Abstract
Central auditory circuits are influenced by the somatosensory system, a relationship that may underlie tinnitus generation. In the guinea pig dorsal cochlear nucleus (DCN), pairing spinal trigeminal nucleus (Sp5) stimulation with tones at specific intervals and orders facilitated or suppressed subsequent tone-evoked neural responses, reflecting spike timing-dependent plasticity (STDP). Furthermore, after noise-induced tinnitus, bimodal responses in DCN were shifted from Hebbian to anti-Hebbian timing rules with less discrete temporal windows, suggesting a role for bimodal plasticity in tinnitus. Here, we aimed to determine if multisensory STDP principles like those in DCN also exist in primary auditory cortex (A1), and whether they change following noise-induced tinnitus. Tone-evoked and spontaneous neural responses were recorded before and 15 min after bimodal stimulation in which the intervals and orders of auditory-somatosensory stimuli were randomized. Tone-evoked and spontaneous firing rates were influenced by the interval and order of the bimodal stimuli, and in sham-controls Hebbian-like timing rules predominated as was seen in DCN. In noise-exposed animals with and without tinnitus, timing rules shifted away from those found in sham-controls to more anti-Hebbian rules. Only those animals with evidence of tinnitus showed increased spontaneous firing rates, a purported neurophysiological correlate of tinnitus in A1. Together, these findings suggest that bimodal plasticity is also evident in A1 following noise damage and may have implications for tinnitus generation and therapeutic intervention across the central auditory circuit.
Keywords: multisensory integration, primary auditory cortex, spike timing-dependent plasticity, somatosensory, tinnitus
tinnitus, the phantom perception of sound in the absence of a physical sound stimulus, has been linked to somatosensory innervation of the central auditory circuitry (Roberts et al. 2010). Somatosensory convergence with auditory neurons as early in the pathway as the dorsal cochlear nucleus (DCN; Kanold and Young 2001; Shore 2005; Zhou et al. 2007) is a potential etiology for tinnitus following noise exposure (Dehmel et al. 2012b; Kaltenbach and McCaslin 1996; Koehler et al. 2011; Koehler and Shore 2013b). Somatosensory-auditory bimodal stimulation results in long-lasting changes in neural firing rates in DCN (Dehmel et al. 2012b) that are stimulus timing dependent (Koehler and Shore, 2013a), consistent with in vitro results showing spike timing-dependent plasticity (STDP) at parallel-fiber synapses (Tzounopoulos et al. 2004). Furthermore, application of noise overexposure leading to tinnitus altered the stimulus timing-dependent rules from Hebbian to anti-Hebbian, with broader windows of enhancement than in sham-controls or noise-exposed animals without tinnitus (Koehler and Shore 2013b). These data implicate alterations in DCN bimodal STDP as an underlying mechanism in tinnitus generation.
Since the first description (Markram et al. 1997; Zhang et al. 1998), STDP has been demonstrated across neural structures (Caporale and Dan 2008; Dahmen et al. 2008; Larsen et al. 2010). In primary auditory cortex (A1) shifts in neuronal frequency selectivity were pairing order and interval dependent, similar to STDP (Dahmen et al. 2008). Similarly, A1 firing rates were increased when a low-frequency tone was preceded by a high-frequency tone, while reversing the pairing order led to no changes in neural firing rates. These studies underscore the importance of the relative timing of sensory input in shaping neural responses and suggest STDP as a key mechanism of plasticity in the auditory system.
While it is evident that somatosensory stimulation can modulate auditory responses in A1 (Allman et al. 2009; Basura et al. 2012; Ghazanfar et al. 2005, 2008; Lakatos et al. 2007; Meredith and Allman 2012; Meredith et al. 2012; Schroeder and Foxe 2002; Schroeder et al. 2001, 2003), it is not known if these effects are stimulus timing dependent or if they adhere to similar timing rule changes, reflecting STDP as in DCN following noise exposure and tinnitus (Koehler and Shore 2013a,b).
The present study tested the hypothesis that stimulus timing-dependent plasticity as observed in DCN (Koehler and Shore 2013a) following bimodal stimulation is also observed in A1, and that it is modified following noise damage (Koehler and Shore 2013b). In A1, bimodal stimulation in sham-animals resulted in stimulus timing-dependent responses similar to those in DCN. Noise exposure shifted timing rules from Hebbian toward anti-Hebbian in animals with and without tinnitus and to greater enhancing rules in animals with tinnitus. Animals with tinnitus demonstrated frequency-specific increases in spontaneous firing rates (SFRs), a tinnitus neural correlate (Eggermont 2015). These findings demonstrate that A1, like the DCN, is also modulated by STDP principles that reflect changes in synaptic strength following noise damage.
MATERIALS AND METHODS
Animals.
Experiments were performed on mature, female pigmented guinea pigs (n = 16; 250–350 g; Elm Hill colony). The guinea pigs used in this study were the same animals used in a recently published report (Koehler and Shore 2013b), in which electrodes were simultaneously placed in A1 and DCN. All procedures were performed in accordance with the National Institutes of Health Guidelines for the Use and Care of Laboratory Animals and approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA).
Experimental design/noise exposures.
The purpose of this study was to evaluate the effects of noise exposure in a tinnitus model on stimulus timing-dependent plasticity of A1 tone-evoked responses and spontaneous firing rates (SFRs) following bimodal stimulation. While STDP in neocortex was initially characterized by manipulating pre- and postsynaptic activity with timed current injections in slices (Markram et al. 1997), sensory stimulation has been used in vivo to induce stimulus timing-dependent plasticity, the macromolecular correlate of STDP (Dahmen et al. 2008). Thus, while stimulus timing-dependent plasticity in vivo in the present data is not, by traditional physiological definitions, STDP, the responses are consistent with the key features of STDP. Following a 2-h unilateral noise exposure (97-dB noise with one-fourth octave band centered at 7 kHz) to the left ears, guinea pigs were tested semiweekly before and after noise using gap-prepulse inhibition of acoustic startle (GPIAS) measures to test tinnitus (Dehmel et al. 2012a; Turner et al. 2006). Animals were anesthetized with ketamine (40 mg/kg) and xylazine (10 mg/kg) during the noise exposure as ketamine has been shown to have no obvious untoward impact on A1 neural frequency tuning (Huang et al. 2013). Ten animals were noise-exposed 3–6 wk following baseline gap detection testing. Six to eight weeks later, each animal was subjected to a second exposure to the same narrowband noise as prior data have suggested that tinnitus development is more likely following repeated noise exposures (Dehmel et al. 2012a,b). The remaining animals were grouped as sham-controls and were only subjected to anesthesia with no noise exposure.
Auditory brain stem response (ABR) thresholds were obtained before initiating gap detection and immediately following the first and second noise exposures to ascertain threshold shifts and 1 wk after noise exposure to measure for threshold recovery (Fig. 1). Four to six weeks after the second noise exposure, single-unit SFRs, rate level functions, and bimodal stimulus timing-dependent plasticity were evaluated in acute A1 recording preparations and compared between sham-controls and noise-exposed animals that did and did not show evidence of tinnitus.
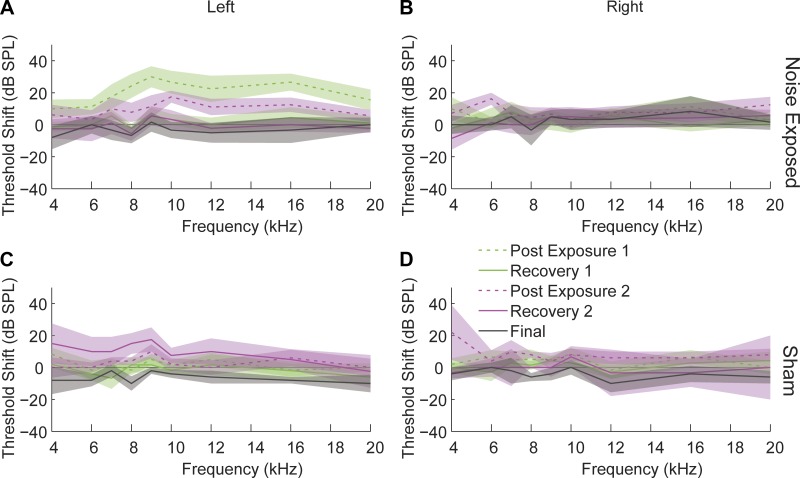
Fig. 1.
