Abstract
With sensory reweighting, reliable sensory information is selected over unreliable information during balance by dynamically combining this information. We used system identification techniques to show the weight and the adaptive process of weight change of proprioceptive information during standing balance with age and specific diseases. Ten healthy young subjects (aged between 20 and 30 yr) and 44 elderly subjects (aged above 65 yr) encompassing 10 healthy elderly, 10 with cataract, 10 with polyneuropathy, and 14 with impaired balance, participated in the study. During stance, proprioceptive information of the ankles was disturbed by rotation of the support surface with specific frequency content where disturbance amplitude increased over trials. Body sway and reactive ankle torque were measured to determine sensitivity functions of these responses to the disturbance amplitude. Model fits resulted in a proprioceptive weight (changing over trials), time delay, force feedback, reflexive stiffness, and damping. The proprioceptive weight was higher in healthy elderly compared with young subjects and higher in elderly subjects with cataract and with impaired balance compared with healthy elderly subjects. Proprioceptive weight decreased with increasing disturbance amplitude; decrease was similar in all groups. In all groups, the time delay was higher and the reflexive stiffness was lower compared with young or healthy elderly subjects. In conclusion, proprioceptive information is weighted more with age and in patients with cataract and impaired balance. With age and specific diseases the time delay was higher and reflexive stiffness was lower. These results illustrate the opportunity to detect the underlying cause of impaired balance in the elderly with system identification.
Keywords: standing balance, elderly, system identification techniques, sensory reweighting, proprioception
impaired standing balance is a common problem in the elderly (Jonsson et al. 2004; Lin and Bhattacharyya 2012) and one of the main causes of falls (Rubenstein 2006). Underlying organ systems, such as the motor, nervous, and sensory systems (i.e., vestibular system, vision, and proprioception), interact with each other to maintain balance in a closed loop, in which cause and effect are interrelated. For example, changes in muscle force have an impact on body sway, and detection of body sway changes by the sensory system has an impact on muscle force. Each underlying system is prone to deterioration with advanced age and is influenced by age-related diseases and medication use (Horak et al. 1989; Maki and McIlroy 1996; Manchester et al. 1989). Systems can partially compensate for each other's deterioration. Failing compensation strategies may eventually result in impaired standing balance, which finally may result in falling.
One possible compensation strategy during standing balance is sensory reweighting between visual, vestibular, and proprioceptive information (Oie et al. 2002; Peterka 2002). According to this strategy, the nervous system prefers reliable sensory information of one sensory system over less reliable information of other sensory systems within a continuous dynamically weighting process. Information of each sensory system is weighted by a weighting factor relative to the contribution of sensory information of the other sensory systems. Deterioration of a sensory system will affect its own weight and the weights of other systems. For example, deficient vestibular information will result in a lower vestibular weight (i.e., downweighting) and will be subsequently compensated by increased reliance on sensory information of other sensory systems (i.e., a higher weight, upweighting) to maintain standing balance (Peterka 2002). The reweighting of sensory information therefore gives more insight into the contribution of the sensory systems in maintaining standing balance. Reweighting of sensory information also depends on environmental conditions, like standing on uneven ground or in a dark room.
Previous research investigated sensory reweighting with posturography by eliminating or disturbing sensory information with external disturbances, such as by closing the eyes or movement of the visual scene or support surface (SS). The ratio of the center of pressure (CoP) or center of mass (CoM) movement with and without external disturbances is used to indicate the diminished reliability of sensory information. These studies showed that healthy elderly individuals rely more on their visual information during standing balance compared with healthy young individuals (Bugnariu and Fung 2007; Faraldo-Garcia et al. 2012). Furthermore, it was shown that with age the nervous system loses ability to adapt to altered sensory conditions (Borger et al. 1999; Bugnariu and Fung 2007; Doumas and Krampe 2010; Eikema et al. 2012; Horak et al. 1989; Jeka et al. 2006; Stelmach and Worringham 1985; Teasdale et al. 1991; Teasdale and Simoneau 2001). Elderly subjects with deteriorated vision (i.e., elderly with cataract or glaucoma) relied more on vestibular and proprioceptive information during standing balance and therefore showed poor performance in altered sensory conditions in which vestibular or proprioceptive information was disturbed (Black et al. 2008; Chen et al. 2012; Friedrich et al. 2008; Kotecha et al. 2012; Ray et al. 2008). Elderly subjects with deteriorated proprioception (i.e., elderly with polyneuropathy) showed increased reliance on the visual system during balance control and therefore showed poor performance in altered sensory conditions in which visual information was disturbed (Bonnet et al. 2009; Boucher et al. 1995; Kars et al. 2009; Lafond et al. 2004; van der Linden et al. 2010).
It is difficult to investigate sensory reweighting with posturography, as changes in CoP and CoM movement are affected by all systems involved in standing balance, i.e., the motor system, the sensory systems, and the nervous system, and also by the use of other compensation strategies, such as cocontraction. Conclusions are only based on changes in CoP and CoM movement, while the contributions of the other underlying systems to these changes are not taken into account. System identification techniques potentially allow identification of the contribution of each individual system in maintaining an upright position and therefore allow investigation of the contribution of each sensory system regardless of changes in the other underlying systems involved in standing balance and compensation strategies used (Allison et al. 2006; Engelhart et al. 2014a; Fitzpatrick et al. 1996; Jeka et al. 2006, 2010; Pasma et al. 2014; Peterka 2002; van der Kooij et al. 2005). Well-known mechanical or sensory disturbances with specific frequency content are used to disturb specific underlying systems, such as proprioception, vision, and the leg or trunk segment. Relating the disturbances with the body response gives a description of the balance control system. Underlying systems, i.e., the sensory systems, the nervous system, and muscles, are quantified by fitting a mathematical model of the balance control system on the response of the human body to the well-known disturbances describing the underlying systems with estimated parameters. Furthermore, by applying sensory disturbances with increasing disturbance amplitude over trials, resulting in less reliable sensory information, it is possible to investigate sensory reweighting (Asslander et al. 2015; Jeka et al. 2010; Pasma et al. 2012; Peterka 2002).
In this study, we applied system identification techniques to assess sensory reweighting of proprioceptive information during standing balance in elderly subjects with disturbances of proprioceptive information of the ankle. For modeling purposes, proprioception is defined as sensory information about leg orientation relative to the SS (Peterka 2002). We investigated sensory reweighting as a function of age and specific diseases interfering with sensory systems to study how these diseases affect the reliance on proprioceptive information and the compensation to proprioceptive disturbances. In addition, to distinguish sensory reweighting from dynamics induced by other underlying systems, e.g., the motor and nervous systems, we used the system identification approach. Healthy elderly individuals were compared with healthy young individuals to investigate the effect of age on proprioceptive reweighting. To investigate the effect of specific sensory deficits, elderly subjects with polyneuropathy and with cataract were compared with healthy elderly individuals. To show the possibility of detecting underlying changes in a heterogeneous population with various causes of impaired balance, elderly subjects with impaired balance were compared with healthy elderly individuals. This is the first study investigating sensory reweighting with system identification techniques in a large group of elderly subjects with specific diseases, i.e., with cataract, polyneuropathy, and impaired balance, in a clinical setting. The results of this study show the applicability of system identification techniques to investigate the use of proprioceptive information and provide more insight into changes in the use of proprioceptive information and the nervous system with age and with deterioration of the sensory systems (i.e., with cataract and with polyneuropathy).
Our hypotheses were based on the sensory reweighting theory and Bayesian decision theory (Kording and Wolpert 2006; Peterka 2002). These paradigms state that more sensory noise results in less reliable sensory information, which can be compensated for by less use of the sensory information and more use of the information of the other sensory systems (i.e., sensory reweighting). Disturbing the sensory systems also results in more sensory noise in the sensory information. It was hypothesized that with age the use of proprioceptive information increases, as the vestibular and visual systems deteriorate more with age compared with the proprioceptive system, resulting in more sensory noise in the vestibular and visual information (Horak et al. 1989; Pasma et al. 2014). In the case of cataract an increased reliance on proprioceptive information was hypothesized as compensation for the more sensory noise in the visual information and therefore a higher proprioceptive weight. In the case of polyneuropathy we hypothesized less reliance on proprioceptive information compared with healthy elderly individuals as compensation for the more sensory noise in the proprioceptive information, resulting in a lower proprioceptive weight. Therefore, we expected the largest contrast between elderly with cataract and elderly with polyneuropathy in the use of proprioceptive information. In elderly subjects with impaired balance, a mix of previous scenarios and a higher interindividual variability in proprioceptive weight was expected, as impaired balance could be the result of deterioration of multiple sensory systems. We expected all groups to downweight proprioceptive information with increasing amplitude in the same way, apart from the elderly subjects with proprioceptive deficits, i.e., with polyneuropathy and impaired standing balance, in which the sensory noise in the proprioceptive information was increased, resulting in less decrease in proprioceptive weight with increasing proprioceptive disturbance.
