-cncr2820741512>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Futakuchi M, Cheng JL, Hirose M, Kimoto N, Cho Y-M, Iwata T, Kasai M, Tokudome S, Shirai T. Inhibition of conjugated fatty acids derived from safflower or perilla oil of induction and development of mammary tumors in rats induced by 2-amino-1-methyl-6-phenylimidazo (4,5-b) pyridine (PhIP) Cancer Lett. 2002;178:131–139. doi: 10.1016/S0304-3835(01)00860-6. [DOI] [PubMed] [Google Scholar]
- Takata T, Minoura T, Takada H, Sakaguchi M, Yamamura M, Hioki K, Yamamoto M. Specific inhibitory effect of dietary eicosapentaenoic acid on N-nitroso-N-methylurea-induced mammary carcinogenesis in female Sprague–Dawley rats. Carcinogenesis. 1990;11:2015–2019. doi: 10.1093/carcin/11.11.2015. [DOI] [PubMed] [Google Scholar]