Auditory brain stem responses (ABR) reveal a temporary threshold shift (TTS) following noise exposure. ABR threshold shifts in noise-exposed animals in the exposed ear (A) and unexposed ear (B) and from sham-control animals from both ears (C and D). ABR thresholds were measured following the initial noise exposure (green), following the second noise exposure (pink), and just prior to in vivo A1 unit recordings (gray). Dashed lines represent ABR thresholds immediately following noise exposure. Solid lines indicate recovered TTS 1 or more wk following noise exposure. Shaded bands represent 95% confidence intervals. [Reproduced from Koehler and Shore 2013b. Copyright 2013 with permission from the Society for Neuroscience].
Gap-prepulse inhibition of acoustic startle (GPIAS).
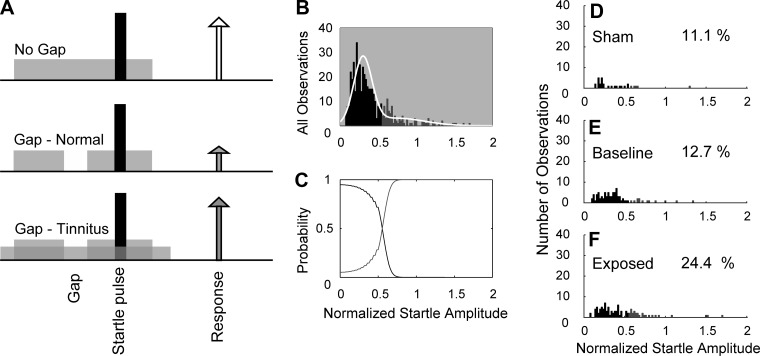
For determining if noise-exposed animals developed tinnitus, the GPIAS for testing tinnitus was utilized as previously described (Dehmel et al. 2012a; Koehler and Shore 2013b). Animals were placed on a piezoelectric force plate to measure movements elicited by a loud broadband noise (startle stimulus: 115 dB; 200-20 kHz). Each trial consisted of a background noise with and without a 50-ms silent gap or pulse placed 50 ms before the startle stimulus (Fig. 2). The background noise (60 dB) was either broadband or bandpass-filtered noise with a 2-kHz band and lower cutoff frequencies of 4, 8, 12, 16 or 20 kHz. The intervals between each trial were randomly selected between 18 and 24 s. Startle response amplitudes were found to only mildly decrease (10%) over multiple testing periods in sham and noise-exposed animals. For each session, the normalized startle response was calculated as the ratio (AG/ANG) where AG is the mean amplitude of the startle response from 10 trials with gap on 1 day and ANG is the mean amplitude of the startle response from 10 trials with no gap on the same day. To evaluate the normalized startle responses within each frequency band for indications of tinnitus, the distribution of all responses was analyzed using Gaussian mixture modeling (Statistics Toolbox; MATLAB release 2012b; MathWorks). The normalized startle responses were placed in the tinnitus group when the probability that the observation belonged to the elevated distribution was >0.55. Using the threshold from the Gaussian mixture model, the distributions of startle responses from all animals were separated into tinnitus and no-tinnitus groups. For statistical comparison, animals with no exposure were assigned to the sham-control group, while noise-exposed animals with no tinnitus were assigned to the Exposed-No Tinnitus (ENT) and those exposed with evidence of tinnitus were assigned to the Exposed-Tinnitus (ET) groups. Last, prepulse inhibition was evaluated in a similar manner as gap detection. All of the animal groups showed no differences in response to prepulse inhibition before and after noise exposure. This finding suggests that baseline temporal processing was unchanged following noise damage, and thus any changes in gap detection were likely a result of the tinnitus percept “filling the gap” and not due to temporal processing or hearing loss.
Fig. 2.
Gap-prepulse inhibition of acoustic startle (GPIAS). A: schematic outlining the startle-based gap-detection assay for tinnitus [Reproduced from Koehler and Shore 2013b. Copyright 2013 with permission from the Society for Neuroscience]. Top row reveals no gap with the bottom two rows showing gap trials (50-ms gap, 50 ms before the startle stimulus). Each trial consisted of continuous 60-dB background noise (gray bar) with a 10-ms, 115-dB startle pulse embedded (black bar). The animal responds to the startle stimulus, with the amplitude of the response shown by the height of each arrow. In noise-exposed animals without tinnitus (ENT), the gap leads to suppression of the startle response (middle row). In animals with noise-induced tinnitus, the gap is filled by tinnitus (gray arrows), and no gap induced reduction in startle response (white arrow). B–F: Gaussian mixture model analysis separating startle distribution into normal and tinnitus. B: histogram of the normalized startle responses (white line) separated into two distributions: no evidence for tinnitus (black bars) and evidence for tinnitus (gray bars). C: probabilities that normalized startle values belong to tinnitus or no tinnitus distributions. D: histogram of the distribution of postexposure normalized startle observations for sham-controls. E: histogram of the distribution of normalized startle observations for baseline (preexposure) observations from sham and noise-exposed animals. F: histogram of the distribution of postexposure startle observations from noise-exposed animals. D–F: percentage of observations placed into the tinnitus group is shown on each panel.
Surgical approach and neural recordings.
Following anesthesia (ketamine 40 mg/kg and xylazine 10 mg/kg), animals were then held in a stereotaxic device (Kopf) with hollow ear bars for sound delivery. Rectal temperature was monitored and core temperature maintained at 38 ± 0.5°C with a thermostatically controlled heating pad. Supplemental anesthesia (0.25–0.5 ml im of initial dose) was given approximately every 30 min, after performing a digital toe-pinch test to elicit paw withdrawal. Craniotomies were performed over the ipsilateral (to the sound source) cerebellum and contralateral auditory cortex to identify the middle cerebral artery as described previously (Basura et al. 2012). After completing neural recordings, the guinea pig was euthanized by intraperitoneal injection of pentobarbital sodium followed by brain removal for histologic analysis and electrode reconstruction to confirm probe placement. To mark the electrode tracks, the recording and stimulating electrodes were dipped in Fluorogold (2%) before being inserted into the brain. After being immersed in 4% paraformaldehyde for 48 h followed by immersion in a 20% sucrose solution (Zhou and Shore 2006), the brain was cryosectioned at 40 μm, placed on slides, and examined under epifluorescence.
Electrode placement.
To stimulate spinal trigeminal nucleus (Sp5) neurons, a concentric bipolar stimulating electrode (FHC, Bowdoin, ME) dipped in Fluorgold for postmortem histologic probe placement confirmation was placed into the left Sp5, using stereotaxic coordinates (0.28 cm left of midline, 0.25–0.3 cm caudal to the transverse sinus, 0.9 cm below the surface of the cerebellum). Five biphasic (100 μs/phase) current pulses (5 pulses, 100/s) at 1,000 Hz were delivered to Sp5 using a custom isolated constant-current source. The current amplitude was set to the highest level (50–70 μA) that did not elicit movement artifact. A four-shank, 32-channel silicon substrate electrode (250 μm between sites, 400 μm between shanks, 177 μm2 site area; NeuroNexus Technologies, Ann Arbor, MI) was used to record activity from the contralateral (to sound source and Sp5 electrode) A1 single- and multiunit clusters. To achieve optimal placement, four-shank probes in in the contralateral A1 penetrated ∼2 mm below the cortical surface based on previously published data (Basura et al. 2012; Wallace et al. 2000). We were able to sample from locations within a small best frequency (BF) range without moving the probe from the brain. When necessary, the electrode was repositioned until robust responses to acoustic stimulation were obtained. After achieving final probe placement the electrodes were connected by a 32-channel preamplifier and digitizer to a TDT data-acquisition system.
Auditory and somatosensory stimulation.
Neural activity in response to unimodal tones or Sp5 stimulation was recorded before and 15 min after the bimodal stimulation protocol (Fig. 3). Tone signals (50-ms duration) gated with a cosine window (2 ms rise/fall time) were generated using Open Ex and an RX8 DSP (TDT, Alachula, FL) with 12-bit precision and sampling frequency set at 100 kHz. Auditory stimuli were delivered to the left ear through a shielded speaker (DT770, Beyer) driven by an HB7 amplifier (TDT, Alachula, FL) coupled to the hollow ear bar using Tucker-Davis Technologies (TDT) system III hardware for digital-to-analog conversion and analog attenuation. Digital signals were generated and delivered to the TDT hardware by TDT software on a PC. Tones were calibrated using a 1/4-in. condenser microphone (Bruel and Kjaer; Mic: 4136; Preamp: 2619; Power Supply: 2804) coupled to the ear bar with a 0.2 ml tube. Equalization to correct for the system response was performed in the frequency domain using digital filters implemented in TDT hardware. The stimulus variable sequences were generated in pseudorandom order in MatLab. The maximum system output was 85 dB SPL.