MATERIALS AND METHODS
Participants
Ten healthy young participants, aged between 20 and 30 yr, and 44 elderly participants, aged above 65 yr, were included. The group of elderly participants was composed of 10 healthy elderly participants, 10 elderly participants with cataract, 10 elderly participants with polyneuropathy, and 14 elderly participants with impaired standing balance. Inclusion criteria for the group of young and healthy elderly participants were applied following the EU-FP7 MYOAGE study (McPhee et al. 2013) to reduce possible confounding by (co)morbidities. Exclusion criteria were being in a dependent living situation, inability to walk a distance of 250 m, presence of comorbidity (neurological disorders, metabolic diseases, rheumatic diseases, recent malignancy, heart failure, severe chronic obstructive pulmonary disease, dementia), use of certain medications (immunosuppressive drugs, insulin, anticoagulation), immobilization for 1 wk during the last 3 mo, and orthopedic surgery during the last 2 yr still causing pain or functional limitation. Inclusion criteria for the other three groups consisted of being scheduled for cataract surgery (cataract group), being diagnosed with polyneuropathy (polyneuropathy group), and being unable to perform a 10-s stance with both feet in one line (i.e., tandem stance) with eyes open (impaired standing balance group) regardless of the underlying cause. This study was performed according to the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Leiden University Medical Center. All participants gave written informed consent to participate in this study.
Participant Characteristics
To characterize the state of health, the participants completed questionnaires that provided information on age, sex, impaired balance experienced, fall incidents, and walking difficulties. Weight and height were measured. Medication use and the presence of diseases were obtained by standardized interviewing before inclusion and checked by reviewing available medical records. Cognition was assessed with the Mini-Mental State Examination (MMSE) (Folstein et al. 1975). Depressive symptoms were assessed with the Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith 1983). Depression was indicated by a score of 8 or more on the HADS depression scale. Physical functioning was assessed by handgrip strength and the Short Physical Performance Battery (SPPB) (Guralnik et al. 1994). Walking speed was measured over 10 m during a 15-m walk at preferred walking speed.
Apparatus
A bilateral ankle perturbator (BAP) (Forcelink, Culemborg, The Netherlands) was used to disturb the proprioceptive information of both ankles by applying SS rotations around the ankle axis (Schouten et al. 2011) (Fig. 1). By these rotations of the feet around the ankle axis, the leg orientation relative to the SS is changed, resulting in disturbed proprioceptive information (Pasma et al. 2012; Peterka 2002). The actual angles of rotation (i.e., motor angles) and the applied torques to both SSs of the BAP (i.e., motor torques) were measured.
Fig. 1.
A: experimental setup with the bilateral ankle perturbator (BAP) with the motor (1), the lever arm (2), and the support surfaces (3) indicated. The participant wore a safety harness to prevent a fall and looked at a poster on the wall. B: schematic figure of the approach showing the support surface (SS) rotation around the ankle axis, the ankle torque (T), and the body sway (BS).
Procedure
During all experiments participants wore antislip socks (Basset home socks). Prior to the experiment, data were recorded for 30 s while the participant kept his/her balance on the BAP without SS rotation (i.e., static condition). During the main experiment, the participant was instructed to stand with the arms crossed over the chest and to keep both feet on the SSs. Both SSs rotated simultaneously after a continuous disturbance signal with increasing zero-to-peak amplitude over trials. Each participant performed three trials with increasing disturbance amplitude, in the range of 0.01, 0.02, 0.04, and 0.08 rad (i.e., zero-to-peak amplitude of 0.57°, 1.15°, 2.29°, and 4.58°). The applied disturbance amplitudes were dependent on the amplitude each participant could maximally withstand. If a participant was not able to perform a trial with amplitude of 0.08 rad, a trial with amplitude of 0.01 rad was performed. Thus all participants performed three trials, including two conditions with disturbance amplitude 0.02 and 0.04 rad and one condition based on the ability of the participant, either 0.01 or 0.08 rad. The trials were presented in random order, and each trial lasted 116.16 s. Before each trial the participant was given ∼30 s to get accustomed to the disturbances. Visual information was standardized by instructing the subject to look at a landscape poster on the wall. Between trials, the participant was offered sufficient resting time depending on individual needs. The participant wore a safety harness to prevent falling, which did not constrain movement and did not provide support or orientation information.
Disturbance Signal
A pseudorandom ternary sequence (PRTS) of numbers was designed (Davies 1970) and used as SS angular velocity. Integration of this velocity signal provided an unpredictable disturbance signal of the SS rotation with a wide spectral bandwidth (Fig. 2). A PRTS signal with a time increment of Δt = 0.12 s was generated, resulting in a signal with a period of 29.04 s. The starting value of the PRTS signal was selected so that ∼76.5% of the rotation disturbance occurred in toe-down direction (Peterka 2002) to prevent initial balance disturbance, as humans tolerate larger angles of ankle rotation in toe-down direction. Each trial consisted of four complete cycles of the disturbance signal (i.e., a total time of 116.16 s).
Fig. 2.

Time signal (top), presented with normalized amplitude, and the corresponding power spectrum (bottom) of the disturbance signal.
Data Recording and Processing
Lower and upper body segmental movements were measured in both anterior-posterior and medio-lateral directions with four draw-wire potentiometers (Sentech SP2, Celesco, Chatsworth, CA) at a sample frequency of 1,000 Hz. The potentiometers were connected to the participant's trunk and right upper leg. The motor angles and motor torques were recorded with a MATLAB interface with a sample frequency of 1,000 Hz. Data analysis was performed with MATLAB (The MathWorks, Natick, MA).
Leg and hip angles were calculated from potentiometer data. Both were calculated relative to the averaged body position during the static condition, i.e., quiet stance, by subtracting the leg and hip angle measured during the static condition from the leg and hip angles measured during disturbances. The leg angle represents the segment angle of the lower leg relative to the vertical, and the hip angle represents the joint angle of the hip, i.e., the angle of the trunk relative to the lower leg. The body sway was represented by the angle of the CoM relative to the vertical, which was calculated using the leg and hip angles and body geometry of individual segments (Winter et al. 1990). The data of the motor angles (i.e., SS rotation) and motor torques were filtered with a second-order low-pass digital Butterworth filter with cutoff frequency of 20 Hz (MATLAB function: filtfilt). The ankle torques (Tl, Tr) were obtained from the recorded motor torques (i.e., the applied torque to the SSs of the BAP) by subtracting the contributions of the mass and inertia of the SSs of the BAP from the measured motor torque. The ankle torques were corrected for the distance between the rotation point of the SS and the real rotation point of the ankle by dividing the ankle torques by the distance between the SS and the rotation point and multiplying it by the distance between the SS and the ankle joint. The time series were segmented into four data blocks of 29.04 s (i.e., the length of the disturbance signal).
Data Analysis
Body sway descriptors.
A description of the response of the leg and hip angle was given by the root mean square (RMS) of the averaged time series of the relative leg and hip angle representing the variance.
Sensitivity functions.
