Abstract
The relative motion between the surface of an object and our fingers produces patterns of skin deformation such as stretch, indentation, and vibrations. In this study, we hypothesized that motion-induced vibrations are combined with other tactile cues for the discrimination of tactile speed. Specifically, we hypothesized that vibrations provide a critical cue to tactile speed on surfaces lacking individually detectable features like dots or ridges. Thus masking vibrations unrelated to slip motion should impair the discriminability of tactile speed, and the effect should be surface-dependent. To test this hypothesis, we measured the precision of participants in discriminating the speed of moving surfaces having either a fine or a ridged texture, while adding masking vibratory noise in the working range of the fast-adapting mechanoreceptive afferents. Vibratory noise significantly reduced the precision of speed discrimination, and the effect was much stronger on the fine-textured than on the ridged surface. On both surfaces, masking vibrations at intermediate frequencies of 64 Hz (65-μm peak-to-peak amplitude) and 128 Hz (10 μm) had the strongest effect, followed by high-frequency vibrations of 256 Hz (1 μm) and low-frequency vibrations of 32 Hz (50 and 25 μm). These results are consistent with our hypothesis that slip-induced vibrations concur to the discrimination of tactile speed.
Keywords: tactile speed perception, speed discrimination, vibrotactile masking, mechanoreceptive afferents, psychophysics
the relative motion between the surface of an object and our fingers produces patterns of mechanical deformation of the skin such as stretch, indentation, and vibrations. Motion-related vibrations not only occur in the direct contact area but also propagate to the hand and the forearm (Delhaye et al. 2012). The vibration frequency spectrum depends on both the texture of the surface and the speed of the scanning movement (Adams et al. 2012; Bensmaia and Hollins 2003; Delhaye et al. 2012; Fagiani et al. 2010). For example, increasing the scanning speed produces an increase in both the frequency and the amplitude of vibrations (Fagiani et al. 2010).
Vibration and motion speed signals are not only tightly coupled in the physical domain but also produce an overlap in the neurophysiological responses of cutaneous mechanoreceptive afferents (Gardner and Kandel 2000). That is, vibrations and other tactile motion cues, such as the rate of skin stretch and indentation, recruit the same type of afferent fibers. Vibrations elicit responses particularly in the fast-adapting afferents (Johansson et al. 1982), which are also highly sensitive to slip motion (Essick and Edin 1995). Low-frequency vibrations are predominantly signaled by fast-adapting type I (RA) afferents, which are most sensitive at about 30 Hz, whereas high-frequency vibrations are predominantly signaled by fast-adapting type II (PC) afferents, which are most sensitive at about 250 Hz (Johansson et al. 1982; Mountcastle et al. 1972; Talbot et al. 1968). The response of these afferents to vibratory stimuli seems to be consistent with their response to the speed of a patterned moving stimulus when both stimuli are coded with respect to their temporal frequency (Goodwin et al. 1989). Accordingly, PC afferents were found to respond preferentially to tactile motion of fine-textured surfaces, whereas RA afferents were most sensitive to motion of surfaces with individually detectable features such as dots or ridges (Srinivasan et al. 1990). In addition to fast-adapting afferents, slowly adapting type I (SA-I) afferents respond preferentially to vibrations in the lower frequency range (Johansson et al. 1982). These afferents are generally less sensitive to motion than RA afferents (Freeman and Johnson 1982; Johansson and Vallbo 1979; LaMotte and Whitehouse 1986) and rather insensitive to changes in motion speed (Goodwin and Morely 1987; Lamb 1983).
When scanning an object, the somatosensory system faces the challenging task of decoding speed information from these multidimensional and correlated signals (Dépeault et al. 2013; Pei and Bensmaia 2014). Skin vibrations, whose frequency spectrum depends on both surface texture and scanning speed, may provide a cue for the observer to discriminate both features of the tactile stimulus. Indeed, vibrations were found to be critical for discriminating fine textures directly with the bare fingertip (Delhaye et al. 2012; Hollins et al. 2001; Weber et al. 2013) or indirectly via a tool (Klatzky and Lederman 1999). Their role in discriminating tactile speed, however, remains to be elucidated.
We hypothesized that motion-induced vibrations provide an important cue for the discrimination of tactile speed, particularly on surfaces lacking individually detectable features such as dots or ridges. Hence, masking vibratory noise should impair speed discrimination. To test this hypothesis, we measured the precision of participants in discriminating tactile speeds of a moving fine-textured surface (experiments 1a and 1b) and of a moving ridged surface (experiment 2). To reduce the reliability of the vibration cue, we masked the “natural” vibrations produced by the slip motion of the surface by simultaneously presenting vibratory noise in the working range of the two fast-adapting afferent types. The effect of masking vibrations was expected to be stronger on the fine-textured surface, because other motion cues are less pronounced on this surface.
MATERIALS AND METHODS
Participants
Overall, we tested 23 naive participants plus author C. J. Dallmann (ages: 18–55 yr, median age: 23 yr; 12/24 women; all right-handed) in the 2 experiments. The sample size was equal to N = 10 participants in experiment 1a and experiment 2, and N = 9 participants in experiment 1b. Four participants took part in more than one experiment. The testing procedures were approved by the ethics committee of Bielefeld University, in accordance with the guidelines of the Declaration of Helsinki for research involving human subjects. Informed written consent was obtained from all participants involved in the study.
Apparatus
Motion devices.