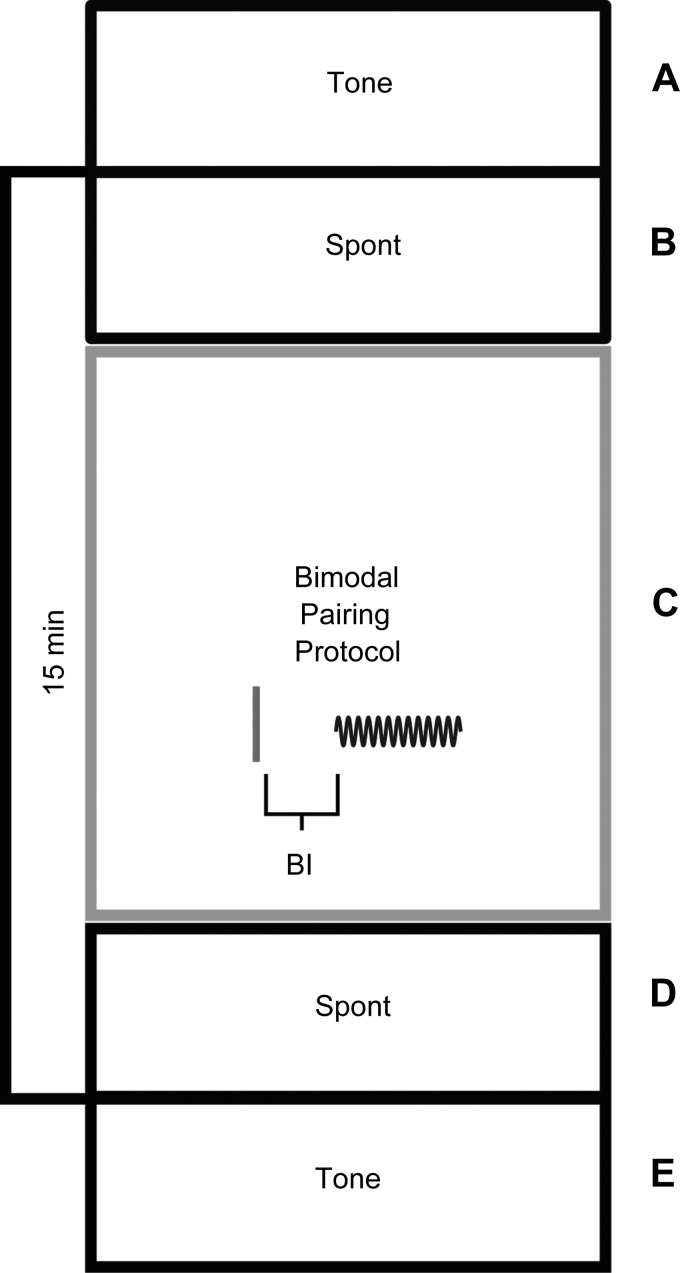
Fig. 3.
Bimodal plasticity protocol where spontaneous neural firing (Spont) and pure tone (Tone) responses were measured before and 15 min after the bimodal pairing protocol. Bimodal pairing consisted of repeated presentations of Sp5 stimulus (solid line) and a pure tone burst (sinusoid) with varied bimodal interval (BI) and order. The BI is defined by the onset time of the tone stimulus and the temporal interval before Sp5 stimulus onset and vice versa. A: tone-alone trial consisting of 50 ms of silence, 50 ms of BF tone burst, 50 ms of silence; repeat × 50. B: silence for 2.5 min. C: bimodal trial consisting of one bimodal pair of Sp5 and 50 ms of BF tone; 300 repetitions. D: silence for 2.5 min. E: tone-alone trial consisting of 50 ms of silence, 50 ms of BF tone burst, 50 ms of silence; repeat × 50.
Experimental design/bimodal stimulation.
First, before any bimodal stimulation, each unit's receptive field was assessed by presenting 50 ms tone stimuli every 200 ms with levels selected from 0- to 85-dB SPL in 5-dB steps and frequencies selected from 200 Hz to 23 kHz in 0.1-octave steps. The bimodal stimulation protocol consisted of 300 trials of 50-ms tones combined with electrical activation of Sp5 presented at 2 Hz. The tone level (range 65–75 dB) and frequency (varied per animal based on best frequency; range 4,000–14,600 Hz) for the bimodal stimulation protocol were fixed for the duration of the recording and were selected to reliably elicit unit responses to sound from most recording sites. All unimodal (BF tone alone without bimodal pairing) tones and rate level functions were at the same frequency used for bimodal stimulation. The entire recording block lasted for 30–35 min with unimodal (either BF tone or Sp5 stimulation alone) recordings at each time point lasting for 5–7 min and the bimodal stimulation protocol lasting for 4–5 min. The recording block (Fig. 3) was repeated for each bimodal interval (BI) tested. The BI was defined as the time elapsed between the tone stimulus onset and the Sp5 stimulus onset. Negative values indicate when tone preceded Sp5 stimulation and positive values denote when Sp5 stimulation preceded tone. To measure stimulus timing dependence, the change in SFR and tone-evoked firing rates before and after bimodal stimulation was measured while varying the BI. The recording parameters were repeated and the sequences in which the various bimodal pairings were tested were randomized from −20, −10, 0, 10, or 20 ms. To ensure that neural responses after bimodal stimulation were not influenced by the lingering effects of the most recent pairing in the randomized set, two time points that showed the greatest neural suppression (0 ms) and enhancement (+10 ms) were each run in separate sham-controls alone and the results recorded 15 min after pairing and compared with the data generated from the randomized animals. To determine the precise timing of the bimodal effect on the circuit, a correction factor was calculated by subtracting the onset of the unimodal (tone or Sp5 alone) response from the respective bimodal pairing. The resultant correction factor is shown in figures in parentheses directly below the BI. Tone-evoked and SFRs to unimodal tone or Sp5 stimulation were presented at the same level as in the bimodal stimulation protocol (300 trials, 5 trials/s) at 15 min after bimodal stimulation (Fig. 3). Responses at 15 min were used for all bimodal plasticity measurements. The responses to tones were determined by calculating the mean firing rates over the 50-ms block corresponding to the tone duration. SFRs were measured by calculating the mean firing rate over 2.5 min of spiking activity in the absence of sound.
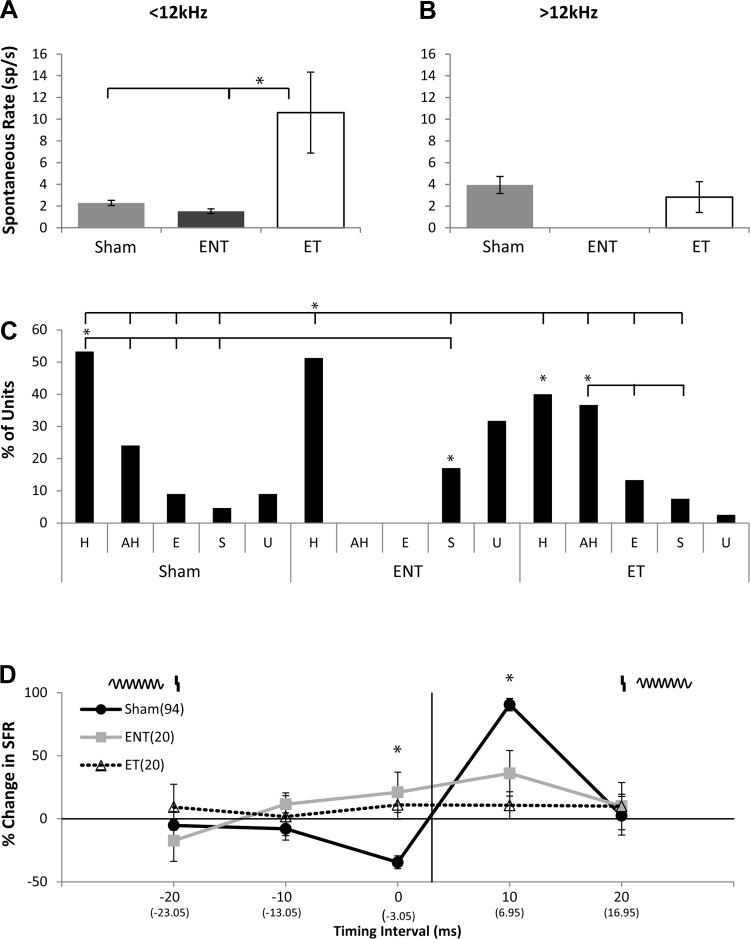
Timing rules were classified as Hebbian-like, anti-Hebbian-like, suppressing or enhancing by comparing the mean change in firing rate (i.e., the firing rate before bimodal stimulation subtracted from the firing rate after bimodal stimulation) when Sp5 stimulation preceded sound and Sp5 stimulation followed sound. As such, Hebbian-like timing rules were defined as a decrease in neural firing rates when sound preceded Sp5 and an increase in firing rate when Sp5 preceded sound while anti-Hebbian was the mirrored effect, respectively (Fig. 4, A and B). Enhanced or suppressed units were defined by a significant (2.5 SD relative to background) increase or decrease in firing rate, respectively, not showing Hebbian or anti-Hebbian-like timing rules. For comparison between sham, ENT, and ET groups, SFRs were measured from the initial recording block prior to the onset of the bimodal stimulation protocol and 15 min after bimodal pairing or repeated unimodal (Sp5 or tones) stimuli.