To indicate the effect of the disturbances on the ankle torque and body sway, frequency response functions (FRFs) were estimated. The SS disturbances, ankle torque, leg and hip angle, and body sway were transformed to the frequency domain. The periodic part of the frequency coefficients was determined by averaging over the data blocks (van der Kooij and de Vlugt 2007). The power spectral densities (PSDs) and cross-spectral densities (CSDs) were computed to calculate the FRFs (van der Kooij et al. 2005). Only the excited frequencies were analyzed (see Disturbance Signal). The FRFs were estimated with the indirect approach (van der Kooij et al. 2005):
In which ΦSS,x represents the CSD of the SS rotation and x, which represents the total ankle torque (Tl + Tr), leg angle (LA), hip angle (HA), or body sway (BS), and ΦSS,SS the PSD of the SS rotation. The FRF magnitude and the FRF phase represent the amplitude ratio and the relative delay, respectively, between the SS rotation and the ankle torque, leg angle, hip angle, or body sway. Four sensitivity functions were estimated: 1) the ankle torque sensitivity function describes the relationship between the SS rotation and the torque exerted by both ankles (SSST); 2) the leg angle sensitivity function describes the relationship between the SS rotation and the leg angle per frequency (SSSLA); 3) the hip angle sensitivity function describes the relationship between the SS rotation and the hip angle per frequency (SSSHA); and 4) the body sway sensitivity function describes the relationship between the SS rotation and the body sway in anterior-posterior direction per frequency (SSSBS). As the corrective torque has to compensate for the gravitational torque, the FRFs of the ankle torque were normalized for the gravitational stiffness, i.e., the participant's mass and the distance from the ankles to the CoM times the gravitational acceleration (mglCoM).
Model Description and Validation
To give physiological meaning to the sensitivity functions, a model of the balance control system was used to describe the sensitivity functions. This model consists of several parameters describing the behavior of the system (Table 1). The present model is based on previous models (Cenciarini et al. 2010; Mahboobin et al. 2007; Peterka 2002; van der Kooij et al. 2005) (Fig. 3, appendix). The balance control system is approached by a one-segmental inverted pendulum model rotating around the ankle joint (Fig. 3), in which the human body is represented by a one-segmental inverted pendulum that is stabilized by a corrective ankle torque. This corrective torque is generated by a neuromuscular controller. The neuromuscular controller is formed by the neural controller, force feedback, the activation dynamics of the muscles, and the sensory channels. Each sensory system (i.e., vision, proprioception, and vestibular system) is presented by a sensory channel consisting of a weighting factor (Wprop, Wves+vis) representing the relative contribution of the sensory systems to maintain balance. The visual and vestibular channels are lumped (Wves+vis), as the individual contributions of these two systems could not be established because participants stood with their eyes open. The sum of the weighting factors always equals 1. The information of the sensory channels is sent to the neural controller representing the nervous system. The controller consists of a PD controller (Kp and Kd) with a time delay (τ) representing the neural delay due to transport and processing time of all sensory information and the reaction time of the motor system. The neural controller processes the sensory signals and sends efferent signals to the muscles. The muscles contract and generate a corrective torque. The muscles are represented by the activation dynamics consisting of a relative damping and natural frequency (β and ω). Furthermore, the force feedback represents the function of the Golgi tendon organ and other force sensors, which gives feedback to the input of the neural control represented by a gain divided by a time constant (Kf/τf).
Table 1.
Overview of estimated model parameters
| Parameter | Abbreviation | Unit | Used in Model Fit | Value |
|---|---|---|---|---|
| Length | lCoM | m | Fixed value | Measured |
| Mass | m | kg | Fixed value | Measured |
| Inertia | I | kgm2/s2 | Calculated | mlCoM2 |
| Vestibular and visual weight | Wves+vis | Calculated | 1 − Wprop | |
| Proprioceptive weight | Wprop | Variable over conditions | ||
| Reflexive stiffness | Kp | Nm/rad | Variable over conditions | |
| Reflexive damping | Kd | Nms/rad | Variable over conditions | |
| Time delay | τ | s | Constant over conditions | |
| Relative damping | β | Fixed value | 0.7 | |
| Natural frequency | ω | rad/s | Fixed value | 5π |
| Force feedback | Kf/τf | rad·Nm−1·s−1 | Constant over conditions |
Fig. 3.
Model of the balance control system in which the body is represented by an inverted pendulum. This inverted pendulum is controlled by the neuromuscular controller, consisting of the weighting factors of the visual, vestibular, and proprioceptive information, a neural controller, force feedback, time delay, and muscle activation dynamics. The torque (T) generated by the neuromuscular controller affects the body sway (BS) angle. The control loop can be disturbed by support surface rotation (SS), resulting in a sensory disturbance of the proprioceptive information.
Balance control modeling.
The parameters describing the sensitivity functions were estimated using the mathematical FRFs of the balance control model (see appendix). The mathematical FRFs of the SS rotation to the ankle torque and to the body sway of the different trials were used to fit the model on the experimental FRFs. To limit the number of unconstrained fit parameters, we used fixed values of relative damping (β = 0.7) and natural frequency (ω = 5π rad/s) (Boonstra et al. 2013) and direct measurements of mass and pendulum length and estimated the inertia by multiplying mass with squared pendulum length (mlCoM2). The time delay and force feedback were kept constant over trials. Of the weighting factors only the proprioceptive weight was estimated, in which the sum of the visual and vestibular weight equals 1 minus the proprioceptive weight. The proprioceptive weight, reflexive stiffness, and reflexive damping were variable between trials. This resulted in an estimation of 11 parameters per participant (Table 1). The model was fitted on all (not normalized) individual experimental FRFs with a nonlinear least-squares fit (MATLAB function: lsqnonlin) by minimizing the vector-valued function:
| (2) |
In which γSS,x represents the coherence between SS rotation and ankle torque or body sway, Hexp the experimental sensitivity function, and Hest the estimated sensitivity function based on the estimated model parameters. The coherence varies between 0 and 1, in which a coherence close to 1 reflects a good signal to noise ratio. To evaluate the goodness of the model fit, the goodness of fit (GOF) in the frequency domain was calculated with Eq. 3:
| (3) |
in which Sest(ω) represents the estimated sensitivity function per frequency and Sexp(ω,p) the experimental sensitivity function per frequency and parameter set. To compare parameters between participants, the parameters Kp and Kd were normalized for the participant's gravitational stiffness (mglCoM).
Statistical Analysis
The characteristics of the participants are represented by mean and standard deviation in case of a Gaussian distribution. Otherwise, median and interquartile range or number and percentage are presented. For statistical analysis, the PSDs and CSDs were averaged within seven frequency bands before the FRFs were calculated, according to the method of Peterka in which the number of points averaged increases with frequency (Peterka 2002), resulting in the frequency bands 0–0.1 Hz, 0.1–0.3 Hz, 0.3–0.7 Hz, 0.7–1.4 Hz, 1.4–2.2 Hz, 2.2–3.1 Hz, and 3.1–4.1 Hz. Subsequently, the magnitude of each FRF was log transformed to make the data normally distributed.
Linear mixed models were used to test significant differences in FRFs between groups and disturbance amplitudes. Frequency band was included as covariate to adjust for differences due to frequencies. Group, disturbance amplitude, and frequency band were fixed effects. Participant intercept was included as a random effect to take the measurement repetitions and differences in conditions into account, as not all participants performed conditions with the same disturbance amplitude.
The proprioceptive reweighting was based on the conditions performed by all participants (i.e., conditions with disturbance amplitude 0.02 and 0.04 rad) and was assessed by fitting individual slopes between disturbance amplitude and proprioceptive weight with linear regression analysis, representing the proprioceptive weight change in response to a 1-rad increase of the disturbance amplitude. A negative value indicated downweighting of proprioceptive information. To test significant differences in estimated parameters (i.e., proprioceptive weight, proprioceptive reweighting, reflexive stiffness and damping, time delay, and force feedback) between groups and disturbance amplitudes, linear mixed models were used, with group and disturbance amplitude as fixed effects and participant intercept as random effect. All analyses were adjusted for age and sex by including those factors in the mixed models. The analyses of the comparison between healthy young and healthy elderly participants were only adjusted for sex. For all tests, significance (α) was set at 0.05. All analyses were performed with SPSS version 20.0 (SPSS, Chicago, IL).
RESULTS
Below the results of this study are presented. Differences between groups in the response to the proprioceptive disturbances are summarized in Table 2.
Table 2.
