Abstract
Although anatomically well described, the functional role of the mammalian efferent vestibular system (EVS) remains unclear. Unlike in fish and reptiles, the mammalian EVS does not seem to play a role in modulation of primary afferent activity in anticipation of active head movements. However, it could play a role in modulating long-term mechanisms requiring plasticity such as vestibular adaptation. We measured the efficacy of vestibuloocular reflex (VOR) adaptation in α9-knockout mice. These mice carry a missense mutation of the gene encoding the α9 nicotinic acetylcholine receptor (nAChR) subunit. The α9 nAChR subunit is expressed in the vestibular and auditory periphery, and its loss of function could compromise peripheral input from the predominantly cholinergic EVS. We measured the VOR gain (eye velocity/head velocity) in 26 α9-knockout mice and 27 cba129 control mice. Mice were randomly assigned to one of three groups: gain-increase adaptation (1.5×), gain-decrease adaptation (0.5×), or no adaptation (baseline, 1×). After adaptation training (horizontal rotations at 0.5 Hz with peak velocity 20°/s), we measured the sinusoidal (0.2–10 Hz, 20–100°/s) and transient (1,500–6,000°/s2) VOR in complete darkness. α9-Knockout mice had significantly lower baseline gains compared with control mice. This difference increased with stimulus frequency (∼5% <1 Hz to ∼25% >1 Hz). Moreover, vestibular adaptation (difference in VOR gain of gain-increase and gain-decrease adaptation groups as % of gain increase) was significantly reduced in α9-knockout mice (17%) compared with control mice (53%), a reduction of ∼70%. Our results show that the loss of α9 nAChRs moderately affects the VOR but severely affects VOR adaptation, suggesting that the EVS plays a crucial role in vestibular plasticity.
Keywords: efferent vestibular system, vestibular adaptation, vestibular plasticity, vestibuloocular reflex, α9-knockout mice
despite considerable effort, the functional role of the mammalian efferent vestibular system (EVS) is poorly understood. Anatomically, the EVS is a well-documented and extensive efferent pathway from the brain stem to the inner ear that can modify afferent output of the peripheral vestibular organs (Gacek and Lyon 1974; Goldberg and Fernandez 1980; Highstein 1991; Marco et al. 1993; Purcell and Perachio 1997). In some nonmammalian species, i.e., fish and reptiles, the EVS prepares the vestibular organs in anticipation of active head movements with gaze shift, presumably in an effort to suppress the vestibuloocular reflex (VOR) response (Brichta and Goldberg 2000; Highstein 1991; Highstein and Baker 1985). However, this does not seem to be the role of the mammalian EVS (Cullen and Minor 2002; Sadeghi et al. 2007). A study by Cullen and Minor (2002) showed that the resting discharge rate and rotational sensitivity of semicircular canal afferents during different conditions of head and eye movement did not change, suggesting that the mammalian EVS did not play a role in modifying primary vestibular afferent signals over the short time course of their experiment.
Two findings support the hypothesis that the mammalian EVS modulates afferent activity during longer processes requiring plasticity, such as vestibular adaptation and compensation. First, contralateral and ipsilateral vestibular efferent neurons have extensive collateral projections to cerebellar flocculus and ventral paraflocculus, two regions known to be crucial for vestibular plasticity (Shinder et al. 2001). Second, changes have been observed in the proportion of tonic vs. phasic central vestibular neurons after unilateral labyrinthectomy, consistent with an increase in the proportion of irregular- to regular-discharging afferents (Beraneck et al. 2003, 2004; Beraneck and Idoux 2012; Cullen et al. 2009; Pfanzelt et al. 2008; Sadeghi et al. 2007; Straka et al. 2005). A prime candidate driving these peripheral changes could be the EVS (Sadeghi et al. 2007).
Minor et al. (1999) suggested that vestibular signal processing predominantly occurs along two pathways: a velocity-sensitive pathway with tonic dynamics and an acceleration-sensitive pathway with phasic dynamics (Clendaniel et al. 2001, 2002; Lasker et al. 1999, 2000; Migliaccio et al. 2003, 2004, 2008; Minor et al. 1999). This model is based on primate data and has been shown to account for the normal VOR response (Migliaccio et al. 2003; Minor et al. 1999), VOR compensation (Lasker et al. 1999, 2000), VOR adaptation (Clendaniel et al. 2001, 2002), and VOR viewing-context modification (Migliaccio et al. 2004, 2008). These studies demonstrated that VOR changes were predominantly mediated by the highly modifiable phasic pathway. The dynamic responses (sensitivity and latency) of these tonic and phasic pathways resemble those of regular- and irregular-discharging vestibular primary afferents (Hullar et al. 2005; Hullar and Minor 1999) as well as vestibular nuclei neurons (Dickman and Angelaki 2004). If the mammalian EVS modulates the relative contribution of the phasic pathway by controlling the proportion of regular/irregular activity, then it could directly control vestibular plasticity. The mammalian EVS was shown to exert its largest effects on irregular-discharging (phasic) afferents, which is consistent with this notion (Goldberg and Fernandez 1980; Marlinski et al. 2004).
We hypothesize that a compromised mammalian EVS results in reduced vestibular plasticity, so we tested VOR adaptation in the α9-knockout mouse. This knockout strain carries a missense mutation of the gene encoding the α9 nicotinic acetylcholine (ACh) receptor (nAChR) subunit found in efferent synapses, which alters peripheral input from the EVS. Apart from a compromised EVS, these mice have no other obvious phenotype and show no difficulties with balance, movement, or vision (Terreros et al. 2015; Vetter et al. 1999). Previous studies have shown that 1) α9 nAChR subunits are specifically expressed in the vestibular and auditory periphery, 2) ACh is the predominant neurotransmitter of vestibular efferents and functions by activating nicotinic receptors (nAChRs) located in the vestibular periphery, and 3) ACh can induce inhibition and/or excitation of vestibular afferents (Anderson et al. 1997; Elgoyhen et al. 1994; Hiel et al. 1996; Holt et al. 2001; Jagger et al. 2000; Katz et al. 2000; Vetter et al. 1999; Zhou et al. 2013). For a review about efferent synaptic mechanisms see Jordan et al. (2013). The cholinergic component of EVS activation likely functions by inhibition of type II hair cells (i.e., strictly a reduction of resting discharge rate and attenuation of sensitivity/gain) via α9 nAChRs coupled to calcium-activated potassium (SK) channels (Holt et al. 2006; Poppi et al. 2014) and excitation of afferents (Boyle and Highstein 1990; Goldberg and Fernandez 1980), through nAChRs that contain α4, α6, and β2 subunits (Holt et al. 2015). In α9-knockout mice this inhibition/excitation dual effect would be partially compromised. Nonfunctional α9 nAChRs would prevent EVS inhibition of type II hair cells. However, because of the presence of alternative types of nAChRs (i.e., α4, α6, and β2 subunits) on calyx-bearing afferents, the excitatory afferent effect could still operate. Our results suggest that the loss of the α9 nAChR subunit moderately affects the VOR but severely affects VOR adaptation, suggesting that the EVS plays a crucial role in vestibular plasticity.
MATERIALS AND METHODS
Animal groups and surgical preparation.
We obtained data from 26 α9-knockout mice and 27 control mice (both sexes, aged 11–14 wk). The mouse strain carrying the α9-knockout mutation has been maintained on a CBA/CaJ × 129/SvEv background line by The Jackson Laboratories (stock no. 005696). We set up an independent colony of hybrid CBA/CaJ × 129/SvEv (here referred to as cba129) mice that we used as the controls. When the homozygous α9-knockout breeders became old, new breeders were selected from different heterozygous (cba129 × α9-knockout) breeding pairs. Both α9-knockout and cba129 mice were randomly assigned to one of three groups: 1) no prior adaptation (i.e., normal gain ≈ 1), 2) gain-increase adaptation (gain = 1.5), or 3) gain-decrease adaptation (gain = 0.5). The baseline VOR response (no prior adaptation) was measured in 22 mice: 11 α9-knockout and 11 cba129 mice. Postadaptation data were acquired from a total of 31 mice, which contributed to one of four groups: cba129-gain increase (n = 8), cba129-gain decrease (n = 8), α9-gain increase (n = 7), and α9-gain decrease (n = 8).