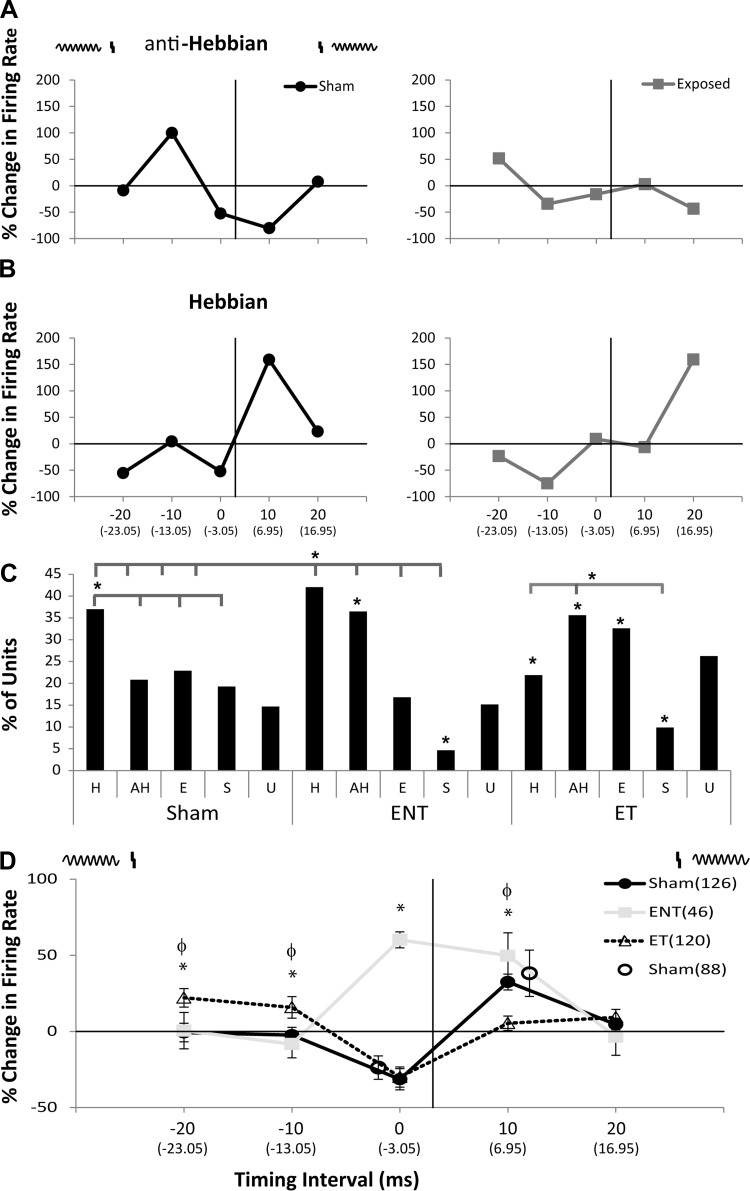
Fig. 4.
Bimodal plasticity shifts from Hebbian to anti-Hebbian-like and enhancing timing rules with less discrete temporal windows in animals with tinnitus and to mostly anti-Hebbian timing rules in guinea pigs without tinnitus. A: two examples of single-unit anti-Hebbian-like timing rules from a sham-control (left) and noise-exposed (right) guinea pig. A schematic at the top of the panel demonstrates the relationship of the Sp5 stimulus (vertical line) and the tone stimulus (sinusoid). At each BI a correction factor was calculated where the timing of the unimodal stimulus onset was subtracted from the bimodal stimulus onset (correction factor listed in parentheses below the respective BI). B: two examples of single-unit Hebbian-like timing rules from a sham-control (left) and noise-exposed (right) animals. C: bar graph showing the percentage of single units that showed Hebbian (H), anti-Hebbian (aH), enhancing (E), and suppressing (S) and undefined (U) timing rules from sham-controls (left), ENT (middle), and ET (right) animals. Within the ET group a larger percentage of anti-Hebbian and enhancing rules were seen compared with shams that predominantly showed Hebbian timing rules. Animals in the ENT group also showed a greater number of anti-Hebbian timing rules compared with sham controls. Significant differences were found between sham, ENT, and ET groups [overlying bar denotes individual comparison (tabs) that is statistically significant compared with the reference (*P < 0.05; Tukey-Kramer's post hoc test); asterisk under bar denotes comparison to sham-controls for that respective timing rule]. D: mean timing rules showing bimodal plasticity of tone-evoked firing rates for units from sham-control (dark line), ENT (gray line), and ET (dashed line) animals. Schematic above represents the order of Sp5 and tone stimuli. The vertical line indicates Sp5 stimulation with the sinusoid representing tone stimulus onset. Mean timing rules were calculated for BIs and stimulus order from across all layers of A1 with error bars that indicate SE. At each BI a correction factor was calculated where the timing of the unimodal stimulus onset was subtracted from the bimodal stimulus onset (correction factor listed in parentheses below the respective BI). To demonstrate the effects of bimodal trials separate from the randomized protocol on sham-control responses using the maximal suppressive (0 ms) and enhancing (10 ms) pairings, the mean change in firing rates (open circles; n = 88 single units) and SE are plotted in relationship to sham-controls at the same BI and order from responses obtained during the randomized set. Note similar responses to each maximal pairing when stimulated alone without a randomized protocol. Significant differences were found between sham-controls, ENT, and ET groups: *P < 0.05; Tukey-Kramer's post hoc test compared with sham controls with ET being significantly different at −20 and −10 ms and ENT at 0 ms; and ϕP < 0.05 compared with the ET group with sham and ENT both significantly different from ET at −20, −10, and +20 ms and the ENT group being different from ET at 0 ms.
Spike detection and sorting.
Recordings were made in a sound-attenuating booth. Voltages from each recording site were digitized by a preamp PZ2 (Fs = 12 kHz, TDT) and band-pass filtered (300 Hz to 3 kHz). An online spike-detection threshold was set independently for each recording channel to 2.5 SDs above the mean background noise voltage (RZ2, TDT). Time stamps and associated waveform snippets were saved to a PC using Open Explorer (TDT, Alachua, FL) and were sorted based on shape and cluster analysis with fixed variance (Plexon Offline Sorter). Single- and multiunits were identified by waveform shape and amplitude. The waveform shapes, amplitudes, and response properties of sorted units were confirmed with pairwise cluster statistics (P > 0.05; Plexon Offline Sorter) and were consistent over the duration of the recording protocols.
Data analysis.
