Abstract
Introduction
Inflammatory breast cancer is a special type of locally advanced mammary cancer that is associated with particularly aggressive behaviour and poor prognosis. The dog was considered the only natural model in which to study the disease because, until now, it was the only species known to present with inflammatory mammary carcinoma (IMC) spontaneously. In the present study we describe clinicopathological and immunohistochemical findings of three cats with IMC, in order to evaluate its possible value as an animal model.
Methods
We prospectively studied three female cats with clinical symptoms of IMC, identified over a period of 3 years. Clinicopathological and immunohistochemical evaluations of Ki-67, and oestrogen, progesterone and androgen receptors were performed.
Results
All three animals presented with secondary IMC (postsurgical) characterized by a rapid onset of erythema, severe oedema, extreme local pain and firmness, absence of subjacent mammary nodules, and involvement of extremities. Rejection of the surgical suture was observed in two of the cats. Histologically, highly malignant papillary mammary carcinomas, dermal tumour embolization of superficial lymphatic vessels, and severe secondary inflammation were observed. The animals were put to sleep at 10, 15 and 45 days after diagnosis. Metastases were detected in regional lymph nodes and lungs in the two animals that were necropsied. All tumours had a high Ki-67 proliferation index and were positive for oestrogen, progesterone and androgen receptors.
Conclusion
Our findings in feline IMC (very low prevalence, only secondary IMC, frequent association of inflammatory reaction with surgical suture rejection, steroid receptor positivity) indicate that feline IMC could be useful as an animal model of human inflammatory breast cancer, although the data should be considered with caution.
Keywords: Animal natural model, feline inflammatory mammary carcinoma
Introduction
Inflammatory mammary carcinoma (IMC) is a special type of locally advanced mammary cancer that is associated with particularly aggressive behaviour and poor prognosis in women (in which case it is termed 'inflammatory breast carcinoma' [IBC]) [1-3] and in the dog (in which case it is termed IMC) [4,5]. Until now these were the only species in which spontaneous inflammatory carcinoma of the mammary gland had been reported [6].
Clinically, IBC and IMC typically present with a rapid onset of symptoms resembling an inflammatory process (erythema, firmness and warmth in the skin of the mammary gland), often without an underlying mass. In fact, this condition can be misdiagnosed as dermatitis or mastitis [1,4,5]. IBC and IMC are clinical diagnoses that can be confirmed pathologically by the observation of neoplastic emboli in dermal lymphatic vessels. This is the only histological criteria for their pathological diagnosis, because several histological types of carcinomas have been described to cause the disease [1,6,7]. In most cases the clinical and pathological diagnoses coincide, but some patients may present with clinical or pathological evidence alone [3,8]. The presence of dermal lymphatic involvement (emboli) in the absence of clinical characteristics of inflammatory carcinoma is called occult inflammatory carcinoma, and this frequently precedes clinical presentation of IBC [9,10] and IMC [5]. Two clinical types of inflammatory carcinoma have been described in women [2,8,10] and in the dog [5,11]: primary (without previous mammary tumour) and secondary (after surgical excision of a malignant mammary tumour) IBC and IMC.
Fortunately, this very aggressive type of cancer is uncommon in humans [12] and in dogs [5]. However, the prevalence of the disease has doubled over the past 15 years in both species [5,12].
In comparison with other types of breast cancer, several differences in the biology of IBC and IMC have been reported, including gene mutations, angiogenesis, angioinvasiveness and endocrine mechanisms [3,6,12-15]. Treatment and research in human IBC remains problematic [3]. In vitro models include cell lines derived from primary IBC tumours; a xenograft murine model has also been developed [3,16]. The dog was proposed as a natural model in which to study the disease, because it was the only species thought to present with IMC spontaneously [6].
Mammary tumours are commonly observed in the female cat. The percentage of malignant mammary tumours is higher in the cat (86%) than in the dog (42%) [17], and the histology of feline mammary tumours is closer to that in human breast cancer [18]. For these reasons, feline mammary cancer might be a more useful model in which to study the disease than the canine model. Nevertheless, IMC has not been described in the cat.
In the present study we describe clinicopathological and immunohistochemical findings in three cats with IMC, and we compare the condition with characteristics previously described in canine IMC.
Methods
Animals and clinical data
This is a prospective study including three female cats that were presented at the Veterinary Teaching Hospital of Madrid with clinical symptoms of IMC over a period of 3 years. The animals ranged in age from 12 to 15 years; one was Siamese (case 1) and two were of short hair common European breed (cases 2 and 3). Cases 1 and 3 were referred from private clinics. In the three cats a complete history was obtained indicating recent mastectomies because of the presence of malignant mammary tumours. In the physical examination mammary glands and regional lymph nodes (axillary and inguinal) were evaluated. The three cats had clinical signs of IMC, as described in the dog [4].
Routine vaccination and antiparasitic drugs had been correctly administered. Serological tests for feline viral diseases (i.e. feline leukaemia virus and feline immunodeficiency virus) had recently been conducted and were negative in the three animals.
