Abstract
Objective
Delirium superimposed on dementia is common and is associated with adverse outcomes. Yet little is known about the patients’ personal delirium experiences. We used quantitative and qualitative methods to assess the delirium superimposed on dementia experience among older patients.
Methods
We conducted a prospective cohort study among patients with delirium superimposed on dementia who were admitted to a rehabilitation ward. Delirium was diagnosed using DSM-IV-TR criteria. Delirium severity and symptoms were evaluated with the Delirium-O-Meter (D-O-M). The experience of delirium was assessed after delirium resolution (T0) and one month later (T1) with a standardized questionnaire and a qualitative interview. Level of distress was measured with the Delirium Experience Questionnaire.
Results
Of the 30 patients included in the study, 50% had mild dementia; 33% and 17% had moderate and severe dementia. Half of the patients had evidence of the full range of D-O-M delirium symptoms. We evaluated 30 patients at T0 and 20 at T1. At T0, half of the patients remembered being confused as part of the delirium episode, and reported an overall moderate level of related distress. Patients reported high distress related to memories of anxiety/fear, delusions, restlessness, hypokinesia, and impaired orientation. Qualitative interviews revealed six main aspects of patients delirium experiences: Emotions; Cognitive Impairment; Psychosis; Memories; Awareness of Change; and Physical Symptoms.
Conclusions
The study provides novel information on the delirium experience in patients with dementia. These findings are key for health care providers to improve the everyday care of this important group of frail older patients.
Keywords: delirium superimposed on dementia, delirium experience, delirium stress
INTRODUCTION
Delirium is a common neuropsychiatric disorder defined by the DSM-51 as a disturbance in attention, awareness, and cognition that develops over a short period of time, and fluctuates over the course of the day. When delirium occurs in patients with dementia, this condition is referred to as delirium superimposed on dementia.2 The prevalence of delirium superimposed on dementia ranges between 22% to 89% in both community and hospital populations, and is associated with higher health care costs, worse functional outcomes, and higher mortality rates when compared to patients with dementia alone.2–6
Clinicians are becoming increasingly aware of the negative effects of delirium superimposed on dementia on clinical outcomes, yet little is known about the patients’, family caregivers’ and hospital staff members’ personal experiences of delirium superimposed on dementia.7 The National Institute for Health and Clinical Excellence (NICE) guidelines for delirium encourage individuals who have experienced delirium to share this experience with their healthcare professionals during recovery in order to describe key components of the delirium experience.8 The literature describing delirium experiences is small, generally qualitative, and involves specific patient populations (i.e., burns, orthopaedic/cardiac surgery, palliative care medicine, and ICU).7 Four quantitative studies describing the delirium experience have been published.9–12 Current evidence indicates that some patients recall delirium, and that the recollections are distressing.7 This distress may result in long-term psychological sequelae, such as prolonged anxiety, or post-traumatic stress disorder.13 However, no previous study has evaluated the experience of delirium specifically in patients with dementia. It is recognized that delirium superimposed on dementia is associated with worse outcomes compared to delirium alone.2–6 Understanding delirium symptoms in dementia can help improve the distinction between the neuropsychiatric symptoms of these conditions. Moreover, this distinction is challenging, and requires a more detailed evaluation of patient perspectives and those of their caregivers, in order to clarify the meaning and significance of the neuropsychiatric symptoms occurring in dementia patients prone to delirium.
In investigating the experience of delirium superimposed on dementia in older patients, we used both quantitative and qualitative methods.
MATERIALS AND METHODS
This was a prospective cohort study of patients 65 years and older with delirium superimposed on dementia who were consecutively admitted to a 27-bed inpatient rehabilitation ward at the Department of Rehabilitation of the Ancelle Hospital (Cremona, Italy) between September, 2013 and May, 2014. Patients are typically admitted either after an acute hospitalization or from home for different clinical reasons. The most frequent reasons for admission are postsurgical interventions (hip fracture surgical repair; hip or knee arthroplasty; abdominal surgery), stroke, heart failure, chronic obstructive pulmonary diseases, Parkinson diseases, or gait and balance disorders owing to a single or mixed etiology, including hypokinetic syndrome. This setting has been fully described previously.6, 14 Briefly, the rehabilitation ward is staffed by full-time geriatricians, physiatrists, nurses, nurse’s aides, physical, speech and occupational therapists. Patients were eligible if they had dementia prior to their hospital admission, and had delirium at the time of admission (prevalent delirium superimposed on dementia), or developed delirium during the hospital stay (incident delirium superimposed on dementia). Patients were excluded if the legal representative refused informed consent, if patients had delirium without dementia, or if patients could not self-report. At enrollment, we obtained informed consent from the patients’ legal representatives. Subsequently, we also obtained consent from patients once they were deemed to be mentally competent. The study was reviewed and approved by the local ethics committee.
Demographics
Patient demographics (e.g., age, sex) and clinical information (e.g., functional status, discharge setting) were recorded at the time of admission. Functional status was assessed with the Barthel Index (BI) through patient and surrogate interviews referring to the month before the acute hospital admission,15, 16 at rehabilitation admission, and at discharge.