Postexperiment data analysis was performed in MatLab. All units were characterized by best frequency and threshold. Response maps were constructed by computing the tone-evoked firing rate during the 50-ms tone stimulus minus SFR measured during the last 50 ms of each trial (200-ms duration). Enhancement or suppression was considered significant when the firing rate was greater than 2.5 SDs above or below the mean spike rate of all trials with no sound. Poststimulus time histograms (PSTHs) were constructed for each unit from 300 trials with the tone level 10–30 dB above threshold and frequency within 0.1 octave of the identified BF. The effect of Sp5 stimulation on the firing rate of the response to BF tones during and after bimodal stimulation was assessed using percent difference in firing rates. The bimodal effect (15 min after pairing) on the response to tone was calculated as follows: 100 × (FRu2 − FRu1)/FRu1, where FRu1 is the average firing rate in response to unimodal stimulation (tones) presented before bimodal stimulation and FRu2 is the average firing rate in response to unimodal stimulation 15 min after bimodal stimulation. Average firing rates of unimodal responses were computed from 300 trials of tone stimulation. Average firing rates of bimodal responses were computed from 300 trials of bimodal stimulation.
Statistics.
Statistical analysis was conducted on single units across all layers of A1 15 min after bimodal stimulation. Significant bimodal plasticity was identified using a paired measurement Student's t-test for the number of spikes measured on each trial before and after bimodal stimulation. Timing rules were constructed and classified as Hebbian, anti-Hebbian, suppressing, or enhancing. The proportion of timing rules from each group (sham, ENT, and ET) was evaluated for significance using a 2 × 2 or 2 × 3 Chi squared test. A 2-way ANOVA with a Tukey-Kramer post hoc test was used to ascertain differences between mean population-timing rules (SPSS 19.0).
RESULTS
Noise exposure-induced temporary threshold shifts (TTS) and tinnitus as measured by GPIAS.
Guinea pigs for each group (sham, ENT, and ET) used in this study for A1 recordings were the same animals that underwent simultaneous recordings in DCN with previously published results (Koehler and Shore 2013b). Noise exposure induced an immediate TTS as demonstrated by ABR thresholds in the noise-exposed ear only that recovered to baseline by 1 wk after exposure (Fig. 1). Maximum threshold elevation was 35 dB at 9 kHz after the first exposure and 19 dB at 10 kHz after the second exposure with thresholds elevated in a band from the exposure frequency to 2 octaves above that frequency. Thresholds measured on ABR in sham-controls were not elevated above baseline.
GPIAS was used to assess each animal for frequency-specific tinnitus. Animals displayed normal gap detection during baseline startle testing with expected reduced responses when a gap was present. Baseline startle responses were determined by comparing the startle amplitude with (AG) and without (ANG) gap prepulse inhibition. Impaired gap detection, indicative of tinnitus, was identified by significantly elevated normalized startle responses. Approximately 60% of exposed animals were found to have tinnitus in the 12- to 14-kHz band, with half at 4–8, 8–10, or 16–18 kHz bands (Fig. 2; see materials and methods). Animals with tinnitus were placed in the ET group and those without evidence of tinnitus in the ENT group, and nonexposed animals were designated as sham-controls.
Animals with tinnitus show shifts from tone-evoked Hebbian plasticity to anti-Hebbian plasticity.
Bimodal plasticity, the persistent change in neural responses following paired bimodal stimuli, was evaluated in vivo by comparing tone-evoked firing rates and SFRs before and 15 min after bimodal stimulation (Sp5 paired with tone) for single units in A1. The order (tone preceding Sp5, or Sp5 preceding tone) and BI (0–20 ms between stimuli; Fig. 3) of Sp5 and tone stimuli in the pairing protocols were randomly varied. Bimodal plasticity was considered stimulus timing-dependent when the tone-evoked and spontaneous firing rates that were facilitated or suppressed following bimodal stimulation were also dependent on the order and timing of the bimodal stimuli. To determine the effects of tinnitus on bimodal stimulus timing-dependent plasticity, tone-evoked responses in sham (n = 126 units), ENT (n = 46 units), and ET (n = 120 units) animals were measured before and 15 min after bimodal stimulation at each BI and pairing order. Bar graph distributions of the timing rules demonstrate a preponderance of Hebbian timing rules in sham-controls (Fig. 4C). In both ENT and ET groups, a significant increase in the number of units showing anti-Hebbian timing rules (* to respective sham aH) is observed, along with a decrease in those showing suppressive rules. A significant decrease in the number of Hebbian, but a significant increase in the number of enhancing, rules is observed in the ET group (Fig. 4C).
Similarly, in sham-controls, the mean timing rule (Fig. 4D) was predominantly Hebbian, with enhancement of tone-evoked firing rates when Sp5 preceded sound stimulation (+10 ms) and suppression when Sp5 stimulation followed sound stimulation. Mean population timing rules for sham-controls were Hebbian, similar to those seen in the DCN (Koehler and Shore 2013b). However, in ET animals, enhancement occurred at BIs of −20 and −10 ms, and suppression occurred at a BI of zero, providing an anti-Hebbian-like plasticity, similar to that in DCN ET animals (Fig. 4D). Conversely, ENT animals showed enhancement at BIs of zero and +10 ms, and no suppressive plasticity (Fig. 4D). The shift from Hebbian in sham animals to anti-Hebbian plasticity in ET animals is consistent with that seen in DCN. However, the shift from Hebbian to bimodal enhancement in ENT animals stands in contrast to the narrow, anti-Hebbian timing rules seen in ENT units in the DCN. An ANOVA revealed significant main effects between sham and ET, F(1.2) (P < 0.015) and not between sham and ENT, F(1.02) (P = 0.4). BIs for which there were significant differences were confirmed by a Tukey-Kramer's post hoc test.
To ensure that the results of bimodal stimulation 15 min after pairing were not influenced by the lingering effects of the repeated pairing in the randomized protocol, separate sham-controls in which only one BI per animal was presented (0 ms or +10 ms) were conducted. The means for results from these individual sham-animals (n = 88 single units) revealed no differences from those seen with the same BI in the randomized set, indicating that the residual effects of the previous pairing in the randomized protocol did not influence the overall effect (Fig. 4D).
Animals with tinnitus show increased frequency-specific SFRs and shifts from Hebbian to less discrete timing rules.
SFRs measured prior to the presentation of bimodal stimuli were significantly elevated in the ET group in frequency regions with TTS and evidence for tinnitus (<12 kHz; Fig. 5A) but not above the tinnitus frequencies (>12 kHz; Fig. 5B). The percent change in SFR was again measured 15 min after various BIs and pairing orders in the three experimental groups. Bar plots reveal mostly Hebbian units for sham-controls and mostly Hebbian and some suppressive units in ENT animals but equivalent numbers of units with anti-Hebbian and Hebbian timing rules in the ET group (Fig. 5C). An ANOVA with sham and ET revealed significant main effects between BI and exposure groups, F(1.78) (P < 0.0001) and with ENT BI, F(1.98) (P < 0.001) (Fig. 5C).
Fig. 5.
Spontaneous firing rates (SFRs) are significantly increased in the ET group but not in ENT animals. A: mean SFRs for units with best frequencies < 12 kHz. Note the significant increase in the ET group compared with sham (*P < 0.003). B: mean SFRs for units with best frequencies > 12 kHz. C: bar graph showing the percentage and distribution of timing rules for SFRs from sham-controls (left), ENT (center), and ET (right) following bimodal stimulation. Overlying bar denotes individual comparison (tabs) that is statistically significant compared with the reference (*P < 0.05; Tukey-Kramer's post hoc test; asterisk under the bar denotes comparison to sham-controls for the respective timing rule). D: mean percent change in SFRs and timing rules revealing bimodal plasticity for single units from sham-controls (black line; 94 units), ENT (gray line; 20 units), and ET (dashed line; 20 units) animals. Mean timing rules were calculated for all measurements across all layers of A1. Error bars indicate SE. Significant differences were found between sham-controls, ENT, and ET groups (*P < 0.05; Tukey-Kramer's post hoc test compared with sham-controls).
As with the tone-evoked results, the mean population timing rules of SFRs in single units from sham (n = 94) animals predominantly showed suppression when sound preceded Sp5 stimulation (BI 0 ms) and enhancement when Sp5 preceded sound (+10 ms) consistent with Hebbian timing rules. There were significant differences between the ENT timing rules (N = 20 single units) and sham-controls at BIs of 0 and +10 ms (Fig. 5D). ET animals (n = 20 single units) exhibited no evidence of plasticity at any of the BIs. This likely reflects a high degree of variability in the responses in this group as the bar graphs showed that individual units did show plasticity but this plasticity was distributed across all timing rule types, as shown in Fig. 5C.