To facilitate head immobilization during VOR recording, we implanted a pedestal onto the skull of each animal on the day of the experiment. The exact implantation technique has been described previously (Hübner et al. 2013; Migliaccio et al. 2005, 2010a). In short, we anesthetized mice with general inhalation anesthesia (isoflurane 2–4%). While mice were under anesthesia we made a midline incision to expose the skull from bregma to lambda. We stripped the periosteum and dried the bony surface with sterile cotton buds. We then drilled three guide holes into the skull (2 lateral of bregma and 1 lateral of lambda) and inserted three stainless steel anchoring screws (no. 0 × 1/8, Micro Fasteners, Thomastown, VIC, Australia). A lightweight countersunk metal screw was placed with the flat head down between the three anchoring screws, and all were embedded in a thick layer of dental composite (Protemp IV, 3M). Also, while mice were still under anesthesia, we shortened eyelashes and vibrissae to minimize irritation and to facilitate placement of the marker arrays onto the eyes immediately prior to VOR testing and after VOR adaptation training. After surgery animals were allowed to recover for 2 h in a separate cage before they were restrained and placed on the rotator platform.
All surgical and experimental procedures were approved by the Animal Care and Ethics Committee of the University of New South Wales.
Adaptation and eye movement recording.
Upon full recovery, mice were restrained in a close-fitting plastic capsule and both capsule and animal were mounted on a rotary platform driven by a high-torque servomotor (GOLDLINE DDR D083, Danaher). The head pedestal was pitched ∼30° “nose down” so that the rotation of the servomotor maximally stimulated the horizontal semicircular canals (Calabrese and Hullar 2006). To evoke adaptation of the VOR we used a custom-built planetarium projector system, which projected a random pattern of light spots onto a dome surrounding the animal. The projector unit was driven by a small high-resolution servomotor, which was synchronized with the rotary platform with an electronic gearing system (latency < 0.1 ms). This system has been successfully used in previous adaptation studies in our laboratory (see Hübner et al. 2014).
For VOR adaptation we chose a sinusoidal vestibular stimulus at 0.5 Hz with peak velocity of 20°/s. These parameters were chosen as optimal based on our experience and reports from other laboratories (De Zeeuw et al. 1998; Kimpo et al. 2005). The visual projector was set to rotate in the opposite direction of the vestibular stimulus with amplitude of 1.5× (gain increase) and 0.5× (gain decrease) of the vestibular stimulus velocity. We kept adaptation training to one 40-min session.
After VOR adaptation was completed, we measured VOR gain in complete darkness with a binocular three-dimensional video-oculography system (Hübner et al. 2013, 2014; Migliaccio et al. 2005, 2010a). To facilitate recording we placed marker arrays onto both eyes, which allowed us to accurately measure VOR eye movement components in all three dimensions: horizontal, vertical, and torsional. Because the marker arrays are affixed to the eye with cyanoacrylate, removal causes temporary corneal swelling that likely affects vision. To ensure ideal vision during adaptation training we recorded preadaptation VOR gains in a separate group of mice, rather than comparing pre- and postadaptation gains within the same mouse.
We tested the VOR in response to horizontal whole body: sinusoidal oscillation at 0.2, 0.4, 0.5, 0.8, 1, 1.6, 2, 5, and 10 Hz with peak velocities of 20, 50, and 100°/s and transient acceleration stimuli at 1,500, 3,000, and 6,000°/s2 reaching a velocity plateau of 100, 150, and 300°/s, respectively. We refer to transient stimulus conditions with the abbreviations 1.5k100, 3k150, and 6k300, respectively.
Data analysis.
To analyze the three-dimensional VOR data, we converted eye movements acquired in eye coordinates into rotation vectors in head coordinates. Eye velocity traces with quick phases removed were inverted so that an ideal VOR would yield a gain (eye velocity/head velocity) of +1 and phase of 0°. Positive phase lead denotes eye velocity leading head velocity. The methods of analysis are similar to those that have been described previously (Hübner et al. 2013, 2014; Migliaccio et al. 2010a, 2010b). Unless otherwise stated, all results are reported as means ± 1 SD.
For sinusoidal rotations, we fit individual cycles of head and eye velocity (10–100, depending on frequency) using least-square pure sine waves with fixed frequency and variable amplitude and phase. VOR gain was then calculated as the average ratio of eye/head velocity peak amplitude of the least-square fits. To analyze the effects caused by changes in stimulus acceleration we calculated the first derivative of the eye and head velocity signal for each stimulus frequency-velocity combination. Peak accelerations that occurred at all three test stimulus peak velocities (20, 50, and 100°/s, at respective frequencies) were compared.
For transient steps of acceleration, we fit least-square linear regressions to the constant-acceleration and constant-velocity part of eye and head velocity traces. Using these fits, we calculated three parameters: acceleration gain (GA), constant-velocity gain (GV), and latency. GA was calculated as the average ratio of eye/head acceleration (using the slopes of the constant-acceleration fit). GV was calculated as the average ratio of eye/head velocity (using the point-by-point offset of the constant-velocity fit) during the 200 ms to 400 ms interval after stimulus onset.
Quick-phase eye movements are closely related to saccadic eye movements, and so their duration and peak velocity can be related to their amplitude to generate a “main sequence” (Stahl et al. 2006). We analyzed quick phases for sinusoidal stimuli at 0.2, 0.4, 0.5, 0.8, and 1 Hz at 20, 50, and 100°/s. Quick phases were either removed or extracted with a semiautomatic desaccading technique that we optimized based on several published algorithms (Faucheux et al. 2007; Migliaccio et al. 2006). However, instead of manually adjusting the acceleration threshold for each individual trace, we automated the process by fixing the acceleration threshold parameter to 5,000°/s2. Amplitude was defined as the difference in horizontal eye position between the quick-phase start and end. Peak velocity was defined as the maxima of the horizontal eye velocity trace during the quick phase. Because quick-phase peak velocity and amplitude histograms were Weibull distributions, we report the median in addition to the mean quick-phase peak velocity and amplitude. The relationship between peak velocity and amplitude was determined by a least-square linear fit. The relationship between amplitude and duration was determined by a nonlinear regression fit of the form
where D stands for quick-phase duration and A for quick-phase amplitude. K and τ are fit constants for the exponential equation and represent the saturated duration and nonlinear component of the fit, respectively (Sakatani and Isa 2007).
Analysis of adaptation selectivity.
Similar to previous studies (Hübner et al. 2014), we compared VOR adaptation selectivity between gain-increase and gain-decrease adaptation training by calculating a generalization index across frequencies and velocities. The generalization index was defined as the fraction of adaptive gain change when the testing stimulus frequency and velocity matched the adaptation training stimulus, compared with the average adaptation at all other testing stimulus frequencies and velocities.
Δgaintraining represents the amplitude of VOR gain adaptation at the same frequency/velocity used during adaptation training. Δgaini represents the amplitude of VOR gain adaptation at one of j stimulus frequencies/velocities other than the adaptation training frequency/velocity (e.g., for adaptation training peak velocity 20°/s, i = {50; 100}°/s, j = 2). A generalization index close or equal to 1 (Δgaini ≈ Δgaintraining) indicates broad generalization of VOR gain adaptation, while a generalization index close or equal to 0 indicates stimulus-specific VOR adaptation.
Statistical analysis.
Because each mouse only contributed to one group (gain increase, gain decrease, or gain baseline) it was not possible to perform a pairwise analysis of adaptation within mice. Instead we used a multivariate ANOVA with mouse strain (α9-knockout and cba129) and adaptation type (gain increase, gain decrease, and gain baseline) as main factors. For sinusoidal rotations, additional independent factors were frequency, peak velocity, and peak acceleration and dependent factors were gain and phase. For transient steps of acceleration, an additional independent factor was stimulus (1.5k100, 3k150, 6k300) and dependent factors were GA and GV. For quick-phase analysis, an additional independent factor was stimulus (0.2 Hz, 0.4 Hz, 0.5 Hz, 0.8 Hz, 1 Hz) and dependent factors were quick-phase amplitude, peak velocity, and duration. All variables were included in the model initially, and those found insignificant were subsequently removed. Post hoc tests were performed with t-tests with multiple-comparison correction in case of significant ANOVA results. Probability values ≥ 0.001 are reported with numeric value; the rest are reported as <0.001. Unless otherwise stated all results are reported as means ± SD.
RESULTS
Baseline VOR response to sinusoidal rotation.
