Abstract
Psoriasis patients exhibit an increased risk of death by cardiovascular disease (CVD) and have elevated levels of circulating intermediate (CD14++CD16+) monocytes. This elevation could represent evidence of monocyte dysfunction in psoriasis patients at risk of CVD, as increases in circulating CD14++CD16+ monocytes are predictive of myocardial infarction and death. An elevation in the CD14++CD16+ cell population has been previously reported in patients with psoriatic disease, which has been confirmed in the cohort of our human psoriasis patients. CD16 expression was induced in CD14++CD16neg classical monocytes following plastic adhesion, which also elicited enhanced β2 but not β1 integrin surface expression, suggesting increased adhesive capacity. Indeed, we found that psoriasis patients have increased monocyte aggregation among circulating PBMCs which is recapitulated in the KC-Tie2 murine model of psoriasis. Visualization of human monocyte aggregates using imaging cytometry revealed that classical CD14++CD16neg monocytes are the predominant cell type participating in these aggregate pairs. Many of these pairs also included CD16+ monocytes, which could account for apparent elevations of intermediate monocytes. Additionally, intermediate monocytes and monocyte aggregates were the predominant cell type to adhere to TNF-α and IL-17A-stimulated dermal endothelium. Ingenuity Pathway Analysis (IPA) demonstrated that monocyte aggregates have a distinct transcriptional profile from singlet monocytes and monocytes following plastic adhesion, suggesting that circulating monocyte responses to aggregation are not fully accounted for by homotypic adhesion, and that further factors influence their functionality.
Keywords: Skin, monocytes, inflammation
Introduction
Psoriasis is a chronic inflammatory disease of the skin affecting 2–3% of the US population in which expression is modified by susceptibility genes and environmental triggers (1). The pathogenesis of psoriatic tissue hyperplasia is thought to be driven by an interplay of macrophages, dendritic cells, and pathogenic and resident memory T cells, with enhanced representation of the IL-23-Th17/Th22 and IL-12-IFN-γ/TNF pathways (2, 3). In addition to an enormous negative impact on quality of life, psoriasis patients exhibit numerous co-morbidities including destructive psoriatic arthritis, stigmatization, depression and anxiety, inflammatory bowel disease, lymphoma, obesity, metabolic syndrome-associated conditions, and notably, increased risk of early death from cardiovascular disease (CVD) (4–9). A mechanism has not yet been elucidated linking psoriasis pathogenesis and onset of CVD, but recent genome-wide association studies (GWAS) found psoriatic individuals have common genetic variants that predispose them to increased risk of dyslipidemia, hypertension, and coronary artery disease (CAD), revealing an association of cardiovascular and metabolic disease genes with psoriasis (10).
Efforts to identify circulating inflammatory transducers of CVD revealed that increases in circulating intermediate monocyte subpopulations are associated with CVD (11), acute ischemic heart failure (12), myocardial infarction (13), peripheral artery disease (14), and acute coronary syndrome associated with HIV (15). Within human peripheral blood, three distinct monocyte populations have been identified and genotyped: classical monocytes (CD14++CD16neg), intermediate monocytes (CD14++CD16+), and non-classical monocytes (CD14+CD16++) (16–22). Psoriasis patients, who also exhibit an increased risk of death by CVD, have been reported to have elevated levels of circulating CD16+ cells which contain the intermediate monocyte population (23, 24). Induction of CD16 on the intermediate monocyte population can occur as a result of platelet interaction (25), which also increases monocyte adhesion to vascular endothelium and subsequent trans-endothelial migration (26). Indeed, circulating monocyte/platelet aggregates (MPAs) are considered a robust marker of platelet activation and indicator of coronary artery disease (CAD) (27), ST segment elevation myocardial infarction (STEMI) (13), and acute myocardical infarction (28), as reviewed in (29).
In murine CVD models, a proinflammatory monocyte subset (CD11b+Ly6Chi) infiltrates murine atherosclerotic plaques and promotes atherogenesis (30) and also plays a role in myocardial infarction (31). Interestingly, in the skin-specific KC-Tie2 murine model of psoriasis, elevated levels of circulating CD11b+Ly6Chi cells are observed and precede the spontaneous formation of aortic root lesions. Moreover, these mice also develop a pro-thrombotic clotting phenotype (32) consistent with the idea that skin-contained chronic inflammation may have the capacity to promote atherothrombosis.
In this report, we demonstrate that psoriasis patients have both a relative and absolute increase in circulating monocyte aggregates as well as an increase in intermediate monocytes, correlating with an increase in disease severity assessed by psoriasis area severity index (PASI), compared to healthy controls. Interestingly, control intermediate monocytes demonstrate increased adhesiveness to Human Dermal Microvascular Endothelial Cells (HMVEC-D) following endothelial cell stimulation with proinflammatory cytokines known to be increased in psoriasis skin (TNF-α and IL-17A). Circulating monocyte-monocyte aggregates are also present in the KC-Tie2 murine psoriasiform model. We also show that monocyte aggregation in humans is associated with a distinct transcriptional profile and can occur in the presence or absence of platelets. Taken together, this data suggests a novel role for monocyte adhesion and subsequent aggregation as a potential link between the pathogenesis of psoriasis and CVD.
Materials and Methods
Human subjects
All studies of human subjects were approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH). Peripheral blood samples and/or punch biopsies were obtained from volunteer healthy controls and psoriasis patients following informed consent. Psoriasis patients were not on any systemic psoriasis medications and those patients using any topical therapeutics discontinued use for at least two weeks prior to entering the study. For patient demographics, see Supplemental Table I.
Cell culture
PBMCs were isolated from 23 controls and 19 psoriasis patients using Ficoll-Paque centrifugation, washed, and red blood cells were lysed using ACK (Invitrogen, Carlsbad, CA) and then immediately stained for surface markers. Experiments were performed using total PBMCs and electronically gated for monocyte subpopulations. For adhesion studies, PBMCs were plated on tissue culture-treated 6 well dishes for 30 minutes, 1 hour, 4 hours, or overnight in RPMI media supplemented with FBS and P/S in 5% CO2 at 37°C.
Flow Cytometry
Flow cytometric data collection was performed using a BD FACS-Aria instrument and analyzed using FlowJo software (Tree Star, Ashland, OR). Monocytes were initially gated using a FSC-A vs SSC-A discrimination plot and then analyzed for specific CD14 versus CD16 staining (see Supplemental Figure 2 for gating strategy and Supplemental Table II for antibody clone and source). Doublet analysis was performed by gating FSC-W versus FSC-H on total events. For experiments that required sorted monocytes, samples were sorted using an 85µm tip at a speed of 1.0 and pressure of 45psi. Surface adhesion analysis of CD11b, CD11c, CD18, VLA-4, VCAM-1, and ICAM-1 was performed on gated classical monocyte populations.
Whole Blood Method
50µl of whole blood from 4 healthy controls and 4 psoriasis patients was stained with mouse anti-human CD14 and CD16 for 15 minutes at RT. The blood was then lysed at RT with 450µl of 1X FACS Lysing Solution (BD, Franklin Lakes, NJ) for 12 minutes and analyzed immediately by flow cytometry.
Monocyte and HMVEC-D co-culture experiments
Monocytes were negatively selected from PBMCs using the Pan-Monocyte kit (Miltenyi, Cologne, Germany). HMVEC-D cells (Lonza, Basel, Switzerland) were cultured in complete media (Lonza) at 37°C, 5% CO2. HMVEC-D cells were stimulated for 4 hours in serum free media at 37°C, 5% CO2 with TNF-α (1ng/ml; R&D, Minneapolis, MN) and IL-17A (100ng/ml; R&D) to mimic a psoriatic and cardiovascular cytokine profile, as published in (33). The cells then rested for 30 minutes before addition of monocytes. HMVEC-D cells were used at P2-P3 and 90–100% confluence in a 6 well dish. 4–5 million monocytes were plated per well and adhered for 1 hour before harvesting the adherent and supernatant fractions and staining for CD14 and CD16 surface markers.
Imaging Flow Cytometry
Heterogeneous doublet population data was collected using an Amnis Imagestream cytometer and analyzed using IDEAS software v6.0. PBMCs were stained for surface expression of CD14, CD16, CD42b, CD3, and CD56 (for antibodies, see Supplemental Table II). Events were first selected on focused events. Next, to gate singlets versus doublets, area vs. aspect ratio of the brightfield channel was plotted. Doublets were defined as having an aspect ratio less than 0.6 with an area of 100–400, while singlets had aspect ratios above 0.6 and an area of 75–200. The singlets and doublets were then separately gated into CD14 APC vs. CD16 FITC for three monocyte subset analysis. From the CD14 vs. CD16 scatter plot of doublets, each subset was plotted again as area vs. aspect ratio of each respective channel. This allowed us to separate out homogeneous versus heterogeneous cell pairs. For example, a doublet that contained two CD14+ cells would have a larger area on the CD14 channel than a doublet that contained one CD14+ cell and one CD14neg cell (such as a T cell).