Radiological evaluation of the thorax (three projections) was performed in order to evaluate the presence of distant metastases. Blood samples were taken to evaluate haematology and biochemistry profiles and clotting times in case 1. Cytological examination with fine-needle aspirates of the mammary area was performed to differentiate neoplastic from inflammatory processes in the three cases.
No other concurrent diseases were found at the time of presentation.
Pathology
Incisional biopsies and histopathological examinations of skin areas with symptoms compatible with IMC were performed in cases 1 and 3. In case 2, previous biopsy material obtained after mastectomy of the primary tumour (before IMC development) was available. Necropsies and corresponding histopathological study were conducted in cases 2 and 3. All the samples were fixed in 10% neutral formalin, embedded in paraffin, cut into sections 4 μm thick, and stained with haematoxylin–eosin. The mammary tumours were diagnosed histopathologically according to the World Health Organization classification for feline mammary gland tumours [18]. In each tumour the histological malignant grade (HMG) was established by scoring tubule formation, nuclear pleomorphism and mitotic rate from 1 to 3 points, according to a human grading system [19].
Immunohistochemistry
Ki-67 proliferation marker was determined, and immunostaining for oestrogen receptor (ER)-α, progesterone receptor (PR) and androgen receptor (AR) were done on deparaffined 4-μm sections, using the streptavidin–biotin complex peroxidase method following a high-temperature antigen unmasking protocol (boiling slides in pressure cooker 2 min, in buffer citrate pH 6). The slides were cooled down in distilled water and washed in Tris-buffered saline (TBS; 0.1 mol/l Tris base, 0.9% NaCl; pH 7.4). Endogenous peroxidase activity was blocked in 1.5 ml H2O2/100 ml methanol for 15 min. Ki-67 immunostaining sections were incubated for 1 hour with primary mouse monoclonal antibody anti-MIB1 nuclear proliferation marker (dilution 1:30; Master Diagnostica, Granada, Spain).
ER-α and PR immunostaining were performed by overnight incubation at 4°C with mouse monoclonal antihuman ER-α (clone CC4-5, NCL-ER-LH2, dilution 1:40; Novocastra Laboratories Ltd, Newcastle upon Tyne, UK) and mouse monoclonal antihuman PR (clone 1A6, NCL-PR-123, dilution 1:40; Novocastra). AR sections were incubated overnight at 4°C with a rabbit polyclonal antibody (RB-1358, dilution 1:15; Neomarkers, Fremont, CA, USA). After incubation with the mouse monoclonal primary antibodies (ER and PR), the slides were incubated with antimouse biotinylated secondary antibody (E04233, dilution 1:200; Dako, Glostrup, Denmark) for 30 min at room temperature. AR slides were subsequently incubated with antirabbit biotinylated secondary antibody (BA1000, 1:400; Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. Afterward, all of the slides were incubated with streptavidin conjugated with peroxidase (P50242, 1:400; Zymed, South San Francisco, CA, USA) for 30 min at room temperature. All washes and dilutions were done in TBS.
The slides were developed for 10 min using a chromogen solution containing 3-3' diaminobenzidine tetrachloride (D5059; Sigma Chemical Co., St Louis, Missouri, USA) and H2O2 in TBS. After washing in distilled water for 10 min, slides were counterstained in haematoxylin (GH5-2-16; Sigma Chemical Co.), washed in tap water, dehydrated, cleared in xylene, and mounted. Negative control slides were made by substituting the primary antibody with TBS.
Normal feline uterus was used as a positive control for ER and PR detection. Adjacent normal mammary gland or hyperplasia was used as an internal positive control in many slides. Normal feline sebaceous glands in the skin were internal positive controls for AR immunostaining. Ki-67 stained mitotic figures were considered positive internal controls for Ki-67 immunostaining. Tumours were considered positive for ER, PR, or positive when more than 10% positive cells were observed in 10 selected fields. In each case, the Ki-67 index was calculated as the mean of the proportion of positive nuclei in 8–10 representative fields. Counting was done with a computer-assisted image analyzer (Olympus Microimage™ Image Analysis, software version 4.0 for Windows, Media Cybernetics, Silver Spring, MD, USA).
Results
History and clinical findings
The three cats were not ovariectomized at diagnosis, and case 1 had been (10 years) receiving progestins routinely to prevent oestrus (medroxyprogesterone acetate 25 mg injected every 6 months). Mastectomies due to malignant mammary tumours had been performed 4 months (case 1) and 1 month (cases 2 and 3) before clinical signs of IMC suddenly appeared (secondary IMC). When clinical signs of IMC were detected, the cats had a slight decrease in appetite and weight loss, but no other complaints were noted apart from those described under clinical signs of IMC (see below). At physical examination the cats had a normal body condition, despite the weight loss observed by the owners, and respiratory movements, hydration, rectal temperature, mucous membranes, pulse and thoracic auscultation were normal. Popliteal lymph nodes of the extremities affected by the mammary tumor were enlarged in all three cases. At diagnosis of the IMC, radiological evaluation of the thorax revealed only a slight interstitial pattern in case 1, but no evidence of thoracic metastasis was found in any cat. Secondary inflammatory carcinoma was diagnosed in the three cats, based on clinical history and signs and histopathological features (see below).
