Abstract
Using a validated air sampling method we found Acinetobacter baumannii in the air surrounding only 1 of 12 patients known to be colonized or infected with A. baumannii. Patients’ closed circuit ventilator status, frequent air exchanges in patient rooms and short sampling time may have contributed to this low burden.
Background
Acinetobacter baumannii causes a variety of healthcare-associated infections with increased morbidity and mortality.1 Studies have demonstrated a potential for airborne transmission of A. baumannii, which has important implications regarding reducing transmission of A. baumannii in the hospital setting. However, lack of detail about air sampling techniques and absence of patient level information make it difficult to draw conclusions.2–4 The aim of this study was to assess air contamination with A. baumannii in an endemic situation using a validated air sampling impaction method and to examine associated patient factors.
Methods
This study was conducted at the University of Maryland Medical Center in Baltimore, Maryland, between May and December 2013. Subjects were enrolled from the medical, surgical, and cardiac surgery and trauma intensive care units (ICUs). All rooms are single patient occupancy. Rooms have at least 6 air changes per hour and a minimum relative humidity of 30% in winter and 60% in summer. Active surveillance screening for A. baumannii, with perianal sampling at each admission, was performed in all study ICUs during the study period per infection prevention policies. Patients were identified as infected or colonized with A.baumannii if they had any culture (surveillance or clinical) positive for growth of A. baumannii within the preceding 10 days.
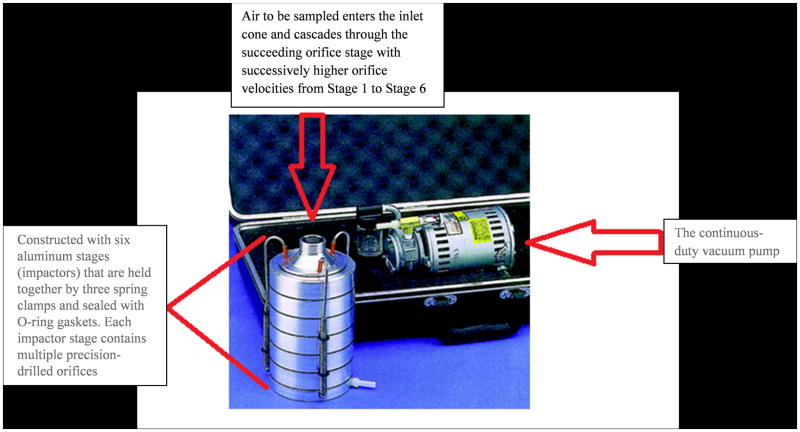
For each patient, air surrounding the patient was sampled for 1 hour, 3 feet from the head of the bed with a Six-Stage Viable Andersen Cascade Impactor (ACI) (ThermoScientific) using standard methodology used in other airborne transmission studies.5 Previous studies have shown that relative humidity is stable during the first hour of air sampling which makes conditions ideal for survival of viable bacteria.5 After that period of time there is a potential for drying of the agar plate which may result in difficulty culturing the bacteria. The Impaction method is designed to separate particles from air flow and embed them onto an agar surface. The Six-Stage Viable Andersen Cascade Impactor has a vacuum pump that draws air through at a speed of 28.3 liters/minute through six layers of agar plates, each layer composed of orifices of decreasing diameter, representing the human respiratory tract (See figure 1). All air sampling was performed between the hours of 9am and 5pm. RambaCHROM™ Acinetobacter selective agar (Gibson Bioscience, KY) plates were used for all air samples, this agar selects for A. baumannii, regardless of susceptibilities.6 Plates were then incubated at 37°C in ambient air for 24 hours. Identification confirmation and susceptibility testing were performed using the Vitek II system (BioMerieux, Marcy Etoile, France).
Figure 1.