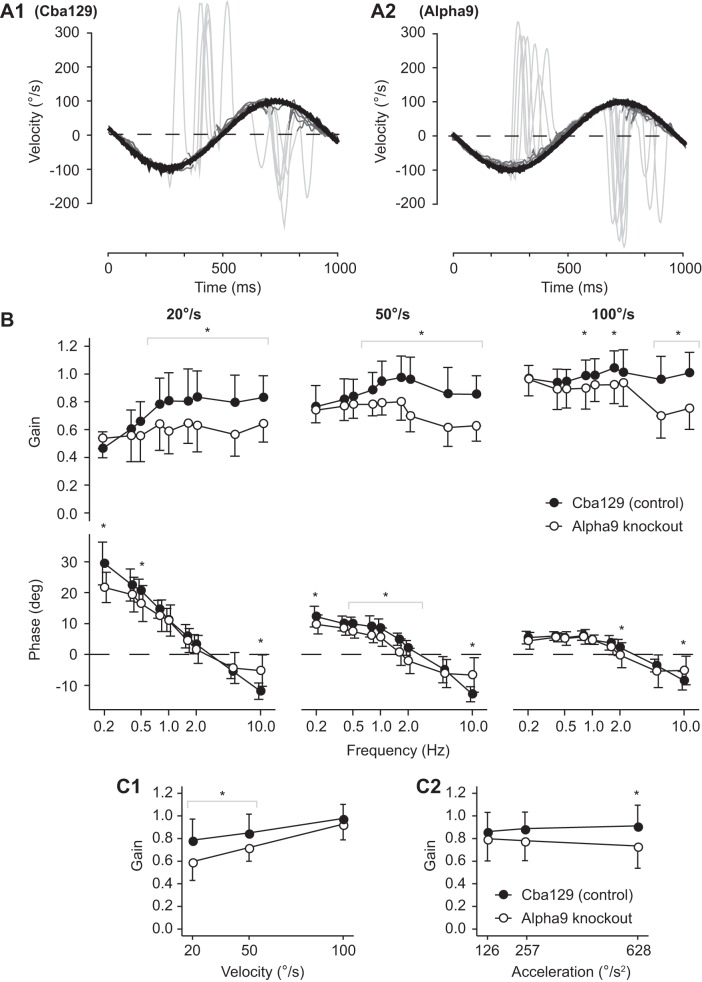
Figure 1, A1 and A2, show superimposed VOR responses to sinusoidal rotations (1 Hz at 100°/s) for one cba129 (control) mouse and one α9-knockout mouse prior to adaptation training. We measured the baseline VOR response (no prior adaptation) in α9-knockout and cba129 mice. Figure 1B shows a direct comparison of cba129 and α9-knockout baseline VOR gain (top) and phase (bottom) across frequencies 0.2–10 Hz and velocities of 20, 50, and 100°/s. For both mouse types the VOR gain significantly increased with stimulus peak velocity [F(1,335) = 547.95, P < 0.001]. In cba129 mice the average VOR gain at 20°/s pooled across frequencies was 0.73 ± 0.21, while at 100°/s the average VOR gain was 0.98 ± 0.13. α9-Knockout mice demonstrated a similar increase in VOR gain with increasing stimulus peak velocity, with average 0.59 ± 0.17 and 0.87 ± 0.16 at 20 and 100°/s, respectively.
Fig. 1.
A: the vestibuloocular reflex (VOR) response to sinusoidal rotations (1 Hz at 100°/s) for 1 cba129 (control) mouse (A1) and 1 α9-knockout mouse (A2) prior to adaptation training. Eye velocity (dark gray) is inverted to allow easier comparison with head velocity. For each stimulus frequency 10–100 individual cycles were superimposed and quick-phase eye movements (light gray) were removed. Least-square pure sine waves were fit to the head velocity stimuli and eye velocity responses to calculate VOR gain and phase. B: baseline VOR gain (top) and phase (bottom) in cba129 control mice and α9-knockout mice. The VOR was measured for frequencies from 0.2 to 10 Hz at peak-velocities of 20, 50, and 100°/s. Error bars indicate mean ± SE. Unlike cba129, α9-knockout mice did not show a characteristic increase in VOR gain for frequencies < 1 Hz at peak velocity of 20 and 50°/s. In addition, α9-knockout mice had significantly lower VOR gains at frequencies > 1 Hz, at all 3 test peak velocities. VOR phase in α9-knockout mice significantly differed from cba129 at low and high frequency extremes. At frequencies < 0.8 Hz at peak velocity 20°/s VOR phase was less than in control mice, while at 10 Hz at all 3 test peak velocities VOR phase was more than in control mice. C: comparison of baseline VOR gain across sinusoidal test stimulus peak velocities (C1) and peak accelerations (C2). Peak acceleration was calculated using the 1st derivative of the velocity stimulus profile. Unlike cba129 mice, the VOR gain decreased as stimulus peak acceleration increased in α9-knockout mice, whereas the VOR gain increased as peak velocity increased in both mouse types but more so in α9-knockout mice. *Significant difference between mouse type baseline gains or phases.
In cba129 mice the VOR gain in response to vestibular stimulation at 20°/s (Fig. 1 B, top left) showed a steady increase with frequency for frequencies <1 Hz. At 0.2 and 1 Hz the VOR gains were 0.46 ± 0.12 and 0.81 ± 0.2, respectively [t(25.56) = −6.08, P < 0.001]. In contrast, α9-knockout mice showed almost no change in VOR gain, with gains of 0.54 ± 0.14 and 0.59 ± 0.16 at 0.2 and 1 Hz, respectively [t(25.94) = −0.91, P = 0.37]. This absence of gain increase with stimulus frequency was also observed when the vestibular stimulus peak velocity was 50°/s. While the VOR gain of cba129 mice climbed from 0.77 ± 0.15 at 0.2 Hz to a maximum of 0.97 ± 0.16 at 1.6 Hz, the VOR gain of α9-knockout mice stayed at 0.74 ± 0.09 and 0.8 ± 0.13 at 0.2 and 1.6 Hz, respectively.
In addition to the difference in VOR gains between mouse types over the 0.2–1 Hz frequency range, α9-knockout mice had significantly lower VOR gains than cba129 mice at frequencies >1 Hz. At 20°/s the VOR gain in α9-knockout mice remained low over the whole range of test frequencies (0.2–10 Hz). In comparison, at 50 and 100°/s there was a frequency threshold of 1.6–2 Hz above which a pronounced VOR gain reduction in α9-knockout mice became apparent (see Fig. 1B, top). The average difference between cba129 and α9-knockout mice at frequencies >2 Hz was 0.23 ± 0.02 (a 25% difference) [t(194.56) = 10, P < 0.001].
A difference between cba129 and α9-knockout mice was also observed when baseline VOR gains were compared across stimulus peak velocities (Fig. 1C1) and peak accelerations (Fig. 1C2). VOR gains of both mouse types increased as peak velocity increased. This effect was more pronounced in α9-knockout compared with cba129 mice. Peak acceleration did not affect VOR gains in cba129 mice but had an effect on the VOR gain of α9-knockout mice. The latter showed significantly decreased VOR gain as peak stimulus acceleration increased.
While the general phase response was similar between cba129 and α9-knockout mice, there were differences in VOR phase at both low and high frequency extremes (see Fig. 1 B, bottom). For stimulus frequencies <0.8 Hz with stimulus peak velocity 20°/s there was a clear decrease in the phase lead of α9-knockout mice. This decrease was most pronounced at 0.2 Hz, the lowest frequency tested, with a phase lead of 29.44 ± 6.93° in cba129 mice vs. 21.70 ± 4.88° in α9-knockout mice [t(32.08) = 3.86, P < 0.001]. A similar effect was observed at 10 Hz at all three test stimulus peak velocities. cba129 mice showed a steadily increasing phase lag with zero-phase crossover at ∼3 Hz and a maximum phase lag of −11.01 ± 3.25° at 10 Hz. α9-knockout mice also demonstrated increasing phase lag with frequency with zero-phase crossover at ∼2 Hz, but the maximum phase lag was only −5.68 ± 5.06° at 10 Hz. This difference of −5.33 ± 0.81° was statistically significant [t(96.56) = −6.58, P < 0.001].
Baseline VOR response to transient steps of acceleration.