In experiment 1, we used the device described in Fritschi et al. (2006) and Moscatelli et al. (2014) to deliver the motion stimuli. The device consisted of a rotating, sandblasted billiard ball (diameter: 6 cm) having a fine-textured surface lacking individually detectable features such as dots or ridges. The fingertip contacted the ball through a small circular aperture (diameter: 2 cm) in the metal cover plate of the device (Fig. 1A). In experiment 2, the motion device consisted of a ridged rubber belt connected to two rotating cylinders and supported by a metal plate in between (Fig. 1B). The ridges of the belt were oriented perpendicularly to its long axis and, unlike the ball, constituted clearly detectable surface features (ridge width: 1 mm; ridge height: 1 mm; distance between ridges: 1 mm). Each of the two devices was actuated by a servomotor (Faulhaber DC-micromotor 2232UO24 combined with MCDC2805 motion controller; maximum slip speed: 39.3 cm/s). The motor was accessed via a serial RS-232 connection from the operating computer and controlled by a custom-written MATLAB script (The MathWorks, Natick, MA). The rotational speed was controlled via an encoder in the driver module.
Fig. 1.
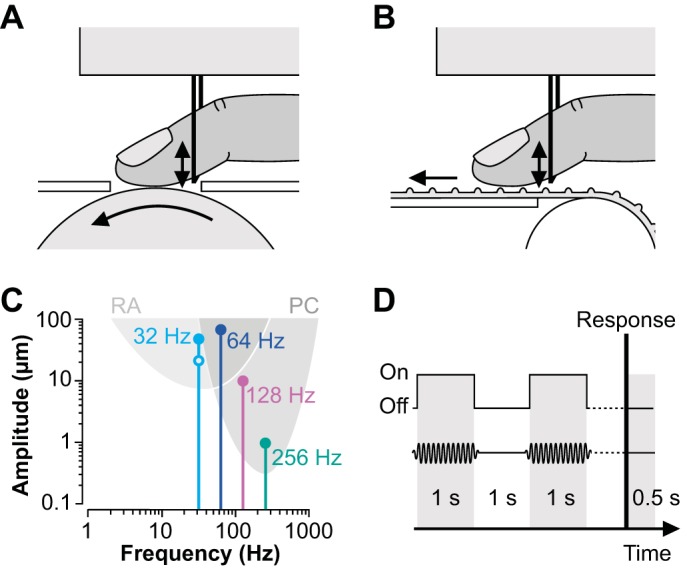
A and B: schematic close-up side view of the experimental setup. The tip of the right index finger contacted either a fine-textured (A; experiment 1) or a ridged surface (B; experiment 2), which was moved from proximal to distal (leftward arrows). Vibrations were induced at the ventral side of the proximal phalanx (vertical arrows) via a metal rod that was rigidly attached to a vibration device above the finger. C: frequencies and amplitudes of the masking vibrations used in the vibratory noise conditions and response ranges of rapidly adapting (RA and PC) afferents (the latter were estimated from Mountcastle et al. 1972). The open circle indicates the masking vibration used in experiment 1b. D: protocol of a single trial. The motion device delivered 2 tactile stimuli of 1 s each, with a 1-s interval in between. Tactile stimuli were masked with vibratory noise (see C). Participants reported whether they had perceived the second stimulus to be faster or slower than the first. The protocol was repeated 0.5 s after the response.
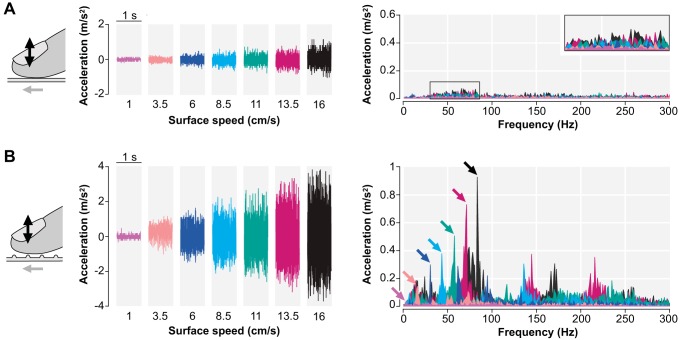
The slip motion of each of the two surfaces induced characteristic vibrations on the fingertip that we analyzed (Fig. 2) to provide comparative information about the two surface textures. Vibrations produced by the rotating ball (experiment 1) increased in amplitude with increasing speed but lacked a dominant frequency (Fig. 2A). To quantify the vibration amplitude, we computed the root mean square of the time series shown in Fig. 2 (acceleration signal). The peak-to-peak amplitude of vibration ranged from 0.07 to 0.29 cm/s2 within the range of the tested slip motion speed. The moving belt (experiment 2) induced vibrations in the fingertip that increased in amplitude with increasing speed, with dominant frequencies occurring at multiples of the spatial period of the belt (Fig. 2B). The peak-to-peak amplitude of vibration ranged from 0.10 to 1.52 cm/s2 within the range of the tested slip motion speed. Thus the amplitude ratio of the vibration generated by the two surfaces (ball:belt) ranged from 0.7 (slowest slip speed) to 0.2 (fastest slip speed).
Fig. 2.
Movement of both the fine-textured surface (A) and the ridged surface (B) induced vibrations in the right index fingertip that increased with increasing surface speed. Accelerations were measured in 1-s time windows during constant surface movement with an accelerometer attached to the fingernail. In contrast to the fine-textured surface, the ridged surface generated vibrations with dominant frequencies, which were reliably shifted toward higher frequencies with increasing speed. Arrows in B mark the dominant frequency for each speed.
Vibration device.
The vibration device (BR-25; Monacor International, Bremen, Germany) delivered sinusoidal vibrations of different frequencies and amplitudes to excite predominantly one or multiple afferent types (below). Vibration amplitudes and frequencies were inferred from accelerometer recordings (ADXL335; Analog Devices, Norwood, MA). We processed the recorded acceleration signal with a fast Fourier transform and estimated the amplitude of displacement by integrating the transformed signal twice, assuming a simple harmonic motion. Before experimentation, we attached the accelerometer directly to the pulp of the distal phalanx and confirmed that vibrations were transmitted from the device to the skin.