Gene Expression Array
RNA was extracted from flow-sorted CD14++CD16neg singlet cells and CD14++doublet cells using the QIAGEN RNeasy Minikit in combination with the QIAGEN QIAshredder kit per the manufacturer's protocol from psoriasis patients (n=5) and controls (n=5). A portion of the CD14++CD16neg singlets were adhered for 4 hours on tissue culture plastic before RNA extraction. cDNA was made using the RT2 First Strand Kit (SA Biosciences, Valencia, CA). cDNA was combined with RT2 SYBR Green Mastermix and dispensed into a PCR Array for Human Extracellular Matrix & Adhesion Molecules (Qiagen) with a 384 (4 × 96) E, G format. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (Golden et al., 2015) and are accessible through GEO Series accession number GSE70327 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE70327). A heat map was generated using R: A Language and Environment for Statistical Computing (Vienna, Austria) with fold changes calculated from ratios using the following equation: =IF(value>1,value,(1/value)*−1). Fold change values of greater than 20 were changed to 20, and fold change values of less than −20 were changed to −20 to normalize the range to the majority of the values and avoid an unclear gradient within the heat map. The psoriasis top doublet network, representing the leukocyte extravasation pathway, was generated through the use of QIAGEN’s Ingenuity Pathway Analysis (IPA®, QIAGEN Redwood City, www.qiagen.com/ingenuity) with an average of all control CD14+-doublets as the comparator.
Immunofluorescence
Frozen 4mm punch biopsies from involved psoriasis plaque of human volunteers were sectioned into 8µm slices and four serial sections were mounted on each slide to provide proper controls for staining. Tissue sections were fixed in acetone for 10 minutes, washed in PBS buffer (HyClone, Thermo Scientific, Waltham, MA), rehydrated using antibody dilutent solution (MP, Santa Ana, CA), and blocked in 20% secondary isotype specific serum (R&D) for 30 minutes. Primary antibodies were incubated either overnight at 4°C or for 1h at room temperature dependent on the target and amplified using an isotype-specific corresponding secondary antibody for 1h at room temperature (see Supplemental Table II). Nuclei were stained using one drop (approximately 15µL) of Prolong Gold anti-fade reagent with DAPI (Molecular Probe, Life Technologies, Eugene, OR).
Fluorescent image acquisition and analysis
All images were acquired using the UltraVIEWVoX™ spinning disk confocal system (PerkinElmer, Waltham, MA) which is mounted on a Leica DMI6000B microscope (Leica Microsystems, Inc., Bannockburn, IL) at 20x magnification. Confocal images were collected using solid state diode lasers, with 640-nm, 488-nm, 561-nm and 405-nm excitation light, respectively, and with appropriate emission filters (see Supplemental Table II for antibodies). All confocal images were analyzed using Volocity™ (PerkinElmer, Waltham, MA), MetaMorph™ Premier software (Molecular Devices Corporation, Sunnyvale, CA), and SigmaPlot™ (Systat Software, Inc., San Jose, CA). The white line designates the dermal-epidermal junction of skin sections.
Mouse studies
The KC-Tie2 binary, tet-repressible psoriasiform mouse model, its genetic engineering, and the characterization of its skin and vascular phenotypes have been described at length previously (32, 34, 35). Mice spontaneously develop a chronic inflammatory skin phenotype following transgenic introduction of the angiopoietin receptor, Tie2, into keratinocytes (using the keratin 5 promoter). The skin inflammation phenocopies human psoriasis (35), is characterized by a robust Th1/Th17 skewed immune response, and is responsive to antibodies targeting TNF-α or antigen cell depletion (34). Mice spontaneously develop systemic inflammation, elevated circulating CD11b+Ly6Chi monocytosis, aortic root vascular inflammation, and are pro-thrombotic; these are reversed following targeted inhibition of the skin inflammation (32). Psoriasis-like inflammation was observed in all animals at the time of experiments.
Spleens from male and female, adult, age-matched KC-Tie2 transgenic mice (1 year old mice on a CD1 outbred background, n=10) and littermate controls (1 year old, n=5) were removed and homogenized in serum-free media containing 50ug/ml DNase I (Sigma, St. Louis, MO) and 2mg/ml collagenase D (Roche, Basil). Red blood cells were lysed using ACK and then cells were pelleted, resuspended, and filtered 2x through a 70µm filter in wash buffer containing 5% FBS. The cells were immediately stained for the cell surface markers Ly6C-Alexa Fluor 700 (eBiosciences, San Diego, CA) and CD11b-eFlour450 (BD), using 7AAD to determine live cells. Cells were gated as previously published (32).
All animal protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee and conformed to the American Association for Accreditation of Laboratory Animal Care guidelines.
Data Analysis
Normality of distributions was tested using the Kolmogorov-Smirnov statistic, and upon non-rejection of this assumption, t-tests for independent samples were used to compare mean values. Equality of variances was tested in order to select the appropriate resultant p-values. The Mann-Whitney test was used when the assumption of normality was not met. Correlations were estimated using Pearson correlation coefficients. Results are expressed as mean ± standard error. Data analysis was done using SPSS v21 and graphs were generated using GraphPad Prism 6.
Results
Psoriasis patients have a higher percentage of circulating CD14++CD16+ intermediate monocytes that correlates with disease severity
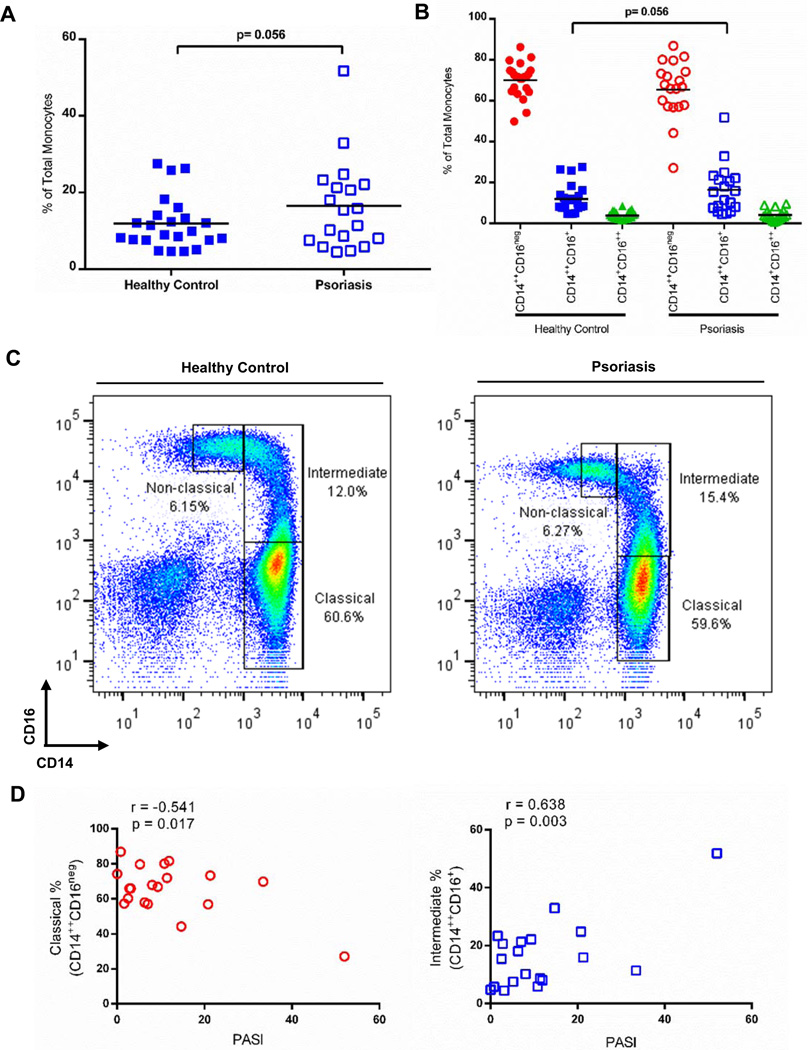
Peripheral blood mononuclear cell (PBMC) preparations were obtained from either psoriasis patients or healthy controls and analyzed for the percentage of circulating monocyte subsets (classical (CD14++CD16neg), intermediate (CD14++CD16+), or non-classical (CD14+CD16++)). Higher percentages of circulating intermediate monocytes were observed among psoriasis patients compared to control (16.5% ± 2.7 vs. 11.9 ± 1.4, n=19, n=23, respectively; p=0.056) (Figure 1A). Classical and non-classical subsets showed no significant differences between psoriasis patients and healthy controls (65.5% ± 3.2 vs. 70.2% ± 1.7, CD14++CD16neg, and 4.1% ± 0.59 vs. 3.8% ± 0.42, CD14+CD16++; Figure 1B). Representative individual scatter plots are shown in Figure 1C. Interestingly, the percentage of circulating classical and intermediate cells correlate with psoriasis disease severity (measured by the Psoriasis Area Severity Index (PASI)) as shown in Figure 1D. While the classical subset negatively correlates with PASI (r=−0.541, p=0.017), the intermediate subset positively correlates with PASI (r=0.638, p=0.003), and non-classical monocytes do not demonstrate a significant correlation (r=−0.082, p=0.738, data not shown). When absolute numbers of cells are calculated, psoriasis patients have increased numbers of total monocytes and doublets compared to healthy control individuals (Table I).
Figure 1. CD14++CD16+ (intermediate) cells are increased in psoriasis patients compared to healthy controls.