Clinical signs of inflammatory mammary carcinoma
The mammary region (previous mastectomy wound and adjacent areas) presented a rapid onset of erythema, severe oedema, extreme local pain, and small areas of skin ulceration and firmness, without subjacent mammary nodules. Severe oedema and rejection of the surgical suture, which were observed in cases 2 and 3 (surgically treated 1 month before), were the most evident clinical signs in these two cases (Fig. 1). Cutaneous signs were also present in the surgical wound in case 1, despite the fact that the surgical procedure had taken place 4 months before and the healing process was completed.
Figure 1.
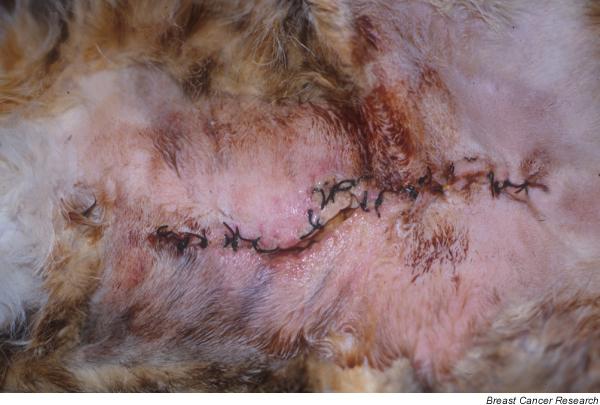
Case 3: secondary inflammatory mammary carcinoma. Erythema and suture rejection after radical mastectomy (surgical excision of primary mammary tumour).
Involvement of the extremities was present in all three cases, consisting of severe oedema and erythema of the medial face of rear extremities (cases 1 and 2) and fore limbs (case 3).
Laboratory results
In cases 2 and 3 examination of fine-needle aspiration cytology samples revealed a severe inflammatory process (neutrophilic and lymphocytic), in which some large isolated or grouped epithelial cells exhibited cytological features of malignancy. In case 1 cytology was nondiagnostic.
In case 1, routine laboratory blood analysis was performed at diagnoses of the IMC. In the other two cats previous analyses obtained 1 month earlier were available and within normal ranges, with the exception of a slight hyperglycaemia (mean 7.8 ± 1.0 mmol/l).
In case 1 haematology revealed an haematocrit of 35%, an haemoglobin of 12 g/dl and a total red cell count of 8.6 × 106/μl (mean corpuscular volume 40.6 fl, mean corpuscular haemoglobin 14.0 pg, mean corpuscular haemoglobin concentration 34.2 g/dl). Total leucocyte count was 22.2 × 103/μl, with elevated neutrophil count (90%) and lymphopaenia (6%). The biochemistry profile revealed the following: glucose 10.5 mmol/l, urea 22.13 mmol/l, creatinine 132.6 μmol/l, total protein 78 g/l, alanine aminotransferase 10 IU/l, potassium 4.6 mmol/l, and corrected calcium 2.5 mmol/l. Clotting times were as follows: prothrombin time 14 s (reference range 9–14 s) and activated partial thromboplastin time 27.5 s (reference range 14–20 s). Fibrinogen concentration was 188 mg/dl (reference range 110–400 mg/dl).
Histopathology
In case 1 incisional biopsy of the skin covering mammary area with IMC signs was obtained. Histology revealed the presence of an infiltrating mammary tumour classified as papillary carcinoma of high HMG (grade III). The pathological features confirmed the presence of an IMC. In case 2 previous biopsy of the mammary tumour (before IMC occurred) was available and showed the presence of a HMG III papillary carcinoma. These pathological characteristics in the absence of clinical signs of IMC, suggested a diagnosis of occult IMC. Three weeks later, signs of clinical IMC appeared. In case 3, incisional biopsy of the skin in the mammary region demonstrated the presence of a highly malignant tubular carcinoma (HMG III) with scattered lipid-rich cells. The pathological diagnosis confirmed the presence of an IMC.
Dermal invasion, dermal tumour embolization of superficial lymphatics (Fig. 2), dermal oedema, and severe secondary inflammation with elevated amounts of neutrophils (case 1), or lymphocytes, plasma cells and macrophages (cases 2 and 3) were also observed.
Figure 2.