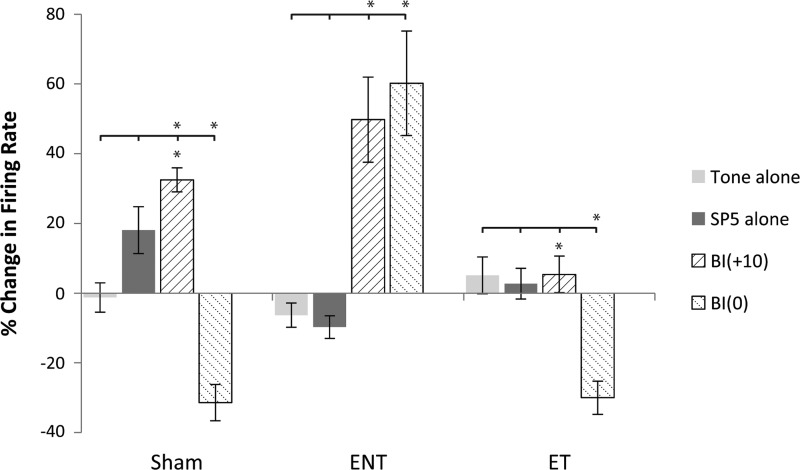
Bimodal stimulation induces more plasticity than unimodal stimulation in A1.
To assess whether unimodal (tone or Sp5 alone) stimulation also produced equivalent persistent responses to bimodal stimulation, tone-evoked responses were measured during protocols in which the bimodal stimulus was replaced by a tone alone or Sp5 stimulation alone. Compared with bimodal stimulation, responses to tone-alone stimulation did not significantly alter neural firing rates (either mean increases or decreases) 15 min after stimulation (Fig. 6). Unimodal Sp5 stimulation produced an increase in tone-evoked firing rates in sham-controls only and had no significant effect in the ENT or ET groups. In all groups, bimodal stimulation at 0 and +10 significantly suppressed or enhanced tone-evoked firing rates compared with unimodal stimulation, respectively (Fig. 6).
Fig. 6.
Bimodal stimulation induces more plasticity than unimodal stimulation A1. Bar graph shows the effects of tone (light gray) and Sp5 (dark gray) alone on the percent change in neural firing rates in all three (sham-control, ENT, and ET) groups in A1. The graph also shows the direct comparison of the unimodal responses to the bimodal responses where maximal enhancement (+10 ms; upward hatch) and suppression (0 ms; downward hatch) were observed. With the exception of a modest increase in neural firing rate in sham-controls following Sp5 alone, neither unimodal stimulus significantly altered neural firing rates alone. Bimodal stimulation results in greater levels of enhancement and suppression of neural firing depending on BI [overlying bar denotes individual comparison between unimodal and bimodal stimulus (tabs) that is statistically significant compared with the bimodal stimulus, *P < 0.05; Tukey-Kramer's post hoc test; asterisk under bar denotes comparison to other bimodal stimulus; *P < 0.05; Tukey-Kramer's post hoc test]. A1, primary auditory cortex; BF, best frequency; PSTH, peri-stimulus time histogram; Sp5, spinal trigeminal nucleus; STDP, spike timing-dependent plasticity.
DISCUSSION
The hypothesis of the present study was that bimodal plasticity in A1 is stimulus timing dependent and displays similar timing rule changes found in DCN (Koehler and Shore 2013b) following noise-induced tinnitus. Extracellular in vivo A1 single-unit responses to tones and SFRs measured before and after bimodal stimulation (paired Sp5-tone) demonstrated stimulus timing dependence similar to that shown in DCN for sham-controls (Koehler and Shore 2013a). Furthermore tone-evoked bimodal plasticity timing rules were altered following noise-induced tinnitus: they were more likely to be Hebbian in sham-control animals and anti-Hebbian-like and enhancing in noise-exposed animals with tinnitus (ET), and somewhere in between in animals without tinnitus (ENT). SFR timing rules remained primarily Hebbian in shams but shifted to multiple timing rule types in animals with tinnitus and to Hebbian and suppressive in animals without tinnitus (ENT). The responses in A1 following bimodal stimulation in sham-controls are consistent with timing rules in DCN (Koehler and Shore 2013b). However, there are some differences in plasticity changes after tinnitus that are observed in A1 and DCN following noise exposure. The changes in A1 appear to be more complex, and demonstrate multiple changes in timing rules in the tinnitus animals. Thus, while A1 appears to demonstrate “already processed” bimodal plasticity in normal animals, additional mechanisms of plasticity change following noise damage appear to be involved in A1.
A1 neurons exhibit bimodal STDP.
To date, few studies investigating STDP in A1 have been conducted. Of those, stimulus timing-dependent plasticity properties in A1 (Dahmen et al. 2008; Kilgard and Merzenich 2002) are similar to those seen in other sensory cortexes (Larsen et al. 2010) including visual (Froemke et al. 2006; Meliza and Dan 2006) and somatosensory (Nevian and Sakmann 2006), showing Hebbian and anti-Hebbian-like timing rules. In the present study, suppression or enhancement of A1 neural firing following combined auditory-somatosensory stimulation that is pairing order- and timing-dependent demonstrates that STDP principles in A1 are not limited to auditory-auditory only paradigms (Dahmen et al. 2008; Kilgard and Merzenich 2002). It is not surprising that nonauditory systems may also modulate auditory circuits since repeated pairing of tones with nucleus basalis, locus ceruleus, or ventral tegmental stimulation increases neural responses to the same tone in A1 (Bakin and Weinberger 1996; Edeline et al. 2011; Froemke et al. 2007; Kilgard and Merzenich 1998; Kilgard et al. 2001). The present study adds support to the contribution of multiple nonauditory systems to A1 processing.
Standard STDP protocols involve single excitatory-postsynaptic potentials (EPSPs) paired with single postsynaptic back-activating action potentials (BAPs; Bi and Poo 1998; Froemke and Dan 2002). Induction of long-term potentiation (LTP) is typically achieved by pre- before postsynaptic pairing, while post- before presynaptic pairing can induce long-term depression (LTD), resulting in a Hebbian-like learning rule (Froemke and Dan 2002). In our model, if A1 is receiving “already-processed” inputs from DCN in which the known pre- and postsynaptic sources are identified (Koehler and Shore 2013b; Tzounopoulos et al. 2004), tone preceding Sp5 (negative values on figures) stimulation would represent post- before pre (Sp5)-synaptic activity resulting in robust suppression of neural firing that is preserved 15 min after pairing, reflecting long-term depression (LTD). Conversely, Sp5 stimulation preceding tone (positive values on figures) represents pre- before postspiking activity, leading to neural facilitation that also persisted 15 min after pairing consistent with long-term potentiation (LTP). Karmarkar and Buonomano (2002) demonstrated these principles in A1 slices where repetitive pairing of pre- before postspiking activity at +10-ms intervals produced LTP and post- before pre-intervals at −40 ms produces LTD at layer II/III cortical synapses. Our results showing predominantly Hebbian-like timing rules from sham-control animals in A1 are consistent with these data. However, variability in population timing rules following bimodal pairings in cortex demonstrate that pre-before postsynaptic stimulation can also lead to less change (Kampa et al. 2006; Markram et al. 1997; Nevian and Sakmann 2006; Sjostrom et al. 2001), LTP (Feldman 2000; Froemke and Dan 2002), or LTD (Zhou et al. 2005) depending on the identity, nature, and location of the synapse. This is exemplified in our data. ENT units showed greater enhancement in neural firing (BIs of 0 and +10) than ET units that displayed a modest, yet significant anti-Hebbian response profile (BIs of −20 and −10 ms; Fig. 4D). This variability may reflect molecular differences in A1 neurons/synapses between ET and ENT groups such as the activity-related gene Arc/Arg3.1. This cytoskeletal protein is mobilized after LTP activity and has been proposed to influence postsynaptic AMPA receptor expression that is required for LTP (reviewed by Knipper et al. 2013). Interestingly, A1 Arc/Arg3.1 following noise exposure was only mobilized in animals without tinnitus and was not changed in those with tinnitus (Ruttiger et al. 2013; Singer et al. 2013). This suggested that increased cortical firing following moderate deafferentation following noise may be the result of enhanced glutamate sensitivity. That study, however, showed more permanent hearing loss in “tinnitus” animals, suggesting that the Arc changes could be a reflection of the hearing loss and not the tinnitus. In our data, all noise-exposed groups only display TTS at the time of testing. It would therefore be interesting to investigate molecular changes that occur in both groups during the threshold shifts before recovery occurred.