Summary of results describing significant differences in body sway descriptors, sensitivity functions, and estimated parameters between groups
| Elderly vs. Young | Cataract vs. Elderly | PNP vs. Elderly | Impaired Balance vs. Elderly | Cataract vs. PNP | |
|---|---|---|---|---|---|
| Body sway descriptors | |||||
| Variance leg angle | = | ↑ | = | = | ↑ |
| Variance hip angle | ↑ | ↑ | = | ↑ | = |
| Sensitivity functions | |||||
| SS to ankle torque | ↑ | ↑ | = | ↑ | ↑ |
| SS to leg angle | = | ↑ | = | ↑ | = |
| SS to hip angle | ↑ | ↑ | = | ↑ | = |
| SS to body sway | ↑ | ↑ | = | ↑ | = |
| Estimated parameters | |||||
| Proprioceptive weight, Wprop | ↑ | ↑ | = | ↑ | = |
| Proprioceptive reweighting, ΔWprop | = | = | = | = | = |
| Time delay, τ | ↑ | ↑ | ↑ | ↑ | = |
| Reflexive stiffness, Kp | ↓ | ↓ | ↓ | ↓ | = |
| Reflexive damping, Kd | ↑ | = | = | = | ↓ |
| Force feedback, Kf/τf | = | = | = | = | = |
Results are summarized by 3 symbols: ↓ representing a significantly lower value, ↑ representing a significantly higher value, and = representing no significant differences between groups, PNP, polyneuropathy; SS, support surface.
Participant Characteristics
Table 3 represents the participant characteristics per group. Healthy elderly participants showed no significant differences in characteristics compared with young participants, except for age and handgrip strength. Between the elderly groups, no differences were found in age. Elderly participants with polyneuropathy experienced more impaired standing balance and showed lower walking speed and lower SPPB score compared with healthy elderly participants. Elderly participants diagnosed with impaired balance showed more medication use, more multimorbidity, lower physical functioning, and more self-reported walking difficulties compared with healthy elderly participants. There were no differences between healthy elderly participants and elderly participants with cataract. Twenty-nine participants (53.7%) were not able to perform the trial with the highest disturbance amplitude of 0.08 rad and therefore performed the trial with a disturbance amplitude of 0.01 rad. This group comprised 1 (10%) healthy elderly, 5 (50%) elderly with cataract, 9 (90%) elderly with polyneuropathy, and 14 (100%) elderly with impaired balance.
Table 3.
Participant characteristics stratified by group
| Elderly |
|||||
|---|---|---|---|---|---|
| Young (n = 10) | Healthy (n = 10) | Cataract (n = 10) | PNP (n = 10) | Impaired balance (n = 14) | |
| Age, yr | 25.4 (2.2) | 76.8 (1.8) | 76.7 (6.8) | 73.7 (8.0) | 83.5 (6.3) |
| Women, n (%) | 6 (60) | 4 (40) | 5 (50) | 3 (30) | 3 (21.4) |
| Health status | |||||
| Multimorbidity, n (%)* | 0 (0) | 0 (0) | 2 (20) | 2 (20) | 4 (28.6) |
| No. of medications [median (IQR)] | 0.5 (0–1) | 1 (0–2) | 1 (0–6.25) | 2 (1–4.5) | 5 (3.8–8) |
| MMSE, points [median (IQR)] | 30 (30–30) | 29.5 (28–30) | 28.5 (28–30) | 29 (27–30) | 29 (28.7–29.3) |
| Depressive symptoms, n (%)† | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) |
| Anthropometry | |||||
| Height, cm | 178 (11) | 171 (9) | 172 (11) | 175 (10) | 174 (9) |
| Weight, kg | 71.1 (6.7) | 73.3 (7.9) | 73.8 (17.0) | 86.4 (10.4) | 79.5 (17.3) |
| Physical functioning | |||||
| Handgrip strength, kg | 44.6 (9.4) | 35.7 (5.9) | 33.8 (10.9) | 37.5 (9.3) | 32.1 (8.9) |
| SPPB score, points [median (IQR)] | 12 (12–12) | 12 (11–12) | 12 (10–12) | 10 (9.5–11.3) | 7.5 (6–10) |
| Walking speed, m/s‡ | 1.48 (0.21) | 1.34 (0.12) | 1.23 (0.22) | 1.07 (0.30) | 1.00 (0.26) |
| Fall incident, n (%) | 0 (0) | 2 (20) | 2 (20) | 3 (30) | 8 (57.1) |
| Impaired standing balance, n (%) | 0 (0) | 0 (0) | 0 (0) | 5 (50) | 8 (57.1) |
| Walking difficulties, n (%) | 0 (0) | 0 (0) | 1 (10) | 8 (80) | 10 (71.4) |
| BAP performance, n (%) | |||||
| Amplitude 0.01 rad | 0 (0) | 1 (10) | 5 (50) | 8 (80) | 14 (100) |
| Amplitude 0.02 rad | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 14 (100) |
| Amplitude 0.04 rad | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 12 (85.7) |
| Amplitude 0.08 rad | 10 (100) | 9 (90) | 5 (50) | 1 (10) | |
All values are presented as means (SD) unless indicated otherwise. IQR, interquartile range; MMSE, Mini-Mental State Examination; SPPB, Short Physical Performance Battery; BAP, bilateral ankle perturbator.
Present in case of 2 or more diseases, including chronic obstructive pulmonary disease, heart failure, rheumatic disorder, dementia, diabetes mellitus, malignancy, Parkinson's disease, (osteo)arthritis, transient ischemic attack, and stroke.
Present with a depression subscore >8 on the Hospital Anxiety and Depression Scale.
Preferred gait speed during a steady-state 10-m walk.
Body Sway Descriptors
Figure 4 shows the RMS of the leg and hip angle for each group and each disturbance amplitude.
Fig. 4.
Root mean square (RMS) of leg and hip angle of each group and each trial. *Significantly different (P < 0.05) compared with young, xsignificantly different (P < 0.05) compared with healthy elderly, +significantly different (P < 0.05) compared with elderly with cataract.
Healthy elderly participants showed a higher variance of the hip angle (P = 0.002) compared with young participants. Comparing elderly participants with cataract and healthy elderly participants, elderly with cataract showed higher variance in both leg and hip angle (leg: P = 0.013, hip: P = 0.012). Between healthy elderly participants and elderly participants with polyneuropathy, there were no significant differences in variance of the leg and hip angle. Elderly participants with impaired balance had higher hip angle variance (P = 0.013) compared with healthy elderly participants. Elderly participants with cataract had only differences in variance of leg angle compared with elderly participants with polyneuropathy (P = 0.017).
In summary, with age the variances of the hip angle are higher. With cataract, the variances of both the ankle and hip angle are higher. With impaired balance, only the variances of the hip angle are higher (Table 2).
Sensitivity Functions
Figure 5 shows the mean sensitivity functions of the ankle torque, leg angle, hip angle, and body sway to the disturbance with amplitude of 0.02 rad averaged over participants for each group. In all groups, the magnitude of all sensitivity functions significantly decreased with increasing disturbance amplitude, indicating a saturation of the ankle torque, leg angle, hip angle, and body sway with increasing disturbance amplitude (P < 0.001 for all sensitivity functions). The magnitude of the sensitivity functions of the ankle torque, hip angle, and body sway were higher in healthy elderly compared with healthy young participants (P < 0.001, P < 0.001, and P < 0.001, respectively), indicating a higher response to the disturbance. No significant difference in the sensitivity of the leg angle was found between young and healthy elderly participants (P = 0.33). All sensitivity function magnitudes were higher in elderly participants with cataract (P = 0.038, P = 0.019, P = 0.004, and P = 0.005, respectively) and in elderly participants with impaired balance (P = 0.012, P = 0.042, P = 0.017, and P = 0.017, respectively) compared with healthy elderly participants. There were no significant differences in the magnitude of the sensitivity functions of the ankle torque, leg angle, hip angle, and body sway between healthy elderly participants and elderly participants with polyneuropathy (P = 0.82, P = 0.54, P = 0.30, and P = 0.43, respectively). Comparing elderly participants with cataract to elderly participants with polyneuropathy showed a higher magnitude of the sensitivity function of the ankle torque in elderly participants with cataract (P = 0.042). No significant differences were found in the other sensitivity functions (leg angle: P = 0.074, hip angle: P = 0.18, and body sway: P = 0.10).
Fig. 5.
Mean sensitivity function of each group of the trial with disturbance amplitude of 0.02 rad. The (normalized) magnitude and phase of the sensitivities of the ankle torque (SSST), the leg angle (SSSLA), the hip angle (SSSHA), and the body sway (SSSBS) to the rotation of the support surface are shown.
In summary, with age the sensitivities of the ankle torque, hip torque, and hip angle are higher. With cataract and impaired balance the sensitivities of both the ankle and hip torque and leg and hip angle are higher (Table 2).