Dementia ascertainment
We ascertained the presence and type of dementia (i.e., Vascular Dementia, Alzheimer Dementia, Lewy Body Dementia, Other) by reviewing patient medical records. Additionally, two expert neuropsychologists (EL, SM) confirmed and rated the severity of dementia by interviewing the caregivers at the time of enrollment using the Clinical Dementia Rating (CDR) Scale17 and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), with a cut-off of 3.3 as indicative of cognitive impairment.18 The CDR score ranges from 1 to 3: 1 (mild dementia); 2 (moderate dementia); 3 (severe dementia).
Delirium evaluation
Two expert geriatricians (AM, RT) diagnosed patients delirium according to the Diagnostic and Statistical Manual of Mental Disorders (4th edition, text revision) (DSM-IV-TR) criteria with a standardized approach.19 Fluctuation of symptoms was ascertained through informant history obtained from nursing staff and from the patients’ caregivers. Attention was evaluated using several methods. First, the patient was asked to state the days of the week forward and backwards, and to count backwards from 20 to 1. Any error in each of these tasks was considered as inattention. An additional test was the SAVEAHAART vigilance task embedded in the Confusion Assessment Method – ICU (CAM-ICU),20 where the assessor recites the sequence of letters slowly and the patient is asked to indicate when the letter ‘A’ is recited. Inattention was defined as the presence of more than two errors, as per CAM-ICU manual. During each of these tasks, the examiner observed the patient’s distractibility, comprehension, and the tendency to lose the thread of conversation. Level of consciousness was assessed using the modified-Richmond Agitation and Sedation Scale (m-RASS).21 The assessment of disorganised thinking was performed by asking the patient a list of pre-defined questions, such as: “Why are you in the hospital?”; “Will a stone float on water?”; “Are there fish in the sea?”. Any error in each of these tasks was considered to indicate disorganised thinking. Additionally, assessors recorded sleep-wake cycle disturbances, psychomotor abnormalities (including abnormal motor behaviour), perceptual disturbances, short and long-term memory disturbances, psychotic symptoms and depressed mood, as derived from the clinical notes and patient interview. These assessments were used in combination against the DSM-IV-TR criteria, with the objective indicators described above, and supplemented by the assessors’ judgement regarding the subjective features. The study geriatricians screened patients with dementia for the presence of delirium at the time of admission, and daily during their hospital stay, if they did not have delirium on admission. Delirium was evaluated twice a day until discharge by the study geriatricians. The motor subtype of delirium (i.e., hypoactive, hyperactive, mixed, non hyperactive-hypoactive delirium) was defined using the modified-Richmond Agitation and Sedation Scale (m-RASS).21 Patients were considered delirium free if they did not have delirium for three consecutive days, as described in the study by Bellelli et al.22 We evaluated the severity of delirium using the Delirium-O-Meter (D-O-M),23 a nurses’ rating scale for monitoring delirium severity in geriatric patients. The D-O-M reliability was high; Cronbach’s alpha values ranged in the validation study from 0.87–0.92; Intra Class Correlation (ICC) ranges were 0.84–0.91 for total scores and 0.40–0.97 for item scores.23 The D-O-M includes twelve symptoms of delirium: Sustained inattention; Shifting attention; Orientation; Consciousness disturbance; Apathy; Hypokinesia/psychomotor retardation; Incoherence; Fluctuation functioning (diurnal variation/sleep wake cycle); Restlessness (psychomotor agitation); Delusions; Hallucinations; and Anxiety/fear. Each symptom is scored on a four-point Likert scale (0=absent; 1=mild; 2=moderate; 3=severe). Total scores range from 0 to 36, with higher scores representing a greater symptom burden. The two study geriatricians completed the D-O-M with patients during the day, and bedside nurses completed it at night.
Quantitative and qualitative evaluation of the experience of delirium
One of the two neuropsychologists (EL, SM) interviewed patients three days after the resolution of delirium, using structured questionnaires, including the Delirium Experience Questionnaire (DEQ).10 The DEQ includes six questions for patients who have recovered from a delirium episode including: 1) “Do you remember being confused? Yes or No”; 2) “If no, are you distressed that you cannot remember? Yes or No”; 3) “How distressed?” 0–4 numerical rating scale with 0=not at all, and 4=extremely; 4) “If you do remember being confused, was the experience distressing? Yes or No”; 5) “How distressing?” 0–4 numerical rating scale; 6) “Can you please describe the experience?” Each answer at this open-ended question was recorded verbatim on paper.
In addition, we asked patients if they could recall experiencing any symptoms included on the D-O-M. For each symptom experienced, we asked patients to rate their level of related distress on a scale from 0–4 (0=no distress; 1=a little; 2=a fair amount; 3= very much; 4=extremely distressed). We also asked patients to rate their overall level of distress on the same scale.
Patients were again evaluated at 1-month follow-up with a phone interview, or in person, if the patients were still in the hospital.
Method of analysis
The characteristics of patients and their families were summarized using descriptive statistics including means, standard deviations, and percentages. We analyzed the characteristics of the patients and the delirium profile according to the ability to remember the delirium episodes at the first evaluation (T0) and at 1-month follow-up (T1). Analysis of variance or the chi-square test were used to examine differences in the patients’ characteristics and the delirium profile. Where significant group effects were detected, the Bonferroni test was applied to indicate post hoc differences between individual groups.