(A) The percentage of intermediate monocytes (CD14++CD16+) is increased in psoriasis patients (n=19, open squares) when compared to healthy controls (n=23, solid squares; p=.056) while (B) the percentage of classical (circles) and non-classical monocytes (triangles) do not differ between psoriasis patients and healthy controls. (C) Representative flow plots showing gates and population distribution. (D) Classical monocytes (red, open circles) negatively correlate with disease severity measured by PASI (r=−0.541, p=0.017), while intermediate monocytes (blue, open square) positively correlate with disease severity (r=0.638, p=0.003).
Table I.
Absolute cell counta
| Doubletsb/ PBMC |
Monocytesc/ Singlet PBMC |
Classicald Monocytes |
Intermediatee Monocytes |
Non-classicalf Monocytes |
|
|---|---|---|---|---|---|
| Control | 0.83 ± 0.12 | 18.8 ± 1.93 | 12.9 ± 1.38 | 2.46 ± 0.40 | 0.69 ± 0.10 |
| Psoriasis | 2.53 ± 0.82 | 32.3 ± 15.9 | 21.6 ± 11.2 | 4.89 ± 2.06 | 1.11 ± 0.45 |
Determined by percentage of monocytes/total PBMCs.
Doublets defined based on a FSC-W vs. FSC-H analysis of all events.
Monocytes defined on a FSC-A vs. SSC-A analysis from the singlet events.
Defined as CD14++CD16neg from the monocyte and singlet gates.
Defined as CD14++CD16+ from the monocyte and singlet gates.
Defined as CD14+CD16++ from the monocyte and singlet gates.
Monocyte doublets are increased in psoriasis patients compared to controls
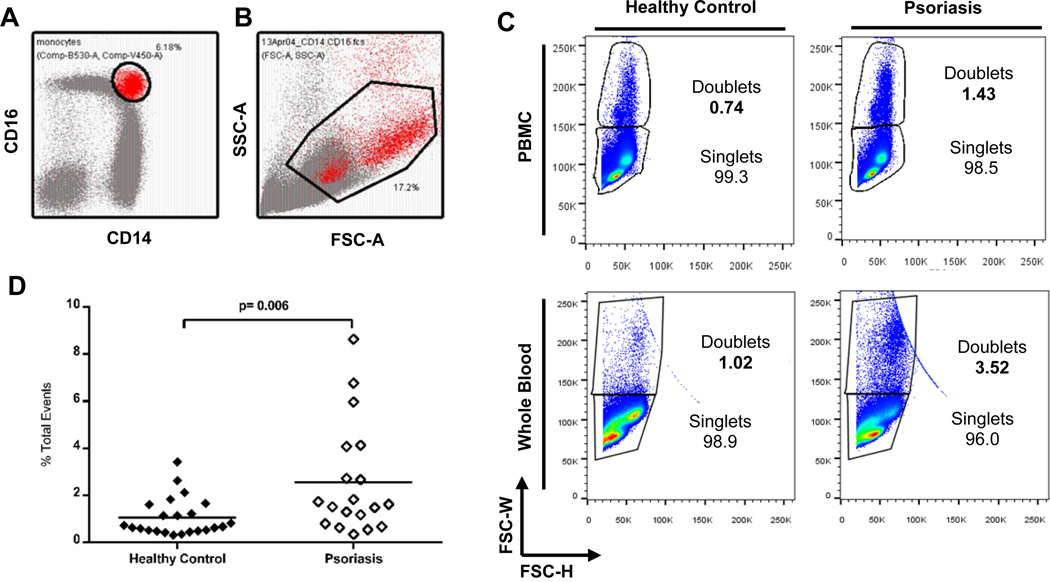
We noted that a prominent population of cells was expressed within the intermediate CD14++CD16+ gate in the scatter plot of psoriatic PBMCs (Figure 2A). Back-gating this population revealed that these cells were larger on FSC-A vs. SSC-A scatter plots and mapped to a region expected to contain doublets (Figure 2B). Indeed, cell width vs. height analysis on forward scatter of total events confirmed the accumulation of doublets (Figure 2C, top row). Doublet discrimination analysis demonstrated that psoriasis patients have a > 2.5 fold increase in total PBMC doublets compared to controls (Figure 2D; 2.56% ± 0.54 vs. 1.06% ± 0.17, n=19, n=23, respectively; p=0.006). The top quartile of psoriasis patients (5/19) with increased doublets have a positive correlation that approaches significance with disease duration (r=.804, p=.101), suggesting that increased doublet percentage may be an additional indicator of disease severity. To ensure that doublet formation was relevant to in vivo psoriasis circulation, we also measured monocyte subset and doublet formation using whole blood, a technique commonly used to quantify the percentage of circulating monocyte subsets (36). As shown in Figure 2C, bottom row, the increase in doublet formation can also be captured in whole blood assays.
Figure 2. Psoriasis patients have increased total doublets within PBMCs.
(A) The prominent intermediate monocyte population in psoriasis patients was selected and in (B), cells were overlaid electronically into a monocyte scatter gate as shown in the FSC-A vs. SSC-A plot. (C) The majority of the cells were doublets, as shown in representative plots of FSC-W vs. FSC-H, demonstrating singlet and doublet populations in PBMCs (n=42) and whole blood (n=8). (D) Analysis of all patients demonstrated that the total doublet percentage is significantly increased in psoriasis patients when compared to controls (open diamonds versus solid diamonds, n=19 and n=23; respectively; p=.006).
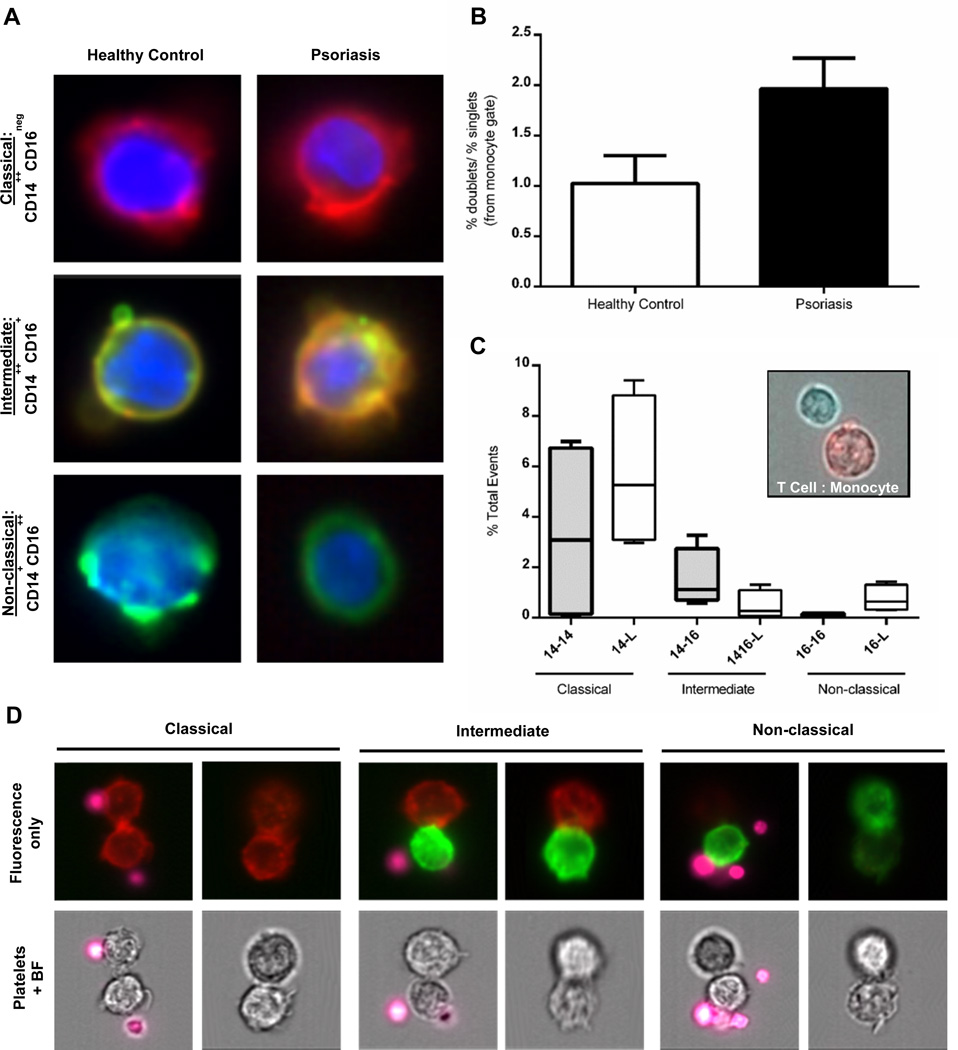
In order to confirm which cells form aggregates, we used an Amnis Imagestream flow cytometer to acquire an image of each cellular event. This analysis is capable of visualizing cellular events by collecting a photographic image that corresponds to each flow cytometric event acquired. PBMCs were stained using mouse anti-human CD14 APC, CD16 FITC, and DAPI to discriminate among classical, intermediate, and non-classical monocytes as shown in Figure 3A. Imagestream analysis recapitulated our standard flow cytometric analysis confirming that monocyte doublets were 2x more likely in psoriasis PBMCs compared to healthy control samples (Figure 3B). Interestingly, analysis of the doublets revealed that monocytes were capable of forming pairs with different subsets of lymphocytes. This included different monocyte subsets such as an intermediate:classical pair (a CD14++CD16+ cell binding to a CD14++CD16neg cell) (Figure 3C, representative images in 3D) as well as monocytes binding to other lymphocytes including T cells (Figure 3C, inset), although monocyte:NK cell binding was not observed. Although some monocyte doublet pairs contain platelets, doublets (monocyte:monocyte) can also be formed in the absence of platelets. In the intermediate gate, the majority of doublets are represented by classical:non-classical (CD14++CD16neg:CD14+CD16++) pairs. Intermediate (CD14++CD16+) monocytes binding to other lymphocytes are also evident, although they represent a minority of the cells comprising the intermediate doublet population. Interestingly, both classical and intermediate monocytes exhibit the capacity to bind either CD14+ or CD16+ monocytes, in addition to other lymphocytes, although the classical monocytes appear to have an enhanced capacity over the intermediate cells to form these pairs (Figure 3C).