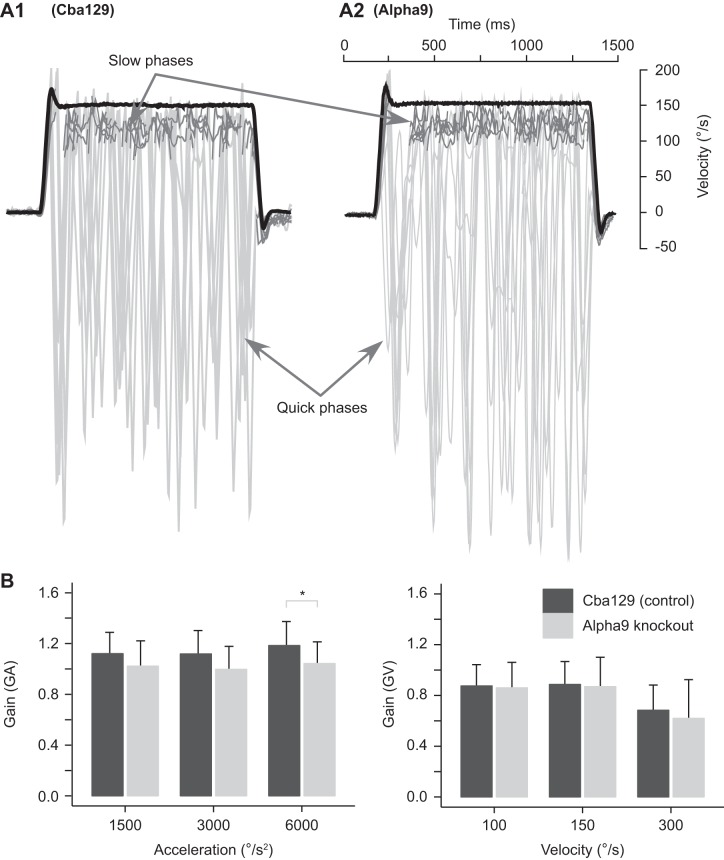
Figure 2A shows horizontal VOR response to transient high-acceleration whole body rotations (acceleration 1.5k and velocity 100°/s) for one cba129 mouse and one α9-knockout mouse prior to adaptation training. We measured the baseline acceleration gain (GAPre) during the initial “constant-acceleration part” of transient step stimuli. cba129 mice had an average GAPre of 1.14 ± 0.19. In contrast, the average GAPre of α9-knockout mice was significantly lower at 1.02 ± 0.21 [F(1,24) = 4.445, P = 0.037] (see Fig. 2B, left). This difference in GAPre between mouse strains was similar for all three stimulus accelerations [interaction “Strain × Acceleration”: F(2,48) = 0.222, P = 0.802].
Fig. 2.
Baseline VOR in response to transient steps of acceleration. A: example of overlaid slow-phase eye velocities (inverted; gray) in response to sudden steps of acceleration (1,500°/s2) followed by a constant velocity plateau (100°/s) in 1 cba129 (control) mouse (A1) and 1 α9-knockout mouse (A2). Individual VOR gains were measured during the constant velocity part and constant acceleration part of the stimulus. Latency of the VOR response was determined with the zero velocity intercept of head acceleration and eye acceleration linear fits. B: acceleration gain (GA; left) and velocity gain (GV; right) in response to 3 acceleration and velocity stimuli. α9-Knockout mice demonstrated significantly lower GA compared with cba129 mice at 6,000°/s2. No difference between mouse types was observed for GV. *Significant difference between mouse type baseline gains.
We also analyzed the preadaptation VOR gain response during the “constant velocity plateau” (GVPre) of transient step stimuli. There was no difference in GVPre between α9-knockout and cba129 mice [factor “Species”: F(1,24) = 2.275, P = 0.144]. Also, the interaction between mouse strain and velocity was not significant [interaction “Strain × Velocity”: F(1,50) = 0.628, P = 0.432]. The average GVPre of cba129 and α9-knockout mice was 0.82 ± 0.2 and 0.79 ± 0.27, respectively. GVPre was similar for stimulus velocities of 100 and 150°/s, with an average gain of 0.87 ± 0.2 (pooled α9-knockout and cba129 mice), but decreased to 0.65 ± 0.26 when tested at 300°/s.
VOR quick-phase main sequence.
Quick-phase eye movements were typically observed during sinusoidal frequencies ≤1 Hz. Figure 1A shows superimposed quick-phase responses to sinusoidal rotations (1 Hz at 100°/s) for one cba129 (control) mouse and one α9-knockout mouse prior to adaptation training.
There was no difference in the number of quick phases per stimulus cycle between α9-knockout and cba129 mice [F(1,29) = 3.83, P = 0.063]. The mean quick-phase amplitude for cba129 and α9-knockout mice was ∼8°, showing no significant difference [t(1798) = 1.676, P = 0.094]. The mean quick-phase peak velocity for cba129 and α9-knockout mice across stimulus conditions was ∼450°/s with median ∼400°/s, showing no significant difference [t(1798) = 0.0502, P = 0.960]. The slope of the linear fit between quick-phase peak velocity and amplitude for cba129 and α9-knockout mice was not significant [t(1798) = −1.11, P = 0.268].
In cba129 mice the mean quick-phase duration was 45.36 ± 22.83 ms, whereas for α9-knockout mice it was ∼10% faster at 41.12 ± 20.93 ms [t(1798) = 4.073, P < 0.001]. The K and τ terms in the nonlinear fit between quick-phase duration and amplitude were similar between cba129 and α9-knockout mice [K: t(1798) = 0.43, P = 0.671; τ: t(1798) = −0.34, P = 0.734].
Sinusoidal VOR response following adaptation training.
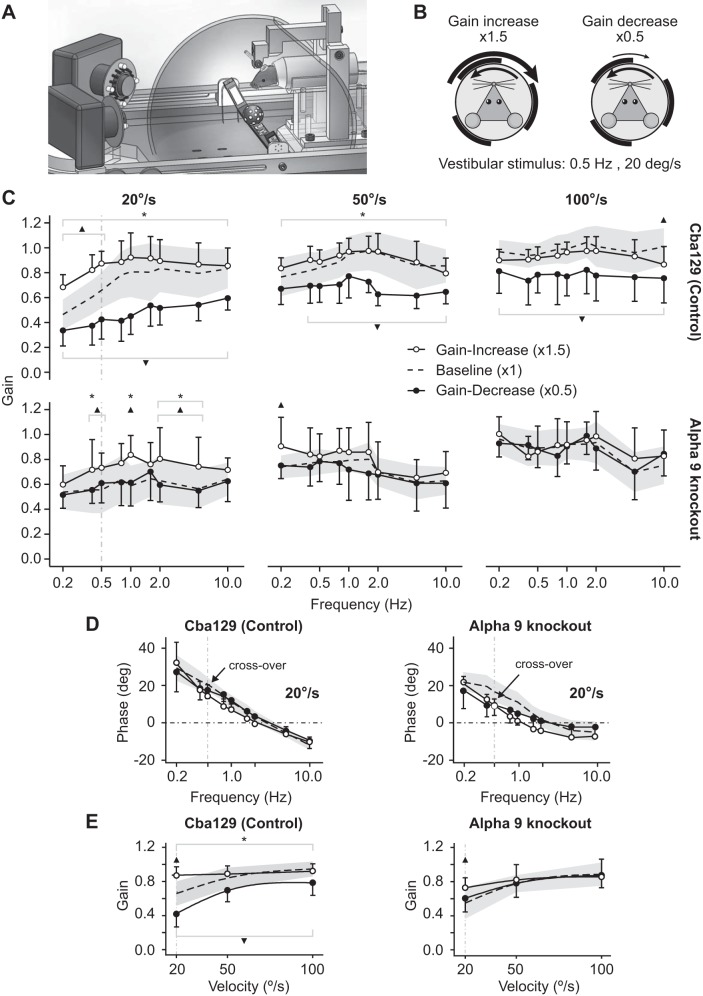
We measured the VOR gain response after 40 min of horizontal sinusoidal gain-increase (1.5×) and gain-decrease (0.5×) visual-vestibular adaptation training with a vestibular stimulus of 0.5 Hz and 20°/s (see Fig. 3, A and B). Figure 3C displays the postadaptation VOR gains for cba129 (Fig. 3C, top) and α9-knockout mice (Fig. 3C, bottom) across frequencies from 0.2 to 10 Hz with peak velocities of 20, 50, and 100°/s. cba129 mice exhibited robust adaptation across all stimulus conditions [F(1,419) = 8.82, P = 0.003]. The adaptation effect for each test peak velocity, calculated as the difference between gain-increase vs. gain-decrease adaptation pooled across frequencies, was maximal at 0.4 ± 0.02 when the test peak velocity matched the adaptation training peak velocity of 20°/s. In contrast, for test peak velocities of 50 and 100°/s the adaptation training only averaged 0.22 ± 0.02 and 0.15 ± 0.02, respectively. This velocity-selective training effect was evident in our statistical model as a highly significant interaction between “Training” and “Velocity” factors [F(1,454) = 69.87, P < 0.001].