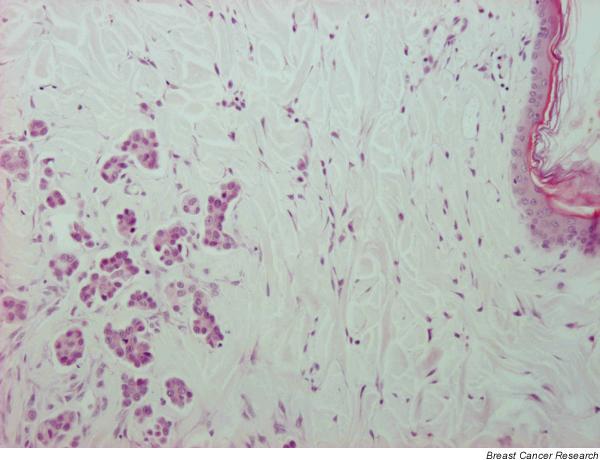
Case 3: incisional biopsy. Tumour embolization of dermal lymph vessels. Haematoxylin–eosin; original magnification 10×.
Treatment and follow-up
According to the therapeutic protocols for IBC and IMC, surgery of the affected area is not a first choice approach. The owners did not consent to administration of chemotherapeutic agents, and only palliative treatment with broad-spectrum antibiotics and anti-inflammatories (piroxicam) was given. The clinical condition of the animals worsened a few days after IMC diagnosis (weakness, anorexia, pain) and they were put down at 10 days (case 2), 15 days (case 3) and 45 days (case 1) after IMC diagnosis, with the owners' consent.
Necropsy findings
The owners gave consent for necropsy procedures in cases 2 and 3.
Case 2
Gross examination of the carcass revealed severe lesions in the skin of the mammary region, consisting of a nonhealing wound with suture rejection and bilateral elevated white plaques in the inguinal area. Severe oedema in both rear limbs (Fig. 3) was observed. Medial muscles of the right rear extremity had multiple small white nodules (less than 0.5 cm). Histopathological examination of the skin and mammary gland revealed HMG III papillary mammary carcinoma, as described previously in the biopsy, with metastases in inguinal lymph nodes, muscles and lungs. Severe signs of inflammatory reaction with an intense vascular congestion of subepidermal neovessels, haemorrhages, extensive neutrophilic infiltration in dermis and epidermal micropustules were present in the necropsy samples. Other histopathological findings were hepatic fatty change, cystic ovaries and mild cystic endometrial hyperplasia.
Figure 3.
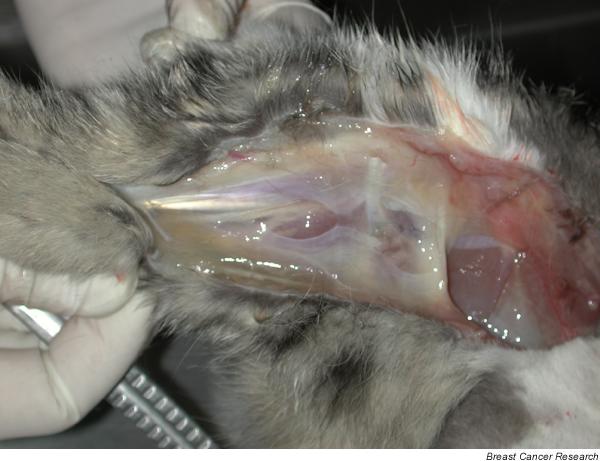
Case 2: severe oedema of rear limbs observed at necropsy.
Case 3
This animal presented with moderate obesity and an open wound because of surgical suture rejection, with very thickened and elevated margins. Moderate subcutaneous and muscular emphysema in the wound area were observed. Small (<0.5 cm diameter), elevated white nodules were noticed in both sides of the wound. Histologically, apart from the carcinoma, the skin of the mammary area exhibited a severe neutrophilic dermatitis with fibrosis, abundant pustules and marked hyperkeratosis. Adipose tissue surrounding the mammary area also had severe fibrosis and neutrophilic panniculitis. Bilateral metastases in inguinal lymph nodes, massive pulmonary tumour embolization, hepatic fatty change, mild multifocal neutrophilic hepatitis, cystic ovaries, and moderate cystic endometrial hyperplasia were also detected. No other significant findings were observed in the remaining organs.
Immunohistochemistry
Ki-67 tumor proliferation index was similarly and markedly elevated in the three animals: in case 1 it was 37.50%, in case 2 it was 37.9%, and in case 3 it was 32.5%. ER immunostaining was positive in case 1 (90% ER+ nuclei) and in case 3 (100% ER+ nuclei). Case 2 was ER-negative in the biopsy (occult IMC; 0% ER+) but ER-positive in the subsequent mammary sample with clinical symptoms of IMC taken at necropsy (75% ER+; Fig. 4). In all three animals the mammary tumours were PR-positive (95–100% PR+ nuclei; Fig. 5). AR immunostaining was very intense and positive in the three tumours (AR+ 100% in case 1, AR+ 80% in case 2, and AR+ 100% in case 3; Fig. 6).
Figure 4.
