Abstract
Our movements are shaped by our perception of the world as communicated by our senses. Perception of sensory information has been largely attributed to cortical activity. However, a prior level of sensory processing occurs in the spinal cord. Indeed, sensory inputs directly project to many spinal circuits, some of which communicate with motor circuits within the spinal cord. Therefore, the processing of sensory information for the purpose of ensuring proper movements is distributed between spinal and supraspinal circuits. The mechanisms underlying the integration of sensory information for motor control at the level of the spinal cord have yet to be fully described. Recent research has led to the characterization of spinal neuron populations that share common molecular identities. Identification of molecular markers that define specific populations of spinal neurons is a prerequisite to the application of genetic techniques devised to both delineate the function of these spinal neurons and their connectivity. This strategy has been used in the study of spinal neurons that receive tactile inputs from sensory neurons innervating the skin. As a result, the circuits that include these spinal neurons have been revealed to play important roles in specific aspects of motor function. We describe these genetically identified spinal neurons that integrate tactile information and the contribution of these studies to our understanding of how tactile information shapes motor output. Furthermore, we describe future opportunities that these circuits present for shedding light on the neural mechanisms of tactile processing.
Keywords: tactile information, spinal cord, sensorimotor integration, genetically identified spinal neurons
the neural control of motor activity is a product of signals arising from three main sources: supraspinal centers, spinal networks, and peripheral sensory afferents. In most general terms, supraspinal centers generate motor commands and select appropriate sequences of motor activity; spinal networks translate descending motor commands into muscle activity. Sensory information conveys important information about the external world (exteroceptive) and about the internal self (interoceptive) to central networks. Whereas basic motor activity can be generated by spinal networks, with or without motor commands from supraspinal networks, the achievement of high complexity, range, accuracy, and fluency of movement within changing and challenging environments is impossible without sensory information (Acevedo and Diaz-Rios 2013; Akay et al. 2014). Sensory information is vital to ensuring appropriate motor execution by evaluating and adjusting motor commands during ongoing motor activity.
Sensory information can be arranged into functional groups or modalities, such as sight, hearing, balance, taste, smell, temperature, nociception, proprioception, and touch. Proprioception contributes to the control of movement by communicating muscle properties and body position. Tactile information is also integral to the control of movement, as it guides movements by providing information about physical features of the external world. This occurs via a process where tactile stimuli are detected and transduced by specialized mechanoreceptors in the skin [for review, see Abraira and Ginty (2013)] and transmitted via afferent fibers from the periphery to the central nervous system (CNS). These signals enter the spinal cord and are directed to the sensory areas of the brain via brain stem nuclei and the thalamus. At the cortical level, tactile information is processed, organized, and interpreted, which in turn, is used to guide behaviors by adjusting descending motor commands. In parallel to the perception of tactile signals generated at the cortical level, tactile signals are also integrated at the level of the spinal cord. That is, in addition to sending projections that travel rostrally up the dorsal column, cutaneous afferents project collaterals that branch out within the spinal cord and terminate onto spinal interneurons (INs) (Brown et al. 1981). Thus the spinal cord serves as a site of active integration of tactile information, putatively for the purpose of shaping tactile perception and/or sensorimotor integration.
With regards to the perception of touch stimuli, little is known about how it is influenced by integration of tactile information at the level of the spinal cord. However, it has been established that movement itself can both stimulate sensory receptors and suppress cutaneous sensation (Prochazka 1989). Indeed, the perceived intensity of cutaneous stimulation is reduced during movement (Duysens et al. 1995; Milne et al. 1988). This may be a result of suppressed sensory transmission at the level of the spinal cord, as suggested by experiments in monkeys where cutaneous sensory-evoked potentials recorded in the spinal cord were reduced during active hand movement (Seki and Fetz 2012). Hence, spinal networks likely contribute to the final tactile perception.
The involvement of tactile information in shaping motor control through spinal circuitry is much more established than its role in tactile perception [briefly reviewed in Panek et al. (2014)]. In humans, motor reflexes can be evoked by electrical stimulation of cutaneous afferents applied through surface or ring electrodes. The early components of these reflexes were revealed to be mediated by spinal circuits rather than through long ascending and descending pathways connecting the brain and the spinal cord (Jenner and Stephens 1982). These circuits denote a strong coupling of low-threshold mechanoreceptors (LTMRs) to both hand and leg motoneurons (Fallon et al. 2005; McNulty et al. 1999). As described below, these observations are part of a growing body of work demonstrating integration of tactile information by spinal circuits involved in motor control. However, the precise outcome of this integration in actually sculpting motor activity has not been well understood. Recent studies have started to shed light on spinal circuits processing tactile signal and their role in motor function (Bourane et al. 2015; Bui et al. 2013; Fink et al. 2014). In this review, we provide an overview of the spinal circuits that have been shown to be involved in sensorimotor integration of tactile information. Furthermore, we discuss the properties of these spinal circuits, including their location, connectivity, and interactions, with spinal motor circuits. We focus on spinal INs that have been identified recently using molecular and genetic studies and that have been ascribed to sensorimotor integration. The study of these INs has added to our understanding of how tactile information can shape motor activity through spinal circuitry. Finally, we outline potential avenues for research using genetically identified spinal neurons to address outstanding questions.
Properties of Spinal Circuits Processing Tactile Information
Location.
The most likely location of spinal INs processing tactile information for motor control can be estimated based on the topographical distribution of the central terminals of LTMRs within the spinal cord. The spinal cord is composed of INs located in Rexed laminae I–VIII and X and motoneurons located in laminae IX (Rexed 1952, 1954). Whereas nociceptive sensory neurons predominantly contact spinal INs in the most superficial laminae (I and II) of the dorsal horn, tracing studies of myelinated cutaneous afferents have described the most dense labeling of terminals to be found in laminae III–V of the spinal cord in rodents (Levinsson et al. 2002; Molander and Grant 1986; Panneton et al. 2005), cats (Brown et al. 1981), and primates (Florence et al. 1991). Consistent with this distribution of tactile afferents within the spinal cord, electrophysiological recordings of touch-evoked sensory inputs to spinal neurons in laminae III–V have been reported in primates (Wagman and Price 1969), rats (Woolf and King 1989), hamsters (Schneider 2005), and cats (Koerber et al. 1990; Sahai et al. 2006), amongst others. Some of these laminae III–V spinal neurons receiving sensory inputs from LTMRs have been identified as premotor INs [i.e., neurons projecting directly to motoneurons (Egger and Wall 1971; Hongo et al. 1989a, b)], whereas some have been observed in more ventral laminae VI and/or VII (Edgley and Jankowska 1987; Moschovakis et al. 1992; Stepien et al. 2010). This anatomical specificity reflects functional differences that are embedded in the spinal network connectivity.
Shaping specific forms of motor activity.
Whereas the precise connectivity between tactile afferents and spinal neurons has not been fully characterized, tactile information has been shown to be particularly important for several motor functions, such as the control of grip strength (Jenner and Stephens 1982; Johansson and Flanagan 2009). During hand grasp, activation of tactile afferents increases hand muscle contraction (Collins et al. 1999; Johansson et al. 1987). Local anesthesia of the fingers or hands precludes human subjects from performing gripping tasks accurately (Augurelle et al. 2003; Johansson and Westling 1984). Furthermore, this deficit could not be compensated by consciously attempting to increase grip strength. Tactile afferents are also involved with locomotor function at the level of the spinal cord. Activation of LTMRs can evoke sets of stereotyped limb reflexes [e.g., tripping perturbations; see Drew and Rossignol (1987); Forssberg et al. (1977); Quevedo et al. (2005a)], as well as modulate the timing (Duysens and Pearson 1976) and levels of muscle activation during locomotion (Duysens and Loeb 1980; Loeb et al. 1987). These reflexes have been suggested to be involved in fine-tuning the timing of locomotor activity (MacKay-Lyons 2002), as well as stabilizing gait in the wake of unexpected perturbations (Bunday and Bronstein 2009; Choi et al. 2013; Zehr et al. 1998). These results underline the importance of the recruitment of spinal circuits by tactile afferents to perform precise motor functions.
Gating of sensory integration at the level of the spinal cord by motor circuits.
As alluded earlier, the relationship between tactile inputs and spinal motor circuits is not strictly unidirectional, where sensory inflow adjusts motor activity through spinal circuitry. Rather, it is bidirectional; there is ample evidence that sensory transmission to the spinal cord from LTMRs is, in turn, modulated by motor activity. For instance, motor reflexes evoked by tactile input are phasically modulated during locomotion in cats (Degtyarenko et al. 1998; Forssberg et al. 1977; Quevedo et al. 2005a, b) and in humans (Baken et al. 2005; Van Wezel et al. 1997; van Wezel et al. 2000; Zehr et al. 1997, 1998). Cutaneous reflexes also seem to be modulated by the type of activity during which they are evoked, as observed in humans, for example, during different gaits (Duysens et al. 1993; Hoogkamer et al. 2012), fine vs. coarse hand movements (Evans et al. 1989), and different limb or hand postures (Garnett and Stephens 1981; Gibbs et al. 1995). The modulation of cutaneous reflexes by motor activity may be due to gating of sensory transmission, which seems to occur at many levels of the CNS, including at the level of the spinal cord (Seki and Fetz 2012). However, there is also evidence suggesting that modulation of spinal INs integrating tactile information for motor control may contribute to gating of cutaneous reflexes (Burke et al. 2001; Quevedo et al. 2005a).