Noise exposure leads to temporary threshold shifts, tinnitus, and changes in bimodal stimulus timing-dependent plasticity.
Narrowband noise exposure (7 kHz) resulted in unilateral TTS and tinnitus in the same animals that also underwent simultaneous recordings from DCN (Koehler and Shore 2013b). In A1, increased SFRs following noise exposure in cats (Eggermont 2007; Komiya and Eggermont 2000), a documented neural physiological correlate of tinnitus (reviewed by Eggermont 2015), were significantly increased only in the tinnitus bands (<12 kHz) compared with sham and noise-exposed animals that did not show evidence of tinnitus. A1 SFRs outside the tinnitus bands (>12 kHz) did not show significant increases in any group. The correlation of increased SFRs with GPIAS measures of tinnitus further validate the use of GPIAS as a method to identify tinnitus in guinea pigs after noise exposure (Dehmel et al. 2012a,b; Koehler and Shore 2013b; Turner et al. 2006).
Tone-evoked timing rules in ET animals shifted from primarily Hebbian-like in sham-controls to primarily anti-Hebbian-like and enhancing rules, with a significant decrease in suppressing timing rules. Animals without tinnitus displayed an equal number of Hebbian and anti Hebbian-like timing rules without a significant change in enhancing timing rules, in between sham-control and ET response profiles. Timing rule classifications for SFRs also shifted for ENT and ET animals compared with sham-controls, where timing rule curves showed a lower magnitude plasticity at all BIs with a widening of the temporal windows. While this effect was observed after 15 min, it is possible that significant changes in plasticity may be seen if recordings were taken for a longer period after pairing. These results confirm that somatosensory inputs have a significant long-term influence on A1 neural activity following noise exposure and tinnitus. These findings are consistent with the neural modulation and changes in bimodal plasticity in DCN following noise exposure and tinnitus and suggest that mechanisms of auditory-somatosensory integration at the brain stem have influence across the central auditory circuit in tinnitus (Koehler and Shore 2013b). This is important to consider given the known extensive cross-modal reorganization of the auditory cortex following noise exposure in which 84% of sampled auditory cortex neurons began to respond to somatosensory stimulation with 76 dB or greater threshold shifts (Allman et al. 2009). The authors of the latter paper acknowledge that their findings may be due, at least in part, to deafness-induced increases in somatosensory inputs to the DCN (Shore et al. 2008; Zeng et al. 2012), the first station of the central auditory relay, which may generate upstream changes seen at the cortical level.
Bimodal stimulation induces more plasticity than unimodal stimulation in A1.
In the present study, with the exception of a modest increase in neural firing rate following Sp5 stimulation alone in sham-controls, unimodal stimulation (tone alone or Sp5 alone) did not significantly suppress or enhance tone-evoked or SFRs, as shown with bimodal stimulation. Sustained plasticity in A1 following bimodal stimulation may reflect principles of temporal coincidence where multisensory interactions are strongest when both (tone-Sp5) modalities are presented (Lakatos et al. 2007). Applying our bimodal plasticity protocol to that model would therefore suggest that somatosensory stimulation (when preceding tone; +10 ms) enhances auditory processing by resetting neural oscillations so that concurrent auditory inputs arrive during a high-excitability phase and are thus amplified/enhanced (Lakatos et al. 2007). Alternatively, those auditory inputs less intimately associated with convergent somatosensory stimuli may have weaker multisensory association (0 ms pairing; when both stimuli are presented simultaneously) and reset neural oscillations to low-excitability phase, resulting in neuron suppression.
Mechanisms of STDP in A1.
Whether Hebbian or anti-Hebbian-like timing rules predominate depends on a number of factors including the dendritic location of the synapses (Letzkus et al. 2006; Sjostrom and Hausser 2006) and order of activity in pre- and postsynaptic cells (Karmarkar and Buonomano 2002; Karmarkar et al. 2002). In some circumstances, the timing rule may be converted from anti-Hebbian to Hebbian in layer II/III cortical pyramidal cell synapses, depending on the timing of pre- and postsynaptic activity. While one STDP learning rule does not “fit all” excitatory synaptic connections, variability in responses in most cases can be explained by differences in levels of postsynaptic depolarization and subsequent Ca2+ influx during the pairing protocol (Zilberter et al. 2009). This concept is supported by data showing that the balance between excitatory and inhibitory synapses dictates neural STDP in A1 in vitro (D'amour and Froemke 2015). When the excitatory to inhibitory synaptic ratio is higher, more Ca2+ channel and NMDA receptor activation occurs during spike pairing due to the increased level of depolarization. Alternatively, lower ratios may mean that inhibition more effectively limits NMDA-based depolarization, limiting excitation. In the present study, the order and timing of bimodal stimulation (Sp5 and tone) may modulate synaptic ratios, ultimately leading to STDP and the observed timing rules. Future studies are needed toward identifying specific timing rules to anatomic layer and cell types across A1.
STDP across auditory circuits.
A variety of learning rules reflecting STDP have been observed in the brain stem (Koehler and Shore 2013a; Tzounopoulos et al. 2004) and cortex (Dahmen et al. 2008; Egger et al. 1999; Letzkus et al. 2006; Schnupp et al. 2006). Bimodal auditory-somatosensory plasticity in DCN modulates spontaneous and tone-evoked activity that is stimulus-timing dependent (Koehler and Shore 2013a). Timing rules from that in vivo study reflected recordings found in vitro (Tzounopoulos et al. 2004), suggesting that bimodal stimulus timing-dependent plasticity in DCN neurons reflects STDP. In 40% of DCN units from sham-controls that exhibited stimulus timing dependency, auditory responses were facilitated after bimodal pairing in which Sp5 stimulation preceded tones but was suppressed if tones preceded Sp5 stimulation, thus exhibiting primarily Hebbian-like timing rules (Koehler and Shore 2013a). These results are consistent with the present findings in A1 sham-controls suggesting consistent mechanisms from brain stem to cortex. This comparison suggests that auditory-somatosensory plasticity works in a similar fashion at both brain levels or that A1 inherits plasticity that has occurred in DCN. Support for a consistent mechanism that displays a variety of timing rules ranging from Hebbian to anti-Hebbian may reflect the diversity of principal cell types within DCN that ultimately dictate physiological control of bimodal plasticity. For example, DCN neurons with less inhibitory influence (Type I receptive fields) were more likely to display Hebbian-like learning rules, while those units with greater inhibitory influence (Type III/IV) more often displayed anti-Hebbian-like stimulus timing dependence (Koehler and Shore 2013a). This is important to consider when interpreting the current A1 results that show evidence of primarily Hebbian STDP similar to DCN in sham-controls. Responses in A1 may therefore reflect greater “processed” sensory information through less inhibitory units (Type I) from DCN conveyed along the non-lemniscal pathway and recorded from layer II/III thalamocortical inputs, vs. “nonprocessed” circuits that require cortical integration (Basura et al. 2012) with different timing rules recorded from remaining A1 layers. Within A1, synapses in layer II/III represent a major site of intracortical processing for inputs arriving from the thalamus where STDP rules could be converted from one mode (Hebbian-like) to another (anti-Hebbian-like) depending on the postsynaptic activity (Zilberter et al. 2009).
Following noise exposure and tinnitus, differences in the proportion of timing rules were observed between DCN and A1. In DCN, increased suppressive plasticity was found in the ENT group that significantly decreased in the ET group. A1 timing rules showed a significant decrease in suppressive units in both noise-exposed groups. While DCN showed a decrease in all units except suppressive rules in ENT animals, both regions showed an increase in anti-Hebbian units in the ET groups. While a consistent mechanism of plasticity may exist in sham-controls between the two regions, different timing rule shifts in A1 suggest that mechanisms underlying multisensory plasticity in A1 and DCN are variably affected by noise exposure and tinnitus. Further evidence for separate multisensory processing in A1 is evident in our data. Maximal changes in neural responses in A1 occurred at BIs of 0 and +10 ms vs. different BIs (−10 to −20 ms and +20 ms; Koehler and Shore 2013b) in DCN, suggesting that discrete processing at the cortical level may also exist. Future studies are needed to investigate changes in timing rules specific to cortical layer and cell types across A1 that may help better identify where bimodal plasticity may be “already processed” by thalamocortical projections vs. layers that are “cortically processed.”