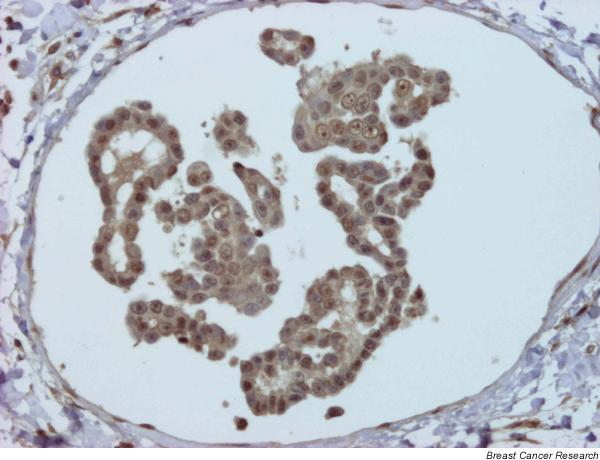
Case 2: positive embolus to oestrogen receptor. Streptavidin–biotin–peroxidase; original magnification 20×.
Figure 5.
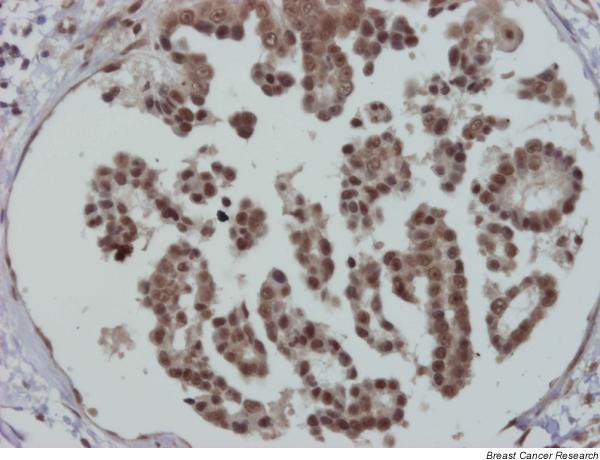
Case 2: positive embolus to progesterone receptor (same embolus as in Fig. 4). Streptavidin–biotin–peroxidase; original magnification 20×.
Figure 6.
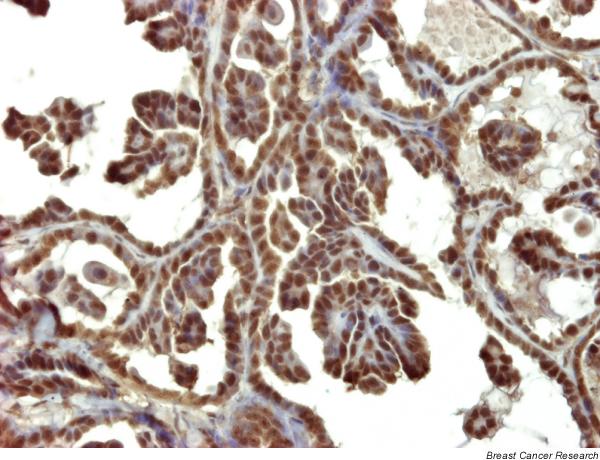
Case 2: strong positive reaction to androgen receptor. Streptavidin–biotin–peroxidase; original magnification 20×.
Discussion
To our knowledge this is the first complete description of feline IMC. The term 'inflammatory mammary carcinoma' is widely used to describe locally advanced mammary carcinoma, which simulates an inflammatory process and is associated with poor prognosis. This condition has been identified in humans (in which case it is termed IBC) [1-3,7-13,15,16] and in the dog (in which case it is termed IMC) [4-6,14]. Similarities among clinical features and prevalence have been found both in women and in the dog [6]. Thus far, spontaneous IMC has only been described in these two species. Although feline mammary malignant tumours are frequent in nonspayed female cats, IMC has not been described in this species. The lack of information on feline IMC may be due to a very low prevalence of the disease in this species (even lower than in the dog) and to difficulties associated with its recognition and accurate diagnosis. In one study on feline mammary tumours, the authors used the term 'inflammatory mammary carcinomas' to include all tumours with any histological lymphatic invasion (not only dermal invasion), which is not the diagnostic criterion for this type of tumour, and data concerning the clinical features were not provided [20].
Two clinical types of presentation of IMC and IBC have been described in humans and in dogs: primary (with no previous mammary tumour) and secondary (postsurgical and non-postsurgical) [2,5,8,10]. Slightly better clinical evolution (survival in days, local pain and pulmonary metastases) and better histological features (lower mitotic count, atypical mitosis and pleomorphism; papillary–tubular versus solid dermal tumour infiltration) have been reported in secondary canine IMC in comparison with primary IMC [5,7]. In humans, neglected locally advanced breast cancer can develop secondary inflammatory characteristics, which may also follow a better clinical course [2]. In the three feline cases presented here, inflammatory carcinoma occurred after surgical excision of a malignant mammary tumour, and therefore they can be classified as secondary postsurgical IMC. In one case, dermal lymphatic emboli were present in the previous biopsy at time of mastectomy (before detection of clinical IMC), indicating the possible presence of an occult inflammatory carcinoma. In our opinion a histopathological diagnosis of occult inflammatory carcinoma, as in case 2 in the present study, may be useful to the clinician because it provides information on the possible evolution of the disease.