It is not clear whether the bidirectional interactions between tactile inputs and motor networks are mediated strictly by spinal circuits or whether they rely on supraspinal control. Indeed, motor control involves hierarchical circuits spanning both spinal and supraspinal networks that enable motor adaptation and motor learning (Kawato et al. 1987). Likewise, sensorimotor integration is distributed across the CNS hierarchy. The distance of a sensorimotor circuit to the musculoskeletal system (spinal circuits being closer, supraspinal circuits being farther) introduces a relative delay to when sensory information influences motor activity (Shadmehr et al. 2010). Therefore, the understanding of at which levels of the CNS sensorimotor interactions occur is important to uncover the roles of these widely distributed sensorimotor circuits. In spite of the truly heroic efforts in earlier decades to demonstrate the presence of spinal circuits integrating tactile afferents, ascribing detailed motor roles to particular groups of spinal INs has been a difficult goal to achieve. The advent of recent genetic approaches to studying spinal neurons has enabled combinations of electrophysiological, cell labeling, and behavioral testing techniques that have provided strong evidence that spinal circuits integrating tactile information play crucial roles in ensuring proper motor control (Bourane et al. 2015; Bui et al. 2013). These genetic approaches are based on the insight that during development, populations of neurons emerge from common progenitor pools that arise from the interaction of inductive signals released by structures, such as the roof plate, floor plate, and notochord (Jessell 2000; Stifani 2014). These signals promote the activation of many transcription factors involved in a diverse set of developmental processes, such as cell cycle progression and exit, cell migration, axon guidance, and establishment of neurotransmitter phenotype, to name a few. Each progenitor pool will express a unique combinatorial set of transcription factors (Fig. 1F). The partitioning of spinal neurons into genetically identified populations has been particularly successful (Alaynick et al. 2011; Francius and Clotman 2014; Lu et al. 2015; Stifani 2014). Herein, we describe those populations of genetically identified spinal neurons that have been determined to integrate tactile afferents for the purpose of shaping motor activity and the fundamental insights that the study of these neurons has provided.
Fig. 1.
Genetically identified spinal interneurons (INs) implicated in microcircuits integrating tactile information for sensorimotor control. A: diagram representing the typical spinal circuitry integrating tactile sensory information, where a low-threshold mechanoreceptor (LTMR) sensory neuron (SN) enters the spinal cord and bifurcates to project to spinal INs and neurons in supraspinal centers. Subpopulations of spinal INs (Spinal INs) receiving tactile inputs project to spinal targets, including motoneurons of the lateral motor column (LMC) and medial motor column (MMC) and/or to supraspinal centers. Roman numerals refer to the Rexed laminae. B–E: diagrams representing dI3 INs (B), retinoic acid receptor-related orphan receptor α (RORα) INs (C), dI4 INs (D), and dI1 INs (E). SNs (blue) connect to spinal IN populations. The established implication of some LTMR subtypes is indicated. IN connections to and from supraspinal centers are indicated. Ascending and descending projections are not represented at their exact location for simplicity purposes. B: dI3 INs project to motoneurons as well as to the lateral reticular nucleus (LRN). Note the weaker connection to MMC motoneurons. vGluT2, vesicular glutamate transporter 2. C: RORα INs project to both V0 cholinergic (V0c) INs (maroon) and motoneurons. RORα INs also receive inputs from the cortex via corticospinal tracts (CST) and the vestibular nucleus via the lateral vestibulospinal tract (LVST). D: dI4 INs are involved in a presynaptic axo-axonic inhibition of sensory projection onto spinal INs (pink). dI4 INs that mediate axo-axonic inhibition of proprioceptive afferents are not depicted. dI4 IN supraspinal connections have yet to be identified. GAD2, glutamate decarboxylase 2; GlyT2, glycine transporter 2; NPY, neuropeptide Y. E: two defined subpopulations of dI1 INs are represented by having either ipsilateral (ipsi; light purple) projections to the cerebellum via the ventral spinocerebellar tract (vSCT) or contralateral (cont; deep purple) projections to the dorsal SCT (dSCT). Subpopulation-specific genetic markers are listed above the IN symbols; a number of shared genetic identifiers are listed below the IN symbol. F: transcriptional code involved in the development of genetically identified spinal INs that integrate tactile information. See Table 1 for more description of the transcription factors listed. Note that the progenitor domain or domains from which RORα INs originate are not known [panel F adapted from Lu et al. (2015) under CC-BY].
dI3 INs
dI3 INs represent one of six identified, early born, dorsally derived populations of INs (dI1–dI6) of the spinal cord (Fig. 1B). These neurons were described initially following the analysis of transcription factors active in the spinal dorsal horn during development, in particular, through the assessment of the effects of gene knockouts on expression patterns of transcription factors (Gross et al. 2002; Muller et al. 2005; Nakada et al. 2004; Wine-Lee et al. 2004). As a result, the dI3 IN population is well characterized by the expression of Brn3a/b, Tlx3, Gsh1/2, and Ascl1, amongst other transcription factors in progenitor and/or postmitotic cells derived from this population (Mizuguchi et al. 2006; Zou et al. 2012). More importantly, they are characterized further by the expression of Isl1, a LIM homeodomain transcription factor that is also expressed in motoneurons but uniquely found in dI3 INs amongst neurons of the dorsal spinal cord (Liem et al. 1997; Pfaff et al. 1996). This exclusive expression of Isl1 amongst dorsal neurons has enabled the application of genetic, electrophysiological, and immunohistochemical methods to characterize dI3 INs and to determine their role in motor control (Bui et al. 2013; Goetz et al. 2015; Pivetta et al. 2014; Stepien et al. 2010).
Conditional expression of yellow fluorescent protein by Isl1 has been used to determine that dI3 INs are primarily located in laminae V–VII at similar densities in cervical and lumbar spinal segments (Bui et al. 2013). Cell labeling and in situ hybridization for vesicular glutamate transporter 2 (vGluT2) mRNA showed that dI3 INs are predominantly glutamatergic with ipsilateral projections (Bui et al. 2013). Retrograde tracing of synaptic inputs to motoneurons using modified rabies virus demonstrated that dI3 INs directly contact motoneurons in the spinal cord (Goetz et al. 2015; Stepien et al. 2010). They are therefore part of a large group of “last-order” spinal INs that control motor activity through direct excitation of motoneurons (Brownstone and Bui 2010).
Of these last-order spinal INs, dI3 INs belong to the subset that receives sensory inputs. Indeed, vGluT1 labeling revealed that dI3 INs receive primary afferent input (Alvarez et al. 2004). A large portion of these vGluT1-immunoreactive afferents were characterized by an absence of expression of parvalbumin, suggesting that much of the sensory input to dI3 INs is not proprioceptive in nature but may rather originate from cutaneous afferents (Bui et al. 2013). Transgenic mice (named dI3OFF)—in which dI3 IN neurotransmission was genetically silenced through conditional ablation of vGluT2, a protein used by these INs for glutamatergic synaptic transmission—were used to define further the nature of the cutaneous afferents that project to dI3 INs (Bui et al. 2013). In control mice, in vivo or in vitro stimulation of the sural nerve, which predominantly carries cutaneous information (Peyronnard and Charron 1982), resulted in strong motor reflexes. The early components of this reflex exhibited latencies characteristic of a disynaptic pathway. Concurrently, recordings of dorsal root potentials demonstrated that the reflex was generated by stimulation of low-threshold myelinated fibers corresponding to low-threshold myelinated Aβ-LTMRs. This reflex response was completely absent in dI3OFF mice, suggesting that dI3 INs are involved in a disynaptic microcircuit linking cutaneous afferents (conveying touch) with motoneurons (Figs. 1B and 2) (Bui et al. 2013).
Fig. 2.
Demonstration of a genetically identified spinal circuit integrating tactile input for motor control. A: scheme of isolated in vitro spinal cord preparation with sural nerve left in continuity used to test for the presence of reflexes evoked by stimulation of tactile inputs. Stimulating electrodes (red) were placed on the sural nerve, which is predominantly cutaneous, and the mixed sensory tibial nerve distal to the sural nerve branchpoint. Recording suction electrodes (green) were placed on the ipsilateral lumbar L5 dorsal (sensory) and L5 ventral (motor) roots. B: to demonstrate the predominantly cutaneous nature of the sural nerve, electroneurogram (ENG) recordings of L5 dorsal root potentials (DRPs), in response to sural nerve or tibial nerve stimulations, were made. In this example, note the longer latency of the sural nerve DRP compared with the tibial nerve DRP, which is due to the proprioceptive component of the tibial nerve. Furthermore, the threshold to evoke a DRP was higher for sural nerve [cutaneous (cut.) DRP; 3 μA] than tibial nerve [proprioceptive (proprio.) DRP; 2 μA] stimulation. C: to test for motor reflexes in response to stimulation of cutaneous afferents, ENG recordings of L5 ventral root responses to multiple stimulation pulses applied to the sural nerve were made. A putative disynaptic reflex response, highlighted in the dashed box, can be observed in control mice but not in animals that had dI3 INs genetically silenced (dI3OFF). D: diagram of experimental design used to test for the presence of motor reflexes evoked by tactile inputs conveyed by the sural nerve and mediated by dI3 INs. Chronically implanted electrodes into gastrocnemius and tibialis anterior muscles of adult control and dI3OFF mice enabled electromyographic (EMG) recordings in awake, behaving mice. E: similar to in vitro tests, EMG recordings of gastrocnemius to multiple stimulation pulses applied to the sural nerve show a putative disynaptic reflex response (dashed box) that is absent in dI3OFF mice. F: diagram representing dI3 INs (dI3) as part of a disynaptic pathway among SNs that are LTMRs, dI3 INs, and motoneurons (MN) [adapted from Bui et al. (2013) with permission from Elsevier]. DRG, dorsal root ganglion.