A total of at least 30 interviews were selected as the sample size for the qualitative analysis, as previously indicated by Creswell.24 We employed content analysis17 to analyze responses to the open-ended questions regarding the overall experience of delirium. Data were analyzed as described by Graneheim and Lundman.25 Four of the authors (EL, SM, AM, RT) began by reading each response several times, making notations to identify various reported aspects of the delirium experience. We then assigned common phrases, or labels (e.g., if the patient reported being confused and disoriented then the label was confusion and disorientation). Next, we grouped common labels into broader categories by topic, (e.g., if the label was confusion and disorientation then the broader category was “cognitive impairment”). We created and modified categories and subcategories (or codes and subcodes) in an iterative process as we read each response. When all data were reviewed and coded, we reapplied the final coding scheme to all responses. Interviews were conducted in Italian and then translated into English by AM.
RESULTS
We screened a total of 37 patients. Two were excluded because their legal representative refused to provide informed consent; therefore, we enrolled a total of 35 patients. Of these, 30 patients completed the T0 interview (three days after resolution of delirium); two patients refused to be evaluated, and three died during the hospital stay. At one-month follow-up, we evaluated 20 patients; four were not testable due to inability to self-report, four were residents of nursing homes –due to inability to perform phone interview–, and two died after the discharge.
Patients are described in Table 1. They were mostly female, with a mean age of 83 (± 5.8) years old, and with a mean CDR of 1.6 (± 0.7), indicative of mild to moderate dementia. The two main types of dementia were Alzheimer’s and vascular dementia. Patients’ mean duration of delirium was 4.1 days (± 4.4 days), with a moderate severity as described by the D-O-M maximum score (16 ± 4.5).
Table 1.
Description of Patients (n=30).
| Patients | N=30 |
|---|---|
| Age | 83 ± 5.8 |
| Gender (female), (n, %) | 20 (66) |
| Barthel Index pre-admission | 73 ± 24 |
| Barthel Index admission | 22 ± 19 |
| Barthel Index discharge | 45 ± 29 |
| Discharge setting (n, %) | |
| - Home | 16 (53) |
| - Nursing home | 6 (20) |
| - Rehabilitation | 3 (10) |
| - Hospice | 4 (13) |
| - In hospital mortality | 1 (3) |
| IQCODE | 4.2 ± 0.9 |
| CDR total | 1.6 ± 0.7 |
| CDR 1 (n, %) | 15 (50) |
| CDR 2 (n, %) | 10 (33) |
| CDR 3 (n, %) | 5 (17) |
| Type of dementia (n, %) | |
| - Alzheimer dementia | 12 (40) |
| - Vascular dementia | 9 (30) |
| - Lewy Body dementia | 3 (10) |
| - Other | 6 (20) |
| Duration of delirium (days) | 4.1 ± 4.4 |
| Hypoactive delirium (n, %) | 20 (67) |
| Hyperactive delirium (n, %) | 9 (30) |
| Non hyper-hypoactive delirium (n, %) | 1 (3) |
| D-O-M maximum score | 16 ± 4.5 |
CDR, Clinical Dementia Rating Scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; D-O-M, delirium-O-meter.
A total of 15 patients at T0-interview and 12 patients at 1-month follow-up remembered being confused. Importantly, we found that patients who remembered being confused had a shorter duration of delirium (2.8 days ± 0.5) compared to those who did not remember being confused (5.4 days ± 1.3) at the T0-interview (Table 2). We found that patients who remembered being confused had a higher severity of delirium and they were more likely to remember symptoms related to problems with shifting attention and apathy, as recorded through the D-O-M evaluation.
Table 2.
Characteristics of Patients at Baseline Interview (T0) By Memory of Being Confused. Patients were categorized into two groups according to the answer to the question “Do you remember being confused?” at the Baseline Interview.
| Variable | Remembered being confused N=15 | Did not remember being confused N=15 | P value |
|---|---|---|---|
| CDR | 1.6 (±0.2) | 1.6 (0.2) | 0.33 |
| IQCODE | 4.3 (±0.2) | 3.9 (±0.3) | 0.21 |
| Category of CDR | 0.77 | ||
| CDR 1 (N, %) | 7 (44%) | 8 (54%) | |
| CDR 2 (N, %) | 7 (44%) | 4 (26%) | |
| CDR 3 (N, %) | 2 (12%) | 3 (20%) | |
| Type of dementia | 0.78 | ||
| -AD | 6 (37) | 6 (46) | |
| -VaD | 5 (31) | 7 (40) | |
| LBD | 1 (6) | – | |
| Other | 4 (25) | 2 (13) | |
| Duration of delirium (days) | 2.8 (± 0.5) | 5.4 (±1.3) | 0.01 |
| Hypoactive delirium, (N, %) | 9 (60) | 11 (73) | 0.50 |
| Hyperactive delirium, (N, %) | 5 (33) | 4 (27) | 0.60 |
| Non hyper-hypoactive delirium | 1 (7) | – | |
| D-O-M max | 16.5 (±1.2) | 15.1 (1.2) | 0.05 |
| D-O-M symptoms (N, %)* | |||
| Sustained attention | 10 (66) | 1 (6) | 0.08 |
| Shifting attention | 9 (60) | 2 (13) | 0.05 |
| Orientation | 7 (46) | 3 (20) | 0.52 |
| Consciousness | 8 (53) | 3 (20) | 0.25 |
| Apathy | 9 (60) | 1 (6) | 0.02 |
| Hypokinesia/Psychomotor retardation | 5 (33) | 2 (13) | 0.07 |
| Incoherence | 6 (40) | 1 (6) | 0.22 |
| Fluctuation in functioning | 6 (40) | 1 (6) | 0.13 |
| Restlesness | 7 (46) | 1 (6) | 0.07 |
| Delusions | 6 (40) | 0 (0) | 0.08 |
| Hallucinations | 4 (26) | 0 (0) | 0.09 |
| Anxiety/fear | 6 (40) | 1 (6) | 0.13 |
CDR, Clinical Dementia Rating Scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; D-O-M, delirium-O-meter; AD, Alzheimer’s Dementia; VaD, Vascular Dementia; LBD, Lewy Body dementia.