Stimulus and Procedure
Motion stimulus.
In all experiments, tactile motion stimuli were delivered to the tip of the right index finger (Fig. 1, A and B), which has the highest density of mechanoreceptive afferents (Vallbo and Johansson 1984). The direction of motion of the surface was proximal to distal relative to the finger. Each motion stimulus consisted of a trapezoidal motion profile with a steep acceleration/deceleration (±180 cm/s2). Using the motion encoder, we confirmed that target speeds (see Experimental procedure) were always reached at the plateau. Because the acceleration/deceleration was kept constant across trials, the shear force on the fingertip was not informative about the motion speed. Instead, the duration of the acceleration/deceleration phase (6–88 ms) could in principle provide a cue to motion speed, if participants were able to discriminate the acceleration phase from the speed plateau. However, this additional cue would not account for a potential effect of masking vibrations because the acceleration/deceleration did not vary across experimental conditions.
Vibratory stimulus.
Vibrations were delivered to the ventral side of the distal phalanx of the right index finger, a few millimeters proximal to the skin area contacting the moving surface (Fig. 1, A and B). The pulp of the distal phalanx rested on a metal rod (diameter: 1 mm), which was rigidly attached to the vibration device above the finger. In different experimental conditions, the device delivered vibrations of 32, 64, 128, and 256 Hz. Peak-to-peak amplitudes were 50, 65, 10, and 1 μm, respectively. In the control condition, the vibration device was in place but produced no vibrations. Amplitudes and frequencies of vibration were chosen according to the tuning function of the different afferent fiber types (Fig. 1C). Specifically, vibration amplitudes and frequencies were designed to predominantly target RA afferents (32 Hz), RA and PC afferents (64 Hz), or predominantly PC afferents (128 and 256 Hz) (Johansson et al. 1982; Mountcastle et al. 1972). As illustrated in Fig. 1C, the tuning curves of RA and PC afferents largely overlap in the lower frequency range. To further address their contribution to the participants' response, we additionally tested, on the fine-textured surface only, the effect of small-amplitude (25 μm), low-frequency (32 Hz) masking vibrations (experiment 1b). Before each experiment, participants confirmed that they could clearly detect all masking vibrations.
Experimental procedure.
Participants were comfortably seated in front of the setup table, with the right forearm and hand supported on a padded board. They kept the right finger stationary during the entire experimental session, in contact with the motion device. An opaque plane between the right and the left arm occluded the vision of the motion device. In addition, room light was limited to the indirect light of a computer screen displaying experimental instructions. Pink auditory noise was delivered to the participants via earphones throughout each experimental session to mask external sounds from the motion and the vibration device.
Using a forced-choice procedure, we assessed the ability of participants to discriminate the speed of the fine-textured and ridged surface under different vibratory noise conditions. In each test trial, we successively presented two motion stimuli (the standard and the comparison, in pseudorandom order) and simultaneously applied the masking vibrations (Fig. 1D). Participants then reported whether they had perceived the second motion stimulus to be faster or slower than the first one by pressing either the up (faster) or down (slower) button on a standard keyboard with their left hand. Each motion stimulus lasted 1 s with an interstimulus interval of 1 s. The vibration stimulus started 0.1 s before the motion stimulus and ended 0.1 s after the motion stopped. The speed of the standard stimulus was always 8.5 cm/s. In each vibratory noise condition, the standard was presented first in 50% of the trials. The speed of the comparison stimulus was pseudorandomly chosen among seven speeds: 1, 3.5, 6, 8.5, 11, 13.5, or 16 cm/s. Tested speeds were within the recommended maximum speed of the servomotor (39.3 cm/s). In experiments 1a and 2, each comparison stimulus was presented 20 times per vibratory noise condition, resulting in a total of 700 trials per experiment. In experiment 1b, we presented each comparison stimulus 40 times per condition, resulting in a total of 560 trials. Participants completed all trials within 1 h. To keep concentration levels high, participants took a 5-min break after completing half of the trials.
Data Analysis
We modeled the responses of all participants by means of a generalized linear mixed model (GLMM). GLMMs are conditional models designed for the analysis of clustered data (Agresti 2002; Moscatelli et al. 2012). In our case, a cluster is the collection of repeated responses from a given participant. In the GLMM framework, the observed response is modeled as a linear combination of the systematic effect of the experimental variables (the surface speed and the vibratory noise), the random variability between participants, and the residual error within participants (the latter arising from the binomial process in the forced-choice procedure). The GLMM accounts separately for the experimental effects and the variability between participants by means of random- and fixed-effect parameters, respectively (Moscatelli et al. 2012). By combining both parameters, we obtained the model predictions at the level of single participants, which are highly valuable for evaluating the model fit to the data (Figs. 3A, 4A, and 5A).
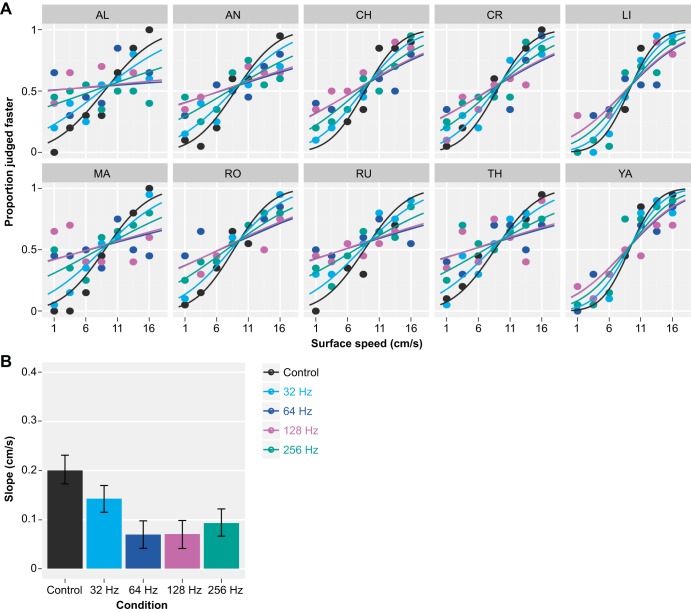
Fig. 3.