To determine the functional role of the tactile sensorimotor circuit mediated by dI3 INs, motor function of the dI3OFF mice was tested in a number of behavioral tests. dI3OFF mice demonstrated greater difficulty with the ladder test and displayed an increase in missteps compared with control littermates (Bui et al. 2013). Even so, the most striking deficit was an inability to mediate grip strength in the face of increasing loads—their own weight—during the wire hang test (Bui et al. 2013). Both of these handicaps resulted from the silencing of dI3 INs. Whereas the above experiments did not identify exact types of LTMRs contacting dI3 INs, it is possible to exclude a major contribution from Merkel cells, as experiments in which Merkel cells were eliminated from mice did not result in any deficits in grasping, as tested by the wire hang test (Maricich et al. 2012).
The genetic silencing studies and the previous immunohistochemical and electrophysiological experiments lead us to believe that dI3 INs integrate cutaneous information necessary for motor behaviors. The most evident involvement of this circuitry is in grasping; however, their full involvement in other motor behaviors has not been fully determined.
Related Orphan Receptor INs
To identify putative populations of spinal neurons that process tactile information for motor control, Del Barrio and colleagues (2013) performed a preliminary transcription factor screening to probe the molecular identities of INs in laminae III–V, which are predominantly innervated by LTMRs that transduce cutaneous mechanosensory information. From this screen, INs expressing the protein retinoic acid receptor-related orphan receptor α (RORα) were targeted and studied further using transgenic methods (Bourane et al. 2015). The RORα IN population is characterized by its expression of RORα, MafA, c-Maf, and Lmx1b, amongst other transcription factors, where the latter can mark excitatory INs in the dorsal spinal horn (Ding et al. 2004). To confirm the excitatory nature of these cells, in situ hybridization for vGluT2 mRNA revealed that RORα INs are predominantly glutamatergic.
The mapping of RORα INs demonstrated that they are mostly located between inner lamina II (IIi) and lamina III. RORα INs are primarily innervated by LTMRs and receive little to no nociceptive input. The combination of recent advances in recombinant rabies virus techniques to label genetically identified neurons (Weible et al. 2010) and identification of molecular markers for LTMRs (Li et al. 2011; Luo et al. 2009) allowed for characterization of the sources of sensory input to RORα INs. Interestingly, these INs were demonstrated to be innervated by Meissner corpuscles in glabrous skin and Merkel cells, D-hair terminals, and transverse lanceolate endings in hairy skin (Bourane et al. 2015). Furthermore, putative evidence suggests the implication of Ruffini endings as sensory input; however, the full extent of the innervation of RORα INs by LTMRs innervating this class of sensory receptors requires further investigation. These anatomical experiments were substantiated further by whole cell recording of these neurons, which demonstrated the presence of monosynaptic excitatory potentials mostly in response to electric recruitment of low-threshold myelinated Aβ and high-threshold myelinated Aδ fibers (Bourane et al. 2015).
To determine whether RORα INs play a role in motor control, projections to spinal neurons involved in motor activity were sought (Bourane et al. 2015). Indeed, spinal RORα INs were found to project directly on motoneurons in the lumbar spinal cord. Furthermore, contacts were found on V0 cholinergic (V0c) neurons, which are known to modulate the excitability of motoneurons, suggesting perhaps that RORα INs possess the ability to modulate motor output through the intermediary of V0c neurons (Zagoraiou et al. 2009). Additionally, RORα INs were found to be innervated by corticospinal tract neurons in motor cortex and by lateral vestibulospinal tract (LVST) neurons. LVST neurons are known to originate in the pons and project downwards to the spinal cord carrying information about posture and balance (Markham 1987). Collectively, these results demonstrate that RORα INs are spinal neurons that are implicated in integrating tactile inputs to shape motor activity (Fig. 1C).
To study the specific role of RORα INs in motor control, ablation of RORα INs in the caudal spinal cord was achieved by inducing selective expression of the diphtheria toxin receptor in RORα INs and subsequent treatment with diphtheria toxin (Bourane et al. 2015). The resulting mice were subjected to a battery of sensory tests. Most interestingly, these mice displayed an impairment in sensing dynamic and static light touch on the plantar surface of the foot. Nonetheless, the RORα IN-ablated mice showed no difference in pain sensation, suggesting that RORα INs mainly contribute to light-touch perception without compromising responsiveness to other sensory modalities. RORα IN-ablated mice did not show any significant locomotor differences to control littermates when tested on a treadmill or on a horizontal ladder. However, when fine motor control during locomotion was accessed by the raised beam test (Crawley 2008), a significant increase in hindlimb missteps was reported. These results suggest that RORα INs mediate cutaneous sensory information for use in motor commands involved in corrective foot movements.
The ablation and tracing studies showed that RORα INs integrate cutaneous sensory information from LTMRs with descending cortical inputs into motor commands for corrective feet movements; however, the mechanisms by which these neurons integrate this descending cortical input have yet to be studied.
dI4 INs
So far, we have described two populations of genetically identified spinal neurons that integrate tactile afferents. These two populations are predominantly composed of excitatory neurons. However, integration of tactile afferents by spinal cord circuitry may also involve inhibition. For instance, stimulation of low-threshold cutaneous inputs can gate the transmission of tactile information within the spinal cord by shunting the excitability of other cutaneous afferents through axo-axonic contacts in a phenomenon known as presynaptic inhibition (Rudomin 2009).
The origin of GABAergic projections to spinal terminals of sensory neurons mediating presynaptic inhibition was discovered to originate commonly from dI4 INs, a class of dorsally derived spinal INs marked by the expression of the transcription factor Ptf1a (Fig. 1D). In fact, most, if not all, GABAergic boutons contacting sensory terminals within the spinal cord were found to derive from dI4 INs (Betley et al. 2009). GABAergic boutons apposed to sensory afferent terminals were demarked from GABAergic boutons apposed to dendrites or somas by the expression of the protein glutamate decarboxylase 2 (Gad2) (Betley et al. 2009). Remarkably, even though Gad2-immunoreactive GABAergic boutons contacting cutaneous afferent terminals and those contacting proprioceptive afferent terminals originate from dI4 INs, only the former shows GlyT2, enkephalin, and neuropeptide Y expression (Betley et al. 2009). Therefore, subpopulations of dI4 INs clearly exist, which can be distinguished by expression of selective proteins at synaptic terminals and more importantly, by the type of sensory afferents they target.
The role of presynaptic inhibition in motor control has recently been investigated using genetic techniques. Genetic silencing of Gad2-immunoreactive GABAergic boutons mediating presynaptic inhibition has been shown to disrupt smooth-reaching movements (Fink et al. 2014). However, this effect was attributed to loss of presynaptic inhibition of proprioceptive afferents rather than of cutaneous afferents. Therefore, the role of presynaptic inhibition of tactile afferents or the role of presynaptic inhibition driven by tactile afferents remains an open question.
dI1 INs
Defined by expression of Atoh1 (formerly Math1), dI1 INs are also a population of dorsally derived neurons that migrate ventrally to be positioned in the deep dorsal horn of the spinal cord (Bermingham et al. 2001). These neurons form three distinct spinocerebellar tracts (SCTs), by which sensory feedback is relayed to the cerebellum. In more rostral spinal cord segments (cervico-thoracic), dI1 INs form the rostral SCT (rSCT), whereas in more caudal spinal cord segments (thoraco-lumbo-sacral), dI1 INs are found in two clusters that form the dorsal SCT (dSCT) and the ventral SCT (vSCT) (Miesegaes et al. 2009) (Fig. 1E). The SCTs convey sensory information from muscle afferents and to a lesser degree, from LTMRs to the cerebellum (Edgley and Jankowska 1988; Randic et al. 1981), a region important for correcting movements and for motor learning (Brownstone et al. 2015; Kawato et al. 1987). Therefore, these neurons are involved with sensorimotor integration at supraspinal levels. There is less information about their interaction with motor circuitry at the spinal cord level; however, neurons of the dSCT are contacted by corticospinal inputs, suggesting that the sensory feedback that they relay is shaped by descending motor commands (Hantman and Jessell 2010).