Fig. 3.
VOR response following visual-vestibular mismatch adaptation. A: a custom-built planetarium projector unit mounted beneath the head of the mouse was used to project a pattern of random light spots onto a surrounding dome surface. After adaptation this dome was removed and 2 high-speed cameras were used to record binocular 3D eye movements. B: the projected light spots moved synchronously with the head but in the opposite direction. During gain-increase and gain-decrease adaptation the angular velocity of the projected light spots was set to 1.5 × and 0.5 × the head velocity stimulus. C: both cba129 (top) and α9-knockout mice (bottom) demonstrated maximal adaptation at 20°/s, the velocity used during adaptation training. However, in α9-knockout mice the overall adaptation effect (difference between gain-increase vs. gain-decrease VOR gains) was ∼70% smaller compared with cba129. This was true across all test stimulus frequencies and velocities. D: VOR phase in both mouse types showed a crossover between gain-increase and gain-decrease responses at 0.5 Hz and 20°/s, the same stimulus parameters used during adaptation training (black arrow). E: comparison of gain-increase (○) and gain-decrease (●) VOR gains across all test velocities (frequency pooled). The baseline VOR response is illustrated as a dashed line (mean) surrounded by gray shading (SD). cba129 mice showed strong velocity-selective VOR adaptation. At 20°/s the effect of adaptation (difference between gain-increase vs. gain-decrease VOR gains) was maximal. As velocity increased the effect of adaptation continuously decreased. Similar velocity selective effects were also observed in α9-knockout mice. However, in α9-knockout mice the adaptation effect was minimal at 20°/s and disappeared at higher velocities. *Significant difference between post gain-decrease and post gain-increase adaptation training gain. ▲Significant difference between baseline gain and post gain-increase adaptation training gain. ▼Significant difference between baseline gain and post gain-decrease adaptation training gain.
Velocity- and frequency-selective effects were analyzed separately for gain-increase and gain-decrease adaptation. Velocity selectivity was most evident for gain-increase adaptation. The generalization index across velocities, a measure of the degree of velocity selectivity, was 0.03 ± 0.26 (highly selective) for gain-increase adaptation and 0.77 ± 0.4 (generalized across velocities) for gain-decrease adaptation [t(11.85)= −4.22, P = 0.001]. Velocity selectivity during gain-increase adaptation is best seen in Fig. 3E. At 20°/s, the VOR gain difference (adaptation effect) between the baseline and gain-increase VOR and the difference between the baseline and gain-decrease VOR were nearly equal in magnitude. No gain-increase adaptation training was observed at 50 and 100°/s [F(1,17) = 0.21, P = 0.65].
α9-Knockout mice, compared with cba129 mice (controls), showed significantly reduced adaptation across all test frequencies and velocities. At 0.5 Hz and 20°/s, the same stimulus used during adaptation training, we measured an overall effect of adaptation training of 0.45 ± 0.05 in cba129 mice but only 0.12 ± 0.07 in α9-knockout mice, a reduction of 0.33 (73%) [t(12.93) = −1.76, P = 0.10]. The average reduction at 20°/s when calculated across all tested frequencies was 0.26 (65%). Similar to cba129, α9-knockout mice demonstrated velocity-selective adaptation behavior. The largest overall adaptation effect was measured at 20°/s, which was the velocity used during adaptation training [F(1,13) = 3.90, P = 0.07]. Frequency did not affect adaptation [F(1,112) = 0.33, P = 0.57]. Overall adaptation was governed purely by gain-increase adaptation. No gain-decrease adaptation was observed at either velocity. At 50°/s overall adaptation was minimal at 0.11 ± 0.03 [F(1,13) = 1.03, P = 0.33], a reduction of 0.11 (50%) compared with cba129 mice. At 100°/s no adaptation occurred [F(1,13) = 0.09, P = 0.76]. The generalization index for gain-increase adaptation across test velocities was 0.49 ± 0.34, which is considerably more generalized compared with results in cba129 mice.
Unlike the VOR gain response, there was no significant difference in VOR phase between cba129 and α9-knockout mice [F(1,27) = 0.01, P = 0.93]. We did not observe an independent effect of adaptation training on VOR phase [F(1.27) = 1.56, P = 0.22]. However, for both mouse types there was a phase-crossover between their gain-increase and gain-decrease response curves at 0.5 Hz and 20°/s, the exact stimulus parameters used during adaptation training (Fig. 3D) [F(1,534) = 2.81, P = 0.094], which was not seen at stimulus peak velocities of 50 and 100°/s.
VOR response to transient steps of acceleration following adaptation training.
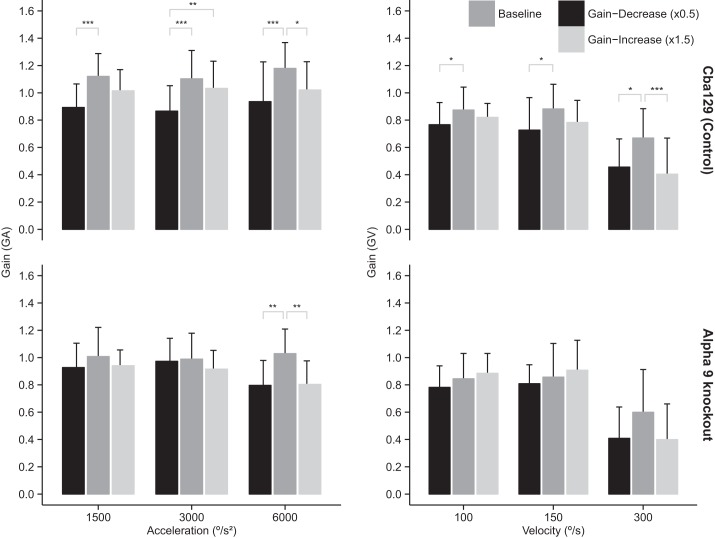
In cba129 mice we observed a pronounced adaptation effect for the acceleration gain GA for all three acceleration stimuli (see Fig. 4, top left). The average difference of GA between gain-increase vs. gain-decrease adaptation was 0.12 ± 0.03 [t(145.93) = −4.12, P < 0.001]. Post hoc pairwise comparison revealed that this difference was mostly due to gain-decrease adaptation resulting in GA significantly lower than baseline for all three acceleration stimuli (1.5k: P < 0.001, 3k: P < 0.001, 6k: P < 0.001). In contrast, α9-knockout mice demonstrated adaptation of GA compared with baseline only at the 6k acceleration stimulus (gain decrease: P < 0.01, gain increase: P < 0.01; Fig. 4, bottom left), with GA decreasing after both gain-decrease and gain-increase adaptation training. Analysis using ANOVA indicated that the interaction effect between “Mouse Strain” and “Adaptation Training” was significant [F(4,98) = 2.57, P = 0.043].
Fig. 4.
Acceleration gain (GA; left) and constant-velocity gain (GV; right) after sinusoidal visual-vestibular mismatch adaptation training. cba129 mice (top) demonstrated pronounced changes in GA after adaptation training with a significant effect of adaptation, measured as the difference between gain-increase and gain-decrease conditions. This difference was mostly due to gain-decrease adaptation resulting in significantly lower than baseline gains at all stimulus conditions. In contrast, α9-knockout mice (bottom) showed adaptation of GA only at 6k acceleration but none for GV at any stimulus condition. Significant difference: *P < 0.05, **P < 0.01, ***P < 0.001.
In cba129 mice we observed a pronounced adaptation effect for the velocity gain GV for all three velocity stimuli (Fig. 4, top right). Post hoc pairwise comparison revealed that this difference was mostly due to gain-decrease adaptation resulting in GV significantly lower than baseline for all three velocity stimuli (100: P < 0.05, 150: P < 0.05, 300: P < 0.05). In contrast, α9-knockout mice did not demonstrate adaptation of GV at any test velocity stimulus.