STDP and tinnitus.
Although the timing rules and neural changes in A1 in the present study were similar to those in DCN in sham-controls, differences were seen between the two regions after noise exposure in animals with and without evidence for tinnitus. Compared with the findings from DCN in the same animals under the same recording conditions, common changes in timing rules (shifts from Hebbian to anti-Hebbian) were observed in noise-exposed animals with tinnitus (Koehler and Shore 2013b). Changes in A1 STDP following noise exposure and tinnitus suggest that processing following bimodal stimulation may be implicated in tinnitus generation and can be potentially harnessed to ameliorate the percept. The observation that STDP is altered in both DCN and A1 following noise-induced tinnitus creates inroads for therapeutic design to target STDP mechanisms within both regions to simultaneously and noninvasively ameliorate neural tinnitus correlates and, ultimately, perception.
GRANTS
This work was supported by National Institutes of Health Grants P01-DC-00078, R01-DC-004825 (S. E. Shore), R03-DC-009893-01 (G. J. Basura) and by the American Otological Society (G. J. Basura).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.J.B., S.D.K., and S.E.S. conception and design of research; G.J.B. and S.D.K. performed experiments; G.J.B. analyzed data; G.J.B. and S.E.S. interpreted results of experiments; G.J.B. prepared figures; G.J.B. drafted manuscript; G.J.B., S.D.K., and S.E.S. edited and revised manuscript; G.J.B., S.D.K., and S.E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Wiler for expert technical assistance and M. Issa for statistical help.
REFERENCES
- Allman BL, Keniston LP, Meredith MA. Adult deafness induces somatosensory conversion of ferret auditory cortex. Proc Natl Acad Sci USA 106: 5925–5930, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA 93: 11219–11224, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Koehler SD, Shore SE. Multi-sensory integration in brainstem and auditory cortex. Brain Res 1485: 95–107, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18: 10464–10472, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci 31: 25–46, 2008. [DOI] [PubMed] [Google Scholar]
- D'amour JA, Froemke RC. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron 86: 514–528, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DEH, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J Neurosci 28: 13629–13639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Eisinger D, Shore S. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci 6: 42, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Koehler SD, Bledsoe S, Shore S. Noise over-exposure alters long-term somatosensory auditory processing in the dorsal cochlear nucleus—possible basis for tinnitus-related hyperactivity? J Neurosci 32: 1660–1671, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Hennevin E. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res 274: 75–84, 2011. [DOI] [PubMed] [Google Scholar]
- Egger V, Feldmeyer D, Sakmann B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat Neurosci 2: 1098–1105, 1999. [DOI] [PubMed] [Google Scholar]
- Eggermont J. Neural substrates of tinnitus in animal and human cortex: Cortical correlates of tinnitus. HNO 63: 298–301, 2015. [DOI] [PubMed] [Google Scholar]
- Eggermont J. Correlated neural activity as the driving force for functional changes in auditory cortex. Hear Res 229: 69–80, 2007. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron 27: 45–56, 2000. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature 416: 433–438, 2002. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Tsay IA, Raad M, Long JD, Dan Y. Contribution of individual spikes in burst-induced long-term synaptic modification. J Neurophysiol 95: 1620–1629, 2006. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nat Lett 15: 425–429, 2007. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Chandrasekaran C, Logothetis NK. Interactions between the superior temporal sulcus and auditory cortex mediate dynamic face/voice integration in rhesus monkeys. J Neurosci 28: 4457–4469, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK. Multisensory integration of dynamic faces and voices in rhesus monkey auditory cortex. J Neurosci 25: 5004–5012, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Bai L, Zhao Y, Xiao Z. Comparison of tonal response properties of primary auditory cortex neurons of adult rats under urethane and ketamine anesthesia. J South Med Iniv 33: 785–793, 2013. [PubMed] [Google Scholar]
- Kaltenbach JA, McCaslin DL. Increases in spontaneous activity in the dorsal cochlear nucleus following exposure to high intensity sound: a possible neural correlate of tinnitus. Aud Neurosci 3: 57–78, 1996. [PMC free article] [PubMed] [Google Scholar]
- Kampa BM, Stuart GJ. Calcium spikes in basal dendrites of layer 5 pyramidal neurons during action potential bursts. J Neurosci 26: 7424–7432, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci 21: 7848–7858, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. A model of spike-timing dependent plasticity: one or two coincidence detectors? J Neurophysiol 88: 507–513, 2002. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Najarian MT, Buonomano DV. Mechanisms and significance of spike-timing dependent plasticity. Biol Cybern 87: 373–382, 2002. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science 279: 1714–1718, 1998. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci USA 99: 3205–3209, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol 86: 326–338, 2001. [DOI] [PubMed] [Google Scholar]
- Knipper M, Van Dijk P, Nunes I, Ruttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol 111: 17–33, 2013. [DOI] [PubMed] [Google Scholar]
- Koehler SD, Pradhan S, Manis PB, Shore SE. Somatosensory inputs modify auditory spike timing in dorsal cochlear nucleus principal cells. Eur J Neurosci 33: 409–420, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SD, Shore SE. Stimulus-timing dependent multisensory plasticity in guinea pig dorsal cochlear nucleus. PLOS One 8: 1–11, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci 33: 19647–19656, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol 120: 750–756, 2000. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron 53: 279–292, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RS, Rao D, Manis PB, Philpot BD. STDP in the developing sensory neocortex. Front Synaptic Neurosci 2: 1–11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci 26: 10420–10429, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275: 213–215, 1997. [DOI] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron 49: 183–189, 2006. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Allman BL. Early hearing-impairment results in crossmodal reorganization of ferret core auditory cortex. Neural Plast 601591: 1–13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Keniston LP, Allman BL. Multisensory dysfunction accompanies crossmodal plasticity following adult hearing impairment. Neuroscience 214: 136–148, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci 26: 11001–11013, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci 10: 14972–14979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttiger L, Singer W, Panford-Walsh R, Matsumoto M, Lee SC, Zuccotti A, Zimmermann U, Jaumann M, Rohbock K, Xiong H, Knipper M. The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PLoS One 8, e57247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Foxe JJ. The timing and laminar profile of converging inputs to multisensory areas of the macaque neocortex. Brain Res Cogn Brain Res 14: 187–198, 2002. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lindsley RW, Specht C, Marcovici A, Smiley JF, Javitt DC. Somatosensory input to auditory association cortex in the macaque monkey. J Neurophysiol 85: 1322–1327, 2001. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Smiley JF, Fu KG, McGinnis T, O'Connell MN, Hackett TA. Anatomical mechanisms and functional implications of multisensory convergence in early cortical processing. Int J Psychophysiol 50: 5–17, 2003. [DOI] [PubMed] [Google Scholar]
- Schnupp JW, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci 26: 4785–4795, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci 21: 3334–3348, 2005. [DOI] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci 27: 155–168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Zuccotti A, Jaumann M, Lee SC, Panford-Walsh R, Xiong H, Zimmermann U, Franz C, Geisler HS, Köpschall I, Rohbock K, Varakina K, Verpoorten S, Reinbothe T, Schimmang T, Ruttiger L, Knipper M. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol Neurobiol 47: 261–279, 2013. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Hausser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron 51: 227–238, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32: 1149–1164, 2001. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120: 188–195, 2006. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7: 719–725, 2004. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Identification and localisation of auditory areas in guinea pig cortex. Exp Brain Res 132: 445–456, 2000. [DOI] [PubMed] [Google Scholar]
- Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci 32: 15791–15801, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tao H, Holt C, Harris W, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature 395: 37–44, 1998. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Acker CD, Netoff TI, Sen K, White JA. Increasing Ca2+ transients by broadening postsynaptic action potentials enhances timing-dependent synaptic depression. Proc Natl Acad Sci USA 102: 19121–19125, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vesicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol 500: 777–787, 2007. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol 495: 100–112, 2006. [DOI] [PubMed] [Google Scholar]
- Zilberter M, Holmgren C, Shemer I, Silberberg G, Grillner S, Harkany T, Zilberter Y. Input specificity and dependence of spike timing-dependent plasticity on preceding postsynaptic activity at unitary connections between neocortical layer 2/3 pyramidal cells. Cereb Cortex 19: 2308–2320, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]