Abstract
PURPOSE
Current first-line chemotherapy for patients with metastatic adrenocortical cancer (ACC) includes doxorubicin, etoposide, cisplatin, and mitotane with a reported response rate of only 23.2%. New therapeutic leads for patients with refractory tumors are needed; there is no standard second-line treatment.
METHODS
Samples from 135 ACC tumors were analyzed by immunohistochemistry, in situ hybridization (FISH or CISH), and/or gene sequencing at a single commercial reference laboratory (Caris Life Sciences) to identify markers associated with drug sensitivity and resistance.
RESULTS
Overexpression of proteins related to demonstrated chemotherapy sensitivity or resistance included topoisomerase 1, progesterone receptor, and topoisomerase 2-alpha in 46%, 63%, and 42% of cases, respectively. Loss of excision repair cross-complementary group 1 (ERCC1), phosophatase and tensin homolog, O(6)-methylguanine-methyltransferase, and ribonucleotide reductase M1 (RRM1) was identified in 56%, 59%, 71%, and 58% of cases, respectively. Other aberrations included overexpression of programmed death-ligand 1 or programmed cell death protein 1 tumor-infiltrating lymphocytes in >40% of cases. In all, 35% of cases had a mutation in the canonical Wnt signaling pathway (either CTNNB1 or APC) and 48% had a mutation in TP53. No other genomic alterations were identified.
CONCLUSION
Biomarker alterations in ACC may be used to direct therapies, including recommendations for and potential resistance of some patients to traditional chemotherapies, which may explain the low response rate in the unselected population. Limited outcomes data support the use of mitotane and platinum therapies for patients with low levels of the proteins RRM1 and ERCC1.
Keywords: adrenocortical cancer, molecular profiling, next-generation sequencing, targeted therapy
Introduction
Adrenocortical cancer (ACC) is a rare malignancy occurring in approximately two out of a million people in the US.1 At the time of initial presentation with ACC, 20%–50% of patients have metastases or stage IV disease.2,3 Patients with localized disease, who are able to undergo surgical resection with curative intent, often have a recurrence.2,4 Systemic treatment, therefore, remains a key component of therapy for most patients with ACC. The rarity of this disease has made it challenging to conduct clinical trials to inform efficacious therapy. First-line standard therapy consists of mitotane, either alone or in combination with etoposide, doxorubicin, and cisplatin (EDP-M). In a previous phase II trial, this combination had a superior response rate of 48.6% and an overall survival of 28.5 months when compared with other chemotherapy schemes.5–11 Unfortunately, in the recently completed FIRM-ACT phase III trial, EDP-M was associated with a response rate of only 23.2% and a median survival of 14.8 months.12 Furthermore, the regimen was associated with significant toxicities, including leukopenia and neurologic side effects. There is no established standard second-line treatment for refractory ACC.
A significant advancement in the treatment of ACC would be made if physicians could accurately predict, for a particular individual, their tumor’s sensitivity and resistance to various chemotherapeutic treatments. Patients whose tumors are unlikely to respond could be spared the toxicity associated with an ineffective treatment and receive an alternative treatment that has a higher chance of success. Recent studies have shown that for patients with refractory tumors, treatment selected on the basis of tumor profiling may result in better outcomes when compared to treatment based on physicians’ choice without molecular analysis.13,14
Previous evidence in human studies has shown that loss of protein or low expression levels of ribonucleotide reductase M1 (RRM1) and P-glycoprotein (PGP) are correlated with positive response to mitotane15 and doxorubicin,16 while overexpression of topoisomerase 2-alpha (TOPO2A) is correlated with a positive response to etoposide.17 Therefore, in this study, we investigated whether one could identify molecular markers in ACC, such as RRM1, PGP, and TOPO2A expression levels, which might explain why a patient’s tumor might or might not respond to standard chemotherapy and identify alternative treatment approaches in patients with refractory ACC. We assayed markers of drug resistance as well as targets for anti-cancer drugs in a set of 135 tumor samples from patients with primary, recurrent, or metastatic ACC. We found that a majority of patients had elevated markers suggestive of drug resistance to standard first-line ACC chemotherapies. Encouragingly, we also identified potential second-line therapeutic options by observing the expression of a number of markers for sensitivity to other agents. Additionally, in a limited set of patients for whom treatment and outcomes data were available, we evaluated response to treatment based on biomarker status.