Figure 3. Amnis Imagestream visualization of cell surface expression of CD14 (APC) and CD16 (FITC) and monocyte-monocyte doublets.
(A) Representative images of each type of monocyte (classical, intermediate, and non-classical) as imaged by the Amnis imagestream cytometer. CD14 (red) is expressed on the surface of the classical cells, CD16 (green) is expressed on the surface of the non-classical cells, while both CD14 and CD16 (orange) co-localize to the surface of the intermediate monocyte. (B) Psoriasis patients (n=3) have increased monocyte doublets when compared to controls (n=3) (black bar versus white bar, respectively; as expressed by a ratio of (% monocyte doublets/% monocyte singlets)). (C) Monocytes form aggregate pairs in the form of monocyte:monocyte or monocyte:lymphocyte. The classical CD14+ cells participate in the most doublet formation as a homogenous pair (CD14+ binding to another CD14+) or as a heterogeneous pair (CD14+ binding to a lymphocyte). The CD14+ cells within the intermediate doublet gate also participate in homo- and heterogeneous pairs, but not to the same extent. Additionally, double positive CD14+CD16+ monocytes participate in binding to CD3+ lymphocytes (as shown in D). Non-classical CD16+ cells rarely participate in homogenous doublet pairs. (D) Representative images of the doublet pairs described in C. Platelets (labeled with CD42b (pink)) participate in doublet formation but are not necessary for monocyte:monocyte aggregation.
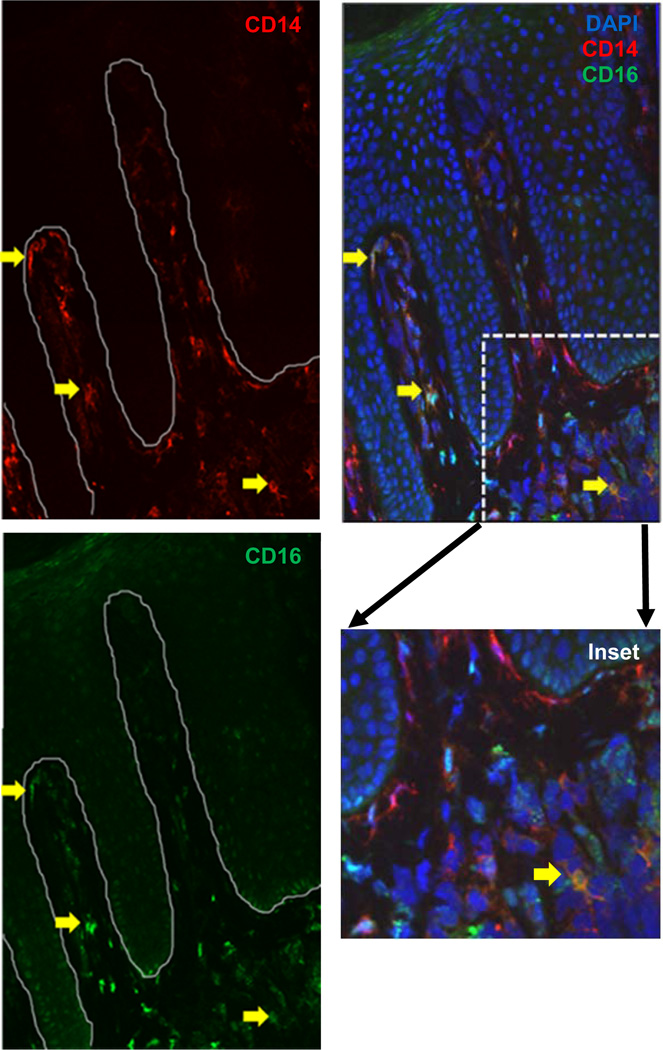
Intermediate CD14++CD16+ cells are detectable in psoriasis tissue
Although intermediate CD14++CD16+ monocytes have been proposed to be dendritic cell (DC) precursors, the evolution/differentiation of these cells remains controversial (37). As shown in Figure 4, intermediate monocytes (yellow arrows) can indeed be detected in psoriasis involved papillary dermal perivasculature, as confirmed by co-localization with CD31 (Supplemental Figure 1A), indicating that they do gain entry into lesional tissue. Interpersonal variation in the number of intermediate monocytes was observed, however all patients had detectable intermediate monocytes within lesional skin. Some monocytes appear to be undergoing differentiation to DCs as evidenced by co-expression with DEC-205 (Supplemental Figure 1C), or to macrophages based on CD68 co-expression (Supplemental Figure 1B). Given the known criticality of T cells in psoriasis, we also identified CD3+ cells present in the plaque adjacent to, but not overlapping with, CD14+ cells (Supplemental Figure 1D).
Figure 4. CD14++CD16+ cells are present and detectable in psoriatic plaques.
Frozen involved human psoriatic plaque stained for CD14 (red) and CD16 (green) with nuclei stained by DAPI (blue). Yellow arrows represent CD14++CD16+ (intermediate) monocytes. Representative image from a psoriasis patient with a PASI score of 33.4 (n=7).
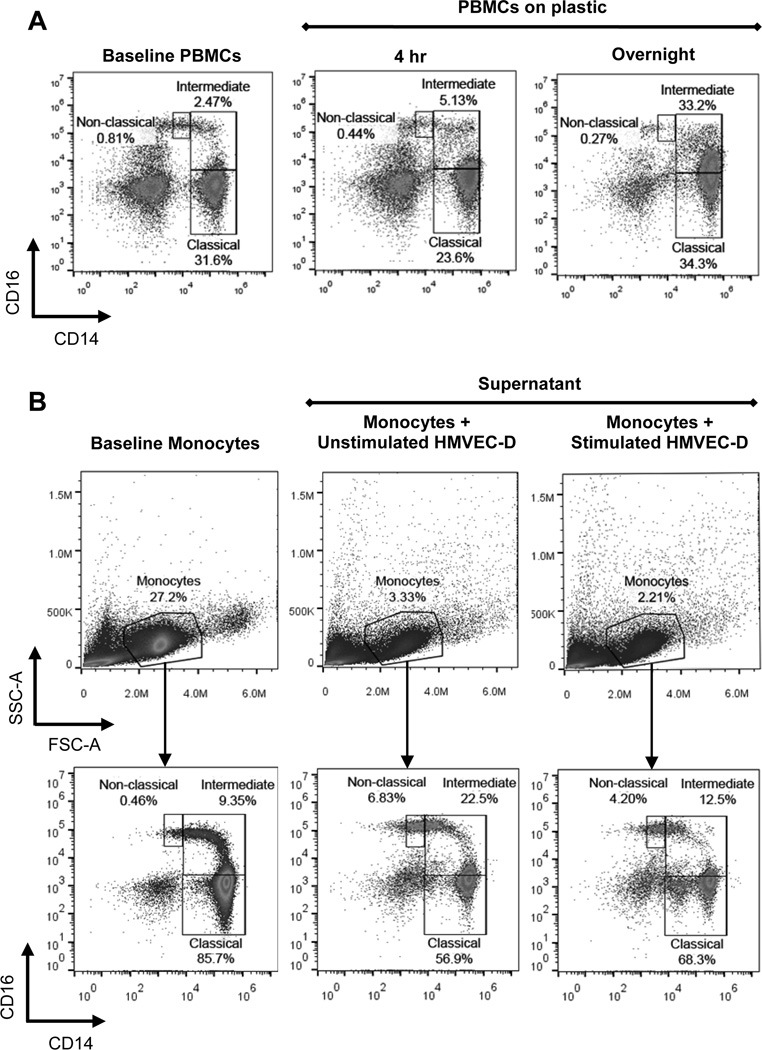
Monocyte modulation of CD16 following adherence
Based on our results demonstrating increased intermediate monocytes in psoriasis circulating blood and tissue and the participation of CD14+ cells in monocyte:monocyte and monocyte:lymphocyte doublets, we anticipated that these monocytes would demonstrate increased adhesiveness. Indeed, exposure of monocytes to tissue culture plastic significantly increases the expression of CD16, confirming previously published observations (23). The classical monocyte population is significantly diminished, and the majority of monocytes take on an intermediate monocyte phenotype beginning as early as 4 hours and becoming nearly exclusively CD14++CD16+ following overnight adherence to plastic (Figure 5A). Interestingly, monocytes derived from either psoriasis or healthy control peripheral blood exhibited the capacity to up-regulate CD16 expression upon binding. To examine CD16 induction on a more physiologically relevant substrate, we co-cultured control monocytes on pre-stimulated HMVEC-D endothelial cells (TNF-α and IL-17A) and compared them to monocytes cultured on unstimulated HMVEC-D cells. After one hour, the supernatants of unstimulated HMVEC-D (Figure 5B, middle panels) or stimulated HMVEC-D cells (Figure 5B, right panels) were isolated and stained for CD14 and CD16 expression on monocytes. Although a brief exposure to endothelial cells did not induce CD16 to the same extent as culturing on plastic, intermediate monocytes exhibited an average 2-fold increase in adhesion to stimulated HMVEC-D cells, based on monocyte number in the supernatant population, compared to unstimulated HMVEC-D cells (23.9% ± 4.5% vs. 12.4% ± 1.2%, respectively, n=4, p=.029; representative images in Figure 5B, middle and right panels, supernatant intermediate population).