Symptoms remembered by the patients at the T0 interview.
Quantitative Results
Using the D-O-M, we found differences in the prevalence of delirium symptoms between delirium episodes (Table 3). All patients reported deficits in sustained and shifting attention, orientation, incoherence, and fluctuation in functioning. Almost half of the patients reported delusions, hallucinations, anxiety/fear, and restlessness. It is noteworthy that in patients who remembered being confused (n=15) at the T0 interview, the most frequently remembered symptoms were deficits in sustained (33%) and shifting attention (30%), orientation (23%), consciousness (27%), apathy (30%) and restlessness (23%). At the 1-month follow-up, of the 13 patients who remembered being confused, the most frequently remembered symptoms were deficits in sustained (20%) and shifting attention (20%), apathy (20%), hypokinesia/psychomotor retardation (20%), and anxiety/fear (20%).
Table 3.
Memories of each D-O-M item in patients at the first evaluation (T0) and at 1-month follow-up.*
| Variable | Recorded during delirium (N=30)* | Remembered by patient T0 (N=15)** | Remembered by patient at follow-up (N=12)** |
|---|---|---|---|
| Sustained attention, (N, %) | 30 (100) | 10 (33) | 6 (20) |
| Shifting attention, (N, %) | 30 (100) | 9 (30) | 6 (20) |
| Orientation, (N, %) | 30 (100) | 7 (23) | 5 (17) |
| Consciousness, (N, %) | 26 (87) | 8 (27) | 3 (10) |
| Apathy, (N, %) | 25 (83) | 9 (30) | 6 (20) |
| Hypokinesia/Psychomotor retardation, (N, %) | 28 (93) | 5 (17) | 6 (20) |
| Incoherence, (N, %) | 30 (100) | 6 (20) | 2 (6) |
| Fluctuation in functioning, (N, %) | 30 (100) | 6 (20) | 2 (6) |
| Restlessness, (N, %) | 16 (53) | 7 (23) | 3 (10) |
| Delusions, (N, %) | 16 (53) | 6 (20) | 2 (6) |
| Hallucinations, (N, %) | 15 (50) | 4 (13) | 3 (10) |
| Anxiety/fear, (N, %) | 17 (57) | 6 (20) | 6 (20) |
Items recorded during delirium assessment by the investigators in the 30 patients evaluated at the first evaluation after delirium resolution.
Items reported by the patients at the at the Baseline (T0) and Follow-Up (T1) interview. If patients remembered being confused then the neuropsychologists asked patients if they could recall experiencing any symptoms included on the D-O-M.
The overall mean distress at T0-interview and at 1-month follow-up was 2.3 ± 1.8 and 2.4 ± 1.3, respectively. A score of 2 on the scale of 0 to 4 indicates a “fair amount” of distress. At the T0-interview, patients reported higher levels of distress associated with memories of anxiety/fear, delusions, restlessness, hypokinesia, and deficits in orientation (Figure 1). At the 1-month follow-up, a higher level of distress was reported related to anxiety/fear and restlessness (Figure 1).
Figure 1.
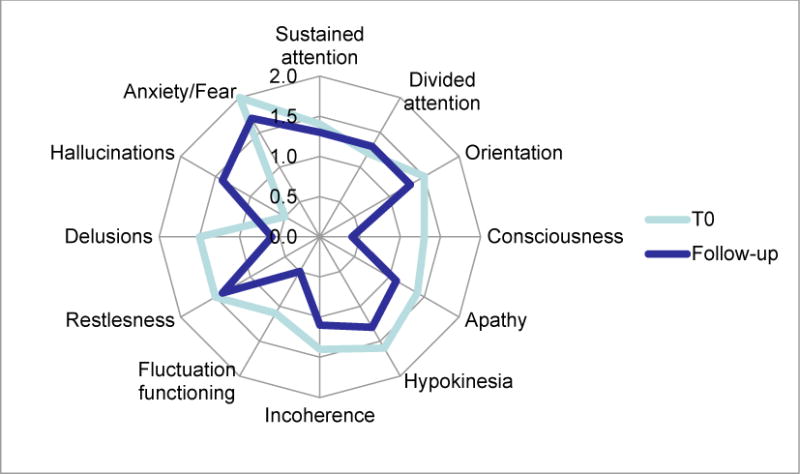
Radar plot of mean stress for each D-O-M items in patients at the first evaluation (T0-interview) and at 1-month follow-up.