Patient demographic and clinical data was obtained. Multidrug-resistant A. baumannii was defined as susceptible to ≤ 2 classes of antibiotics, a standard definition used in other studies.7 For patients with a positive sputum sample for A. baumannii, presence of pneumonia as defined by CDC/NHSN criteria was noted.8
Results
Air surrounding twelve patients known to be infected or colonized with A. baumannii was sampled. A. baumannii was identified from the air samples surrounding 1(8%) of the 12 patients. Table 1 gives the characteristics of all patients sampled, including patient 1 who had the positive air sample. The mean age of the group was 59 years, 33% were women. The majority of patients (7 out of 12, 58%) were transferred from another acute care hospital, 1 (8%) patient from long term care and the remainder from home. MDR A. baumannii was found in 7 (58%) of 12 patients. Sputum culture was positive for A. baumannii in 9 (75%) of 12 patients and 2 (22%) of these patients met criteria for pneumonia. Closed circuit mechanical ventilation was present in 7 (58%) of 12.
Table 1.
Characteristics of patients infected or colonized with A. baumannii who had surrounding air sampled.
| Patient | LOS1 | Days from Culture2 | Culture Site3 | PNA4 | MDR5 | Antibiotic6 | MV7 | UC8 | CVC9 | Diarrhea |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | 7 | CA, S | No | Yes | NS | Yes | Yes | Yes | Yes |
| 2 | 17 | 7 | PA, S | No | Yes | S | No | Yes | No | No |
| 3 | 19 | 8 | S | No | Yes | NS | No | No | No | Yes |
| 4 | 8 | 1 | S | No | Yes | S | Yes | No | No | Yes |
| 5 | 11 | 8 | PA, S | Yes | No | S | Yes | Yes | Yes | Yes |
| 6 | 16 | 5 | S | No | No | S | Yes | Yes | Yes | Yes |
| 7 | 3 | 3 | PA, S | Yes | Yes | S | Yes | Yes | Yes | No |
| 8 | 10 | 4 | PA | - | Yes | NS | No | Yes | Yes | No |
| 9 | 10 | 7 | PA, W | - | Yes | NS | Yes | Yes | Yes | No |
| 10 | 65 | 6 | S | No | No | S | Yes | No | Yes | Yes |
| 11 | 6 | 6 | B | - | No | S | Yes | Yes | Yes | No |
| 12 | 11 | 5 | S | No | No | S | Yes | Yes | No | No |
LOS = Length of stay from hospital admission to time of air sample, in days
Days from time of most recent Acinetobacter baumannii positive culture to time of air sample
Culture site, where B = Blood culture; CA = Catheter tip culture; PA = Perianal surveillance culture; S = Sputum culture; W=wound
PNA = Presence of pneumonia as defined by the National Healthcare Surveillance Network/Centers for Disease Control (8)
MDR = Multidrug-resistant A. baumannii; an isolate was considered multidrug resistant if it was non-susceptible to at least one agent in at least three antimicrobial classes
All patients were receiving antibiotics at the time of air sampling; S = indicated the antibiotic received was susceptible and NS = non-susceptible 7
MV = Mechanical ventilation at the time of air sampling; all ventilation was closed-circuit
UC = Urinary catheter present at the time of air sampling
CVC = Central venous catheter present at the time of air sampling
Discussion
We cultured A. baumannii from air surrounding only 1 of 12 patients who were infected or colonized with A. baumannii.1 This patient had the longest length of stay at 77 days and it is possible that his environment may have been more saturated due to time. However, the lack of A. baumannii found differs from the small number of other studies where A. baumannii has been found more frequently in the air. 2–4 Published studies on this topic are few; use differing air sampling techniques, and note minimal patient level data making it difficult to draw conclusions.2–4
Air sampling methods are categorized as passive or active.9 The use of settle plates is a common method of passive air sampling. Uncovered agar plates are exposed to the air and when the plate is cultured one can identify which bacteria fell from the air onto the plate.10 This technique is simple, inexpensive but not sensitive and gives no quantitative impression of bacteria in the air. Conversely, an active method, such as the air impactor used in this study, is more beneficial if trying to assess a concentration of inhalable viable particles.10 Settle plates, however, have been used to identify A. baumannii in the air in outbreak settings. These studies are infrequent and provide little detail regarding patient level factors. An A. baumannii outbreak investigation in 1987 using this technique was the first to suggest that it may be aerially disseminated.2 A recent study of trauma-ICU patients colonized or infected with MDR A. baumannii found that in 52% of cases the air surrounding patients was contaminated by use of settle plates.3 A 2011 study performed in China, using a similar air impactor to what we used in this study, found A. baumannii in 16 air samples.4 Air was sampled for just 10 minutes using the 6 stage Anderson impactor. However, information regarding number of air samples taken, proximity of sampler to patients or patient factors was not provided.