Results of the speed discrimination task on the fine-textured surface (experiment 1a). A: individual responses with generalized linear mixed model (GLMM) fits. Vibratory noise reduced the precision (slope) of the response similarly in all participants. B: vibratory noise of 64 Hz (dark blue) and 128 Hz (magenta) reduced the precision most strongly, followed by vibratory noise of 256 Hz (green) and 32 Hz (light blue). Vertical error bars show 95% confidence intervals.
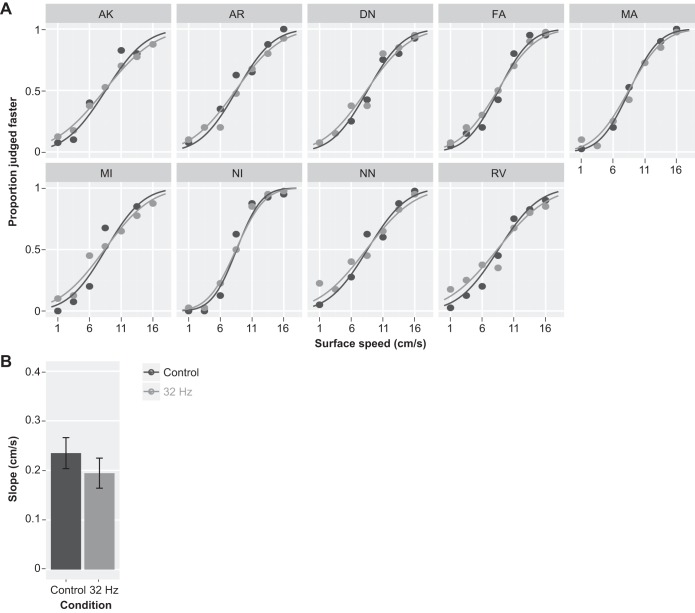
Fig. 4.
Results of the speed discrimination task on the fine-textured surface with 32-Hz masking vibrations of reduced amplitude (25 μm; experiment 1b). A: individual responses with GLMM fits. Vibratory noise reduced the precision (slope) of the response similarly in all participants. B: the effect of vibratory noise (light gray) was small but significant. Vertical error bars show 95% confidence intervals.
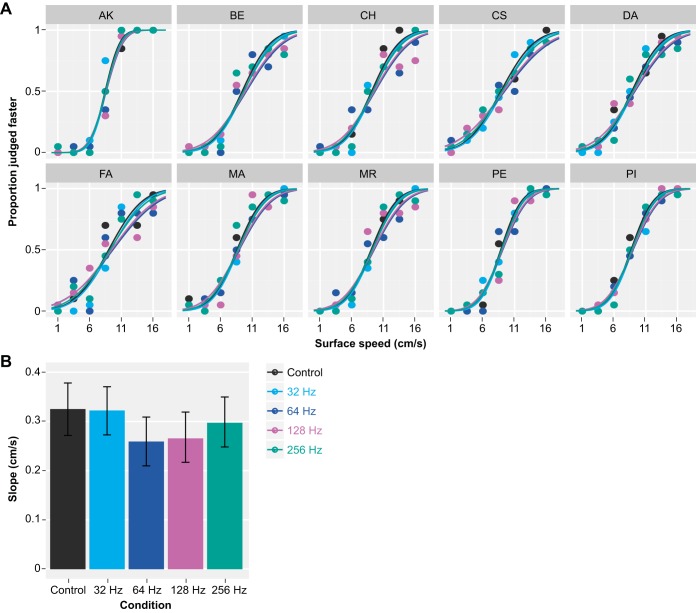
Fig. 5.
Results of the speed discrimination task on the ridged surface (experiment 2). A: individual responses with GLMM fits. In contrast to the fine-textured surface, vibratory noise reduced the precision (slope) of the response only moderately on the ridged surface. B: albeit much weaker, vibratory noise of 64 Hz (dark blue) and 128 Hz (magenta) again had the strongest effect on the precision, followed by vibratory noise of 256 Hz (green) and 32 Hz (light blue). Vertical error bars show 95% confidence intervals.
In experiment 1a, random-effect parameters were denoted with the lowercase letter u{0,1} and fixed-effect parameters with the Greek letter θ{1, . . ., 9}. The model equation was the following:
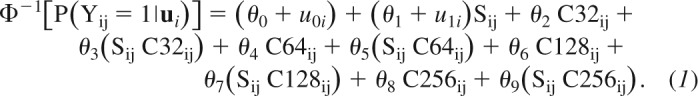 |
This model was chosen among several nested GLMMs based on the Akaike information criterion (AIC). The left side of the equation is the probability that participant i in trial j reported that the comparison was moving faster than the standard, in symbols P(Yij = 1|ui), with Φ−1[.] being the probit transform of this probability (i.e., the inverse of the cumulative Gaussian function). The right side of the equation is a linear combination of the fixed- and random-effect predictors. Specifically, sij is the speed of the comparison stimulus, and C32ij,. . ., C256ij are the categorical predictors coding for the vibratory noise condition. We used a dummy coding for the vibratory noise condition; therefore, the control condition (without vibratory noise) is the baseline in this model. The fixed-effect parameters θ0, . . ., θ9 estimate the effect of the experimental variables (i.e., surface speed and vibratory noise), common to all participants. The fixed-effect parameters θ0 and θ1 correspond to the intercept and the slope of the response function in the control condition, respectively.