DISCUSSION
We sought to determine whether the EVS affects vestibular adaptation. Our findings suggest that the EVS has minimal to no effect on the oculomotor system, moderately affects the VOR gain, but severely affects VOR adaptation. The main sequence was similar between α9-knockout and control mice (cba129), suggesting that the quick-phase/saccadic oculomotor system is not affected by loss of α9 nAChR function. The α9-knockout mice had significantly lower baseline VOR gains (1×) compared with cba129 mice. This difference in gain widened with increasing stimulus frequency. At stimulus frequencies < 1 Hz the difference was ∼5% compared with ∼25% at frequencies > 1 Hz. These findings are consistent with a recent study showing that calcitonin gene-related peptide (CGRP)-knockout mice (CGRP is expressed in vestibular efferents and important for EVS function) also had a reduction (∼50%) in baseline VOR gain (Luebke et al. 2014). In contrast, the baseline VOR phase between α9-knockout and control mice was similar and did not change as a result of VOR adaptation training. The baseline VOR gain in the cba129 mouse was almost identical to that observed in the wild-type C57BL/6 mouse tested under similar conditions (Hübner et al. 2014). The capacity for vestibular adaptation, measured as the difference in mean VOR gains between gain-increase (1.5×) and gain-decrease (0.5×) adaptation training groups, was significantly smaller in α9-knockout mice compared with cba129. The VOR gains of cba129 mice ranged from ∼0.41 after gain-decrease adaptation to ∼0.88 after gain-increase adaptation, a difference of (0.47) ∼53%. In contrast, the VOR gains of α9-knockout mice ranged from ∼0.60 to ∼0.73, a difference of only (0.13) ∼17%. This represents a 70% reduction of vestibular adaptation in the α9-knockout mouse. Could this reduction be due to poor vision and/or poor generation of a retinal image slip signal to drive adaptation? We did not measure optokinetic function in these mice, but there are several lines of evidence suggesting that these mice have adequate vision and slip signal for adaptation. First, qualitatively these mice react to light and visual movement in the same way as other mice we have tested. The fact that the α9-knockout mice were able to perform a baseline visual discrimination task described by Terreros et al. (2015) just as well as the wild-type mice also suggests that they have normal vision. Second, the α9-knockout mice we tested did show adaptation, especially during gain-increase training when the test stimulus peak-velocity was 20°/s, suggesting they have an image slip signal large enough to drive adaptation. Third, we recently demonstrated in C57BL/6 mice that VOR gain changes as a result of adaptation training are maximal when the stimulus testing velocity matches the adaptation training velocity (Hübner et al. 2014). In the present study we observed similar velocity-selective adaptation in α9-knockout and cba129 mice. We also observed in both mouse types stronger velocity-selective adaptation for gain-increase compared with gain-decrease adaptation, which also matches our previous observations in the C57BL/6 mouse (Hübner et al. 2014).
Can changes in EVS activity affect the proportion of irregular- to regular-firing vestibular primary afferents?
It is important to note that dimorphic afferents account for ∼75–80% of all mammalian vestibular afferents and receive input from both type I and type II hair cells (Baird et al. 1988). As shown in turtles (Holt et al. 2006), and more recently in mammals (C57BL/6 and cba129 mice: Poppi et al. 2014), EVS activation, specifically the cholinergic component, is thought to have a dual effect. One effect of EVS activation is the inhibition of type II hair cells (i.e., strictly a reduction of resting discharge rate and attenuation of sensitivity/gain) via α9 nAChRs coupled to SK channels (Holt et al. 2006; Poppi et al. 2014). The other effect is the excitation of afferents (Boyle and Highstein 1990; Goldberg and Fernandez 1980), through nAChRs that contain α4, α6, and β2 subunits (Holt et al. 2015). In α9-knockout mice this inhibition/excitation dual effect would be partially compromised. Since α9 nAChRs are nonfunctional in these knockout mice, this would prevent EVS inhibition of type II hair cells seen in control mice. This lack of inhibition would allow the normally suppressed type II hair cells to “contribute” to the overall afferent activity (particularly dimorphs) during EVS activation in α9-knockout mice. Simultaneously, because of the presence of alternative types of nAChRs (i.e., α4, α6, and β2 subunits) on calyx-bearing afferents, the excitatory EVS effect would still be operating in α9-knockout mice. In short, the predicted overall effect of EVS activation on dimorphic afferents in α9-knockout mice is increased afferent discharge but with an additional input from normally EVS-suppressed type II hair cells (i.e., increased type II hair cell gain and resting discharge rate compared with controls) leading to an increase in afferent regularity and corresponding shift in afferent dynamic response. This hypothesis is supported by a preliminary single-unit vestibular afferent study in the α9-knockout mouse (Han et al. 2007) that compared the single-unit vestibular afferent response during 2-Hz whole body rotations between α9-knockout and C57BL/6 mice (note that C57BL/6 is not the appropriate control background strain for the α9 knockout). That study reported three major differences between these two mouse types. First, regularly (tonic) discharging afferents (defined as having a CV* < 0.1; CV* is the normalized coefficient of variation in the interspike interval of afferent background discharge) were more sensitive to head rotations, i.e., for the same head velocity the firing rates of regular discharging afferents were higher in α9-knockout compared with C57BL/6 mice. Second, irregularly (phasic) discharging afferents (defined as having a CV* > 0.1) were less sensitive to head rotations in α9-knockout compared with C57BL/6 mice. Third, the ratio of regularly vs. irregularly afferents was higher in the α9-knockout mouse.
Can changes in EVS activity affect central adaptation mechanisms?
In addition to the vestibular sensory neuroepithelium, EVS neurons project collaterals to the flocculus and ventral paraflocculus bilaterally (Shinder et al. 2001). These collateral projections suggest that the EVS may directly influence the part of the cerebellum involved with regulation and adaptation of the VOR, specifically, the Purkinje cells of the flocculus. Purkinje cells in the floccular lobe of the cerebellum receive information about head and eye movement through parallel fiber synapses and information about image motion through climbing fibers from the inferior olive and therefore are well positioned to sense and reduce VOR error resulting in retinal slip (Ito 1982), i.e., via the modifiable pathways involving the floccular target neurons (FTNs) (Lisberger and Pavelko 1986). A well-tested theory is that cerebellar learning depends on long-term depression (LTD) of synapses from parallel fibers onto Purkinje cells (for review see De Zeeuw and Yeo 2005; Gittis and du Lac 2006). Targeted genetic disruption of LTD in Purkinje cells has little effect on baseline oculomotor function but impairs short-term learning in the VOR that is induced by visual-vestibular mismatch training (de Zeeuw et al. 1998), such as ours, so a possibility is that the EVS affects LTD in Purkinje cells. It has also been proposed that the EVS could vary the proportion of irregular to regular afferent signal input going to the FTNs (Lisberger 1994), i.e., similar to the effect the EVS might have on the firing of vestibular primary afferents. Varying the signal going to the FTNs in this way could be important for encoding and processing signals across a wide range of stimuli, i.e., avoiding signal cutoff or saturation (Goldberg 2000; Shinder et al. 2001). In addition, the proportion of irregular to regular afferent signal might need to be modified under different behavioral circumstances (Chen-Huang and McCrea 1998) or to compensate for differences in the dynamic loads of the various reflexes (Boyle et al. 1992). Therefore, the difference in VOR adaptation between α9-knockout and control mice may be due to not only differing primary (peripheral) vestibular afferent signals but also changes in central adaptation mechanisms.
Can decrease in proportion of irregular- to regular-firing vestibular primary afferents explain decrease in baseline high-frequency VOR response and decrease in adaptation?
The observed differences in the baseline VOR response between α9-knockout and cba129 mice may be explained by differences in EVS activity, i.e., reduced EVS activity in the α9-knockout mouse leading to vestibular dimorphic afferents with discharge rate that is more regular (tonic). The nonlinear (phasic) and linear (tonic) components of the behavioral VOR can be considered as two pathways: one irregular, consisting of predominantly irregularly discharging afferents and mostly irregularly discharging (type B) medial vestibular neurons, the other regular, consisting of predominantly regularly discharging afferents and mostly regularly discharging (type A) medial vestibular neurons (e.g., Beraneck et al. 2004). This idea is supported by the observation that the transfer function of regularly discharging canal afferents fits well with the tonic component of the behavioral VOR that is predominantly dependent on stimulus (head) velocity (Minor et al. 1999). Similarly, the transfer function of irregularly discharging canal afferents fits well with the phasic component of the behavioral VOR that has a dependence on both stimulus velocity and frequency corresponding closer to head acceleration (Minor et al. 1999). It is this acceleration signal that causes the VOR gain to increase when the stimulus frequency, and consequently acceleration, increases (Minor and Lasker 2009). Thus the phasic pathway significantly “augments” the VOR response during high-acceleration stimuli, such as those with frequencies > 2 Hz. The phasic pathway may also contribute, albeit to a lesser extent, to the low-frequency VOR response; however, it has been shown that during low-frequency/amplitude sinusoids the contribution of irregularly discharging afferents is minimal (Minor and Goldberg 1991). If a compromised EVS (which we hypothesize is similar to an inhibited EVS) results in a decrease in the ratio and sensitivity of irregular-firing afferents leading to a decrease in the phasic pathway, then one would predict that the high-frequency VOR would be affected most, which is what we observed in the α9-knockout mouse. However, a decrease in the phasic pathway would minimally affect velocity selectivity, because velocity selectivity is likely to be mediated mostly by tonic (velocity sensitive) pathways (Hübner et al. 2014). This may explain why velocity selectivity in α9-knockout mice was similar to that in cba129 mice.