Qualitative Findings
All patients responded to the open-ended questions at the T0-interview and at the 1-month follow-up. Initially, we analyzed the two time points separately, but we identified the same aspects at both times, therefore we coded six main categories of patient experiences with delirium along with their constituent subcategories (Table 4): Emotions (concern, anxiety, fear, anger, threat, shame); Cognitive Impairment (confusion, disorientation, difficulties in comprehension, altered perception of time); Psychosis (disturbing and rambling thoughts, hallucinations, delusions, nightmares, depersonalization, feeling confined); Memories (memories of parents, delightful memories); Awareness of Change (sudden change, change back to reality, loss); and Physical Symptoms (restrained, falls, constraint, drowsiness). We report on each category and its subcategories below.
Table 4.
Key Aspects of the Delirium Experience As Reported By Patients After Delirium Resolution (n= 30)
| Category | Subcodes |
|---|---|
| Emotions | Concern, anxiety, fear, anger, threat, shame |
| Cognitive impairment | Confusion, disorientation, difficulties in comprehension, altered perception of time |
| Psychosis | Disturbing and rambling thoughts, hallucinations, delusions, nightmares, depersonalization, feeling confined |
| Memories | Memories of parents, delightful memories |
| Awareness of change | Sudden change, change back to reality, loss |
| Physical symptoms | Restrained, falls, constraint, drowsiness |
Emotions
Patients reported experiencing different emotions as part of their delirium experience. Emotions included concern, anxiety: “I was extremely … I didn’t know anything about my future”; fear: “I was just so afraid of everything around me..”; “I remember I was seeing men around me but they were not there… I was afraid…”; anger: “Instead of saying one word I was saying another one and I realized it only when people around me would tell me I was confused… this situation was making me angry”; threat: “I was afraid that somebody could take advantage of this situation”; and shame: “I remember I was ashamed to come here because I choose the wrong day”. Emotions were predominantly negative, with the exception of concern, which may be a more neutral emotion.
Cognitive Impairment
Cognitive impairment included changes in cognitive function, including reports of confusion, difficulties in comprehension: “I was feeling confused… I could not understand anything…”; “my head was shaking…”; “…I remember that when I was sick my head was confused…”; disorientation, and altered perception of time: “I only remember I couldn’t speak… and then it was like a temporary distraction… it lasted for three days…”; “… I don’t even remember when they moved me here… I only remember that when I first came here I didn’t even remember where I was…”; “…I felt like the time was fixed…”.
Psychosis
Patients reported disturbing and rambling thoughts, feeling confined: “Men were getting into my bed and they were trying to hurt me…”; “I remember I was in a narrow street and I was waiting for my husband… nothing serious… people were running around…”; “…I was like in a dream…”; delusions, depersonalization, nightmares, hallucinations: “I only realized it afterwards… I only described it to my little grandchild because it was so unreal… dragons were flying… people were waving… I felt like it was real but it didn’t belong to me.”; “I don’t remember being confused but the dreams… I was always like in prison and I was trying to escape.”
Memories
Memories fell into two main subcategories: memories of parents and delightful memories. One patient described remembering her mother, who was long deceased: “I had memories of my mother… She died 40 years ago…” Another patient: “They told me I was confused… I can’t remember it.. I don’t believe it. I have such good memories of my hospital stay and of the health care staff…”
Awareness of change
Patients reported sudden change, change back to reality, loss: “…So different from my usual life.”; “I understand there is something wrong and different.”; “It happens every time I change the environment around me…”; “now it’s gone…”; “it didn’t happen again…”
Physical symptoms
Patients also recalled less pleasant experiences such as being restrained, falls, constraint, drowsiness: “I remember I fell down and I called the housekeeper…”; “… there was a time… ten days ago… my head was spinning… the usual headache… I was weak…”; “I was confused… my stomach was upset… my head was well…”; “my body was like tightened up and I couldn’t get out of it…”
DISCUSSION
This is the first study to describe, both qualitatively and quantitatively, the personal experiences of patients with delirium superimposed on dementia who were admitted to a rehabilitation setting three days after the resolution of delirium and at 1-month follow-up. Interestingly, about half of the patients interviewed remembered being confused and they had an overall “fair amount” of distress related to the delirium episode at the T0-interview and at 1-month follow-up. Higher levels of distress were reported in association with memories of anxiety/fear, delusions, restlessness, hypokinesia, and deficit in orientation. Most patients experienced a wide range of delirium symptoms (i.e., deficits in sustained and shifting attention, orientation, incoherence, and fluctuation in functioning, delusions, hallucinations, anxiety/fear, and restlessness). We identified six main categories of patient experience: Emotions, Cognitive Impairment, Psychosis, Memories, Awareness of Change, and Physical Symptoms.