The slope provides an estimate of the discriminability of the stimulus: the higher the slope, the higher the discriminability. The fixed-effect parameters θ2, θ4, θ6, and θ8 test whether the slope in a given experimental condition was significantly different from the control, which was the main focus of the present study. The slope in a given condition “*” was equal to the algebraic sum of θ1 and θ*. For example, the slope in C32 was θ1(C32) = θ1 + θ3. If the discriminability of the stimulus was not significantly different from the control, then θ1(C32) would be not significantly different from θ1, and hence θ3 not significantly different from zero (the null hypothesis). The other fixed-effect parameters θ3, θ5, θ7, and θ9 provided a minor adjustment to the intercept in each vibratory condition. The random-effect parameters u0i and u1i estimated the heterogeneity between participants. By using two random-effect parameters, the model allowed the intercept and the slope of the response to vary in a random fashion between participants. We applied an analogous model in experiment 1b and experiment 2. The fixed-effect parameters were denoted with the Greek letters β and η, respectively.
In all experiments, the significance of the slope parameters of the GLMMs were tested using the likelihood ratio test, as explained in Moscatelli et al. (2012). The P values were adjusted for multiple comparisons using the Holm method (Holm 1979). For comparison with previous studies, we also estimated the just noticeable difference (JND) in each condition, which is an inverse function of the slope. We estimated the parameter using the bootstrap method described in Moscatelli et al. (2012). All statistical analyses were carried out in R (R Core Team; https://www.r-project.org). We used the lme4 package to fit the GLMM and the MERpsychophysics package (https://mixedpsychophysics.wordpress.com) to estimate the JND.
RESULTS
Participants discriminated the speed of a fine-textured surface (experiments 1a and 1b) and a ridged surface (experiment 2) moving across the stationary fingertip. Our leading hypothesis was that motion-induced vibrations provide a cue for discriminating tactile speeds. If so, vibratory noise would mask the cue and impair speed discrimination. Therefore, we expected the slope parameter of the GLMM (which estimates the discriminability of the stimulus; see materials and methods) to be smaller if vibratory noise was presented simultaneously with the motion stimulus. A systematic error (a shift of the response curve) was not expected, because vibratory noise was delivered with both the standard and the comparison motion stimulus.
Vibratory Noise Impairs Speed Discrimination on the Fine-Textured Surface (Experiment 1)
In experiment 1 (fine-textured surface), all participants were able to discriminate tactile speeds in the control condition (Fig. 3A). The JND in control condition was 3.10 ± 0.23 cm/s (estimate ± SE) in experiment 1a and 2.9 ± 0.19 cm/s in experiment 1b. This corresponded to a Weber fraction of 0.36 and 0.34, respectively, with the Weber fraction being the ratio of the JND to the standard speed.
In accordance with our hypothesis, masking the motion stimuli with vibratory noise impaired speed discrimination significantly. In experiment 1a, the slope of the response was significantly reduced in all experimental conditions compared with the control (P < 0.001; Table 1 and Fig. 3B). Vibrations of 64 and 128 Hz had the strongest effect, followed by vibrations of 256 and 32 Hz. That is, the slopes in the 64- and 128-Hz conditions were both significantly smaller than in the 256- and 32-Hz condition (P < 0.001, except P < 0.01 when comparing 128 and 32 Hz). Inspection of the raw data and the model fits shows the effects appeared to be consistent across participants (Fig. 3A). The slope of the fitted response was 0.20 cm/s (0.17–0.23 cm/s; bootstrap-based 95% confidence interval, unless stated otherwise) in the control condition. Instead, it was equal to 0.07 cm/s (0.04–0.10 cm/s) in the 64- and 128-Hz conditions, 0.09 cm/s (0.07–0.12 cm/s) in the 256-Hz condition, and 0.14 cm/s (0.11–0.17 cm/s) in the 32-Hz condition (Fig. 3B).
Table 1.
Fixed-effect parameters of GLMM for fine-textured surface (experiment 1a)
| Estimate | SE | zValue | Pr(> z) | |
|---|---|---|---|---|
| θ0 (intercept) | −1.77 | 0.16 | −11.07 | <0.001 |
| θ1 (speed) | 0.20 | 0.02 | 12.19 | <0.001 |
| θ2 (32 Hz) | 0.58 | 0.13 | 4.44 | <0.001 |
| θ3 (speed: 32 Hz) | −0.06 | 0.01 | −4.29 | <0.001 |
| θ4 (64 Hz) | 1.28 | 0.13 | 10.2 | <0.001 |
| θ5 (speed: 64 Hz) | −0.13 | 0.01 | −10.17 | <0.001 |
| θ6 (128 Hz) | 1.25 | 0.13 | 9.99 | <0.001 |
| θ7 (speed: 128 Hz) | −0.13 | 0.01 | −10.09 | <0.001 |
| θ8 (256 Hz) | 1.04 | 0.13 | 8.22 | <0.001 |
| θ9 (speed: 256 Hz) | −0.11 | 0.01 | −8.26 | <0.001 |
See text for description of parameters.
In experiment 1b, the slope of the response was also significantly smaller in the 32-Hz condition compared with the control condition (P < 0.001; Table 2 and Fig. 4). The effect was consistent across participants. The slope of the fitted response was 0.23 cm/s (0.20–0.27 cm/s) in the control condition and 0.19 cm/s (0.16–0.22 cm/s) in the 32-Hz condition.