Estimated Model Parameters
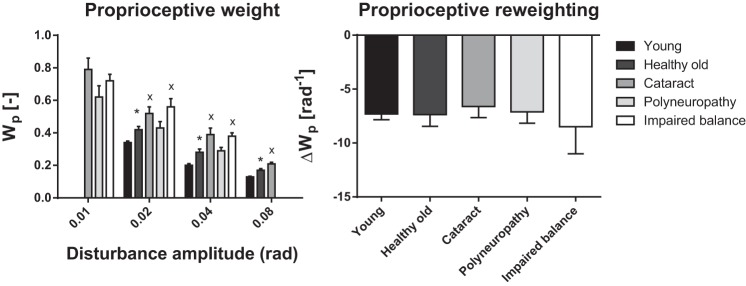
Table 4 shows the GOF per trial per group representing the goodness of the model fits. The GOF was higher in trials with higher disturbance amplitude. The GOF of the fitted model in elderly with cataract, elderly with polyneuropathy, and elderly with impaired balance was lower compared with young and healthy elderly participants. Figure 6 shows both the estimated proprioceptive weight of each disturbance amplitude and proprioceptive reweighting for each group. In all groups, the proprioceptive weight significantly decreased with increasing disturbance amplitude (P < 0.001), as also shown by the sensory reweighting parameter. Healthy elderly participants showed higher proprioceptive weight (P < 0.001) and no differences in proprioceptive reweighting (P > 0.99) compared with young participants. Compared with healthy elderly participants, elderly participants with cataract showed a higher proprioceptive weight (P = 0.018) and no significant difference in proprioceptive reweighting (P = 0.64). Elderly participants with polyneuropathy showed no significant differences in proprioceptive weight (P = 0.42) and proprioceptive reweighting (P = 0.90) compared with healthy elderly participants. Elderly participants with impaired balance showed a higher proprioceptive weight (P = 0.005) and no differences in proprioceptive reweighting (P = 0.77) compared with healthy elderly participants. There were no significant differences between elderly with cataract and elderly with polyneuropathy in proprioceptive weight (P = 0.13) and proprioceptive reweighting (P = 0.87).
Table 4.
Goodness of fit of frequency response function SSST model fits per trial per group
| Elderly |
|||||
|---|---|---|---|---|---|
| Young | Healthy | Cataract | PNP | Impaired balance | |
| 0.01 rad | 58.7 (8.2) | 53.0 (4.7) | 55.1 (5.4) | ||
| 0.02 rad | 78.5 (2.4) | 76.2 (6.0) | 78.4 (1.7) | 68.6 (3.7) | 69.2 (4.3) |
| 0.04 rad | 86.0 (1.4) | 84.7 (2.8) | 79.7 (3.8) | 68.4 (9.7) | 79.9 (3.0) |
| 0.08 rad | 90.1 (0.4) | 85.4 (2.2) | 87.5 (3.1) | ||
All values (in %) are presented as means (SE).
Fig. 6.
Proprioceptive weight (Wp) and reweighting (ΔWp) per radian increase of disturbance amplitude of each group and each trial. *Significantly different (P < 0.05) compared with young, xsignificantly different (P < 0.05) compared with healthy elderly, +significantly different (P < 0.05) compared with elderly with cataract.
Figure 7 represents the other estimated parameters for each group and each disturbance amplitude. Healthy elderly participants showed lower reflexive stiffness (P = 0.004), higher reflexive damping (P = 0.003), and higher time delay (P < 0.001) compared with young participants. No significant differences in force feedback (P = 0.80) were found. Compared with healthy elderly participants, elderly participants with cataract showed a lower reflexive stiffness (P = 0.013) and a higher time delay (P = 0.009). No differences in reflexive damping (P = 0.12) and force feedback (P = 0.81) were found. Elderly participants with polyneuropathy had a lower reflexive stiffness (P = 0.003) and a higher time delay (P = 0.007) compared with healthy elderly participants. There were no significant differences in reflexive damping (P = 0.51) and force feedback (P = 0.42). Elderly participants with impaired balance showed a lower reflexive stiffness (P = 0.027) and a higher time delay (P = 0.002) compared with healthy elderly participants and no difference in reflexive damping (P = 0.67) and force feedback (P = 0.36). Compared with elderly participants with cataract, elderly participants with polyneuropathy showed a higher reflexive damping (P = 0.029). There were no significant differences between elderly participants with cataract and elderly participants with polyneuropathy in reflexive stiffness (P = 0.25), time delay (P = 0.46), and force feedback (P = 0.39).
Fig. 7.
Normalized reflexive stiffness and damping, time delay, and force feedback of each group and each trial. *Significantly different (P < 0.05) compared with young, xsignificantly different (P < 0.05) compared with healthy elderly, +significantly different (P < 0.05) compared with elderly with cataract.
In summary, with age, cataract, and impaired balance the proprioceptive weight is higher. Furthermore, with age and disease the reflexive stiffness is lower, while the time delay is higher. There are no differences in the change of proprioceptive weight (Table 2).
DISCUSSION
As summarized in Table 2, the results of this study showed that the proprioceptive weight was higher with age and higher with cataract and impaired balance and showed no differences in proprioceptive reweighting with age and specific diseases. Results are consistent with the hypothesis that sensory reweighting is an adaptive process to prevent loss of balance in case of deficits in the underlying sensory systems; increasing the disturbance amplitude of the proprioceptive information resulted in a decrease in proprioceptive weight, i.e., downweighting. The change in proprioceptive reweighting depends on the deteriorated sensory system. Deficits of the visual system were compensated by an increase in proprioceptive weight and changes in the nervous system, while deficits of the proprioceptive system only showed changes in the nervous system.
More Use of Proprioceptive Information with Age
We demonstrated that healthy elderly participants rely more on their proprioceptive information during standing balance compared with healthy young participants, represented by a higher proprioceptive weight. Previous studies showed contradictory results according to the use of sensory information in the elderly, in which studies using posturography showed more use of visual information (Bugnariu and Fung 2007; Faraldo-Garcia et al. 2012) and studies using system identification techniques showed more use of proprioceptive information (Cenciarini et al. 2010). In this study, the proprioceptive downweighting is comparable between age groups; healthy elderly participants have the same ability to compensate for unreliable sensory information as young participants. This is in accordance with previous studies in which sensory reweighting of visual information was investigated in healthy young and elderly subjects (Jeka et al. 2006) but in contrast with others (Borger et al. 1999; Bugnariu and Fung 2007; Doumas and Krampe 2010; Eikema et al. 2012; Horak et al. 1989; Stelmach and Worringham 1985; Teasdale et al. 1991; Teasdale and Simoneau 2001). Compared with previous studies, we included elderly participants with a higher age, i.e., 75 yr or older instead of 65 yr or older.
The increased use of proprioceptive information compared with young participants follows expectations, as explained by the deterioration of the other sensory systems with age (Horak et al. 1989; Pasma et al. 2014). Previous studies argued that the vestibular system deteriorates the most with age (Barin and Dodson 2011; Faraldo-Garcia et al. 2012; Horak et al. 1989; Sturnieks et al. 2008), which will result in less reliable vestibular information. This may be compensated for by upweighting of the other sensory information, resulting in a higher visual and/or proprioceptive weight. However, others have argued that vestibular information plays an important role during standing balance by showing a larger vestibular-evoked balance response in healthy elderly men compared with healthy young men (Dalton et al. 2014). However, to investigate whether both vestibular and proprioceptive information are used relatively more by healthy elderly compared with healthy young, experiments with multiple simultaneous sensory disturbances must be performed. This makes it possible to determine the contribution of each sensory system separately during standing balance. Our results showed that the nervous system is still able to compensate for disturbances of the proprioceptive information, represented by similar proprioceptive downweighting in young and healthy elderly participants. However, when the higher proprioceptive weight of the healthy elderly participants is taken into account, the elderly participants downweight their proprioceptive information relatively less compared with young participants.
More Use of Proprioceptive Information with Cataract
In patients with cataract we found a higher proprioceptive weight compared with healthy elderly participants, which means that the elderly with cataract rely more on their proprioceptive information. This is in accordance with previous studies, which showed that the elderly with visual problems rely more on vestibular and proprioceptive information (Black et al. 2008; Chen et al. 2012; Friedrich et al. 2008; Kotecha et al. 2012; Ray et al. 2008). Furthermore, these studies showed that the elderly have more problems with maintaining balance during trials in which proprioceptive information is more disturbed, which is in accordance with our results. However, we found no differences in proprioceptive downweighting compared with healthy elderly participants in case of proprioceptive disturbances.