The clinical condition of the animals in the present study was better than that described in canine IMC. This might be due to a slightly more favourable course of feline IMC than occurs in canine IMC, but it might also be due to the better clinical evolution that is typical of the secondary type of IMC as compared with primary IMC. As the disease progressed the clinical status of the cats worsened as well. The clinical signs of IMC are similar to those described in the dog, but erythema, ulceration and inflammatory reaction, with rejection of the surgical suture, appear to be the most evident signs in the cat in comparison with those observed in the dog. Rejection of the surgical suture is not a common finding among dogs with secondary IMC.
Haematology and biochemistry results at the time of IMC occurrence were normal in one cat, with the exception of slight leucocytosis and prolonged coagulation times. In the other two cats, laboratory results obtained one month before IMC occurrence were normal. Unfortunately, these data were not available at the time of IMC presentation. Prolonged clotting times have been observed in dogs with advanced mammary carcinoma and with distant metastases, suggesting a disseminated intravascular coagulation [21]. Probably in the cat with advanced IMC, disseminated intravascular coagulation takes place, but more clinical studies are necessary to confirm this.
There are few reports concerning histological aspects of human IBC. It is well known that IBC is not a specific histological subtype; infiltrating ductal carcinomas, other carcinomas and unspecified malignant tumours have been found to be involved with IBC [1,3,22,23]. In canine IMC, histology revealed several types of carcinomas (solid, tubular, papillary and adenosquamous) and lipid-rich carcinomas [6]. The diagnosis of lipid-rich carcinoma was established in canine IMC, according to human pathology criteria [1], when more than 80% of the tumour cells were lipid producing. The three feline IMC cases presented here were histologically papillary/tubular mammary carcinomas of high histological malignancy. In case 3, a low proportion of lipid-rich cells was found (<80%). Lipid-rich carcinoma is considered a rare type of mammary carcinoma in humans [1] and dogs [18,24]. Lipid droplets in lipid-rich carcinomas have been associated with steroids and special neoplastic endocrine mechanisms in canine IMC [14]. In the cat, this type of carcinoma has not previously been described and it is not included in the histological classifications.
The presence of neoplastic emboli in superficial dermal lymph vessels (the hallmark of histological diagnosis of IBC) was seen in all feline cases, confirming similarities among the three species. In contrast, feline IMC had a severe inflammation in dermis and adipose tissue, which is is unusual in human and canine cases, and accounts for the vascular reaction observed in one case (severe vascular congestion and proliferation of subepithelial sanguineous capillaries). Two types of neoplastic dermal infiltration have been described in canine IMC: papillary type (characteristic of secondary IMC) and sarcomatous type (more malignant and typical of primary IMC, the most aggressive type) [6]. In the feline cases studied here the infiltration was always of the papillary type, and this is coincident with the secondary IMC that the animals had developed. At necropsy the presence of pulmonary metastases (not detected previously by radiology) and cystic ovaries with endometrial cystic hyperplasia are common findings in dogs (unpublished data) and cats with IMC.
ER-α immunohistochemistry was positive in the tumours of cats with clinical IMC. This is different than in the dog, in which all the studied tumours were ER-negative [6]. Although the antibody and the technique used was the same in feline and canine IMC, there might be stronger similarities between human and feline ER-α with respect to the particular epitope recognized by the monoclonal antibody used. In addition, comparisons between hormonal analyses in these two species should be considered with caution. Presentation of canine IMC has been found to be significantly associated with the luteal phase [5]. The luteal phase is shorter (mean duration 36 days in cats) and is less frequent in female cats than in female dogs, because in the cat coitus-induced ovulation is required for initiation of the luteal phase. The three cats in the present study had no coital exposure, and IMC appeared during the inter-oestrus period.
Comparison of ER status of IMC tumours between species cannot be properly done using immunohistochemical procedures only. A detailed molecular study of ER would be desirable. The high positive immune reaction to PR and AR found in feline IMC cases is comparable to that described in canine IMC [6] and suggests, as in the dog, the possible involvement of special endocrine mechanisms in IMC development [14]. In this sense, it is interesting to note the reversion in the ER immunoexpression status in feline IMC case 2, which was ER-negative when the animal did not have inflammatory signs and ER-positive in the samples corresponding with clinical IMC. There is no explanation for this ER reversion, but it could be related to a special endocrine cellular phenotype related to the 'inflammatory' type. Several studies on ER and PR expression in human IBC have yielded variable results [3]. ER-positive cases varied from 22% to 52% and PR varied from 16% to 34% [25-28]. In metastatic IBC the proportion of steroid receptors was low, with the PR being more expressed than the ER (8.7% ER+ and 17.4% PR+) [29]. Other authors consider most IBC cases to be ER and PR negative [3]. There are no studies concerning AR expression in human IBC.
