Asthma is a common chronic disease that affects care during pregnancy in approximately 8% of pregnant women.1 Asthma during pregnancy has adverse effects on both mothers and infants, including increased risk for preeclampsia, pregnancy-induced hypertension, preterm delivery, and birth weight of less than 2,500 g.2,3 A commonly quoted generalization based on data from several small and select study populations is that the course of asthma worsens in one-third of pregnant women, improves in one-third, and remains unchanged in one-third.4
In a large population-based cohort, we studied 8,149 pregnant women with asthma from a larger group of 112,171 pregnant white and black women aged 15 to 44 years who were enrolled in TennCare, Tennessee’s Medicaid managed care program from 1995 to 2001 and who had at least 180 days of continuous enrollment before their last menstrual period. The 112,171 eligible women represented 48% of all deliveries to women enrolled in Tennessee Medicaid and 21% of all live births in Tennessee during the study period. Data were obtained from linked Tennessee Medicaid database and vital records files, providing information on maternal demographics, International Classification of Diseases, Ninth Revision (ICD-9) diagnoses, and pharmacy claims. The protocol was approved by the institutional review boards of Vanderbilt University and the Tennessee Department of Health. A more complete discussion of these methods is contained in the article by Enriquez et al.5 Asthma control was defined by use of short-acting β-agonists. Pharmacy claims containing short-acting β-agonist dose and days of supply were available for all study participants from 25 weeks before last menstrual period until the end of pregnancy. These data were used to determine short-acting β-agonist use for each week from 20 weeks before last menstrual period through the last full week of pregnancy or 40 weeks. Women were classified as current users for each week that was within 30 days of their last dispensed prescription; otherwise, they were classified as nonusers for that week. In Figure 1 (bottom), 95% confidence bands were estimated by bootstrap simulations.6
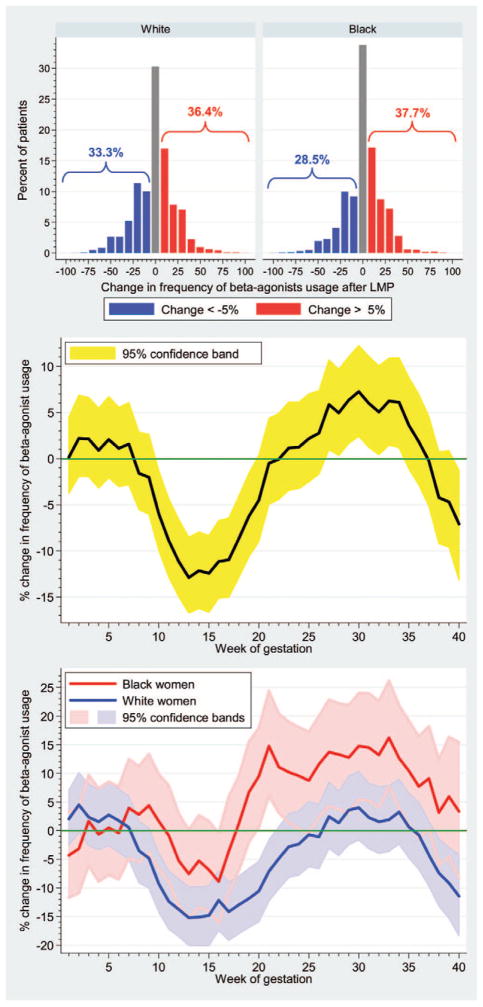
Figure 1.
Effect of pregnancy on asthma control as measured by short-acting β-agonist use. Top, Proportion of white and black women whose β-agonist use increased or decreased during pregnancy. Black women had a significantly greater increase in β-agonist use than did white women (P = .002). Center and Bottom, Change in percentage of study participants actively using short-acting β-agonists each week compared with the average percentage using each week for the 20 weeks before their last menstrual period (baseline). Center, Data for all study subjects; the yellow band gives the 95% confidence interval. Asthma was significantly improved in weeks 10 to 19 and was significantly exacerbated in weeks 27 to 34 (P < .05). Bottom, Same data subdivided by race.
Among the 8,149 pregnant asthmatic women, 77% used short-acting β-agonists during follow-up. Overall, pregnant women had an initial decrease in β-agonist use in the first trimester before experiencing peak use in the third trimester, with 31.8% experiencing worsening asthma control, 31.4% experiencing unchanged asthma control (defined as <5% variation from baseline), and 36.8% experiencing improved asthma control during pregnancy. Changes in use for black and white women are given in Figure 1 (top). Figure 1 (center) shows that asthma control improves during the first half of pregnancy and then worsens. Among pregnant white women control improves during the first half of pregnancy and then reverts to control similar to baseline (Figure 1). However, black women show little change in asthma control in the first 20 weeks and then experience worsening control in the last half of their pregnancy.
The initial decrease in albuterol use early in pregnancy is consistent with previous work, from both our group and other groups, showing that despite national guidelines that recommend continuation of maintenance asthma medications,7 use of inhaled corticoste-roids and rescue medications decreases overall during pregnancy.5 Alternatively, consistent with prior work,8 the group whose asthma improved may have experienced the improvement early in pregnancy, resulting in decreased overall use in this interval. The rebound in albuterol use early in the third trimester may be attributable to previously stopping use of controller medications or may be partly attributable to the physiologic effects of pregnancy on asthma control.4 Finally, the racial differences in worsening asthma control during the third trimester compared with prepregnancy seen in blacks but not whites is important and requires further study.
We present the first study of the time course and trends in asthma control during pregnancy in a large population-based cohort. This study affirms the classic adage about change in asthma severity during pregnancy, with approximately one-third of pregnant women showing improved control, one-third experiencing worsened control, and one-third remaining unchanged, as measured by change in pharmacy claims for short-acting β-agonist prescriptions. This pattern is similar for both blacks and whites; however, compared with their prepregnancy states, black women were more likely to have worsened asthma control during pregnancy than white women.
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health (NIH) Mid-Career Investigator Award K24 AI 077930 (to Dr Hartert) and NIH U01 NIH HL (project principal investigator, Dr Hartert).
We are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration and the Tennessee Department of Health, Office of Policy, Planning & Evaluation for providing the data.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol. 2003;13:317–324. doi: 10.1016/s1047-2797(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 2.Kallen B, Rydhstroem H, Aberg A. Asthma during pregnancy—a population based study. Eur J Epidemiol. 2000;16:167–171. doi: 10.1023/a:1007678404911. [DOI] [PubMed] [Google Scholar]
- 3.Demissie K, Breckenridge MB, Rhoads GG. Infant and maternal outcomes in the pregnancies of asthmatic women. Am J Respir Crit Care Med. 1998;158:1091–1095. doi: 10.1164/ajrccm.158.4.9802053. [DOI] [PubMed] [Google Scholar]
- 4.Schatz M. Interrelationships between asthma and pregnancy: a literature review. J Allergy Clin Immunol. 1999;103:S330–S336. doi: 10.1016/s0091-6749(99)70258-7. [DOI] [PubMed] [Google Scholar]
- 5.Enriquez R, Wu P, Griffin MR, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol. 2006;195(1):149–53. doi: 10.1016/j.ajog.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 6.Moulton LH, Zeger SL. Analyzing repeated measures on generalized linear models via the bootstrap. Biometrics. 1989;45:381–394. [Google Scholar]
- 7.NAEPP Expert Panel Report. Managing asthma during pregnancy: recommendations for pharmacologic treatment—2004 update. J Allergy Clin Immunol. 2005;115:34–46. doi: 10.1016/j.jaci.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988;81(3):509–17. [PubMed] [Google Scholar]