A key characteristic of this study is the inclusion of a relatively large population of older patients with dementia, including an assessment of dementia type and severity. Few studies have previously investigated the experience of delirium in geriatric patients admitted to Intensive Care Units, orthopedic, cardiac surgery, palliative care and geriatric wards,7, 26–32 One of these studies specifically excluded patients with dementia.33 The largest studies included 50 older patients with delirium in orthopedic care,34 and 101 patients who suffered from delirium while hospitalized with cancer.10 None of the prior studies specifically reported whether or not patients with dementia were included, and there were no measures of dementia severity, nor type of dementia. Only one study reported a history of dementia in 18 patients who were included in a larger study of 101 patients with cancer.10
About half of the patients in our study remembered being confused at the T0-interview and at 1-month follow-up. This is interesting since our study included only patients with dementia and one might expect that in this population memories of a delirium episode might indeed be absent, especially after 1-month after delirium resolution. The ability to remember a delirium episode has been quite variable. Previous studies ranged from describing the majority of patients having no recall of delirium28 to reporting a recall of delirium in more than half of the patients.10, 11, 29, 30, 32, 34 The ability to recall a delirium episode has been associated with delirium severity, perceptual disturbances and dementia in patients hospitalized with cancer.10 In particular, patients with dementiahaving more severe delirium and hallucinations were less likely to remember the delirium episodes.10 Recall of delirium was not significantly different according to delirium subtype (hypoactive, hyperactive, and mixed-type).10, 11 Interestingly, in a previous epidemiological study,35 35% of the patients with delirium were aware of their cognitive deficits, and the acuity of onset and the presence of disorientation were correlated with the ability of patients to recognize the presence of delirium. In our study we did not find a clinical difference in the ability to recall delirium according to type and severity of dementia, subtypes, duration and severity of delirium. However, this might have been due to the sample size of our population and should be further investigated.
The distress related to delirium experience in our study was indicative of a “fair amount” of distress (i.e. level of 2 on a scale from 0 to 4), and is similar to previous studies in cancer patients which reported moderate to severe distress related to the recall of delirium.10–12 Additionally, our patients reported a higher level of distress associated with memories of anxiety/fear, delusions, restlessness, hypokinesia, and deficit in orientation. These findings support and extend those of previous studies of cancer patients for whom the presence of delusions and psychomotor agitation were important predictors of severity of distress.10, 11
From the qualitative evaluation, we identified six main categories of patient experiences: Emotions, Cognitive impairment, Psychosis, Memories, Awareness of change, and Physical symptoms. Two recent reviews26, 27 of the qualitative literature on delirium experience summarized three main themes regarding the experience of delirium: incomprehension and feelings of discomfort (e.g. incomprehension and feelings of discomfort, fluctuation between reality and fantasy, lucidity and confusion, a world between past and present; lack of control; hallucinations; delusions; fear; anger); the need to keep one’s distance and to protect oneself (i.e., feeling threatened; attacked; acting aggressively), and interventions that diminish suffering (i.e., feeling understood; feeling protected by the presence of family members). The studies included in these reviews26, 27 enrolled a total of 184 patients in orthopaedic and surgical units, burn units, intensive care units, geriatric wards, and hospice. The smallest study included five older patients who recovered from delirium and the main themes reported by the patients were “being temporarily confused, experience threat, being suspicious, need to escape from experience, fear of occurrence, feeling shame, guilt, humiliation, and looking for an explanation.”36 The largest qualitative study evaluated fifty older patients (67–96 years of age) who developed delirium during an hospitalization in an orthopaedic unit.37 The authors did not mention if they have included patients with dementia. The patients’ recall of the delirium experience was a mix of patient life history, the present situation, and an overall difficulty in communicating their emotional state and experience during and after the delirium episode.
The categories of delirium experience reported by our patients were similar to the previous studies, especially concerning emotions (i.e., anger, fear, threat, shame), cognitive impairment (i.e., difficulties in comprehension, disorientation, altered perception of time,), psychosis (i.e., delusions, hallucinations, disturbing and rambling thoughts, nightmares,), memories (i.e., memories of parents and delightful memories), awareness of change (i.e., change back to reality, sudden change,), and physical symptoms (i.e., restrained, constraint, drowsiness).
Finally, in this study we did not find significant differences in the recollection of delirium or of specific items according to the type and severity of cognitive impairment. This might likely be due to the sample size of the population included in our study. One in fact might expect that patients with milder cognitive impairment would be more likely to remember a delirium episode, and in particular, they would be more likely to report specific delirium symptoms, such as hallucinations, and agitation. Future research should explore the ability of dementia patients to remember a delirium episode and the related experience according to the type and severity of dementia.
Clinical Implications
The evaluation and discussion of the delirium experience has been advocated in the NICE guidelines for delirium.8 MacLullich and colleagues38 recently underlined how a “delirium-friendly hospital” requires specific staff training to help patients dealing with, and understanding of an episode of delirium. High quality delirium care is complex and time consuming and special expertise is required to optimize patient care. Health care staff–including physicians, nurses, nurses-aids, physical therapists–should be aware of the main features of delirium during and after an episode of delirium. Questions should be proactively asked of the patients to understand if they can remember the delirium episodes, and whether or not they have frightening memories. Patients who experience delirium with perceptual disturbances are often reluctant to mention this to staff.26 Patients reported the importance of knowing that unreal experiences were common,39 and stated that knowledge about events and plans for their ongoing care helped them feel safe and reassured.31, 33 Health care staff can help patients understand their experience, and provide support to minimize stress experienced during both the acute and recovery phases.7 Future research is required to design specific protocols to guide the staff on the timing and type of intervention and for possible psychological support.
Limitations
Our study presents important strengths along with limitations. It is the first study to evaluate the quantitative and qualitative experience of delirium in patients with dementia with a rigorous approach after three days of delirium resolution and at 1-month follow-up. We were able to collect information on the stage and type of dementia, with daily evaluations of delirium. Limitations include the single center nature of the study, the relatively small number of patients in each individual dementia severity group, thus, limiting the ability to specifically evaluate delirium experience in different stages and type of dementia. Additionally, the psychologists might have influenced patients in the recollection of the delirium symptoms during the interview. The psychologists listed each specific symptoms, and the patients were asked if they could remember that symptom and its related stress. Finally, we did not gather information on the pharmacological delirium treatment (e.g, antipsychotics use). Future larger studies should evaluate the possible effects of specific drugs on the delirium experience.