Figure 5. Upregulation of CD16 expression and enhanced adhesion.
(A) Representative images: PBMCs at baseline (left panel) were adhered for 4 hours (middle panel) or overnight (right panel) to plastic and then stained for CD14 and CD16 (n=3). After 4 hours, expression of CD16 begins to increase; after overnight culture almost the entire classical population has upregulated CD16 and transitioned into the intermediate gate. (B) Representative images of monocyte gating of negatively selected control monocytes at baseline (left panels) and after 1 hour culture with unstimulated endothelial HMVEC-D cells (middle panels) and HMVEC-D cells stimulated with TNF-α (1ng/ml) and IL-17A (100ng/ml) (right panels); (n=4).
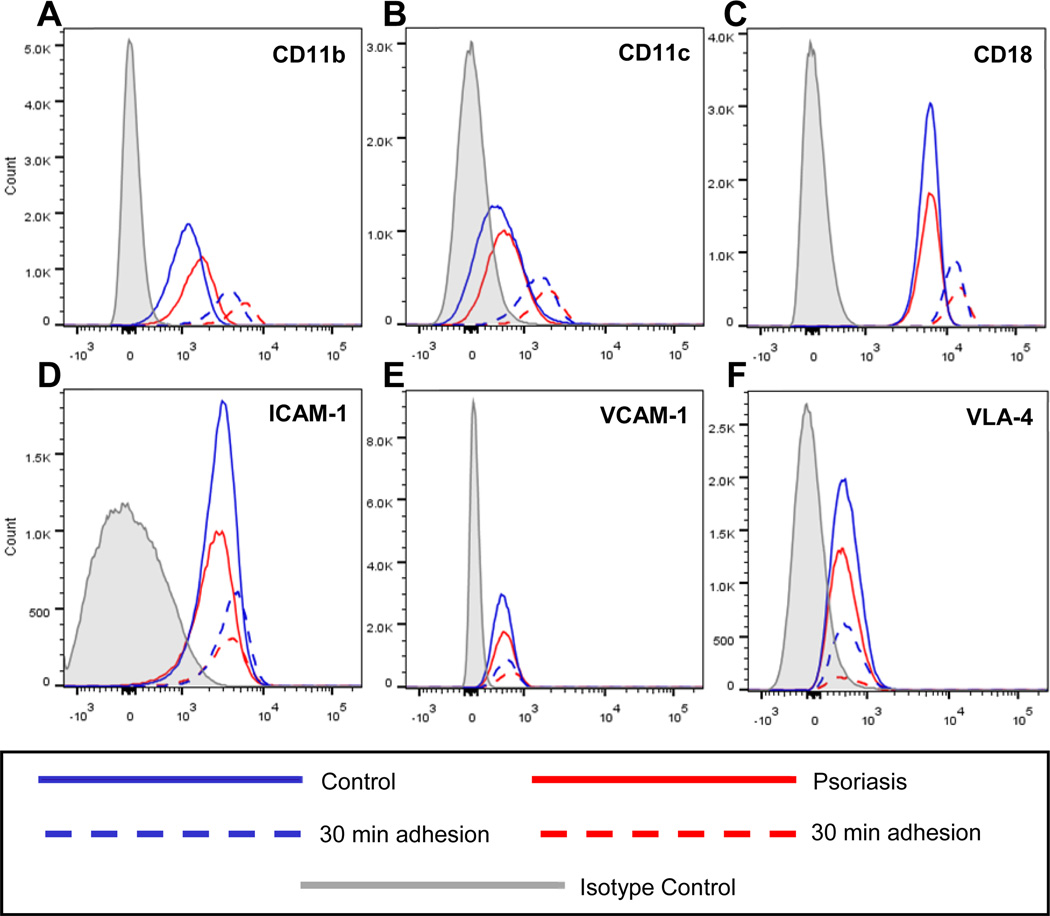
Psoriatic classical monocytes have increased β2 integrin expression at baseline
After determining that monocytes are capable of forming homogeneous pairs, and adhesion to plastic significantly increased CD16 expression, we asked whether or not psoriasis classical monocytes (Figure 6, solid red lines; n=3) had a different adhesion marker profile compared to classical cells from healthy controls (Figure 6, solid blue lines; n=3) at baseline. Psoriasis patients had moderately increased surface expression of the β2 integrins CD11b (Figure 6A) and CD11c (Figure 6B) with a slight elevation of CD18 (Figure 6C and Table II), while psoriasis and control patients had similar levels of ICAM-1 (Figure 6D), VCAM-1 (Figure 6E), and VLA-4 (Figure 6F) expression. Isotype control curves are shown in gray.
Figure 6. Increased β2 integrin expression on psoriasis classical monocytes.
At baseline, psoriasis patients (solid red lines; n=3) demonstrate increased surface expression of (A) CD11b and (B) CD11c with a slight upregulation of (C) CD18 on the classical population when compared to controls (solid blue lines; n=3). After a 30 minute plastic adhesion, control (dashed blue lines) and psoriasis (dashed red lines) classical monocytes upregulate (A) CD11b, (B) CD11c, and (C) CD18, while there is no change in (D) ICAM-1, (E) VCAM-1, or (F) VLA-4. Isotypes are shown on all panels in grey.
Table II.
MFI changes
| Adhesion Molecule | Baselinea Psoriasis/ Control |
Controlb 30min/ Baseline |
Psoriasisc 30min/ Baseline |
|---|---|---|---|
| ICAM-1 | 1.0 | 1.3 | 1.2 |
| CD11b | 1.4 | 2.3 | 1.9 |
| CD11c | 1.5 | 2.6 | 2.2 |
| CD18 | 1.4 | 1.8 | 1.7 |
| VLA-4 | 1.2 | 1.1 | 1.0 |
| VCAM-1 | 1.2 | 1.1 | 1.1 |
Change in mean fluorescence intensity (MFI) of psoriasis classical monocytes compared to control classical monocytes. These cells were not adhered to plastic.
Change in MFI of control classical monocytes after a 30 min adhesion relative to baseline expression.
Change in MFI of psoriasis classical monocytes after a 30 min adhesion relative to baseline expression.
Although control and psoriasis classical monocyte subsets upregulate CD11b and CD11c at baseline, control monocytes induce greater upregulation of these markers after a 30 minute adhesion (Figure 6, dashed blue lines) when compared to psoriasis monocytes (Figure 6, dashed red lines). This suggests that the psoriasis cells may already be “primed” to adhere, resulting in less upregulation of CD11b and CD11c than controls. CD18 is moderately upregulated in both control and psoriasis cells after adhesion, whereas levels of ICAM-1, VCAM-1, and VLA-4 do not change, suggesting that these markers are not likely to mediate the observed adhesion phenotype.
PCR analysis demonstrates a different mRNA expression profile for singlet, doublet, and adhered monocytes
In an attempt to interrogate the adhesive interaction observed in doublets, we compared the mRNA expression profiles of singlet classical monocytes (CD14++CD16neg) to adherent monocytes (singlet classical monocytes adhered to tissue culture plastic for 4 hours), and monocytes forming doublets in peripheral blood using an RNA array specific for adhesion molecules (SA Biosciences Extracellular Matrix and Adhesion Array) in both healthy controls (n=5) and psoriasis patients (n=5).