Cullen and Minor (2002) showed in primates that the resting discharge rate and rotational sensitivity of semicircular canal afferents do not change for different conditions of head and eye movement, suggesting that tonic EVS activity does not change in mammals such as mice, at least over the short time course of an experiment such as ours. Therefore, VOR adaptation as observed in our control mice is unlikely to be due to EVS-induced short-term changes in type II hair cell gain or afferent background discharge rate as shown to occur in fish and reptiles. Rather, the adaptation differences are likely due to preexisting differences in EVS activity before training (which affects the regularity of afferents) and due to differences in the way irregular and regular pathway signals are processed centrally. Rather than inducing short-term changes in the behavioral VOR, we hypothesize that the normal EVS shifts the regularity of dimorphic afferents over a long time course as part of the process of maintenance and calibration (Sadeghi et al. 2007), i.e., not during short-term adaptation or other tasks requiring immediate changes in VOR response (e.g., Cullen and Minor 2002).
Taken together, our data suggest that unlike the EVS in fish and reptiles that modulates the primary afferent firing rate during large or active head movements, the mammalian EVS changes the proportion of irregular- to regular-discharging afferents. We hypothesize that the EVS changes occur over a long time course as part of the process of maintenance and calibration. When the EVS activity is significantly reduced, the contribution from the highly plastic phasic pathway is also reduced, and this leads to a reduction in behavioral vestibular plasticity as shown in this study. Our results not only provide a pivotal contribution toward understanding the role of the mammalian EVS but also identify for the first time a crucial part of the vestibular system that upon stimulation, e.g., electrically or via EVS agonists, could potentially boost vestibular plasticity.
GRANTS
A. A. Migliaccio and this work were supported by National Health and Medical Research Council of Australia (NHMRC) Biomedical Career Development Award CDA-568736 and NHMRC Project Grant APP1010896. P. P. Hübner was supported by a University of New South Wales (UNSW) International Research Scholarship and a Neuroscience Research Australia (NeuRA) supplementary scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.P.H. and S.I.K. performed experiments; P.P.H., S.I.K., and A.A.M. analyzed data; P.P.H. and A.A.M. interpreted results of experiments; P.P.H. prepared figures; P.P.H. and A.A.M. drafted manuscript; P.P.H., S.I.K., and A.A.M. edited and revised manuscript; P.P.H., S.I.K., and A.A.M. approved final version of manuscript; A.A.M. conception and design of research.
ACKNOWLEDGMENTS
We thank Alan M. Brichta for his help in revising the manuscript.
REFERENCES
- Anderson AD, Troyanovskaya M, Wackym PA. Differential expression of alpha2-7, alpha9 and beta 2–4 nicotinic acetylcholine receptor subunit mRNA in the vestibular end organ and Scarpa's ganglia of the rat. Brain Res 778: 409–413, 1997. [DOI] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60: 182–203, 1988. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Hachemaoui M, Idoux E, Ris L, Uno A, Godaux E, Vidal PP, Moore LE, Vibert N. Long-term plasticity of ipsilesional medial vestibular nucleus neurons after unilateral labyrinthectomy. J Neurophysiol 90: 184–203, 2003. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Idoux E. Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front Neurol 3: 25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraneck M, Idoux E, Uno A. Unilateral labyrinthectomy modifies the membrane properties of contralesional vestibular neurons. J Neurophysiol 92: 1668–1684, 2004. [DOI] [PubMed] [Google Scholar]
- Boyle R, Goldberg JM, Highstein SM. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in squirrel monkey vestibular nuclei. III. Correlation with vestibulospinal and vestibuloocular output pathways. J Neurophysiol 68: 471–484, 1992. [DOI] [PubMed] [Google Scholar]
- Boyle R, Highstein SM. Efferent vestibular system in the toadfish: action upon horizontal semicircular canal afferents. J Neurosci 10: 1570–1582, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Responses to efferent activation and excitatory response-intensity relations of turtle posterior-crista afferents. J Neurophysiol 83: 1224–1242, 2000. [DOI] [PubMed] [Google Scholar]
- Calabrese DR, Hullar TE. Planar relationships of the semicircular canals in two strains of mice. J Assoc Res Otolaryngol 7: 151–159, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B, Adams DJ. Analgesic α-conotoxins Vc1.1 and RgIA inhibit N-type calcium channels in sensory neurons of α9 nicotinic receptor knockout mice. Channels (Austin) 4: 51–54, 2010. [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, McCrea RA. Contribution of vestibular nerve irregular afferents to viewing distance-related changes in the vestibulo-ocular reflex. Exp Brain Res 119: 116–130, 1998. [DOI] [PubMed] [Google Scholar]
- Clendaniel RA, Lasker DM, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. IV. Responses after spectacle-induced adaptation. J Neurophysiol 86: 1594–1611, 2001. [DOI] [PubMed] [Google Scholar]
- Clendaniel RA, Lasker DM, Minor LB. Differential adaptation of the linear and nonlinear components of the horizontal vestibuloocular reflex in squirrel monkeys. J Neurophysiol 88: 3534–3540, 2002. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci 22: RC226, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Minor LB, Beraneck M, Sadeghi SG. Neural substrates underlying vestibular compensation: contribution of peripheral versus central processing. J Vestib Res 19: 171–182, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron 20: 495–508, 1998. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol 15: 667–674, 2005. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Angelaki DE. Dynamics of vestibular neurons during rotational motion in alert rhesus monkeys. Exp Brain Res 155: 91–101, 2004. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79: 705–715, 1994. [DOI] [PubMed] [Google Scholar]
- Faucheux S, Schwaller B, Buizza A. Automatic detection and removal of fast phases from nystagmographic recordings by optimal thresholding. Biomed Signal Process Control 2: 144–150, 2007. [Google Scholar]
- Gacek RR, Lyon M. The localization of vestibular efferent neurons in the kitten with horseradish peroxidase. Acta Otolaryngol 77: 92–101, 1974. [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Curr Opin Neurobiol 16: 385–390, 2006. [DOI] [PubMed] [Google Scholar]
- Goldberg GM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130: 277–297, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol 43: 986–1025, 1980. [DOI] [PubMed] [Google Scholar]
- Han GC, Lasker DM, Vetter DE, Minor LB. Extracellular recordings from semicircular canal afferents in mice that lack the alpha 9 nicotinic acetylcholine receptor subunit (Abstract). ARO Midwinter Meeting, p. 1–2, Denver, 2007. [Google Scholar]
- Hiel H, Elgoyhen AB, Drescher DG, Morley BJ. Expression of nicotinic acetylcholine receptor mRNA in the adult rat peripheral vestibular system. Brain Res 38: 347–352, 1996. [DOI] [PubMed] [Google Scholar]
- Highstein SM. The central nervous system efferent control of the organs of balance and equilibrium. Neurosci Res 12: 13–30, 1991. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Baker R. Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J Neurophysiol 54: 370–384, 1985. [DOI] [PubMed] [Google Scholar]
- Holt JC, Lioudyno M, Athas G, Garcia MM, Perin P, Guth PS. The effect of proteolytic enzymes on the alpha9-nicotinic receptor-mediated response in isolated frog vestibular hair cells. Hear Res 152: 25–42, 2001. [DOI] [PubMed] [Google Scholar]
- Holt JC, Lioudyno M, Guth PS. A pharmacologically distinct nicotinic ACh receptor is found in a subset of frog semicircular canal hair cells. J Neurophysiol 90: 1526–1536, 2003. [DOI] [PubMed] [Google Scholar]