Table 2.
Fixed-effect parameters of GLMM for control experiment with fine-textured surface (experiment 1b)
| Estimate | SE | zValue | Pr(> z) | |
|---|---|---|---|---|
| β0 (intercept) | −2.02 | 0.14 | −14.38 | <0.001 |
| β1 (speed) | 0.24 | 0.016 | 14.82 | <0.001 |
| β2 (32 Hz) | 0.38 | 0.09 | 4.00 | <0.001 |
| β3 (speed: 32 Hz) | −0.04 | 0.01 | −4.10 | <0.001 |
See text for description of parameters.
These results demonstrate that the discrimination of tactile speed is impaired by vibratory masking noise in a frequency- and amplitude-dependent manner.
Effects of Vibratory Noise are Weaker on the Ridged Surface (Experiment 2)
In experiment 2 (ridged surface), participants discriminated speeds with higher precision compared with the fine-textured surface (Fig. 5A). A better performance can readily be seen by comparing the responses of participants that took part in both experiments (AK, CH, FA, and MA). The JND was 2.07 ± 0.20 cm/s (estimate ± SE), which is about one-third smaller than that for the fine-textured surface. This corresponded to a Weber fraction of 0.24, which is comparable to the Weber fraction of 0.25 estimated in a previous study (Essick et al. 1988).
Masking the motion stimuli with vibratory noise also impaired speed discrimination on the ridged surface. However, the effects were much weaker compared with the fine-textured surface (Fig. 5A). The slope of the response was significantly reduced with respect to the control only with 64-Hz (P < 0.001) and 128-Hz (P < 0.05) vibrations (Table 3 and Fig. 5B), which also had the strongest effect on the fine-textured surface. The slopes in these two conditions were also significantly smaller compared with those in the 256-Hz (both P < 0.05) and 32-Hz conditions (both P < 0.001). Vibratory noise at 32 and 256 Hz, on the other hand, had no significant effect on the slope. The slope of the fitted response was 0.33 cm/s (0.27–0.38 cm/s) in the control condition. It declined to 0.26 cm/s (0.21–0.31 cm/s) with 64-Hz vibrations and to 0.27 cm/s (0.22–0.32 cm/s) with 128-Hz vibrations. With 256- and 32-Hz vibrations, the slope decreased only to 0.30 cm/s (0.25–0.35 cm/s) and 0.32 cm/s (0.27–0.37 cm/s), respectively.
Table 3.
Fixed-effect parameters of GLMM for ridged surface (experiment 2)
| Estimate | SE | zValue | Pr(> z) | |
|---|---|---|---|---|
| η0 (intercept) | −2.88 | 0.26 | −10.95 | <0.001 |
| η1 (speed) | 0.33 | 0.03 | 10.42 | <0.001 |
| η2 (32 Hz) | −0.05 | 0.18 | −0.29 | 0.77 |
| η3 (speed: 32 Hz) | −0.01 | 0.02 | −0.15 | 0.88 |
| η4 (64 Hz) | 0.48 | 0.16 | 2.92 | <0.001 |
| η5 (speed: 64 Hz) | −0.07 | 0.02 | −3.93 | <0.001 |
| η6 (128 Hz) | 0.45 | 0.16 | 2.75 | 0.01 |
| η7 (speed: 128 Hz) | −0.06 | 0.02 | −3.53 | 0.001 |
| η8 (256 Hz) | 0.20 | 0.17 | 1.19 | 0.23 |
| η9 (speed: 256 Hz) | −0.03 | 0.02 | −1.59 | 0.22 |
See text for description of parameters.
These results suggest that the effects of vibratory noise were overall weaker in the presence of clearly detectable surface features, as is the case with the ridged surface. Notably, however, the effects tended to be of similar relative strength to those with the fine-textured surface: 32-Hz vibrations had the weakest effect, followed by 256-Hz vibrations and vibrations of 64 and 128 Hz.
DISCUSSION
In this study, we have shown that tactile speed discrimination is impaired when masking vibratory noise is presented simultaneously with slip motion. Low-frequency vibrations of 32 Hz (50-μm amplitude) had the smallest effect, followed by high-frequency vibrations of 256 Hz (1 μm) and intermediate vibrations of 64 (65 μm) and 128 Hz (10 μm). Low-frequency vibrations of 32 Hz with reduced amplitude (25 μm) also produced a small effect, which was statistically significant. The effects of vibratory noise were strong during speed discrimination of a fine-textured surface (Figs. 3 and 4). A weaker, yet significant effect was found during speed discrimination of a ridged surface with clearly detectable surface features (Fig. 5). In the two following paragraphs we present two alternative hypotheses on the functional mechanism of this masking effect.
PC-Induced Inhibition
Previous studies showed that high-frequency masking vibrations impaired the detection of tactile vibrations (Ferrington et al. 1977) and finger movement (Weerakkody et al. 2007). These studies proposed that the impairment resulted from PC afferents inhibiting the input of the other afferent types at synaptic relays of the sensory pathway. Given the vibration amplitudes in our experiments, PC afferents were likely activated by vibrations of 64, 128, and 256 Hz (Fig. 1C; Johansson et al. 1982; Mountcastle et al. 1972). Therefore, PC-induced inhibition could account for the drop in performance in this frequency range. It is less clear, however, to what extent PC-induced inhibition alone can explain the drop in performance in the lower frequency range. Given their high sensitivity, PC afferents could have been activated by the 32-Hz vibrations used in the present study, along with RA afferents and possibly slowly adapting afferents (Bolanowski et al. 1988; Johansson et al. 1982; Mountcastle et al. 1972). However, it is worth noting that in investigating PC-induced inhibition, Ferrington et al. (1977) and Weerakkody et al. (2007) found no effect of 30-Hz masking vibrations with rather high amplitudes (50- and 100-μm peak-to-peak amplitude, respectively). In contrast, 32-Hz vibrations significantly impaired speed discrimination in our experiments, even with lower vibration amplitudes (experiment 1). Given the overlap of the response functions of the different afferent types in the low-frequency range and their stochastic variation across individuals and trial repetitions, it is difficult to reliably predict afferent responses solely based on the behavioral data in the psychophysical task. Thus further studies including afferent recordings might help to clarify whether PC-induced inhibition alone can account for our results.