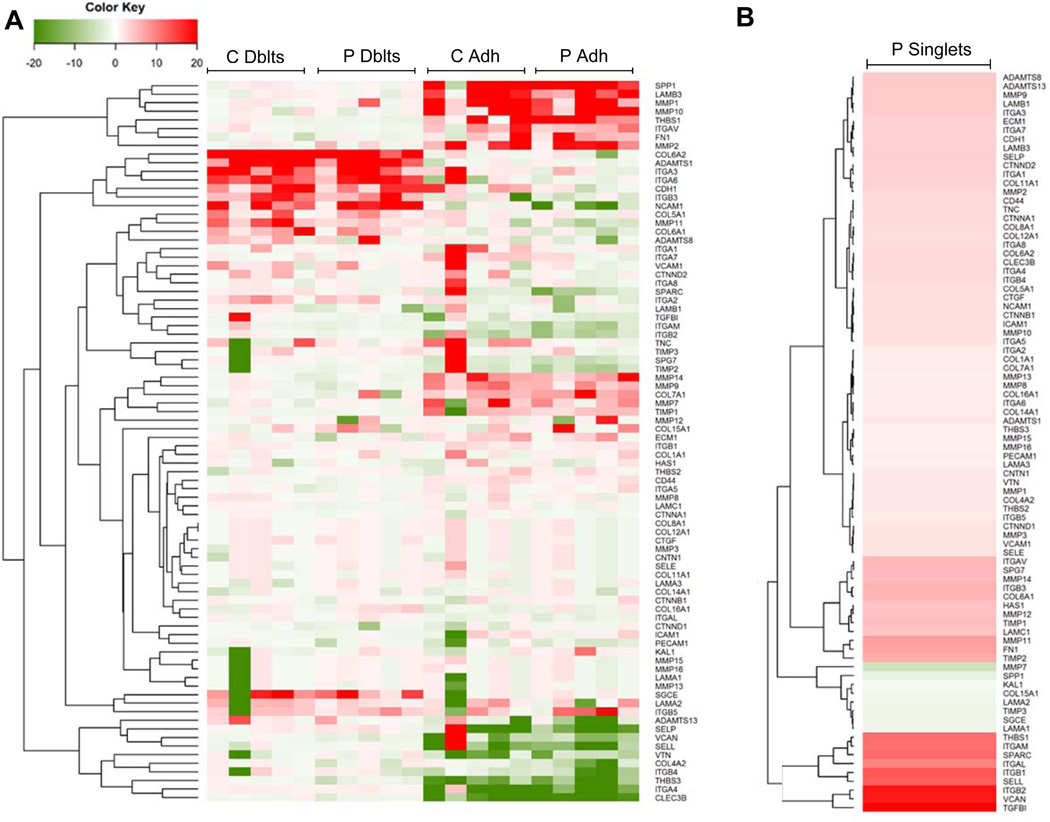
Interestingly, we found doublet cells (sorted from CD14+ cells) have an mRNA expression pattern that is distinct from classical monocytes that have been adhered to tissue culture plastic (Figure 7A, heatmap). As shown, doublet monocyte pairs (both psoriasis and healthy controls) express an upregulated cluster of genes distinct from post-plastic adherent monocytes including integrins (ITGA3, ITGA6, ITGB3), disintegrins (ADAMTS1, ADAMTS8, ADAMTS13), a matrix metalloproteinase (MMP11), collagens (COL5A1, COL6A1, COL6A2), and other cellular adhesion molecules (NCAM1, CDH1, LAMA2). Similarly, post-plastic adherent monocytes have a unique upregulated gene set compared to baseline singlet monocytes and monocyte doublets that included numerous matrix metalloproteinases (MMP1, MMP2, MMP9, MMP10, MMP14), fewer integrins (ITGAV), a laminin (LAMB3), and an adhesive glycoprotein (THBS1). Interestingly, adhered singlet monocytes from healthy control individuals also appear to downregulate several cellular adhesion genes including an intercellular adhesion molecule (ICAM-1), a sarcoglycan (SGCE), an extracellular matrix protein (KAL1), and a laminin (LAMA3), when compared to psoriasis adhered singlet monocytes. Each individual patient’s classical singlet monocytes were used as the comparator. A comparison of the average psoriasis classical singlet monocytes to the average expression of control classical singlet monocytes also revealed a distinct gene expression profile (Figure 7B), although the changes were not as robust as those observed between doublet and adherent cell populations.
Figure 7. Gene networks in doublets and adhered classical monocytes.
(A) Heat map of different gene expression patterns of CD14+-doublets (Dblt) and tissue culture adhered classical singlet monocytes (Adh) in control (C; n=5) and psoriasis (P; n=5) individuals. Each individual patient’s singlet classical monocytes were used as the comparator. (B) Heat map of gene expression patterns of psoriasis singlet classical monocytes. An average of the classical singlet monocytes was used as the comparator.
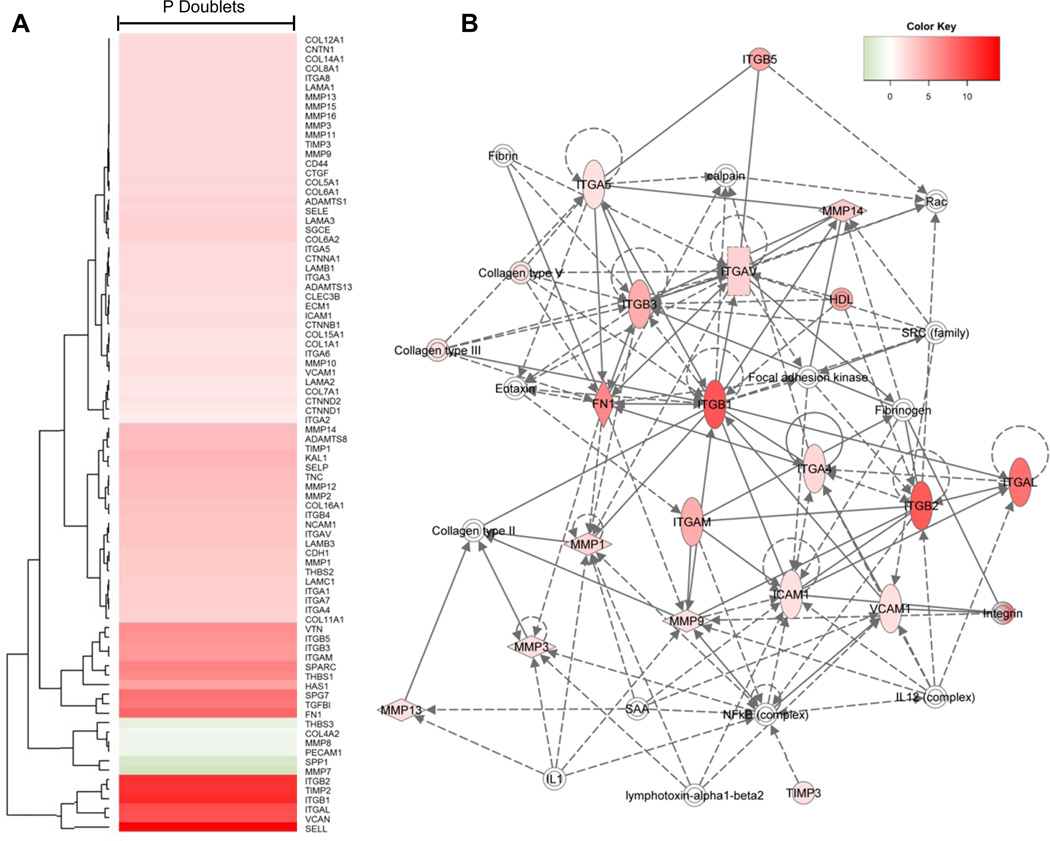
Analysis of the psoriasis versus control doublets indicates upregulation of several integrins, L-selectin (SELL), extracellular matrix proteins (VCAN, FN1, HAS1), and TGFB1 (Figure 8A). IPA analysis of the psoriasis doublet population identified the leukocyte extravasation pathway (shown graphically in Figure 8B) as the top canonical pathway. Network analysis from this pathway identified several directly upregulated alpha-integrins (ITGAV, ITGAL, ITGAM, ITGA4, ITGA5), beta-integrins (ITGB1, ITGB2, ITGB3, ITGB5) and cellular adhesion molecules (ICAM1, VCAM1) as well as several imputed genes of interest in psoriasis versus control doublets, including IL-1, the NFkB complex, the IL-12 complex, and genes known to play a role in cell surface adhesion such as fibrinogen, fibrin, collagen type II, and focal adhesion kinase (FAK).
Figure 8. Gene network of psoriasis doublets.
(A) Psoriasis doublet gene network generated using the average of all psoriasis doublets (n=5); an average of all control doublets (n=5) is the comparator. (B) Leukocyte extravasation signaling pathway network of psoriasis doublets generated by IPA.
Monocyte doublets are increased in KC-Tie2 mice
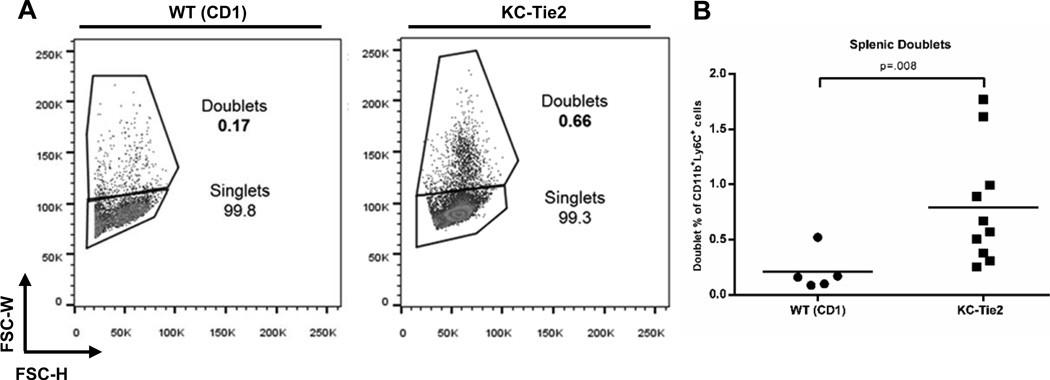
In order to address what mediates the doublet formation, we used the KC-Tie2 skin-specific psoriasiform mouse model. KC-Tie2 mice have been previously shown to develop elevated systemic monocytosis comprised of circulating, proinflammatory CD11b+Ly6Chi monocytes (32); this precedes the spontaneous development of aortic root vascular inflammation. This CD11b+Ly6Chi monocyte population has been previously correlated with the classical and intermediate human monocyte populations (22), thus we were curious whether KC-Tie2 mice would also demonstrate increases in circulating monocyte-monocyte doublets. Similar to our observations in psoriasis patient blood, KC-Tie2 mice also demonstrated an approximate 4 fold increase in monocyte:monocyte doublet formation compared to wild type (WT) controls (0.79% ± 0.17 vs. 0.20% ± 0.08, n=10, n=5, respectively; p=.008; Figure 9B, representative images in Figure 9A), indicating that chronic skin-specific inflammation may influence circulating monocyte aggregation.