Key Insights and Open Questions
Much work has been dedicated in the past to revealing the presence of spinal neurons that integrate tactile input and in the process, sculpt ongoing motor activity. The recent studies of genetically defined spinal INs that we have described (summarized in Fig. 1) build on the work of the past decades and serve to highlight a number of key features of integration of tactile inputs within the spinal cord. First, there are populations of spinal neurons defined by the expression of specific combinations of transcription factors and molecular markers that receive inputs from cutaneous afferents conveying tactile information (Table 1). Second, based on the types of LTMRs involved and specificity of the connectivity with spinal and supraspinal motor networks, these genetically defined populations of spinal INs form specialized networks. Manipulation of genetically defined spinal INs revealed that they indeed play roles in distinct aspects of motor control. Finally, although sensorimotor integration also occurs at the brain level, integration by spinal INs is vital in ensuring proper motor control. Below, we offer a short and nonexhaustive list of further avenues of investigation into sensorimotor integration of tactile information, taking advantage of genetically defined spinal INs.
Table 1.
Molecular markers expressed by spinal interneurons involved in sensorimotor integration of tactile information
| Common Name | Approved Name | UniProt ID | References |
|---|---|---|---|
| Ascl1 | Achaete-scute family bHLH transcription factor 1 | P50553 | dI3 IN, dI4 IN: Mizuguchi et al. (2006) |
| BarH1 | BarH-like homeobox 1 | Q9BZE3 | dI1 IN: Bermingham et al. (2001) |
| Brn3a | POU class 4 homeobox 1 | Q01851 | dI1 IN, dI3 IN: Zou et al. (2012) |
| Brn3b | POU class 4 homeobox 2 | Q12837 | dI3 IN: Zou et al. (2012) |
| Cbln2 | Cerebellin 2 precursor | Q8IUK8 | dI1 IN: Miesegaes et al. (2009) |
| c-Maf | v-maf Avian musculoaponeurotic fibrosarcoma oncogene homolog | O75444 | RORα IN: Bourane et al. (2015) |
| Drg11 | Dorsal root ganglia homeobox | A6NNA5 | dI3 IN: Rebelo et al. (2010) |
| Enkephalin | Prodynorphin | P01213 | dI4 IN: Betley et al. (2009) |
| Gad2 | Glutamate decarboxylase 2 (pancreatic islets and brain, 65 kDa) | Q05329 | dI4 IN: Betley et al. (2009) |
| GlyT2 | Solute carrier family 6 (neurotransmitter transporter), member 5 | Q9Y345 | dI4 IN: Betley et al. (2009) |
| Gsh1 | GS homeobox 1 | Q9H4S2 | dI4 IN: Mizuguchi et al. (2006) |
| Gsh2 | GS homeobox 2 | Q9BZM3 | dI3 IN, dI4 IN: Mizuguchi et al. (2006) |
| Isl1 | ISL LIM homeobox 1 | P61371 | dI3 IN: Liem et al. (1997); Pfaff et al. (1996) |
| Lhx1 | LIM homeobox 1 | P48742 | dI4 IN: Pillai et al. (2007) |
| Lhx2 | LIM homeobox 2 | P50458 | dI1 IN: Bermingham et al. (2001) |
| Lhx5 | LIM homeobox 5 | Q9H2C1 | dI4 IN: Pillai et al. (2007) |
| Lhx9 | LIM homeobox 9 | Q9NQ69 | dI1 IN: Bermingham et al. (2001) |
| Lmx1b | LIM homeobox transcription factor 1, beta | O60663 | RORα IN: Bourane et al. (2015) |
| Math1 | Atonal homolog 1 (Drosophila) | Q92858 | dI1 IN: Bermingham et al. (2001) |
| NPY | Neuropeptide Y | P01303 | dI4 IN: Betley et al. (2009) |
| Pax2 | Paired box 2 | Q02962 | dI4 IN: Glasgow et al. (2005) |
| Ptf1a | Pancreas transcription factor 1 subunit alpha | Q7RTS3 | dI4 IN: Glasgow et al. (2005) |
| RORa | RAR-related orphan receptor A | P35398 | RORα IN: Del Barrio et al. (2013) |
| Smarca2 | SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 2 | P51531 | dI1 IN: Miesegaes et al. (2009) |
| Tag-1 | Contactin 2 (axonal) | Q02246 | dI1 IN: Miesegaes et al. (2009) |
| Tlx3 | T Cell leukemia homeobox 3 | O43711 | dI3 IN: Cheng et al. (2005); Mizuguchi et al. (2006) |
| vGluT2 | Solute carrier family 17 (vesicular glutamate transporter), member 6 | Q9P2U8 | dI3 IN: Bui et al. (2013); RORα IN: Bourane et al. (2015) |
bHLH, basic helix-loop-helix; IN, interneuron; RORα, retinoic acid receptor (RAR)-related orphan receptor α; SWI/SNF, switch/sucrose nonfermentable. Sources: http://www.genenames.org/ and http://www.uniprot.org/.
Identification of Other Spinal Populations/Subpopulations that Integrate Tactile Information
A long-standing question in neuroscience is how to classify populations of neurons (DeFelipe et al. 2013). Classes of neurons can be defined based on single or combinations of intrinsic properties, such as protein expression, morphology, anatomical location, neurotransmitter phenotypes, synaptic inputs, and/or outputs. Alternatively, neurons can be classified along functional lines, such as firing behavior and involvement with certain neuronal or physiological forms of activity. Needless to say, the classification of neurons is an ongoing, fluid exercise that is necessary for the proper study of neural systems. For the immediate purpose of discussing classes of spinal neurons involved with the integration of tactile information for motor control, it is necessary to highlight that each of the canonical populations of genetically identified spinal INs (dI1–dI6; dIL,A-B; V0–V3) and motoneurons is likely to consist of many subpopulations of neurons that are differentiated along molecular and/or functional lines. For instance, the V2 population of ipsilaterally projecting ventral neurons, which are characterized by Lhx3 expression, has been parsed out to consist of V2a, V2b, and V2c, based on Chx10, Gata2, and Sox1 expression (Al-Mosawie et al. 2007; Lundfald et al. 2007; Panayi et al. 2010; Zhou et al. 2000). These neurons are also distinguished by different neurotransmitter phenotypes and likely, functional roles as well (Crone et al. 2008; Zhong et al. 2010). Not surprisingly, there is some degree of functional heterogeneity within each subpopulation (Dougherty et al. 2013). Clearly, the identification of the functional roles of spinal neurons from common progenitor pools is only the starting step to understanding fully the roles of spinal circuits in processing tactile information. The identification of subpopulations within these cardinal cell populations, based on molecular, anatomical, and functional properties, will refine the classification further. With the consideration that spinal INs receiving both proprioceptive and cutaneous afferents have long been identified in deep dorsal horn and intermediate laminae of the spinal cord (Edgley and Jankowska 1987; Moschovakis et al. 1992), it would not be altogether surprising if subpopulations of ventrally derived spinal neurons were found to integrate tactile information for motor control. A prime candidate for ventrally derived spinal neurons integrating tactile information is a subpopulation of the V3 IN population, which consists of commissural INs, involved in maintaining locomotor stability (Zhang et al. 2008), found to migrate during development and to be ultimately positioned in the deep dorsal horn (Blacklaws et al. 2015; Borowska et al. 2013).
The work on spinal RORα INs is a prime example of the need for studying subpopulations as well. It is not clear whether these neurons form a subpopulation of neurons that arise from a common progenitor domain or whether they consist of neurons from several progenitor domains. The latter would reveal that functional roles may be distributed amongst spinal neurons originating from a broad distribution of progenitor pools. A precedent for the distribution of functional roles amongst neurons arising from different progenitor pools was demonstrated when the spinal neurons responsible for reciprocal inhibition mediated by Ia afferents were found to arise from both V1 and V2b populations (Siembab et al. 2010; Zhang et al. 2014). The screening for genes commonly expressed in spinal lamina III–V, where sensory neurons innervating mechanoreceptors are most abundantly found, has revealed a number of candidate proteins that may mark other classes of functionally related spinal neurons that integrate tactile information and shape motor activity (Del Barrio et al. 2013). It would not be altogether surprising to discover that neurons belonging to the dI1, dI3, and dI4 IN populations consist of several subpopulations, as is already suggested for dI1 INs, based on anatomical location and ascending tract formation (Miesegaes et al. 2009). The dissection of these canonical populations of sensorimotor INs is a crucial step to understanding further the integration of tactile information for motor control.
Connecting Spinal Circuits for Sensorimotor Integration of Tactile Information
It is clear that dI3 INs and RORα INs both use tactile information but play distinct roles in motor control. For instance, when dI3 INs were silenced, mice showed deficits on the ladder test as well as in controlling grip strength (Bui et al. 2013), whereas ablation of caudal populations of RORα INs did not impact performance on the ladder test but resulted in impairments on the raised beam test (Bourane et al. 2015). However, this does not preclude the possibility that to serve their respective motor functions, these two populations of neurons interact. Along the same lines, dI4 INs may be linked to dI3 INs and RORα INs through presynaptic inhibition of cutaneous afferents to the two groups of excitatory neurons.
In addition to studying the interactions between genetically identified spinal INs involved in sensorimotor integration of tactile inputs, their interactions with other spinal neurons involved in complementary aspects of motor function must be determined. For example, whereas dI3 INs have been implicated with controlling grip strength, they do not seem to be critical for other aspects of hand control, such as skilled reach (unpublished observation). On the other hand, the V2a population of spinal neurons has been shown to be intimately involved with skilled reaching (Azim et al. 2014b). A subset of dI4 INs is also involved in ensuring smooth hand trajectory (Fink et al. 2014). Evidently, dI3 INs, dI4 INs, and V2a INs seem to operate concurrently to achieve proper hand control (Azim et al. 2014a). Therefore, as the individual contributions of genetically identified spinal INs to motor control and to sensorimotor integration of tactile inputs are revealed, the mutual connectivity between spinal neurons has to be mapped to understand fully how tactile information truly tailors motor output across the spectrum of possible motor activities.