The higher proprioceptive weight could be explained by the compensation for less reliable visual information in cataract patients; less reliable visual information is downweighted, which is accompanied by upweighting of the sensory information of the other sensory systems, i.e., the proprioception and/or vestibular system (Black et al. 2008; Friedrich et al. 2008; Kotecha et al. 2012; Ray et al. 2008). An explanation for the comparable downweighting in healthy elderly participants could be that the nervous system still can compensate for the unreliable proprioceptive information by more use of the vestibular information instead of the visual information. This will result in the same amount of downweighting of the proprioceptive information despite deterioration of the visual system. When we take the already higher proprioceptive weight into account, elderly participants with cataract downweight their proprioceptive information relatively less compared with healthy elderly participants. This could also explain why the elderly with cataract are not able to perform conditions with high disturbance of proprioceptive information.
No Changes in Use of Proprioceptive Information with Polyneuropathy
It was expected that participants with polyneuropathy would rely less on proprioception because of the expected deficits in this particular sensory channel. However, we did not detect differences in the weight of proprioceptive information between healthy elderly participants and elderly participants with polyneuropathy. This is in contradiction with previous studies, which showed more reliance on vestibular and visual information during standing balance in this population group (Boucher et al. 1995; Kars et al. 2009; van der Linden et al. 2010; Vaugoyeau et al. 2011). However, participants included in these studies were younger compared with the participants included in our study. Furthermore, no differences in downweighting of proprioceptive information in elderly participants with polyneuropathy compared with healthy elderly participants were found.
That we did not find differences between elderly participants with polyneuropathy and healthy elderly participants could be explained by the variation in degree of polyneuropathy resulting in a high group variability. It might be that the small tactile nerves are damaged earlier compared with larger afferent nerves of the muscle spindles and the Golgi tendon organs, resulting in a small difference between healthy elderly and elderly with polyneuropathy. Furthermore, this could also mean that the processing of sensory information still works sufficiently well and therefore is still able to downweight proprioceptive information. However, it is remarkable that the elderly with polyneuropathy show less ability to maintain standing balance in more demanding test conditions. This implies deterioration of other underlying systems involved in standing balance, such as the nervous system.
In contrast with our expectations, we did not find a difference between elderly participants with cataract and elderly participants with polyneuropathy in the use of proprioceptive information. This could be explained by the high variability in the group of elderly participants with polyneuropathy, as mentioned above.
More Use of Proprioceptive Information with Impaired Balance
In elderly participants with impaired balance the results showed a higher proprioceptive weight compared with healthy elderly participants and no differences in proprioceptive downweighting. The included elderly participants with impaired balance had characteristics comparable to those of elderly with a history of falls included in previous studies investigating sensory integration (Allison et al. 2006; Barrett et al. 2013; Jeka et al. 2006). Previous studies found a comparable sensory downweighting of visual information between fall-prone elderly participants with a history of unexplained falls and healthy elderly participants (Jeka et al. 2006), which is in accordance with our study. Taking the higher proprioceptive weight at the lowest disturbance amplitude into account, the results show that elderly participants with impaired balance downweight their proprioceptive information relatively less compared with healthy elderly participants. A higher proprioceptive weight with a higher disturbance amplitude means a higher sensitivity to the proprioceptive disturbance. This increases the chance of a too large body sway with proprioceptive disturbance, which may result in falls. This could also explain why elderly participants with impaired balance are less able to perform the condition with the highest perturbation amplitude.
Nervous System Changes with Age and Sensory Deficits
Using system identification, we could detect changes in sensory reweighting with age and sensory deficits but also changes in the nervous system, i.e., reflexive stiffness, reflexive damping, and neural time delay. We found that healthy elderly participants had a higher neural time delay, consisting of transport and processing time of all sensory information and the reaction time of the motor system, compared with healthy young participants, which is consistent with previous studies (Davidson et al. 2011; Doumas and Krampe 2010). This could be explained by slow nerve conduction speed in afferent or efferent pathways, a slow muscle activation, or slow central processing time due to a decrease in the number of neurons and loss of myelination, both of which occur with age (Barin and Dodson 2011; Horak et al. 1989; Sturnieks et al. 2008). In healthy elderly participants, we found a lower reflexive stiffness and higher reflexive damping compared with healthy young participants. This means that healthy elderly participants had a lower reflexive response to maintain balance compared with young participants. This is in contrast with Cenciarini et al. (2010), who found an increase of both reflexive parameters. Davidson et al. (2011) only found a higher reflexive damping in elderly participants compared with young participants.
In elderly participants with cataract, polyneuropathy, and impaired balance we found a lower reflexive stiffness compared with healthy elderly participants, indicating a lower response of ankle torque as a result of changes in body sway to maintain balance compared with healthy elderly participants. In addition, we found a higher neural time delay in these groups compared with healthy elderly participants, probably due to the sensory deficits. The neural time delay represented the transport and processing time of all sensory information as well as the transport time of motor commands to the muscles, i.e., the individual time delays are lumped. Deficits in sensory systems could result in longer conduction (Fischer et al. 1979) and processing time (Stelmach and Worringham 1985; Whipple et al. 1993) and therefore a higher neural time delay. A higher reflexive damping found in elderly participants with polyneuropathy compared with healthy elderly participants could be a strategy to overcome the higher neural time delay, as a higher reflexive damping could result in less influence of the time delay on the response. The changes found in the nervous system might explain the lower ability to maintain standing balance during more demanding test conditions (i.e., higher disturbance amplitude of the proprioceptive information).
Methodological Considerations
In this study system identification techniques were used to identify cause and effect relations, allowing us to distinguish the underlying changes in proprioceptive reweighting during standing balance from changes in compensation strategy and deterioration of other underlying systems (Pasma et al. 2014). Previous studies used system identification techniques in healthy young participants, healthy elderly participants, and patients with dysfunction of the vestibular organ to assess sensory reweighting (Cenciarini et al. 2010; Cenciarini and Peterka 2006; Mahboobin et al. 2007; Peterka 2002). Compared with these studies, we found a lower proprioceptive weight in young and healthy elderly participants. This could be explained by differences in conditions in which visual information was eliminated by closing the eyes or disturbed by visual stimulations, both resulting in a higher proprioceptive weight. In the present study participants stood with their eyes open, as conditions with eyes closed were too difficult to perform for our study population. This had no consequences for our conclusion, as we were only interested in the reweighting of proprioceptive information.
The models used in previous studies (Cenciarini et al. 2010; Peterka 2002) formed the basis for the model used in the present study. Intrinsic properties were not included in the present model, as inclusion did not result in better fits and gave unrealistic values for the intrinsic parameters. The effect of excluding the intrinsic dynamics from the model on the other estimated parameters and the GOFs was small and therefore did not affect the conclusions drawn in this study. It only resulted in somewhat higher reflexive stiffness; previous studies showed that reflexive dynamics were dominant over intrinsic dynamics (Cenciarini and Peterka 2006; Peterka 2002) and that the human body, an unstable system, could not be controlled by intrinsic dynamics alone (Loram and Lakie 2002; Morasso and Schieppati 1999; Vlutters et al. 2015). Estimated reflexive stiffness and reflexive damping of the present study are within the ranges previously found in the literature (Cenciarini et al. 2010; Cenciarini and Peterka 2006; Davidson et al. 2011; Mahboobin et al. 2007; Peterka 2002) varying from 898 Nm/rad to ∼1,500 Nm/rad for reflexive stiffness and from 288 to ∼480 Nms/rad for reflexive damping.
In extension of previous models, muscle activation dynamics were added in the present model, which resulted in better model fits. However, inclusion of muscle activation dynamics interferes with the neural time delay, as the reaction time of the motor system (i.e., electromechanical delay) is included. Therefore, the estimated neural time delay is not comparable with the neural time delay found in previous studies (Cenciarini et al. 2010; Cenciarini and Peterka 2006; Davidson et al. 2011; Mahboobin et al. 2007; Peterka 2002). Probably the activation dynamics used were too slow, resulting in low values of the neural time delay. As we assumed that the activation dynamics were the same in all groups, possible differences in activation dynamics between the groups also showed up in the neural time delay.