Vibrations as a Cue to Tactile Speed
As an alternative explanation, we propose that the tactile system combines motion-induced vibrations with other time-varying cues for the discrimination of slip motion speed. According to this hypothesis, external vibrations masking the “natural” vibrations generated by the moving surfaces (Fig. 2) impaired speed discrimination in our experiments. That is, external vibrations decreased the signal-to-noise ratio of the vibration cue to speed. The vibration cue could be integrated with other motion cues, like the indentation wave produced by the displacement of traceable surface features (such as ridges or dots) across the skin. Vibration cues might then be critical on fine-textured surfaces, lacking these traceable features.
Our results are consistent with this hypothesis. The difference in the reliability for the response between the fine-textured surface (experiment 1) and the ridged surface (experiment 2) was in accordance with an optimal integration of the different motion cues (Ernst and Banks 2002). That is, participants would have integrated multiple cues such as vibrations, feature tracking, and tangential skin stretch when estimating the speed of the ridged surface, resulting in a reliable response. In contrast, estimating the speed of the fine-textured surface resulted in a noisier response because of the lack of the feature-tracking cue. A better speed discrimination in the presence of individually detectable surface features also confirms previous findings (Dépeault et al. 2008).
A mechanism of speed discrimination based on motion-induced vibrations could rely on an intensive code (Dépeault et al. 2013; Essick and Edin 1995), which encodes higher relative speed as higher vibration amplitude. In our experiments, vibration amplitude increased with increasing speed on both the ridged and the fine-textured surfaces (Fig. 2), consistent with previous findings (Fagiani et al. 2010). On the fine-textured surface, the amplitude of vibrations (acceleration signal) increased approximately fourfold within the tested range of slip motion speed (see materials and methods and Fig. 2A). On the ridged surface, it increased ∼15-fold (Fig. 2B). At the same tangential speed, the movement of the ridged surface induced vibrations of higher amplitude than the fine-textured surface. Hence, the signal-to-noise ratio (i.e., the ratio of slip-induced vibrations to masking vibrations) was higher on the ridged surface compared with the fine-textured surface (Fig. 2), which can also account for the difference in the effect size between experiment 1 and experiment 2. Furthermore, if vibration amplitude were used as a motion cue, a rough or a ridged surface would be perceived as moving faster than a smooth surface moving at the same physical speed. A recent study demonstrates that this is indeed the case (Dépeault et al. 2008).
In principle, all vibration-sensitive afferents could provide an intensive code. Given their high sensitivity and large receptive fields, PC afferents are likely to play a central role in conveying this cue (Srinivasan et al. 1990; Vallbo and Johansson 1984), although SA and RA afferents could contribute as well (Johansson et al. 1982; Talbot et al. 1968). According to recent studies (Dépeault et al. 2013, Harvey et al. 2013), the integration of multiple tactile cues could occur in the primary somatosensory cortex (S1). Neurons of S1 were found to be sensitive to moving tactile stimuli of the types used in the present study (Dépeault et al. 2013). Moreover, they encoded vibration amplitude in the strength of their response and responded to a wide range of vibration frequencies, suggesting they receive input from PC and other afferents (Harvey et al. 2013).
In conclusion, we propose that skin vibrations are integrated with other motion cues for the discrimination of tactile speed, and that vibration cues are particularly important in the absence of clearly detectable surface features. Along a similar line of reasoning, Yao and Hayward (2006) showed that simulated vibrations produced the vivid sensation of a ball rolling inside a tube held in the hand. In their study, participants were able to estimate the length of the motion path of the object on the basis of the simulated vibrations, which required inferring the motion kinematics. An integration of slip-induced vibrations with other tactile motion cues would further support the emerging view of submodality convergence in the tactile system (Jörntell et al. 2014; Saal and Bensmaia 2014).
GRANTS
This study was supported by the European Commission FP7/2007–2013 Project 601165 WEARHAP (WEARable HAPtics for Humans and Robots) and the Cluster of Excellence Cognitive Interaction Technology “CITEC” (EXC 277) funded by the German Research Foundation (DFG).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.D., M.O.E., and A.M. conception and design of research; C.J.D. performed experiments; C.J.D. and A.M. analyzed data; C.J.D., M.O.E., and A.M. interpreted results of experiments; C.J.D. and A.M. prepared figures; C.J.D. and A.M. drafted manuscript; C.J.D., M.O.E., and A.M. edited and revised manuscript; C.J.D., M.O.E., and A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marian Rosenstengel, Alexandra Kassis, and Janina Röckner for laboratory assistance and Marieke Rohde for helpful comments on an earlier version of the manuscript.
Present address of C. J. Dallmann: Department of Biological Cybernetics, Bielefeld University, Universitätsstrasse 25, 33615 Bielefeld, Germany.