Connecting Spinal Circuits with Supraspinal Circuits for Sensorimotor Integration of Tactile Information
Whereas spinal circuits involved in sensorimotor integration are able to operate in isolation without supraspinal input (e.g., tendon reflex), cortical and subcortical regions of the brain, as well as the cerebellum, are involved with motor processes, such as motor planning, motor learning, and motor adaptation. In addition, several regions of the brain stem are involved in the control of motor activity, such as locomotion (Grillner and Shik 1973) and hand function (Soteropoulos et al. 2012), through direct recruitment of spinal circuitry. Therefore, spinal circuits are likely to operate in conjunction with descending inputs from supraspinal areas. Indeed, cortical stimulation has been shown to modulate hindlimb cutaneous reflexes (Bretzner and Drew 2005). Similarly, cutaneous reflexes may modulate corticospinal efficacy through ascending reflex pathways (Christensen et al. 1999). RORα INs have been shown to receive supraspinal inputs from motor cortex and the cerebellum, the latter through the intermediary of projections originating from the lateral vestibular nuclei (Bourane et al. 2015). In addition, they project to ascending postsynaptic dorsal column neurons that may link these neurons with the cerebellum (Bourane et al. 2015). We also expect dI3 INs to receive supraspinal inputs based on clinical observations. For instance, dI3 INs mediate a palmar grasp reflex at early postnatal stages (Bui et al. 2013) that resembles a postnatal grasp reflex in humans. This reflex disappears in time (Mestre and Lang 2010), in parallel with the maturation of descending pathways to the spinal cord (Clarac et al. 2004). In addition, abnormal grasp reflexes in human patients have often been associated with frontal cerebral lesions (Mestre and Lang 2010), perhaps due to loss of supraspinal regulation of dI3 INs or of an analogous population in humans. Supraspinal regions may also be targeted by dI3 INs, as revealed by their innervation of the lateral reticular nucleus, a precerebellar nuclei (Pivetta et al. 2014). Therefore, the sources of supraspinal inputs to these neurons will be an active area of investigation. In addition, the role of these supraspinal projections in shaping sensorimotor integration of tactile information at the level of the spinal cord will be important. A prominent role for supraspinal projections may be the gating of sensory information during motor activity (see above). Therefore, how supraspinal inputs influence the gating of tactile information at the spinal level and during which forms of motor activity may be answered through the study of supraspinal projections to genetically identified spinal neurons.
Alternatively, spinal circuits may also influence the processing of tactile information by supraspinal regions dedicated to somatosensation, as demonstrated by findings that dI1 INs, dI3 INs, and RORα INs are part of ascending pathways from the spinal cord to the brain. Postsynaptic dorsal column neurons contacted by RORα INs send ascending projections that may ultimately be relayed to somatosensory cortex. Indeed, mice lacking RORα INs showed delays in detecting an irritant taped to their paws (Bourane et al. 2015). In addition to shaping somatosensation, spinal circuits may shape the motor strategies used to probe the external world. Indeed, perception and action are often processes that overlap (Hsiao et al. 2011). Classically, the relationship between sensory and motor systems has been viewed as sensory circuits informing motor systems. However, motor systems can shape sensory processing. For example, by changing whisking strategies, an animal can change the rate of tactile sensory inflow (Saig et al. 2012). By virtue of their access to motor circuits, both dI3 INs and RORα INs may influence tactile perception by shaping motor processes involved with sensing the external world. Therefore, the mutual interactions between spinal and supraspinal circuits must be investigated to understand further the processes by which tactile information shapes motor activity, and spinal circuits shape somatosensation and the motor strategies used to acquire sensory information.
Neural Coding
One of the many achievements of Steven Hsiao (Hsiao and Yau 2008) in the field of tactile perception was the discovery of neural codes for the perception of object features, such as texture, size, and shape. Whereas much focus has been paid to the role of the somatosensory cortex in this process, recent studies in humans provide evidence that sensory neurons innervating glabrous skin of the hand can perform higher-order feature detection, such as edge detection (Pruszynski and Johansson 2014). In light of this, we propose that spinal neurons receiving inputs from cutaneous receptors may further process features detected by sensory neurons for the purpose of motor control.
Indeed, spinal neurons have characteristics that hint at their capability to detect object features. For instance, the response of dorsal spinal neurons to tactile stimuli applied to the skin has been shown to depend on the nature of the stimuli and the receptive field. Responses can be graded (Fitzgerald 1985; King et al. 1990; Schneider 2005), excitatory, inhibitory, or a mixture of both (Hillman and Wall 1969; Kato et al. 2011; Kolmodin and Skoglund 1960; Woolf and King 1989), subthreshold or suprathreshold (Woolf and King 1989), and short or sustained (De Koninck and Henry 1994; Schneider 2005). The wide ranges of response patterns of spinal neurons are a reflection of the range of activation patterns of LTMRs (Abraira and Ginty 2013; Johansson and Flanagan 2009), as well as heterogeneity in intrinsic properties of spinal neurons (Ku and Schneider 2011).
Recordings of genetically identified spinal neurons can further establish whether detection of features of objects can occur at the spinal level. We know that dI3 INs have a number of ionic currents that alter the latency and the duration of their response to sensory inputs (Bui et al. 2013). As well, both dI3 INs and RORα INs receive inputs from multiple sources of sensory information. In fact, they also exhibit a number of different firing responses to electrical stimulation of sensory afferents (Bourane et al. 2015; Bui et al. 2013). To determine fully whether these neurons are capable of detecting object features, studies detailing the receptive fields of individual INs and their response to different forms of tactile stimuli, as well as the integration of stimuli applied to multiple sites on the skin, various tactile receptors, or other sensory modalities, need to be undertaken.
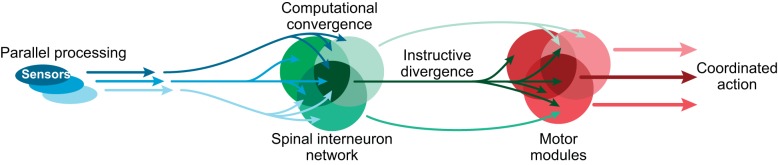
Another important aspect of feature detection and sensory processing, in general, is the representation of sensory information at different neural layers as information flows centrally through the nervous system. Studies of other sensory systems, such as the visual, olfactory, and electrosensory systems, suggest the involvement of common mechanisms of sensory information processing that may apply to sensorimotor integration of tactile information within the spinal cord as well (Babadi and Sompolinsky 2014). First, sensory processing is characterized by an expansion of sensory representations, whereby as sensory information travels centrally, it is processed by an increasing number of neurons. A second commonly observed mechanism of sensory processing is sparse representation, whereby as sensory information travels centrally, it only projects to a subset of downstream neurons (Bui and Brownstone 2015), despite the larger number of downstream neurons devoted to the processing of sensory information (see expansion). These two mechanisms are illustrated in the fly olfactory system, where ∼150 projection neurons, relaying signals from olfactory receptor neurons, project to 2,500 Kenyon cells in the fly mushroom body (Turner et al. 2008). However, as a consequence of limited convergence of inputs from projection neurons to Kenyon cells, each individual Kenyon cell responds only to a small sample of possible odors captured by olfactory receptor neurons. In the context of the flow of tactile information from the skin to spinal circuits, an expansion of tactile signals conducted by sensory neurons in exclusive parallel fibers would imply a projection of these signals to a wide array of spinal neurons. These sensory fibers account for specific sensory modalities but additionally, for their intensity and spatiotemporal distribution. The requirement for sparseness would dictate that the tactile signals relayed to the spinal cord would converge toward a network of spinal INs composed of nonoverlapping and/or partially overlapping populations. The study of genetically identified spinal neurons involved in sensorimotor integration could reveal whether there is expansion and sparseness in the representation of tactile information at the spinal level. Finally, to shape motor control through an integration of tactile information, these spinal INs must project a set of instructive motor commands. We suggest that these motor commands diverge to activate spinal circuits or motor modules dedicated to specific aspects of motor control (Giszter and Hart 2013). The activity of a single or multiple motor modules generates a coordinated motor response to the tactile stimuli (Fig. 3).
Fig. 3.
Conceptual model of sensorimotor neural processing in the spinal cord. Proposed scheme in which sensory inputs from multiple modalities are conveyed in parallel to partially overlapping spinal IN populations. The converging sensory information is processed, and sets of instructive motor commands are generated. These commands diverge to motor modules that generate individual motor actions, which together, form the desired coordinated motor response. We propose that this anatomical and functional structure provides a biological framework to neuronal computations underlying spinal sensorimotor integration.