We fitted the model on three conditions at the same time with restrictions on the variability over conditions, i.e., neural time delay and force feedback were assumed constant over the conditions with increasing disturbance amplitude, which is supported by the literature (Peterka 2002). Furthermore, it was previously shown that reflexive stiffness and time delay were related (Peterka 2002); when reflexive stiffness increases, the time delay decreases. This could also explain why we did not find a variation of reflexive stiffness with increasing disturbance amplitude in all groups; the time delay was kept constant over conditions restricting changes in reflexive stiffness over conditions.
In this study we modeled the human body by a one-segmental inverted pendulum. However, the results showed that during the disturbances both ankle and hip strategies were used to maintain an upright position, which differed between groups and over conditions. Previous studies tried to eliminate movement around the hip joint by using a rigid backboard (Pasma et al. 2012; Peterka 2002). However, this was less feasible in this population of elderly participants. Peterka (2002) compared sensory reweighting results with and without the use of a backboard and found no differences in sensory reweighting between the conditions. To identify the control of the ankle and the hip independently with system identification techniques, two independent mechanical disturbances are required (Engelhart et al. 2014b). This allows detection of the underlying changes in the use of the ankle and hip joint (Engelhart et al. 2014a). This is a subject for further study.
Furthermore, the model could not perfectly fit all experimental data, which was expressed by the reduced GOF. Especially, the model fitted less well on participants with specific diseases. A possible explanation for the reduced GOF could be that participants with specific diseases are less constant, i.e., they show more noisy and time-variant behavior. This was supported by the observation that the model fitted better on experimental data obtained with a higher disturbance amplitude, where the response is larger, resulting in a higher signal-to-noise ratio. As mentioned above, another extension of the model could be to model the human body by a double inverted pendulum, especially as participants with diseases also moved more (variable) around the hip joint.
In this study we were interested in the contribution of proprioceptive information to standing balance. To determine which information is upweighted in case of downweighting of proprioceptive information further research has to be done, in which disturbances of proprioceptive information must be combined with disturbances of visual or vestibular information to unravel the contribution of all sensory systems separately in standing balance.
Conclusions
This study showed that it is possible to detect differences in proprioceptive weight during standing balance with age and specific diseases regardless of changes in the nervous system. The reliance on proprioceptive information relative to other sensory information is higher with aging, with visual deficits, and with impaired balance. No changes in proprioceptive downweighting in response to increased proprioceptive disturbances were found with age and disease. Furthermore, with age and sensory deficits the nervous system changed, represented by a higher neural time delay and a lower reflexive stiffness. The results of this study give more insight in the underlying changes with age and deterioration of sensory systems and indicate the opportunities provided by system identification techniques in detecting the underlying cause of impaired standing balance and therefore in applying targeted interventions to improve standing balance.
GRANTS
This research (BalRoom; project no. 10737) is supported by the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO) and which is partly funded by the Ministry of Economic Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.H.P., D.E., A.B.M., A.C.S., H.v.d.K., and C.G.M.M. conception and design of research; J.H.P. performed experiments; J.H.P. analyzed data; J.H.P., D.E., A.C.S., H.v.d.K., and C.G.M.M. interpreted results of experiments; J.H.P. prepared figures; J.H.P. and D.E. drafted manuscript; J.H.P., D.E., A.B.M., A.C.S., H.v.d.K., and C.G.M.M. approved final version of manuscript; A.B.M., A.C.S., H.v.d.K., and C.G.M.M. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank the Department of Ophthalmology (Prof. G. P. M. Luyten), the Department of Neurology (Dr. M. T. Tonk), and the Department of Geriatrics (Prof. G. J. Blauw) of the Bronovo Hospital, the Hague, The Netherlands, for recruiting participants. Furthermore, we thank the seventh framework program MYOAGE (HEALTH-2007-2.4.5-10) for the use of the subject database to recruit young and healthy elderly participants.
Appendix
A model of the balance control system was used to estimate parameters describing the behavior of the system. The human body is approached by a one-segmental inverted pendulum rotating around the ankle joint. The linearized transfer function from ankle torque to body sway of the inverted pendulum is given by Eq. A1:
| (A1) |
in which I represents the inertia, m the mass, and lCoM the pendulum length.
The human body is controlled by a neuromuscular controller consisting of the nervous system, muscles, force feedback, and sensory systems. The nervous system is represented by a PD controller with a neural time delay and the muscles by the muscle activation, as described in Eq. A2:
| (A2) |
in which Kp represents the reflexive stiffness, Kd the reflexive damping, τ the time delay, β the relative damping, and ω the natural frequency of the muscle activation.
The force feedback represents the force sensors in the tendon and muscles. The force feedback is represented by the transfer function as described in Eq. A3:
| (A3) |
in which Kf represents the gain of the force feedback and τf the time constant. As τf is much larger than Kf, this equation could be simplified to Eq. A4:
| (A4) |
The sensory systems are described by weighting factors, which indicate how much the information of each sensory system is used. As we are interested in the use of the proprioceptive weight (Wprop) we made use of proprioceptive disturbances. The sum of all weighting factors always equals 1. The vestibular and visual weight together (Wves+vis) therefore equals 1 − Wprop. The transfer function of the proprioceptive disturbance, i.e., the SS rotation, to the ankle torque and of the SS rotation to the body sway can now be described as in Eq. A5:
 |
REFERENCES
- Allison LK, Kiemel T, Jeka JJ. Multisensory reweighting of vision and touch is intact in healthy and fall-prone older adults. Exp Brain Res 175: 342–352, 2006. [DOI] [PubMed] [Google Scholar]
- Asslander L, Hettich G, Mergner T. Visual contribution to human standing balance during support surface tilts. Hum Mov Sci 41: 147–164, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barin K, Dodson EE. Dizziness in the elderly. Otolaryngol Clin North Am 44: 437–454, 2011. [DOI] [PubMed] [Google Scholar]
- Barrett MM, Doheny EP, Setti A, Maguinness C, Foran TG, Kenny RA, Newell FN. Reduced vision selectively impairs spatial updating in fall-prone older adults. Multisens Res 26: 69–94, 2013. [DOI] [PubMed] [Google Scholar]
- Black AA, Wood JM, Lovie-Kitchin JE, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci 85: 489–497, 2008. [DOI] [PubMed] [Google Scholar]
- Bonnet C, Carello C, Turvey MT. Diabetes and postural stability: review and hypotheses. J Mot Behav 41: 172–190, 2009. [DOI] [PubMed] [Google Scholar]
- Boonstra TA, Schouten AC, van der Kooij H. Identification of the contribution of the ankle and hip joints to multi-segmental balance control. J Neuroeng Rehabil 10: 23, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger LL, Whitney SL, Redfern MS, Furman JM. The influence of dynamic visual environments on postural sway in the elderly. J Vestib Res 9: 197–205, 1999. [PubMed] [Google Scholar]
- Boucher P, Teasdale N, Courtemanche R, Bard C, Fleury M. Postural stability in diabetic polyneuropathy. Diabetes Care 18: 638–645, 1995. [DOI] [PubMed] [Google Scholar]
- Bugnariu N, Fung J. Aging and selective sensorimotor strategies in the regulation of upright balance. J Neuroeng Rehabil 4: 19, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenciarini M, Loughlin PJ, Sparto PJ, Redfern MS. Stiffness and damping in postural control increase with age. IEEE Trans Biomed Eng 57: 267–275, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenciarini M, Peterka RJ. Stimulus-dependent changes in the vestibular contribution to human postural control. J Neurophysiol 95: 2733–2750, 2006. [DOI] [PubMed] [Google Scholar]