The main differences observed between canine IMC and feline IMC may be summarized as follows. Secondary postsurgical presentation is the only clinical presentation of IMC observed in the cat thus far. The most severe clinical signs of IMC in the cat are inflammatory signs with oedema, ulceration of the skin and rejection of surgical suture, with no healing process completed after surgery (observed in two out of three cases). This latter feature is not commonly present in the dog. Histologically, apart from the typical neoplastic embolization of superficial dermal lymphatics (which are characteristic of IMC), a severe inflammatory infiltrate composed of large amounts of neutrophils and other inflammatory cells was seen in the three feline cases. This histologically severe inflammation is not common in canine IMC or human IBC, and cannot be accounted for only by an inflammatory response in the wound healing area, because in one cat (case 1) the repair process had completed. A specific antineoplastic immune reaction should be considered in feline cases.
In general, spontaneously occurring canine and feline mammary tumours can be used as models of human cancer [30]. On the basis of histological characteristics and clinical outcome, feline mammary tumours are better models of human breast cancer than are canine mammary tumours. However, the information we have obtained thus far in feline IMC (lower prevalence, only secondary IMC described, associated with wound infection, ER+) appears to indicate that, in this particular type of mammary cancer, canine IMC is a better model. Feline IMC should be viewed with caution as a natural model of human IBC.
Conclusion
The information provided herein regarding feline IMC (very low prevalence, only secondary IMC, frequently associated with wound infection, steroid receptor positivity) indicate that feline IMC could be useful as a natural model of human IBC, but the findings should be considered with caution.
Competing interests
None declared.
Abbreviations
AR = androgen receptor; ER = oestrogen receptor; HMG = histological malignant grade; IBC = inflammatory breast cancer; IMC = inflammatory mammary carcinoma; PR = progesterone receptor; TBS = Tris-buffered saline.
Acknowledgments
Acknowledgements
We thank the veterinarians caring for the referred cases (Veterinary Clinics 'Indra' for case 3 and 'Mi Mascota' for case 1) and Pedro Aranda and Mario Hernando for their technical support.
Contributor Information
M Dolores Pérez-Alenza, Email: mdpa@vet.ucm.es.
Ángeles Jiménez, Email: lothie@terra.es.
Ana I Nieto, Email: ananieto@vet.ucm.es.
Laura Peña, Email: laurape@vet.ucm.es.
References
- Tavassoli FA. In Pathology of the Breast. 2. New York: McGraw-Hill; 1999. Infiltrating carcinoma: special types. Inflammatory carcinoma; pp. 538–541. [Google Scholar]
- Giordano SH, Hortobagyi GN. Inflammatory breast cancer: clinical progress and the main problems that must be addressed. Breast Cancer Res. 2003;5:284–288. doi: 10.1186/bcr608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423–429. doi: 10.1186/bcr89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaneck SJ, Allen TA, Hoopes J, Withrow SJ, Macy DW. Inflammatory mammary carcinoma in the dog. J Am Animal Hosp Assoc. 1983;9:971–976. [Google Scholar]
- Perez-Alenza MD, Tabanera E, Pena L. Inflammatory mammary carcinoma in dogs: 33 cases (1995–1999) J Am Vet Med Assoc. 2001;219:1110–1114. doi: 10.2460/javma.2001.219.1110. [DOI] [PubMed] [Google Scholar]
- Pena L, Perez-Alenza MD, Rodriguez-Bertos A, Nieto A. Canine inflammatory mammary carcinoma: histopathology, immunohistochemistry and clinical implications of 21 cases. Breast Cancer Res Treat. 2003;78:141–148. doi: 10.1023/A:1022991802116. [DOI] [PubMed] [Google Scholar]
- Elis DL, Teitelbaum SL. Inflammatory carcinoma of the breast: a pathologic definition. Cancer. 1974;33:1045–1047. doi: 10.1002/1097-0142(197404)33:4<1045::aid-cncr2820330422>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bonnier P, Charpin C, Lejeune C, Romain S, Tubiana N, Beedassy B, Martin PM, Serment H, Piana L. Inflammatory carcinomas of the breast: a clinical, pathological, or a clinical and pathological definition? Int J Cancer. 1995;62:382–385. doi: 10.1002/ijc.2910620404. [DOI] [PubMed] [Google Scholar]
- Saltzstein SL. Clinically occult inflammatory carcinoma of the breast. Cancer. 1974;34:382–388. doi: 10.1002/1097-0142(197408)34:2<382::aid-cncr2820340223>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Amparo RS, Angel CD, Ana LH, Antonio LC, Vicente MS, Carlos FM, Vicente GP. Inflammatory breast carcinoma: pathological or clinical entity? Breast Cancer Res Treat. 2000;64:269–273. doi: 10.1023/A:1026512722789. [DOI] [PubMed] [Google Scholar]