Figure 9. Monocyte doublets are increased in KC-Tie2 mice.
(A) Representative figures of doublet gating strategy. Percentages represent doublets within the CD11b+Ly6Chi gate. (B) Splenic doublets are significantly increased in KC-Tie2 mice (n=10) when compared to littermate WT controls (n=5) (p=.008).
Discussion
Psoriasis is an immune-mediated inflammatory autoimmune disease (IMIAD) that has been demonstrated at the epidemiologic level to place patients at a higher risk for cardiovascular complications (6). The linkage of a number of IMIAD’s to cardiovascular disease (CVD) points to a common pathology, but there is a major gap in understanding how cellular inflammation at distant sites predisposes vascular tissue to CVD. The advanced state of validation of specific psoriasis pathogenesis pathways via biologic therapies provides a unique opportunity to link pathomechanisms of IMIADs with CVD. Intervention with current biological therapeutics for psoriasis has demonstrated that psoriasis may be dependent upon LFA-1 expressing leukocytes (myeloid cells and T cells), TNF-producing cells (monocytes, T cells, others), IL-23 (monocytes and DCs), and IL-17 (Th17 cells) (38–44). In humans, a newly defined intermediate monocyte subset (CD14++CD16+) is predictive of CVD, myocardial infarction, and death (11–13). Several publications have implicated CD14++CD16+ intermediate cells as critical mediators of inflammation (45–47). Importantly, there is compelling epidemiologic evidence connecting increases in intermediate monocytes as predictive of fatal cardiovascular complications (11) as well as compelling epidemiologic evidence connecting psoriasis to cardiovascular risk (4, 5). Several relevant review articles have described the critical relationship among monocytes and cardiovascular disease (30, 48) and the concept of chronic inflammation driving cardiovascular outcomes has been validated in numerous murine models, including one psoriasiform mouse (32, 49, 50). The potential cellular mechanism(s) connecting psoriasis with elevated CVD risk has not been definitively addressed. As elevated levels of the intermediate subset have been shown to contribute to CVD (51), we hypothesized that increases in this CD14++CD16+ subset seen in human psoriasis patients may also mediate a link between psoriasis disease pathology and its associated CVD co-morbidities. Increased MPAs in psoriasis patients suggest that circulating monocytes in these individuals may have increased adhesive properties and may play a potential role in the common pathology between psoriasis progression and CVD.
In this study, we asked if psoriasis patients possessed elevated levels of intermediate monocytes and if these monocytes demonstrated elevated adherence properties compared to healthy controls. Increased levels of intermediate (CD14++CD16+) cells in psoriasis circulation confirms a recent study among psoriasis patients (24) as well as a previously reported increase in total CD16++ cells in patients with psoriatic arthritis (23). We demonstrate here that intermediate monocytes correlate with disease severity, are present in involved psoriatic plaque tissue, and preferentially bind to stimulated endothelial HMVEC-D cells. Upon back-gating of the circulating intermediate monocyte population, we noted that many of these cells appear consistent with larger cell populations judged by side scatter. Additionally, when areas where doublets appear were included in the gating strategy, the intermediate population was more prominent in these larger forward scatter areas. Since intermediate monocytes are believed to contribute to, or correlate with, numerous co-morbid conditions for psoriasis, we hypothesized that doublets made up of two intermediate monocytes would be predominant in psoriasis pathology and plaque formation.
Using Amnis Imagestream technology, we demonstrated that monocytes can form monocyte:monocyte and monocyte:lymphocyte pairs in the presence or absence of platelets. Specifically, the predominant monocyte:monocyte doublet pairs consist of one CD14++CD16neg classical monocyte binding to either another CD14++CD16neg classical monocyte, a CD14++CD16+ intermediate monocyte, or a lymphocyte. To better understand which adhesion molecules may mediate the monocyte doublet formation, we screened control and psoriasis PBMCs using a panel of typical surface adhesion markers. Monocytes from psoriasis patients express moderately increased levels of β2 integrins at baseline levels and do not upregulate expression of these adhesion markers to the same extent as healthy control monocytes upon adherence to plastic, suggesting that psoriatic monocytes may be previously “primed” by the circulating psoriasis milieu and are not able to be further stimulated. This potential priming and baseline elevation of CD11b and CD11c may lead to the observed increase in circulating monocyte:monocyte doublets of psoriasis patients. Additionally, co-culture of control monocytes on TNF-α and IL-17A-stimulated HMVEC-D endothelial cells results in an average 2-fold increase in adhesion of intermediate monocytes to stimulated HMVEC-D cells when compared to monocytes cultured on unstimulated HMVEC-D cells, indicating that myeloid cell interaction with activated psoriatic endothelium may contribute to observed enhanced adhesiveness.
To further define the gene expression pattern of classical singlet monocytes, doublets containing CD14+ cells, and monocytes post-adherence, we used an mRNA expression array specific for extracellular matrix and adhesion gene expression and compared monocytes from doublet pairs or monocytes post-adherence on tissue culture plastic to singlet classical monocytes. After adherence to tissue culture plastic, singlet classical monocytes isolated from healthy individuals upregulate genes involved in cellular adhesion including integrins (ITGA3, ITGA7, ITGA8), a cadherin (CDH1), and VCAM-1 to a greater extent than psoriasis monocytes, suggesting some disease-specific adhesion gene response profiles. Singlet plastic-adherent classical monocytes from both control and psoriasis patients upregulate several matrix metalloproteinases (MMP-1, −2, −10, and −14), thrombospondin, and secreted phosphoprotein 1 (SPP1), while downregulating P-selectin (SELP), indicating that plastic adhesion is distinct from cell-cell adhesion. The observed upregulation of MMPs in plastic adhesion when compared to the doublet cell populations suggests that the cell-cell interaction of monocyte-doublet pairs initiates signaling pathways that are distinct from a monocyte plastic-adherence phenotype. MMPs may play an important role in this adhesion as they are known to participate in atherosclerotic plaque stability (52) and are predominantly upregulated upon cellular contact with extracellular matricies. A major difference in gene expression patterns among psoriasis patients compared to healthy individuals occurs primarily following adherence of classical monocytes to tissue culture plastic.
A readily apparent difference between monocytes in doublet pairs compared to monocytes post-plastic adherence highlights upregulation in doublets of integrins, sarcoglycan, collagen type VI, alpha 1 and 2, and disintegrins. Interestingly, upregulation of CD56 (NCAM1) is also observed in the doublet pairs compared to plastic-adherent monocytes. CD56 expression may indicate upregulation of the CD56+ monocyte population (53), previously demonstrated in other autoimmune disorders (54). Specific genes upregulated in doublet pairs, such as ITGA-3 and ADAMTS1 (a disintegrin), suggest that integrins may participate in the cell-cell adhesion we observed using Amnis Imaging technology. Although the function of ADAMTS1 in monocytes is unclear, one report showed that ADAMTS1 expression can be induced during monocyte to macrophage differentiation (55). Interestingly, a polymorphism in the ADAMTS1 allele has been linked to an increased risk of fatal coronary disease, indicating that it may play a role in mediating CVD (56). IPA pathway network analysis of the leukocyte extravasation pathways in psoriasis doublets identified several imputed genes of interest, including genes known to play a role in cell surface adhesion such as fibrin, fibrinogen, a collagen, and focal adhesion kinase, while also including molecules known to participate in important signal transduction pathways impinging upon STAT3 regulation such as the NFκB signaling complex. At baseline, comparison of singlet classical monocytes from psoriasis and healthy control patients demonstrates a distinct gene expression pathway in psoriasis monocytes that may account for their increased propensity to form doublets.
To determine what was mediating the doublet formation, we took advantage of the KC-Tie2 mouse, a psoriasiform model that exhibits chronic skin-specific inflammation and is known to have increases in circulating monocytes and thrombosis formation, as well as to develop aortic root vascular lesions. We show here that these mice also have increased circulating monocyte-monocyte doublets, suggesting that skin inflammation may drive cell differentiation and aggregation, although the precise mechanisms mediating this outcome require further study. Cumulatively, the data suggest that cells forming doublets in circulation upregulate distinct adhesion molecules that potentiate cellular adhesion and may increase the risk of cardiovascular co-morbidities observed in psoriasis patients.
Supplementary Material
Acknowledgements
We thank Mr. Mike Sramkoski of the Case Comprehensive Cancer Center Cytometry Core Facility, Dr. Howard Meyerson and Ms. Amy Graham of University Hospitals Case Medical Center Clinical Pathology Laboratory for their assistance with Amnis flow cytometry. We would also like to thank Mr. Vai Pathak in the Gene Expression & Genotyping Core Facility of the Case Comprehensive Cancer Center for his assistance in collecting the PCR data and Ms. Doina Diaconu for her assistance in mouse breeding.