- Chen EW, Fu AS, Chan KM, Tsang WW. Balance control in very old adults with and without visual impairment. Eur J Appl Physiol 112: 1631–1636, 2012. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Blouin JS, Allen MD, Rice CL, Inglis JT. The altered vestibular-evoked myogenic and whole-body postural responses in old men during standing. Exp Gerontol 60: 120–128, 2014. [DOI] [PubMed] [Google Scholar]
- Davidson BS, Madigan ML, Southward SC, Nussbaum MA. Neural control of posture during small magnitude perturbations: effects of aging and localized muscle fatigue. IEEE Trans Biomed Eng 58: 1546–1554, 2011. [DOI] [PubMed] [Google Scholar]
- Davies WD. System Identification for Self-Adaptive Control. London: Wiley-Interscience, 1970. [Google Scholar]
- Doumas M, Krampe RT. Adaptation and reintegration of proprioceptive information in young and older adults' postural control. J Neurophysiol 104: 1969–1977, 2010. [DOI] [PubMed] [Google Scholar]
- Eikema DJ, Hatzitaki V, Tzovaras D, Papaxanthis C. Age-dependent modulation of sensory reweighting for controlling posture in a dynamic virtual environment. Age (Dordr) 34: 1381–1392, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart D, Pasma JH, Schouten AC, Meskers CG, Maier AB, Mergner T, van der Kooij H. Impaired standing balance in elderly: a new engineering method helps to unravel causes and effects. J Am Med Dir Assoc 15: 227.e1–6, 2014a. [DOI] [PubMed] [Google Scholar]
- Engelhart D, Schouten AC, Aarts RG, van der Kooij H. Assessment of multi-joint coordination and adaptation in standing balance: a novel device and system identification technique. IEEE Trans Neural Syst Rehabil Eng (November 20, 2014b). 10.1109/TNSRE.2014.2372172. [DOI] [PubMed] [Google Scholar]
- Faraldo-Garcia A, Santos-Perez S, Crujeiras-Casais R, Labella-Caballero T, Soto-Varela A. Influence of age and gender in the sensory analysis of balance control. Eur Arch Otorhinolaryngol 269: 673–677, 2012. [DOI] [PubMed] [Google Scholar]
- Fischer W, Reichel G, Rabending G, Bruns W, Haubenreiser H, Sodemann K, Zander G. [The diabetic polyneuropathy. I. Relation between impaired function in peripheral nerves and clinical findings.] Endokrinologie 74: 207–220, 1979. [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol 76: 3994–4008, 1996. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Grein HJ, Wicher C, Schuetze J, Mueller A, Lauenroth A, Hottenrott K, Schwesig R. Influence of pathologic and simulated visual dysfunctions on the postural system. Exp Brain Res 186: 305–314, 2008. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49: M85–M94, 1994. [DOI] [PubMed] [Google Scholar]
- Horak FB, Shupert CL, Mirka A. Components of postural dyscontrol in the elderly: a review. Neurobiol Aging 10: 727–738, 1989. [DOI] [PubMed] [Google Scholar]
- Jeka J, Allison L, Saffer M, Zhang Y, Carver S, Kiemel T. Sensory reweighting with translational visual stimuli in young and elderly adults: the role of state-dependent noise. Exp Brain Res 174: 517–527, 2006. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Allison LK, Kiemel T. The dynamics of visual reweighting in healthy and fall-prone older adults. J Mot Behav 42: 197–208, 2010. [DOI] [PubMed] [Google Scholar]
- Jonsson R, Sixt E, Landahl S, Rosenhall U. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res 14: 47–52, 2004. [PubMed] [Google Scholar]
- Kars HJ, Hijmans JM, Geertzen JH, Zijlstra W. The effect of reduced somatosensation on standing balance: a systematic review. J Diabetes Sci Technol 3: 931–943, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kording KP, Wolpert DM. Bayesian decision theory in sensorimotor control. Trends Cogn Sci 10: 319–326, 2006. [DOI] [PubMed] [Google Scholar]
- Kotecha A, Richardson G, Chopra R, Fahy RT, Garway-Heath DF, Rubin GS. Balance control in glaucoma. Invest Ophthalmol Vis Sci 53: 7795–7801, 2012. [DOI] [PubMed] [Google Scholar]
- Lafond D, Corriveau H, Prince F. Postural control mechanisms during quiet standing in patients with diabetic sensory neuropathy. Diabetes Care 27: 173–178, 2004. [DOI] [PubMed] [Google Scholar]
- Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope 122: 1858–1861, 2012. [DOI] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Direct measurement of human ankle stiffness during quiet standing: the intrinsic mechanical stiffness is insufficient for stability. J Physiol 545: 1041–1053, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboobin A, Loughlin PJ, Redfern MS. A model-based approach to attention and sensory integration in postural control of older adults. Neurosci Lett 429: 147–151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Postural control in the older adult. Clin Geriatr Med 12: 635–658, 1996. [PubMed] [Google Scholar]
- Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol 44: M118–M127, 1989. [DOI] [PubMed] [Google Scholar]
- McPhee JS, Hogrel JY, Maier AB, Seppet E, Seynnes OR, Sipila S, Bottinelli R, Barnouin Y, Bijlsma AY, Gapeyeva H, Maden-Wilkinson TM, Meskers CG, Paasuke M, Sillanpaa E, Stenroth L, Butler-Browne G, Narici MV, Jones DA. Physiological and functional evaluation of healthy young and older men and women: design of the European MyoAge study. Biogerontology 14: 325–337, 2013. [DOI] [PubMed] [Google Scholar]
- Morasso PG, Schieppati M. Can muscle stiffness alone stabilize upright standing? J Neurophysiol 82: 1622–1626, 1999. [DOI] [PubMed] [Google Scholar]
- Oie KS, Kiemel T, Jeka JJ. Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Brain Res Cogn Brain Res 14: 164–176, 2002. [DOI] [PubMed] [Google Scholar]
- Pasma JH, Boonstra TA, Campfens SF, Schouten AC, van der Kooij H. Sensory reweighting of proprioceptive information of the left and right leg during human balance control. J Neurophysiol 108: 1138–1148, 2012. [DOI] [PubMed] [Google Scholar]
- Pasma JH, Engelhart D, Schouten AC, van der Kooij H, Maier AB, Meskers CG. Impaired standing balance: the clinical need for closing the loop. Neuroscience 267: 157–165, 2014. [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol 88: 1097–1118, 2002. [DOI] [PubMed] [Google Scholar]
- Ray CT, Horvat M, Croce R, Mason RC, Wolf SL. The impact of vision loss on postural stability and balance strategies in individuals with profound vision loss. Gait Posture 28: 58–61, 2008. [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 35, Suppl 2: ii37–ii41, 2006. [DOI] [PubMed] [Google Scholar]
- Schouten AC, Boonstra TA, Nieuwenhuis F, Campfens SF, van der Kooij H. A bilateral ankle manipulator to investigate human balance control. IEEE Trans Neural Syst Rehabil Eng 19: 660–669, 2011. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Worringham CJ. Sensorimotor deficits related to postural stability. Implications for falling in the elderly. Clin Geriatr Med 1: 679–694, 1985. [PubMed] [Google Scholar]
- Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin 38: 467–478, 2008. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Simoneau M. Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture 14: 203–210, 2001. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Stelmach GE, Breunig A, Meeuwsen HJ. Age differences in visual sensory integration. Exp Brain Res 85: 691–696, 1991. [DOI] [PubMed] [Google Scholar]
- van der Kooij H, de Vlugt E. Postural responses evoked by platform pertubations are dominated by continuous feedback. J Neurophysiol 98: 730–743, 2007. [DOI] [PubMed] [Google Scholar]
- van der Kooij H, van Asseldonk E, van der Helm FC. Comparison of different methods to identify and quantify balance control. J Neurosci Methods 145: 175–203, 2005. [DOI] [PubMed] [Google Scholar]
- van der Linden MH, van der Linden SC, Hendricks HT, van Engelen BG, Geurts AC. Postural instability in Charcot-Marie-Tooth type 1A patients is strongly associated with reduced somatosensation. Gait Posture 31: 483–488, 2010. [DOI] [PubMed] [Google Scholar]
- Vaugoyeau M, Hakam H, Azulay JP. Proprioceptive impairment and postural orientation control in Parkinson's disease. Hum Mov Sci 30: 405–414, 2011. [DOI] [PubMed] [Google Scholar]
- Vlutters M, Boonstra TA, Schouten AC, van der Kooij H. Direct measurement of the intrinsic ankle stiffness during standing. J Biomech 48: 1258–1263, 2015. [DOI] [PubMed] [Google Scholar]
- Whipple R, Wolfson L, Derby C, Singh D, Tobin J. Altered sensory function and balance in older persons. J Gerontol 48 Spec No: 71–76, 1993. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Frank JS. Assessment of balance control in humans. Med Prog Technol 16: 31–51, 1990. [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370, 1983. [DOI] [PubMed] [Google Scholar]