- Holt JC, Lysakowski A, Goldberg JM. Mechanisms of efferent-mediated responses in the turtle posterior crista. J Neurosci 26: 13180–13193, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Kewin K, Jordan PM, Cameron P, Klapczynski M, McIntosh JM, Crooks PA, Dwoskin LP, Lysakowski A. Pharmacologically distinct nicotinic acetylcholine receptors drive efferent-mediated excitation in calyx-bearing vestibular afferents. J Neurosci 35: 3625–3643, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. Velocity-selective adaptation of the horizontal and cross-axis vestibulo-ocular reflex in the mouse. Exp Brain Res 232: 3035–3046, 2014. [DOI] [PubMed] [Google Scholar]
- Hübner PP, Lim R, Brichta AM, Migliaccio AA. Glycine receptor deficiency and its effect on the horizontal vestibulo-ocular reflex: a study on the SPD1J mouse. J Assoc Res Otolaryngol 14: 249–259, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol 93: 2777–2786, 2005. [DOI] [PubMed] [Google Scholar]
- Hullar TE, Minor LB. High-frequency dynamics of regularly discharging canal afferents provide a linear signal for angular vestibuloocular reflexes. J Neurophysiol 82: 2000–2005, 1999. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar control of the vestibulo-ocular reflex—around the flocculus hypothesis. Annu Rev Neurosci 5: 275–296, 1982. [DOI] [PubMed] [Google Scholar]
- Jagger DJ, Griesinger CB, Rivolta MN, Holley MC, Ashmore JF. Calcium signalling mediated by the alpha 9 acetylcholine receptor in a cochlear cell from the immortomouse. J Physiol 527: 49–54, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Wedemeyer C, Vetter DE, Adachi R, Holley MC, Elgoyhen AB, Marcotti W. Cholinergic efferent synaptic transmission regulates the maturation of auditory hair cell ribbon synapses. Open Biol 3: 130163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PM, Parks XX, Contini D, Holt JC. A review of synaptic mechanisms of vestibular efferent signaling in turtles: extrapolation to efferent actions in mammals. J Vestib Res 23: 161–175, 2013. [DOI] [PubMed] [Google Scholar]
- Katz E, Verbitsky M, Rothlin CV, Vetter DE, Heinemann SF, Elgoyhen AB. High calcium permeability and calcium block of the alpha9 nicotinic receptors. Hear Res 141: 117–128, 2000. [DOI] [PubMed] [Google Scholar]
- Kimpo RR, Boyden ES, Katoh A, Ke MC, Raymond JL. Distinct patterns of stimulus generalization of increases and decreases in VOR gain. J Neurophysiol 94: 3092–3100, 2005. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Backous DD, Lysakowski A, Davis GL, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. II. Responses after canal plugging. J Neurophysiol 82: 1271–1285, 1999. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Hullar TE, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. III. Responses after labyrinthectomy. J Neurophysiol 83: 2482–2496, 2000. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Neural basis for motor learning in the vestibulo-ocular reflex of primates. III. Computational and behavioral analysis of the sites of learning. J Neurophysiol 72: 974–979, 1994. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA. Vestibular signals carried by pathways subserving plasticity of the vestibulo-ocular reflex in monkeys. J Neurosci 6: 346–354, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke AE, Holt JC, Jordan PM, Wong YS, Caldwell JS, Cullen KE. Loss of α-calcitonin gene-related peptide (αCGRP) reduces the efficacy of the vestibulo-ocular reflex (VOR). J Neurosci 34: 10453–10458, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Vetter DE, Liberman MC. A novel effect of cochlear efferents: in vivo response enhancement does not require α9 cholinergic receptors. J Neurophysiol 97: 3269–3278, 2007. [DOI] [PubMed] [Google Scholar]
- Marco J, Lee W, Suárez C, Hoffman L, Honrubia V. Morphologic and quantitative study of the efferent vestibular system in the chinchilla: 3-D reconstruction. Acta Otolaryngol 113: 229–234, 1993. [DOI] [PubMed] [Google Scholar]
- Marlinski V, Plotnik M, Goldberg JM. Efferent actions in the chinchilla vestibular labyrinth. J Assoc Res Otolaryngol 5: 126–143, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Cremer PD, Aw ST, Halmagyi MG, Curthoys IS, Minor LB, Todd MJ. Vergence-mediated changes in the axis of eye rotation during the human vestibulo-ocular reflex can occur independent of eye position. Exp Brain Res 151: 238–248, 2003. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, MacDougall HG, Minor LB, Della Santina CC. Inexpensive system for real-time 3-dimensional video-oculography using a fluorescent marker array. J Neurosci Methods 143: 141–150, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Della Santina CC, Carey JP, Minor LB, Zee DS. The effect of binocular eye position and head rotation plane on the human torsional vestibuloocular reflex. Vision Res 46: 2475–2486, 2006. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Meierhofer R, Della Santina CC. Characterization of the 3D angular vestibulo-ocular reflex in C57BL6 mice. Exp Brain Res 210: 489–501, 2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Carey JP. Vergence-mediated modulation of the human horizontal vestibulo-ocular reflex is eliminated by a partial peripheral gentamicin lesion. Exp Brain Res 159: 92–98, 2004. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Carey JP. Vergence-mediated modulation of the human angular vestibulo-ocular reflex is unaffected by canal plugging. Exp Brain Res 186: 581–587, 2008. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Della Santina CC. Adaptation of the vestibulo-ocular reflex for forward-eyed foveate vision. J Physiol 588: 3855–3867, 2010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci 11: 1636–1648, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor LB, Lasker DM. Tonic and phasic contributions to the pathways mediating compensation and adaptation of the vestibulo-ocular reflex. J Vestib Res 19: 159–170, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor LB, Lasker DM, Backous DD, Hullar TE. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I. Normal responses. J Neurophysiol 82: 1254–1270, 1999. [DOI] [PubMed] [Google Scholar]
- Murthy V, Taranda J, Elgoyhen AB, Vetter DE. Activity of nAChRs containing alpha9 subunits modulates synapse stabilization via bidirectional signaling programs. Dev Neurobiol 69: 931–949, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanzelt S, Rössert C, Rohregger M, Glasauer S, Moore LE, Straka H. Differential dynamic processing of afferent signals in frog tonic and phasic second-order vestibular neurons. J Neurosci 28: 10349–10362, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppi LA, Tabatabaee H, Callister RJ, Lim R, Brichta AM. Cholinergic activity of the peripheral efferent vestibular system. J Vestib Res 24: 53–140, 2014.24837206 [Google Scholar]
- Purcell I, Perachio A. Three-dimensional analysis of vestibular efferent neurons innervating semicircular canals of the gerbil. J Neurophysiol 78: 3234–3248, 1997. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97: 1503–1514, 2007. [DOI] [PubMed] [Google Scholar]
- Sakatani T, Isa T. Quantitative analysis of spontaneous saccade-like rapid eye movements in C57BL/6 mice. Neurosci Res 58: 324–331, 2007. [DOI] [PubMed] [Google Scholar]
- Shinder ME, Purcell IM, Kaufman GD, Perachio AA. Vestibular efferent neurons project to the flocculus. Brain Res 889: 288–294, 2001. [DOI] [PubMed] [Google Scholar]
- Stahl JS, James RA, Oommen BS, Hoebeek FE, De Zeeuw CI. Eye movements of the murine P/Q calcium channel mutant tottering, and the impact of aging. J Neurophysiol 95: 1588–1607, 2006. [DOI] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76: 349–392, 2005. [DOI] [PubMed] [Google Scholar]
- Terreros G, Jorratt P, Elgoyhen AB, Delano P. Selective attention to visual stimuli in α9-nicotinic acetylcholine receptor knock-out mice (Abstract). Association for Research in Otolaryngology MidWinter Meeting Program 38: 32, 2015. [Google Scholar]
- Turcan S, Slonim DK, Vetter DE. Lack of nAChR activity depresses cochlear maturation and up-regulates GABA system components: temporal profiling of gene expression in α9 null mice. PLoS One 5: e9058, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23: 93–103, 1999. [DOI] [PubMed] [Google Scholar]
- Zhou T, Wang Y, Guo C, Zhang W, Yu W, Zhang K, Kong W. Two distinct channels mediated by m2mAChR and alpha9nAChR co-exist in type II vestibular hair cells of guinea pig. Int J Mol Sci 14: 8818–8831, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]