REFERENCES
- Adams MJ, Johnson SA, Lefèvre P, Lévesque V, Hayward V, André T, Thonnard JL. Finger pad friction and its role in grip and touch. J R Soc Interface 10: 20120467, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti A. Categorial Data Analysis. New York: Wiley, 2002. [Google Scholar]
- Bensmaia SJ, Hollins M. The vibrations of texture. Somatosens Mot Res 20: 33–43, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanowski SJ, Gescheider GA, Verrillo RT, Checkosky CM. Four channels mediate the mechanical aspects of touch. J Acoust Soc Am 84: 1680–1694, 1988. [DOI] [PubMed] [Google Scholar]
- Delhaye B, Hayward V, Lefèvre P, Thonnard JL. Texture-induced vibrations in the forearm during tactile exploration. Front Behav Neurosci 6: 37, 1–10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dépeault A, Meftah EM, Chapman CE. Tactile speed scaling: contributions of time and space. J Neurophysiol 99: 1422–1434, 2008. [DOI] [PubMed] [Google Scholar]
- Dépeault A, Meftah EM, Chapman CE. Neuronal correlates of tactile speed in primary sensory cortex. J Neurophysiol 110: 1554–1566, 2013. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002. [DOI] [PubMed] [Google Scholar]
- Essick GK, Edin BB. Receptor encoding of moving tactile stimuli in humans. II. The mean response of individual low-threshold mechanoreceptors to motion across the receptive field. J Neurosci 15: 848–864, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essick GK, Franzen O, Whitsel BL. Discrimination and scaling of velocity of stimulus motion across the skin. Somatosens Mot Res 6: 21–40, 1988. [DOI] [PubMed] [Google Scholar]
- Fagiani R, Massi F, Chatelet E, Berthier Y, Sestieri A. Experimental analysis of friction-induced vibrations at the finger contact surface. Proc Inst Mech Eng Part J J Eng Tribol 224: 1027–1035, 2010. [Google Scholar]
- Ferrington DG, Nail BS, Rowe M. Human tactile detection thresholds: modification by inputs from specific tactile receptor classes. J Physiol 272: 415–433, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AW, Johnson KO. Cutaneous mechanoreceptors in macaque monkey: temporal discharge patterns evoked by vibration, and a receptor model. J Physiol 323: 21–41, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi M, Ernst MO, Buss M. Integration of kinesthetic and tactile display-a modular design concept. In: Proc Eurohaptics 2006. Paris, France: EuroHaptics Society, 2006, p. 607–612. [Google Scholar]
- Gardner EP, Kandel ER. Touch. In: Principles of Neural Science, edited by Kandel ER, Schwartz JH, Jessell TM. New York: McGraw-Hill, 2000, p. 451–471. [Google Scholar]
- Goodwin AW, John KT, Sathian K, Darian-Smith I. Spatial and temporal factors determining afferent fiber responses to a grating moving sinusoidally over the monkey's fingerpad. J Neurosci 9: 1280–1293, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AW, Morley JW. Sinusoidal movement of a grating across the monkey's fingerpad: representation of grating and movement features in afferent fiber responses. J Neurosci 7: 2168–2180, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Saal HP, Dammann JF 3rd, Bensmaia SJ. Multiplexing stimulus information through rate and temporal codes in primate somatosensory cortex. PLoS Biol 11: e1001558, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins M, Bensmaia SJ, Washburn S. Vibrotactile adaptation impairs discrimination of fine, but not coarse, textures. Somatosens Mot Res 18: 253–262, 2001. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70, 1979. [Google Scholar]
- Johansson RS, Landström U, Lundström R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244: 17–25, 1982. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol 286: 283–300, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Bengtsson F, Geborek P, Spanne A, Terekhov AV, Hayward V. Segregation of tactile input features in neurons of the cuneate nucleus. Neuron 83: 1444–1452, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzky RL, Lederman SJ. Tactile roughness perception with a rigid link interposed between skin and surface. Percept Psychophys 61: 591–607, 1999. [DOI] [PubMed] [Google Scholar]
- Lamb GD. Tactile discrimination of textured surfaces: peripheral neural coding in the monkey. J Physiol 338: 567–587, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Whitehouse J. Tactile detection of a dot on a smooth surface: peripheral neural events. J Neurophysiol 56: 1109–1128, 1986. [DOI] [PubMed] [Google Scholar]
- Moscatelli A, Mezzetti M, Lacquaniti F. Modeling psychophysical data at the population-level: the generalized linear mixed model. J Vis 12: pii: 26, 2012. [DOI] [PubMed] [Google Scholar]
- Moscatelli A, Naceri A, Ernst MO. Path integration in tactile perception of shapes. Behav Brain Res 274: 355–364, 2014. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, LaMotte RH, Carli G. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J Neurophysiol 35: 122–136, 1972. [DOI] [PubMed] [Google Scholar]
- Pei YC, Bensmaia SJ. The neural basis of tactile motion perception. J Neurophysiol 112: 3023–3032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal HP, Bensmaia SJ. Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci 37: 689–697, 2014. [DOI] [PubMed] [Google Scholar]
- Srinivasan MA, Whitehouse JM, LaMotte RH. Tactile detection of slip: surface microgeometry and peripheral neural codes. J Neurophysiol 63: 1323–1332, 1990. [DOI] [PubMed] [Google Scholar]
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31: 301–334, 1968. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol 3: 3–14, 1984. [PubMed] [Google Scholar]
- Weber AI, Saal HP, Lieber JD, Cheng JW, Manfredi LR, Dammann IIIJF, Bensmaia SJ. Spatial and temporal codes mediate the tactile perception of natural textures. Proc Natl Acad Sci USA 110: 17107–17112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody NS, Mahns DA, Taylor JL, Gandevia SC. Impairment of human proprioception by high-frequency cutaneous vibration. J Physiol 581: 971–980, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HY, Hayward V. An experiment on length perception with a virtual rolling stone. In: Proc Eurohaptics 2006. Paris, France: EuroHaptics Society, 2006, p. 325–330. [Google Scholar]