Concluding Remarks
Most of the studies of genetically identified spinal INs were performed in mice, testing the cutaneous afferents either on hairy or glabrous skins. Therefore, caution should be exercised in attempting to generalize these finding to other species. However, many of the transcription factors and molecular markers studied are phylogenetically conserved and expressed in spinal neurons (e.g., Isl1 in chicks and in zebrafish) (Avraham et al. 2010; Uemura et al. 2005), leading us to believe that there are some universal mechanisms of tactile integration at the spinal level that are shared among various species, perhaps also including humans. However, before insights into tactile sensorimotor integration at the spinal level gleaned from studies of animal models can be confidently related to our understanding of human function, it is instructive to consider the directions taken in human studies and how the studies using animal models can complement or supplement the former.
Human studies are currently limited in terms of their access to spinal circuitry. However, compared with animal models, a greater range of sensorimotor tasks can be studied, and there is much greater access to peripheral sensory neurons that have frequently been recorded in human studies. The linkage between various tactile afferents and muscle activity in hands and feet (Fallon et al. 2005; McNulty et al. 1999) has been demonstrated. Furthermore, recordings of different cutaneous afferents during specific motor tasks have revealed complex patterns of activity. In particular, studies of hand grasp suggest that there is an intricate spatiotemporal pattern of activation of different types of tactile afferents distributed across the hand (Dimitriou and Edin 2008; Johansson and Flanagan 2009; Macefield et al. 1996). The ability to study these spatiotemporal patterns of sensory transmission during specific motor tasks in human and nonhuman primates is a powerful technique that remains relatively inaccessible in nonprimate animal models. These techniques enable the investigation of tactile integration at the spinal level during motor activity, motor learning, and motor adaptation. They provide a detailed understanding of the sensory signals used to inform certain movements, and they hint at certain central mechanisms (supraspinal and spinal) by which these signals are processed. However, current technical limitations prevent a better understanding of the neural mechanisms operating within the human CNS, by which complex patterns of tactile information shape motor control. To understand these phenomena further requires a dissection of the different neural circuitry within the periphery, the spinal cord, and supraspinal regions.
Therein lies the strengths of the animal model. The genetic identification of spinal neurons has spurred the application of genetic techniques to expand our understanding of how spinal circuitry controls movement. Of the many outcomes of this approach is the characterization of a number of spinal neuron populations that integrate tactile information and the identification of their role in shaping motor control as reviewed above. Each identified population of spinal INs presents further opportunities to probe mechanisms by which sensorimotor integration of tactile input occurs. Whereas this approach is conditioned by our ability to 1) identify and 2) manipulate defined neuronal populations, recent technical advances are meeting these challenges. Cellular RNA expression profiling, for example, has been used successfully to identify new neuronal populations (Del Barrio et al. 2013), whereas optogenetic and chemogenetic techniques, such as channelrhodopsins (Zhang and Oertner 2007) or designer receptors exclusively activated by designer drugs (DREADDs) (Coward et al. 1998; Lechner et al. 2002), offer exquisite approaches for the manipulation of genetically identified neurons. The greater popularization of advanced molecular techniques, such as intersectional genetics, virus-based mapping, and optogenetics, to name just a few, can lead to significant advances in manipulating and addressing the respective roles of spinal neuronal populations involved in processing tactile information. The integration of tactile information is complex and requires multiple circuits distributed within the spinal cord and supraspinal regions. Recent technological advances, such as two-photon in vivo and in operandi recordings, combined with post hoc dimensionality reduction, will be invaluable tools in tackling the complexity of network activity in the spinal cord.
The greatest challenge in relating the findings from studies of genetically identified spinal neurons in animal models to our understanding of human function is in determining whether analogous, genetically defined spinal circuits are present in the human spinal cord. However, genetic techniques have already been applied in nonhuman primate models to dissect the spinal circuitry associated with hand function (Kinoshita et al. 2012). The importance of bridging the gap between our understanding of how tactile information shapes motor control through spinal circuitry in human and nonhuman animal models goes beyond understanding how sensorimotor control is implemented by the nervous system. In addition to applying genetic techniques for mechanistic studies, genetic identification of spinal neurons offers the potential for targeted control of spinal circuits for biomedical applications. As pointed out by Steven Hsiao (Hsiao et al. 2011) in a review detailing the use of sensory feedback for upper-limb prostheses, electrical and optical stimulation of the fibers associated with specific sensory pathways will be required for optimal prostheses design. The potential involvement of tactile information in recovery of motor function following spinal cord injury is beginning to come to light (Bouyer and Rossignol 2003; Frigon et al. 2012; Hurteau et al. 2015; Slawinska et al. 2012). Moreover, as mentioned previously, the process of motor control encompasses a number of sensory modalities that are being studied, for the most part, in isolation. Better understanding of the circuitry implicated in the integration of tactile sensation is paramount to understanding the role of multimodal integration during movement (Kim et al. 2015). Genetic identification of spinal circuits, along with sensory neurons (Abraira and Ginty 2013) and supraspinal circuitry, will therefore be critical in all of these endeavors.
GRANTS
Support for T. V. Bui was provided by a grant from the Natural Sciences and Engineering Research Council (RGPIN-2015-06403).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.V.B. analyzed data; T.V.B. and N.S. interpreted results of experiments; T.V.B., N.S., I.P., and C.F. prepared figures; T.V.B., N.S., and C.F. drafted manuscript; T.V.B., N.S., I.P., and C.F. edited and revised manuscript; T.V.B., N.S., I.P., and C.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Frédéric Bretzner and Andrew Pruszynski for their invaluable comments on a prior draft of this manuscript. The authors also thank William Alaynick for permission to adapt a number of his published figures and Rob Brownstone for helpful comments through this process.
REFERENCES
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 79: 618–639, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo JM, Diaz-Rios M. Removing sensory input disrupts spinal locomotor activity in the early postnatal period. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 199: 1105–1116, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci USA 111: 16877–16882, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci 26: 3003–3015, 2007. [DOI] [PubMed] [Google Scholar]
- Alaynick WA, Jessell TM, Pfaff SL. SnapShot: spinal cord development. Cell 146: 178–178.e171, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004. [DOI] [PubMed] [Google Scholar]
- Augurelle AS, Smith AM, Lejeune T, Thonnard JL. Importance of cutaneous feedback in maintaining a secure grip during manipulation of hand-held objects. J Neurophysiol 89: 665–671, 2003. [DOI] [PubMed] [Google Scholar]
- Avraham O, Hadas Y, Vald L, Hong S, Song MR, Klar A. Motor and dorsal root ganglion axons serve as choice points for the ipsilateral turning of dI3 axons. J Neurosci 30: 15546–15557, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Fink AJ, Jessell TM. Internal and external feedback circuits for skilled forelimb movement. Cold Spring Harb Symp Quant Biol 79: 81–92, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508: 357–363, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babadi B, Sompolinsky H. Sparseness and expansion in sensory representations. Neuron 83: 1213–1226, 2014. [DOI] [PubMed] [Google Scholar]
- Baken BC, Dietz V, Duysens J. Phase-dependent modulation of short latency cutaneous reflexes during walking in man. Brain Res 1031: 268–275, 2005. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on Math1. Neuron 30: 411–422, 2001. [DOI] [PubMed] [Google Scholar]
- Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 139: 161–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklaws J, Deska-Gauthier D, Jones CT, Petracca YL, Liu M, Zhang H, Fawcett JP, Glover JC, Lanuza GM, Zhang Y. Sim1 is required for the migration and axonal projections of V3 interneurons in the developing mouse spinal cord. Dev Neurobiol 75: 1003–1017, 2015. [DOI] [PubMed] [Google Scholar]
- Borowska J, Jones CT, Zhang H, Blacklaws J, Goulding M, Zhang Y. Functional subpopulations of V3 interneurons in the mature mouse spinal cord. J Neurosci 33: 18553–18565, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourane S, Grossmann KS, Britz O, Dalet A, Del Barrio MG, Stam FJ, Garcia-Campmany L, Koch S, Goulding M. Identification of a spinal circuit for light touch and fine motor control. Cell 160: 503–515, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol 90: 3640–3653, 2003. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Drew T. Motor cortical modulation of cutaneous reflex responses in the hindlimb of the intact cat. J Neurophysiol 94: 673–687, 2005. [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE, Rose PK, Snow PJ. Spinal cord collaterals from axons of type II slowly adapting units in the cat. J Physiol 316: 469–480, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Bui TV. Spinal interneurons providing input to the final common path during locomotion. Prog Brain Res 187: 81–95, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Bui TV, Stifani N. Spinal circuits for motor learning. Curr Opin Neurobiol 33: 166–173, 2015. [DOI] [PubMed] [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, Brownstone RM. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron 78: 191–204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Brownstone RM. Sensory-evoked perturbations of locomotor activity by sparse sensory input: a computational study. J Neurophysiol 113: 2824–2839, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Bronstein AM. Locomotor adaptation and aftereffects in patients with reduced somatosensory input due to peripheral neuropathy. J Neurophysiol 102: 3119–3128, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Degtyarenko AM, Simon ES. Patterns of locomotor drive to motoneurons and last-order interneurons: clues to the structure of the CPG. J Neurophysiol 86: 447–462, 2001. [DOI] [PubMed] [Google Scholar]
- Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, Ma Q. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci 8: 1510–1515, 2005. [DOI] [PubMed] [Google Scholar]
- Choi JT, Lundbye-Jensen J, Leukel C, Nielsen JB. Cutaneous mechanisms of isometric ankle force control. Exp Brain Res 228: 377–384, 2013. [DOI] [PubMed] [Google Scholar]
- Christensen LO, Morita H, Petersen N, Nielsen J. Evidence suggesting that a transcortical reflex pathway contributes to cutaneous reflexes in the tibialis anterior muscle during walking in man. Exp Brain Res 124: 59–68, 1999. [DOI] [PubMed] [Google Scholar]
- Clarac F, Brocard F, Vinay L. The maturation of locomotor networks. Prog Brain Res 143: 57–66, 2004. [DOI] [PubMed] [Google Scholar]
- Collins DF, Knight B, Prochazka A. Contact-evoked changes in EMG activity during human grasp. J Neurophysiol 81: 2215–2225, 1999. [DOI] [PubMed] [Google Scholar]
- Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H, Conklin BR. Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci USA 95: 352–357, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron 57: 809–818, 2008. [DOI] [PubMed] [Google Scholar]
- Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, Sharma K. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 60: 70–83, 2008. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Henry JL. Prolonged GABAA-mediated inhibition following single hair afferent input to single spinal dorsal horn neurones in cats. J Physiol 476: 89–100, 1994. [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, Gonzalez-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvarday Z, Kubota Y, Lewis DA, Marin O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamas G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14: 202–216, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Norden-Krichmar T, Burke RE. Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat. J Neurophysiol 79: 447–463, 1998. [DOI] [PubMed] [Google Scholar]
- Del Barrio MG, Bourane S, Grossmann K, Schule R, Britsch S, O'Leary DD, Goulding M. A transcription factor code defines nine sensory interneuron subtypes in the mechanosensory area of the spinal cord. PLoS One 8: e77928, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Discharges in human muscle receptor afferents during block grasping. J Neurosci 28: 12632–12642, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Yin J, Kania A, Zhao ZQ, Johnson RL, Chen ZF. Lmx1b controls the differentiation and migration of the superficial dorsal horn neurons of the spinal cord. Development 131: 3693–3703, 2004. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O. Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 80: 920–933, 2013. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. A kinematic and electromyographic study of cutaneous reflexes evoked from the forelimb of unrestrained walking cats. J Neurophysiol 57: 1160–1184, 1987. [DOI] [PubMed] [Google Scholar]
- Duysens J, Loeb GE. Modulation of ipsi- and contralateral reflex responses in unrestrained walking cats. J Neurophysiol 44: 1024–1037, 1980. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res 24: 245–255, 1976. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Nawijn S, Berger W, Prokop T, Altenmuller E. Gating of sensation and evoked potentials following foot stimulation during human gait. Exp Brain Res 105: 423–431, 1995. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Trippel M, Dietz V. Increased amplitude of cutaneous reflexes during human running as compared to standing. Brain Res 613: 230–238, 1993. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol 389: 647–674, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol 397: 81–97, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger MD, Wall PD. The plantar cushion reflex circuit: an oligosynaptic cutaneous reflex. J Physiol 216: 483–501, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AL, Harrison LM, Stephens JA. Task-dependent changes in cutaneous reflexes recorded from various muscles controlling finger movement in man. J Physiol 418: 1–12, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JB, Bent LR, McNulty PA, Macefield VG. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J Neurophysiol 94: 3795–3804, 2005. [DOI] [PubMed] [Google Scholar]
- Fink AJ, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509: 43–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J Physiol 364: 1–18, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. Central projections from the skin of the hand in squirrel monkeys. J Comp Neurol 311: 563–578, 1991. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res 132: 121–139, 1977. [DOI] [PubMed] [Google Scholar]
- Francius C, Clotman F. Generating spinal motor neuron diversity: a long quest for neuronal identity. Cell Mol Life Sci 71: 813–829, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Thibaudier Y, Johnson MD, Heckman CJ, Hurteau MF. Cutaneous inputs from the back abolish locomotor-like activity and reduce spastic-like activity in the adult cat following complete spinal cord injury. Exp Neurol 235: 588–598, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett R, Stephens JA. Changes in the recruitment threshold of motor units produced by cutaneous stimulation in man. J Physiol 311: 463–473, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Cutaneomuscular reflexes recorded from the lower limb in man during different tasks. J Physiol 487: 237–242, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Hart CB. Motor primitives and synergies in the spinal cord and after injury—the current state of play. Ann N Y Acad Sci 1279: 114–126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow SM, Henke RM, Macdonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development 132: 5461–5469, 2005. [DOI] [PubMed] [Google Scholar]
- Goetz C, Pivetta C, Arber S. Distinct limb and trunk premotor circuits establish laterality in the spinal cord. Neuron 85: 131–144, 2015. [DOI] [PubMed] [Google Scholar]
- Grillner S, Shik ML. On the descending control of the lumbosacral spinal cord from the “mesencephalic locomotor region”. Acta Physiol Scand 87: 320–333, 1973. [DOI] [PubMed] [Google Scholar]
- Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron 34: 535–549, 2002. [DOI] [PubMed] [Google Scholar]
- Hantman AW, Jessell TM. Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 13: 1233–1239, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman P, Wall PD. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res 9: 284–306, 1969. [DOI] [PubMed] [Google Scholar]
- Hongo T, Kitazawa S, Ohki Y, Sasaki M, Xi MC. A physiological and morphological study of premotor interneurones in the cutaneous reflex pathways in cats. Brain Res 505: 163–166, 1989a. [DOI] [PubMed] [Google Scholar]
- Hongo T, Kitazawa S, Ohki Y, Xi MC. Functional identification of last-order interneurones of skin reflex pathways in the cat forelimb segments. Brain Res 505: 167–170, 1989b. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Massaad F, Jansen K, Bruijn SM, Duysens J. Selective bilateral activation of leg muscles after cutaneous nerve stimulation during backward walking. J Neurophysiol 108: 1933–1941, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao S, Yau J. Neural basis of haptic perception. In: Human Haptic Perception: Basics and Applications. Basel, Switzerland: Birkhäuser Basel, 2008, p. 103–112. [Google Scholar]
- Hsiao SS, Fettiplace M, Darbandi B. Sensory feedback for upper limb prostheses. Prog Brain Res 192: 69–81, 2011. [DOI] [PubMed] [Google Scholar]
- Hurteau MF, Thibaudier Y, Dambreville C, Desaulniers C, Frigon A. Effect of stimulating the lumbar skin caudal to a complete spinal cord injury on hindlimb locomotion. J Neurophysiol 113: 669–676, 2015. [DOI] [PubMed] [Google Scholar]
- Jenner JR, Stephens JA. Cutaneous reflex responses and their central nervous pathways studied in man. J Physiol 333: 405–419, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1: 20–29, 2000. [DOI] [PubMed] [Google Scholar]
- Johansson H, Sjolander P, Sojka P, Wadell I. Fusimotor reflexes to antagonistic muscles simultaneously assessed by multi-afferent recordings from muscle spindle afferents. Brain Res 435: 337–342, 1987. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 10: 345–359, 2009. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56: 550–564, 1984. [DOI] [PubMed] [Google Scholar]
- Kato G, Kosugi M, Mizuno M, Strassman AM. Separate inhibitory and excitatory components underlying receptive field organization in superficial medullary dorsal horn neurons. J Neurosci 31: 17300–17305, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M, Furukawa K, Suzuki R. A hierarchical neural-network model for control and learning of voluntary movement. Biol Cybern 57: 169–185, 1987. [DOI] [PubMed] [Google Scholar]
- Kim SS, Gomez-Ramirez M, Thakur PH, Hsiao SS. Multimodal interactions between proprioceptive and cutaneous signals in primary somatosensory cortex. Neuron 86: 555–566, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Thompson SW, Woolf CJ. Characterization of the cutaneous input to the ventral horn in vitro using the isolated spinal cord-hind limb preparation. J Neurosci Methods 35: 39–46, 1990. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T. Genetic dissection of the circuit for hand dexterity in primates. Nature 487: 235–238, 2012. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Brown PB, Mendell LM. Correlation of monosynaptic field potentials evoked by single action potentials in single primary afferent axons and their bouton distributions in the dorsal horn. J Comp Neurol 294: 133–144, 1990. [DOI] [PubMed] [Google Scholar]
- Kolmodin GM, Skoglund CR. Analysis of spinal interneurons activated by tactile and nociceptive stimulation. Acta Physiol Scand 50: 337–355, 1960. [DOI] [PubMed] [Google Scholar]