CONCLUSIONS
This study provides novel information to improve the care of patients with delirium and dementia. Specifically, about half of the patients interviewed remembered being confused, and they report an overall “fair amount” of distress related to the delirium episode. Higher levels of distress were reported regarding specific memories of anxiety/fear, delusions, restlessness, hypokinesia, and deficit in orientation. The qualitative interviews identified several important categories of patient experiences. This information can help health care providers in understanding the burden of delirium in patients with dementia and assist in efforts to minimize the negative impact of symptoms, both during delirium episodes and in the post-delirious recovery phase.
Acknowledgments
Fundings: Dr. Inouye, Dr. Schmitt and Dr. Schulman-Green are supported by R01AG044518 (SKI) and K07AG041835 (SKI) from the National Institute on Aging.
Footnotes
Conflict of interest: None.
Contributor Information
Elena Lucchi, Email: elena.lucchi@ancelle.it.
Renato Turco, Email: renatoturco@virgilio.it.
Sara Morghen, Email: morghen-sara@ancelle.it, sara.morghen@ancelle.it.
Fabio Guerini, Email: fabio.guerini@ancelle.it.
Simona Gentile, Email: simona.gentile@ancelle.it.
David Meagher, Email: david.meagher@ul.ie.
Philippe Voyer, Email: philippe.voyer@fsi.ulaval.ca.
Donna Fick, Email: dmf21@psu.edu.
Eva M. Schmitt, Email: EvaSchmitt@hsl.harvard.edu.
Sharon K. Inouye, Email: sharoninouye@hsl.harvard.edu.
Marco Trabucchi, Email: trabucchi.m@grg-bs.it.
Giuseppe Bellelli, Email: giuseppe.bellelli@unimib.it.
Bibliography
- 1.Association AP. Diagnostic and statistical manual of mental disorders. fifth. Arlington, VA: American Psychiatric Association; [Google Scholar]
- 2.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: A systematic review. Journal of the American Geriatrics Society. 2002;50:1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 3.Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: Prospective cohort study of prevalence and mortality. The British journal of psychiatry: the journal of mental science. 2009;195:61–66. doi: 10.1192/bjp.bp.108.055335. [DOI] [PubMed] [Google Scholar]
- 4.Fick DM, Steis MR, Waller JL, Inouye SK. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. Journal of hospital medicine: an official publication of the Society of Hospital Medicine. 2013;8:500–505. doi: 10.1002/jhm.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellelli G, Frisoni GB, Turco R, Lucchi E, Magnifico F, Trabucchi M. Delirium superimposed on dementia predicts 12-month survival in elderly patients discharged from a postacute rehabilitation facility. The journals of gerontology. Series A, Biological sciences and medical sciences. 2007;62:1306–1309. doi: 10.1093/gerona/62.11.1306. [DOI] [PubMed] [Google Scholar]
- 6.Morandi A, Davis D, Fick DM, Turco R, Boustani M, Lucchi E, Guerini F, Morghen S, Torpilliesi T, Gentile S, Maclullich AM, Trabucchi M, Bellelli G. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. Journal of the American Medical Directors Association. 2014;15:349–354. doi: 10.1016/j.jamda.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: What is the effect on patients, relatives and staff and what can be done to modify this? International journal of geriatric psychiatry. 2013;28:804–812. doi: 10.1002/gps.3900. [DOI] [PubMed] [Google Scholar]
- 8.Excellence NIfHaC. Delirium: Diagnosis, prevention and managment. National Clinical Guideline Centre-Acute and Chronic Conditions. 2010 [Google Scholar]
- 9.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Critical care medicine. 2001;29:573–580. doi: 10.1097/00003246-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Breitbart W, Gibson C, Tremblay A. The delirium experience: Delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics. 2002;43:183–194. doi: 10.1176/appi.psy.43.3.183. [DOI] [PubMed] [Google Scholar]
- 11.Bruera E, Bush SH, Willey J, Paraskevopoulos T, Li Z, Palmer JL, Cohen MZ, Sivesind D, Elsayem A. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115:2004–2012. doi: 10.1002/cncr.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grover S, Shah R. Distress due to delirium experience. General hospital psychiatry. 2011;33:637–639. doi: 10.1016/j.genhosppsych.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Drews T, Franck M, Radtke FM, Weiss B, Krampe H, Brockhaus WR, Winterer G, Spies CD. Postoperative delirium is an independent risk factor for posttraumatic stress disorder in the elderly patient: A prospective observational study. European journal of anaesthesiology. 2015:32. doi: 10.1097/EJA.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 14.Bellelli G, Magnifico F, Trabucchi M. Outcomes at 12 months in a population of elderly patients discharged from a rehabilitation unit. Journal of the American Medical Directors Association. 2008;9:55–64. doi: 10.1016/j.jamda.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney Fi BD. Functional evaluation: The barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 16.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 17.Berg L. Clinical dementia rating (cdr) Psychopharmacology bulletin. 1988;24:637–639. [PubMed] [Google Scholar]