Grant numbers and sources of support:
National Institutes of Health (P30AR039750, P50AR055508; R01AR051498 KDC, R01AR063437 NLW, T32AR007569-19 JBG, KDC)
References
- 1.Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harbor perspectives in medicine. 2014;4 doi: 10.1101/cshperspect.a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gniadecki R. Regulation of keratinocyte proliferation. Gen Pharmacol. 1998;30:619–622. doi: 10.1016/s0306-3623(97)00419-9. [DOI] [PubMed] [Google Scholar]
- 3.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 4.Gelfand JM, Azfar RS, Mehta NN. Psoriasis and cardiovascular risk: strength in numbers. J Invest Dermatol. 2010;130:919–922. doi: 10.1038/jid.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelfand JM, Mehta NN, Langan SM. Psoriasis and cardiovascular risk: strength in numbers, part II. J Invest Dermatol. 2011;131:1007–1010. doi: 10.1038/jid.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB, Gelfand JM. Attributable risk estimate of severe psoriasis on major cardiovascular events. The American journal of medicine. 2011;124:775, e771–e776. doi: 10.1016/j.amjmed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A, Baer A, Antigua J, Van Voorhees AS, Torigian DA, Alavi A, Gelfand JM. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031–1039. doi: 10.1001/archdermatol.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, van de Kerkhof P, Stahle M, Nestle FO, Girolomoni G, Krueger JG. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Chen H, Nikamo P, Qi Low H, Helms C, Seielstad M, Liu J, Bowcock AM, Stahle M, Liao W. Association of Cardiovascular and Metabolic Disease Genes with Psoriasis. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Bohm M, Fliser D, Heine GH. CD14++CD16+ Monocytes Independently Predict Cardiovascular Events: A Cohort Study of 951 Patients Referred for Elective Coronary Angiography. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Wrigley BJ, Shantsila E, Tapp LD, Lip GY. CD14++CD16+ monocytes in patients with acute ischaemic heart failure. European journal of clinical investigation. 2013;43:121–130. doi: 10.1111/eci.12023. [DOI] [PubMed] [Google Scholar]
- 13.Tapp LD, Shantsila E, Wrigley BJ, Pamukcu B, Lip GY. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J Thromb Haemost. 2012;10:1231–1241. doi: 10.1111/j.1538-7836.2011.04603.x. [DOI] [PubMed] [Google Scholar]
- 14.Dopheide JF, Obst V, Doppler C, Radmacher MC, Scheer M, Radsak MP, Gori T, Warnholtz A, Fottner C, Daiber A, Munzel T, Espinola-Klein C. Phenotypic characterisation of pro-inflammatory monocytes and dendritic cells in peripheral arterial disease. Thromb Haemost. 2012;108:1198–1207. doi: 10.1160/TH12-05-0327. [DOI] [PubMed] [Google Scholar]
- 15.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, Simon DI, Costa MA, Rodriguez B, Sieg SF, Lederman MM. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today. 1996;17:424–428. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 17.Martinez FO. The transcriptome of human monocyte subsets begins to emerge. J Biol. 2009;8:99. doi: 10.1186/jbiol206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genomics. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 20.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–e61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 21.Zawada AM, Rogacev KS, Schirmer SH, Sester M, Bohm M, Fliser D, Heine GH. Monocyte heterogeneity in human cardiovascular disease. Immunobiology. 2012;217:1273–1284. doi: 10.1016/j.imbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 23.Chiu YG, Shao T, Feng C, Mensah KA, Thullen M, Schwarz EM, Ritchlin CT. CD16 (FcRgammaIII) as a potential marker of osteoclast precursors in psoriatic arthritis. Arthritis Res Ther. 2010;12:R14. doi: 10.1186/ar2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunner PM, Koszik F, Reininger B, Kalb ML, Bauer W, Stingl G. Infliximab induces downregulation of the IL-12/IL-23 axis in 6-sulfo-LacNac (slan)(+) dendritic cells and macrophages. J Allergy Clin Immunol. 2013;132:1184–1193. e1188. doi: 10.1016/j.jaci.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Passacquale G, Vamadevan P, Pereira L, Hamid C, Corrigall V, Ferro A. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS One. 2011;6:e25595. doi: 10.1371/journal.pone.0025595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006;79:499–507. doi: 10.1189/jlb.0605318. [DOI] [PubMed] [Google Scholar]
- 27.Czepluch FS, Kuschicke H, Dellas C, Riggert J, Hasenfuss G, Schafer K. Increased pro-atherogenic monocyte-platelet crosstalk in monocyte subpopulations of patients with stable coronary artery disease. J Intern Med. 2013 doi: 10.1111/joim.12145. [DOI] [PubMed] [Google Scholar]
- 28.Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, Marchese P, Frelinger AL, 3rd, Goldberg RJ, Michelson AD. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–1006. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 29.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85:195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 30.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Gao H, Loyd CM, Fu W, Diaconu D, Liu S, Cooper KD, McCormick TS, Simon DI, Ward NL. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067–2075. doi: 10.1038/jid.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hot A, Lenief V, Miossec P. Combination of IL-17 and TNFalpha induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Annals of the rheumatic diseases. 2012;71:768–776. doi: 10.1136/annrheumdis-2011-200468. [DOI] [PubMed] [Google Scholar]
- 34.Ward NL, Loyd CM, Wolfram JA, Diaconu D, Michaels CM, McCormick TS. Depletion of antigen-presenting cells by clodronate liposomes reverses the psoriatic skin phenotype in KC-Tie2 mice. Br J Dermatol. 2011;164:750–758. doi: 10.1111/j.1365-2133.2010.10129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, Askew D, Gilliam AC, McCormick TS, Ward NL. Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–1458. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heimbeck I, Hofer TP, Eder C, Wright AK, Frankenberger M, Marei A, Boghdadi G, Scherberich J, Ziegler-Heitbrock L. Standardized single-platform assay for human monocyte subpopulations: Lower CD14+CD16++ monocytes in females. Cytometry A. 2010;77:823–830. doi: 10.1002/cyto.a.20942. [DOI] [PubMed] [Google Scholar]
- 37.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubertret L, Sterry W, Bos JD, Chimenti S, Shumack S, Larsen CG, Shear NH, Papp KA and C. M. S. Group. CLinical experience acquired with the efalizumab (Raptiva) (CLEAR) trial in patients with moderate-to-severe plaque psoriasis: results from a phase III international randomized, placebo-controlled trial. Br J Dermatol. 2006;155:170–181. doi: 10.1111/j.1365-2133.2006.07344.x. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, Guzzo C, Xia Y, Zhou B, Li S, Dooley LT, Goldstein NH, Menter A, Group AS. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 40.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 41.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, Baumgartner S. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 42.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S, Dooley LT, Reich K, investigators Ps. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 43.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB, investigators Ps. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 44.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C, Group ES, Group FS. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, Luedde T, Weiskirchen R, Trautwein C, Tacke F. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanai H, Iida T, Takeuchi K, Watanabe F, Yamada M, Kikuyama M, Maruyama Y, Iwaoka Y, Hirayama K, Nagata S, Takai K. Adsorptive depletion of elevated proinflammatory CD14+CD16+DR++ monocytes in patients with inflammatory bowel disease. The American journal of gastroenterology. 2008;103:1210–1216. doi: 10.1111/j.1572-0241.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- 47.Shantsila E, Wrigley B, Tapp L, Apostolakis S, Montoro-Garcia S, Drayson MT, Lip GY. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost. 2011;9:1056–1066. doi: 10.1111/j.1538-7836.2011.04244.x. [DOI] [PubMed] [Google Scholar]
- 48.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nature reviews. Cardiology. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Libby P, Nahrendorf M, Pittet MJ, Swirski FK. Diversity of denizens of the atherosclerotic plaque: not all monocytes are created equal. Circulation. 2008;117:3168–3170. doi: 10.1161/CIRCULATIONAHA.108.783068. [DOI] [PubMed] [Google Scholar]
- 50.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 52.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sconocchia G, Keyvanfar K, El Ouriaghli F, Grube M, Rezvani K, Fujiwara H, McCoy JP, Jr, Hensel N, Barrett AJ. Phenotype and function of a CD56+ peripheral blood monocyte. Leukemia. 2005;19:69–76. doi: 10.1038/sj.leu.2403550. [DOI] [PubMed] [Google Scholar]
- 54.Grip O, Bredberg A, Lindgren S, Henriksson G. Increased subpopulations of CD16(+) and CD56(+) blood monocytes in patients with active Crohn's disease. Inflammatory bowel diseases. 2007;13:566–572. doi: 10.1002/ibd.20025. [DOI] [PubMed] [Google Scholar]
- 55.Ashlin TG, Kwan AP, Ramji DP. Regulation of ADAMTS-1, −4 and −5 expression in human macrophages: differential regulation by key cytokines implicated in atherosclerosis and novel synergism between TL1A and IL-17. Cytokine. 2013;64:234–242. doi: 10.1016/j.cyto.2013.06.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabatine MS, Ploughman L, Simonsen KL, Iakoubova OA, Kirchgessner TG, Ranade K, Tsuchihashi Z, Zerba KE, Long DU, Tong CH, Packard CJ, Pfeffer MA, Devlin JJ, Shepherd J, Campos H, Sacks FM, Braunwald E. Association between ADAMTS1 matrix metalloproteinase gene variation, coronary heart disease, and benefit of statin therapy. Arterioscler Thromb Vasc Biol. 2008;28:562–567. doi: 10.1161/ATVBAHA.107.156653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.