- Attia-Sobol J, Ferriere JP, Cure H, Kwiatkowski F, Achard JL, Verrelle P, Feillel V, De Latour M, Lafaye C, Deloche C. Treatment results, survival and prognostic factors in 109 inflammatory breast cancers: univariate and multivariate analysis. Eur J Cancer. 1993;29:1081–1088. doi: 10.1016/s0959-8049(05)80292-8. [DOI] [PubMed] [Google Scholar]
- Chang S, Parker SL, Pham T, Buzdar A, Hursting SD. Inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program of the National Cancer Institute, 1975–1992. Cancer. 1998;82:2366–2372. doi: 10.1002/(SICI)1097-0142(19980615)82:12<2366::AID-CNCR10>3.3.CO;2-B. [DOI] [PubMed] [Google Scholar]
- van Golen KL, Davies S, Wu ZF, Wang Y, Bucana CD, Root H, Chandrasekharappa S, Strawderman M, Ethier SP, Merajver SD. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511–2519. [PubMed] [Google Scholar]
- Pena L, Silvan G, Perez-Alenza MD, Nieto A, Illera JC. Steroid hormone profile of canine inflammatory mammary carcinoma: a preliminary study. J Steroid Biochem Mol Biol. 2003;84:211–216. doi: 10.1016/S0960-0760(03)00030-X. [DOI] [PubMed] [Google Scholar]
- McCarthy NJ, Yang X, Linnoila IR, Merino MJ, Hewitt SM, Parr AL, Paik S, Steinberg SM, Hartmann DP, Mourali N, Levine PH, Swain SM. Microvessel density, expression of estrogen receptor alpha, MIB-1, p53, and c-erbB-2 in inflammatory breast cancer. Clin Cancer Res. 2002;8:3857–3862. [PubMed] [Google Scholar]
- Alpaugh ML, Tomlinson JS, Shao ZM, Barsky SH. A novel human xenograft model of inflammatory breast cancer. Cancer Res. 1999;59:5079–5084. [PubMed] [Google Scholar]
- Hahn KA, Adams WH. Feline mammary neoplasia: biological behaviour, diagnosis and treatment alternatives. Feline Pract. 1997;25:5–11. [Google Scholar]
- Misdorp W, Else RW, Helmén E, Lipscomb TP. Histological Classification of Mammary Tumors of the Dog and Cat. Washington: Armed Forces Institute of Pathology and World Health Organization; 1999. [Google Scholar]
- Elston EW, Ellis IO. Method for grading breast cancer. J Clin Pathol. 1993;46:189–190. doi: 10.1136/jcp.46.2.189-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Nolte I. Mammary gland tumors in the cat. Prognosis and therapy. Praktische Tierarzt. 1997;78:22–25. [Google Scholar]
- Stockhaus C, Kohn B, Rudolph R, Brunnberg L, Giger U. Correlation of haemostatic abnormalities with tumour stage and characteristics in dogs with mammary carcinoma. J Small Anim Pract. 1999;40:326–331. doi: 10.1111/j.1748-5827.1999.tb03090.x. [DOI] [PubMed] [Google Scholar]
- van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD. RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia. 2000;2:418–425. doi: 10.1038/sj.neo.7900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardivon AA, Viala J, Corvellec RA, Guinebretiere JM, Vanel D. Mammographic patterns of inflammatory breast carcinoma: a retrospective study of 92 cases. Eur J Radiol. 1997;24:124–130. doi: 10.1016/S0720-048X(96)01137-0. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A, Hellmen E, Ramirez GA, Herraez P, Rodriguez F, Ordas J, Millan Y, Lara A, Martin de las Mulas J. Lipid-rich carcinomas of the mammary gland in seven dogs: clinicopathologic and immunohistochemical features. Vet Pathol. 2003;40:718–723. doi: 10.1354/vp.40-6-718. [DOI] [PubMed] [Google Scholar]
- Brooks HL, Mandava N, Pizzi WF, Shah S. Inflammatory breast carcinoma: a community hospital experience. J Am Coll Surg. 1998;186:622–629. doi: 10.1016/S1072-7515(98)00107-0. [DOI] [PubMed] [Google Scholar]
- Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, Theriault RL, Strom EA, Wasaff BJ, Asmar L, Frye D, Hortobagyi GN. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40:321–329. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]
- Fleming RY, Asmar L, Buzdar AU, McNeese MD, Ames FC, Ross MI, Singletary SE. Effectiveness of mastectomy by response to induction chemotherapy for control in inflammatory breast carcinoma. Ann Surg Oncol. 1997;4:452–461. doi: 10.1007/BF02303668. [DOI] [PubMed] [Google Scholar]
- Wilke D, Colwell B, Dewar R. Inflammatory breast carcinoma: comparison of survival of those diagnosed clinically, pathologically, or with both features. Am Surg. 1998;64:428–431. [PubMed] [Google Scholar]
- Atlan D, Chevallier B, Sheng RG. Tamoxifen for the treatment of metastatic inflammatory breast carcinoma. Am J Clin Oncol. 1995;18:74–77. doi: 10.1097/00000421-199502000-00016. [DOI] [PubMed] [Google Scholar]
- Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]