Our findings differ from other most recent studies on the topic.3 Possible reasons for our findings are as follows. The majority (9 of 12, 75%) of patients in this study were on closed circuit mechanical ventilation system at time of sampling. It is plausible that those on closed circuit ventilation systems are less likely to have airborne dissemination. Another potential reason is the dilution effect by air exchanges. Our ICU has at least 6 air exchanges per hour in patient rooms. This may contrast with older studies which may have taken place when ventilation of rooms would not have been as established and air exchange not as frequent.2 Eight (66%) of twelve patients in our study were on antibiotics to which the A. baumannii was susceptible. The antibiotics received may have decreased patient burden of A. baumannii and thus contributed to lack of aerial dissemination. Also air contamination may not be continuous and may not have been captured in our 1 hour sampling time.
This study questions whether A. baumannii is commonly spread by airborne transmission. It is possible due to negative publication bias other studies finding infrequent air contamination with A. baumannii may not have been published. Further research is needed with a larger number of patients, under varying conditions such as mechanical ventilation, with repeated and longer air sampling at different times. It would be important to note activities in the room such as changing of linens and manipulation of the ventilator such as during suctioning. These studies could determine if and which patients are more likely to contaminate the surrounding air and establish a potential role for airborne transmission of this important pathogen.
Acknowledgments
Financial support. KAT is supported by National Institutes of Health (NIH) NIH Career Development Grant 1K23AI08250-01A1.
Financial support. ADH is supported by NIH grant 5K24AI079040-05 and Agency of Healthcare Research and Quality (AHRQ) grant 1 R01 HS022291-01.
Footnotes
Reprints will not be ordered.
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
References
- 1.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 2.Allen KD, Green HT. Hospital outbreak of multi-resistant Acinetobacter anitratus: an airborne mode of spread? J Hosp Infect. 1987;9(2):110–119. doi: 10.1016/0195-6701(87)90048-x. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Fajardo-Aquino Y, Arheart KL, et al. Aerosolization of Acinetobacter baumannii in a trauma ICU*. Crit Care Med. 2013;41(8):1915–1918. doi: 10.1097/CCM.0b013e31828a39c0. [DOI] [PubMed] [Google Scholar]
- 4.Gao J, Zhao X, Bao Y, et al. Antibiotic resistance and OXA-type carbapenemases-encoding genes in airborne Acinetobacter baumannii isolated from burn wards. Burns. 2014;40(2):295–299. doi: 10.1016/j.burns.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Anderson AA. New sampler for the collection, sizing and enumeration of viable airborne particles. J Bacteriol. 1958;76(5):471–484. doi: 10.1128/jb.76.5.471-484.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajao AO, Robinson G, Lee MS, et al. Comparison of culture media for detection of Acinetobacter baumannii in surveillance cultures of critically-ill patients. Eur J Clin Microbiol Infect Dis. 2011;30(11):1425–143. doi: 10.1007/s10096-011-1237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strassle P, Thom KA, Johnsonm JK, et al. The effect of terminal cleaning on environmental contamination rates of multidrug-resistant Acinetobacter baumannii. Am J Infect Control. 2012;40(10):1005–1007. doi: 10.1016/j.ajic.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Srikanth P, Sudharsanam S, Steinberg R. Bio-aerosols in indoor environment: composition, health effects and analysis. Indian J Med Microbiol. 2008;26(4):302–312. doi: 10.4103/0255-0857.43555. [DOI] [PubMed] [Google Scholar]
- 10.Napoli C, Marcotrigiano V, Montagna MT. Air sampling procedures to evaluate microbial contamination: a comparison between active and passive methods in operating theatres. BMC Public Health. 2012;12:594. doi: 10.1186/1471-2458-12-594. [DOI] [PMC free article] [PubMed] [Google Scholar]