- Ku WH, Schneider SP. Multiple T-type Ca2+ current subtypes in electrophysiologically characterized hamster dorsal horn neurons: possible role in spinal sensory integration. J Neurophysiol 106: 2486–2498, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of mammalian neurons. J Neurosci 22: 5287–5290, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinsson A, Holmberg H, Broman J, Zhang M, Schouenborg J. Spinal sensorimotor transformation: relation between cutaneous somatotopy and a reflex network. J Neurosci 22: 8170–8182, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147: 1615–1627, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF Jr, Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91: 127–138, 1997. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Marks WB, Hoffer JA. Cat hindlimb motoneurons during locomotion. IV. Participation in cutaneous reflexes. J Neurophysiol 57: 563–573, 1987. [DOI] [PubMed] [Google Scholar]
- Lu DC, Niu T, Alaynick WA. Molecular and cellular development of spinal cord locomotor circuitry. Front Mol Neurosci 8: 25, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundfald L, Restrepo CE, Butt SJ, Peng CY, Droho S, Endo T, Zeilhofer HU, Sharma K, Kiehn O. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur J Neurosci 26: 2989–3002, 2007. [DOI] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron 64: 841–856, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Hager-Ross C, Johansson RS. Control of grip force during restraint of an object held between finger and thumb: responses of cutaneous afferents from the digits. Exp Brain Res 108: 155–171, 1996. [DOI] [PubMed] [Google Scholar]
- MacKay-Lyons M. Central pattern generation of locomotion: a review of the evidence. Phys Ther 82: 69–83, 2002. [DOI] [PubMed] [Google Scholar]
- Maricich SM, Morrison KM, Mathes EL, Brewer BM. Rodents rely on Merkel cells for texture discrimination tasks. J Neurosci 32: 3296–3300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CH. Vestibular control of muscular tone and posture. Can J Neurol Sci 14: 493–496, 1987. [DOI] [PubMed] [Google Scholar]
- McNulty PA, Turker KS, Macefield VG. Evidence for strong synaptic coupling between single tactile afferents and motoneurones supplying the human hand. J Physiol 518: 883–893, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre T, Lang AE. The grasp reflex: a symptom in need of treatment. Mov Disord 25: 2479–2485, 2010. [DOI] [PubMed] [Google Scholar]
- Miesegaes GR, Klisch TJ, Thaller C, Ahmad KA, Atkinson RC, Zoghbi HY. Identification and subclassification of new Atoh1 derived cell populations during mouse spinal cord development. Dev Biol 327: 339–351, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne RJ, Aniss AM, Kay NE, Gandevia SC. Reduction in perceived intensity of cutaneous stimuli during movement: a quantitative study. Exp Brain Res 70: 569–576, 1988. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat Neurosci 9: 770–778, 2006. [DOI] [PubMed] [Google Scholar]
- Molander C, Grant G. Laminar distribution and somatotopic organization of primary afferent fibers from hindlimb nerves in the dorsal horn. A study by transganglionic transport of horseradish peroxidase in the rat. Neuroscience 19: 297–312, 1986. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Solodkin M, Burke RE. Anatomical and physiological study of interneurons in an oligosynaptic cutaneous reflex pathway in the cat hindlimb. Brain Res 586: 311–318, 1992. [DOI] [PubMed] [Google Scholar]
- Muller T, Anlag K, Wildner H, Britsch S, Treier M, Birchmeier C. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes Dev 19: 733–743, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada Y, Parab P, Simmons A, Omer-Abdalla A, Johnson JE. Separable enhancer sequences regulate the expression of the neural bHLH transcription factor neurogenin 1. Dev Biol 271: 479–487, 2004. [DOI] [PubMed] [Google Scholar]
- Panayi H, Panayiotou E, Orford M, Genethliou N, Mean R, Lapathitis G, Li S, Xiang M, Kessaris N, Richardson WD, Malas S. Sox1 is required for the specification of a novel p2-derived interneuron subtype in the mouse ventral spinal cord. J Neurosci 30: 12274–12280, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek I, Bui T, Wright AT, Brownstone RM. Cutaneous afferent regulation of motor function. Acta Neurobiol Exp (Wars) 74: 158–171, 2014. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Juric R. The central termination of sensory fibers from nerves to the gastrocnemius muscle of the rat. Neuroscience 134: 175–187, 2005. [DOI] [PubMed] [Google Scholar]
- Peyronnard JM, Charron L. Motor and sensory neurons of the rat sural nerve: a horseradish peroxidase study. Muscle Nerve 5: 654–660, 1982. [DOI] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84: 309–320, 1996. [DOI] [PubMed] [Google Scholar]
- Pillai A, Mansouri A, Behringer R, Westphal H, Goulding M. Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development 134: 357–366, 2007. [DOI] [PubMed] [Google Scholar]
- Pivetta C, Esposito MS, Sigrist M, Arber S. Motor-circuit communication matrix from spinal cord to brainstem neurons revealed by developmental origin. Cell 156: 537–548, 2014. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33: 281–307, 1989. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Johansson RS. Edge-orientation processing in first-order tactile neurons. Nat Neurosci 17: 1404–1409, 2014. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Stecina K, Gosgnach S, McCrea DA. Stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol 94: 2045–2052, 2005a. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Stecina K, McCrea DA. Intracellular analysis of reflex pathways underlying the stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol 94: 2053–2062, 2005b. [DOI] [PubMed] [Google Scholar]
- Randic M, Miletic V, Loewy AD. A morphological study of cat dorsal spinocerebellar tract neurons after intracellular injection of horseradish peroxidase. J Comp Neurol 198: 453–466, 1981. [DOI] [PubMed] [Google Scholar]
- Rebelo S, Reguenga C, Lopes C, Lima D. Prrxl1 is required for the generation of a subset of nociceptive glutamatergic superficial spinal dorsal horn neurons. Dev Dyn 239: 1684–1694, 2010. [DOI] [PubMed] [Google Scholar]
- Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100: 297–379, 1954. [DOI] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol 96: 414–495, 1952. [DOI] [PubMed] [Google Scholar]
- Rudomin P. In search of lost presynaptic inhibition. Exp Brain Res 196: 139–151, 2009. [DOI] [PubMed] [Google Scholar]
- Sahai V, Mahns DA, Perkins NM, Robinson L, Rowe MJ. Vibrotactile coding capacities of spinocervical tract neurons in the cat. J Neurophysiol 95: 1465–1477, 2006. [DOI] [PubMed] [Google Scholar]
- Saig A, Gordon G, Assa E, Arieli A, Ahissar E. Motor-sensory confluence in tactile perception. J Neurosci 32: 14022–14032, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP. Mechanosensory afferent input and neuronal firing properties in rodent spinal laminae III–V: re-examination of relationships with analysis of responses to static and time-varying stimuli. Brain Res 1034: 71–89, 2005. [DOI] [PubMed] [Google Scholar]
- Seki K, Fetz EE. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci 32: 890–902, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010. [DOI] [PubMed] [Google Scholar]
- Siembab VC, Smith CA, Zagoraiou L, Berrocal MC, Mentis GZ, Alvarez FJ. Target selection of proprioceptive and motor axon synapses on neonatal V1-derived Ia inhibitory interneurons and Renshaw cells. J Comp Neurol 518: 4675–4701, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska U, Majczynski H, Dai Y, Jordan LM. The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J Physiol 590: 1721–1736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol 590: 4011–4027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron 68: 456–472, 2010. [DOI] [PubMed] [Google Scholar]
- Stifani N. Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci 8: 293, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol 99: 734–746, 2008. [DOI] [PubMed] [Google Scholar]
- Uemura O, Okada Y, Ando H, Guedj M, Higashijima S, Shimazaki T, Chino N, Okano H, Okamoto H. Comparative functional genomics revealed conservation and diversification of three enhancers of the isl1 gene for motor and sensory neuron-specific expression. Dev Biol 278: 587–606, 2005. [DOI] [PubMed] [Google Scholar]
- Van Wezel BM, Ottenhoff FA, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. J Neurosci 17: 3804–3814, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezel BM, van Engelen BG, Gabreels FJ, Gabreels-Festen AA, Duysens J. Abeta fibers mediate cutaneous reflexes during human walking. J Neurophysiol 83: 2980–2986, 2000. [DOI] [PubMed] [Google Scholar]
- Wagman IH, Price DD. Responses of dorsal horn cells of M. mulatta to cutaneous and sural nerve A and C fiber stimuli. J Neurophysiol 32: 803–817, 1969. [DOI] [PubMed] [Google Scholar]
- Weible AP, Schwarcz L, Wickersham IR, Deblander L, Wu H, Callaway EM, Seung HS, Kentros CG. Transgenic targeting of recombinant rabies virus reveals monosynaptic connectivity of specific neurons. J Neurosci 30: 16509–16513, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine-Lee L, Ahn KJ, Richardson RD, Mishina Y, Lyons KM, Crenshaw EB 3rd. Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development 131: 5393–5403, 2004. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Subthreshold components of the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat lumbar spinal cord. J Neurophysiol 62: 907–916, 1989. [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64: 645–662, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol 77: 3311–3325, 1997. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB, Komiyama T. Function of sural nerve reflexes during human walking. J Physiol 507: 305–314, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lanuza GM, Britz O, Wang Z, Siembab VC, Zhang Y, Velasquez T, Alvarez FJ, Frank E, Goulding M. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 82: 138–150, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60: 84–96, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nat Methods 4: 139–141, 2007. [DOI] [PubMed] [Google Scholar]
- Zhong G, Droho S, Crone SA, Dietz S, Kwan AC, Webb WW, Sharma K, Harris-Warrick RM. Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J Neurosci 30: 170–182, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yamamoto M, Engel JD. GATA2 is required for the generation of V2 interneurons. Development 127: 3829–3838, 2000. [DOI] [PubMed] [Google Scholar]
- Zou M, Li S, Klein WH, Xiang M. Brn3a/Pou4f1 regulates dorsal root ganglion sensory neuron specification and axonal projection into the spinal cord. Dev Biol 364: 114–127, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]