- 18.Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the informant questionnaire on cognitive decline in the elderly (iqcode) as a screening test for dementia. Psychological medicine. 1991;21:785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- 19.Bellelli G, Morandi A, Davis DH, Mazzola P, Turco R, Gentile S, Ryan T, Cash H, Guerini F, Torpilliesi T, Del Santo F, Trabucchi M, Annoni G, Maclullich AM. Validation of the 4at, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age and ageing. 2014;43:496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ely EW. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (cam-icu) JAMA: The Journal of the American Medical Association. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 21.Chester JG, Beth Harrington M, Rudolph JL. Serial administration of a modified richmond agitation and sedation scale for delirium screening. Journal of hospital medicine: an official publication of the Society of Hospital Medicine. 2012;7:450–453. doi: 10.1002/jhm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellelli G, Speciale S, Morghen S, Torpilliesi T, Turco R, Trabucchi M. Are fluctuations in motor performance a diagnostic sign of delirium? Journal of the American Medical Directors Association. 2011;12:578–583. doi: 10.1016/j.jamda.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 23.de Jonghe JF, Kalisvaart KJ, Timmers JF, Kat MG, Jackson JC. Delirium-o-meter: A nurses’ rating scale for monitoring delirium severity in geriatric patients. International journal of geriatric psychiatry. 2005;20:1158–1166. doi: 10.1002/gps.1410. [DOI] [PubMed] [Google Scholar]
- 24.Creswll JW. Qualitative inquiry & research design: Choosing among five approaches. 2nd. Sage; Thousand Oaks, CA: 2007. [Google Scholar]
- 25.Graneheim UH, Lundman B. Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse education today. 2004;24:105–112. doi: 10.1016/j.nedt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 26.O’Malley G, Leonard M, Meagher D, O’Keeffe ST. The delirium experience: A review. Journal of psychosomatic research. 2008;65:223–228. doi: 10.1016/j.jpsychores.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Belanger L, Ducharme F. Patients’ and nurses’ experiences of delirium: A review of qualitative studies. Nursing in critical care. 2011;16:303–315. doi: 10.1111/j.1478-5153.2011.00454.x. [DOI] [PubMed] [Google Scholar]
- 28.Duppils GS, Wikblad K. Patients’ experiences of being delirious. Journal of clinical nursing. 2007;16:810–818. doi: 10.1111/j.1365-2702.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- 29.Grover S, Ghosh A, Ghormode D. Experience in delirium: Is it distressing? The Journal of neuropsychiatry and clinical neurosciences. 2014 doi: 10.1176/appi.neuropsych.13110329. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. Journal of palliative care. 2009;25:164–171. [PubMed] [Google Scholar]
- 31.McCurren C, Cronin SN. Delirium: Elders tell their stories and guide nursing practice. Medsurg nursing: official journal of the Academy of Medical-Surgical Nurses. 2003;12:318–323. [PubMed] [Google Scholar]
- 32.Schofield I. A small exploratory study of the reaction of older people to an episode of delirium. Journal of advanced nursing. 1997;25:942–952. doi: 10.1046/j.1365-2648.1997.1997025942.x. [DOI] [PubMed] [Google Scholar]
- 33.Stenwall E, Jonhagen ME, Sandberg J, Fagerberg I. The older patient’s experience of encountering professional carers and close relatives during an acute confusional state: An interview study. International journal of nursing studies. 2008;45:1577–1585. doi: 10.1016/j.ijnurstu.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Andersson EM, Hallberg IR, Edberg AK. Nurses’ experiences of the encounter with elderly patients in acute confusional state in orthopaedic care. International journal of nursing studies. 2003;40:437–448. doi: 10.1016/s0020-7489(02)00109-8. [DOI] [PubMed] [Google Scholar]
- 35.Ryan DJ, O’Regan NA, Caoimh RO, Clare J, O’Connor M, Leonard M, McFarland J, Tighe S, O’Sullivan K, Trzepacz PT, Meagher D, Timmons S. Delirium in an adult acute hospital population: Predictors, prevalence and detection. BMJ open. 2013:3. doi: 10.1136/bmjopen-2012-001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagerberg I, Jonhagen ME. Temporary confusion: A fearful experience. Journal of psychiatric and mental health nursing. 2002;9:339–346. doi: 10.1046/j.1365-2850.2002.00498.x. [DOI] [PubMed] [Google Scholar]
- 37.Andersson EM, Hallberg IR, Norberg A, Edberg AK. The meaning of acute confusional state from the perspective of elderly patients. International journal of geriatric psychiatry. 2002;17:652–663. doi: 10.1002/gps.682. [DOI] [PubMed] [Google Scholar]
- 38.Maclullich AM, Anand A, Davis DH, Jackson T, Barugh AJ, Hall RJ, Ferguson KJ, Meagher DJ, Cunningham C. New horizons in the pathogenesis, assessment and management of delirium. Age and ageing. 2013;42:667–674. doi: 10.1093/ageing/aft148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granberg A, Bergbom Engberg I, Lundberg D. Patients’ experience of being critically ill or severely injured and cared for in an intensive care unit in relation to the icu syndrome. Part i. Intensive & critical care nursing: the official journal of the British Association of Critical Care Nurses. 1998;14:294–307. doi: 10.1016/s0964-3397(98)80691-5. [DOI] [PubMed] [Google Scholar]