Materials and Methods
One hundred thirty-five formalin-fixed paraffin-embedded adrenal cancer samples sent to a commercial molecular profiling laboratory (Caris Life Sciences) for analysis by treating physicians around the world were analyzed using one or more technologies, described later, to identify markers of drug sensitivity and resistance. Biomarkers for analysis varied by case, dependent on tissue availability, physicians’ preference, technology standards over the course of the study, and their potential to be targeted therapeutically and/or based on clinical evidence of a utility in other solid tumors. The specific histology was confirmed by evaluation of hematoxylin and eosin slides. Pathology reports submitted by the treating physicians were further reviewed and verified by board-certified pathologists at Caris. Pediatric patients and patients with either adrenal large cell neuroendocrine carcinosarcomas or pheochromocytomas were excluded from the analysis. The Weiss score was not used, because only 30% of the cases submitted to Caris had details of the microscopic examination available that would allow for Weiss grading/scoring, and because in the absence of a strict education and standardization process, significant interobserver variation in Weiss scores has been demonstrated.18 In accordance with Western Institutional Review Board (IRB) guidelines, because patient identity protection was maintained throughout the study, the study was considered IRB exempt.
Patient demographics
A total of 81% of the patients for whom we received staging information had stage IV disease. Limited treatment information was available for 47 patients; of those, 39 patients had received mitotane at some time during their treatment. Additional patient clinical and pathologic characteristics are shown (Table 1) for the subset of patients regarding whom data were available.
Table 1.
Patient demographics and clinical characteristics (n = 135).
| N | PERCENT (%) | |
|---|---|---|
| Age, yrs. | 135 | |
| ≥50 | 62 | 46 |
| <50 | 73 | 54 |
| Median | 48 | |
| Range | 18–86 | |
| IQR | 20 | |
| Gender | ||
| Female | 80 | 59 |
| Male | 55 | 41 |
| Laterality | ||
| Right | 44 | 33 |
| Left | 51 | 38 |
| Not specified in path report | 40 | 29 |
| Most common metastatic sites (>5%) | ||
| Liver | 23 | 24 |
| Lung | 12 | 13 |
| Abdominal region | 10 | 10 |
| Lymph | 6 | 6 |
| Retroperitoneum/peritoneum | 8 | 8 |
| Documented status at time of profile | ||
| Metastatic | 96 | 71 |
| Recurrent, local | 12 | 9 |
| Not indicated | 27 | 20 |
| Vital status at time of study | ||
| Deceased | 70 | 90 (of known) |
| Alive | 8 | 10 (of known) |
| Not available | 57 | |
| Cases with treatment information provided | 47 | 35 |
Immunohistochemistry
Protein expression was assessed by immunohistochemistry (IHC), as previously described.19 Cancer cells on slides were scored by pathologists. For PD-1, the tumor-infiltrating lymphocytes (TILs) were scored. Protein was considered overexpressed, when the percent staining and intensity were above previously published and validated thresholds specific to each marker, for proteins where increased expression levels were of interest; when loss of protein or underexpression of protein was of interest, percent staining and intensity below previously published and validated levels were reported.19 Antibodies used included: androgen receptor (AR), topoisomerases 1 and 2 (TOPO1, TOPO2A, Leica Biosystems), estrogen receptor (ER), progesterone receptor (PR), cMet, human epidermal growth factor receptor 2 (HER2; Ventana), cKIT, epidermal growth factor receptor (EGFR), phosophatase and tensin homolog (PTEN; Dako), O(6)-methylguanine-methyltransferase (MGMT), PGP, thymidylate synthase (TS; Invitrogen), transducin-like enhancer of split 3 (Santa Cruz), excision repair cross complimentary group 1 (ERCC1; Abcam), RRM1 (Proteintech), SPARC (monoclonal; R&D Systems and polyclonal; Exalpha) and tubulin beta-3 chain (Covance), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1; BD Pharmingen and R&D Systems).
In situ hybridization
Fluorescent in situ hybridization (FISH) and, more recently, chromogenic in situ hybridization were used for evaluation of HER2/neu, EGFR, and cMET, as previously described.19
Mutational analysis
Next-generation sequencing (NGS)
Direct sequence analysis was performed on genomic DNA isolated from formalin-fixed paraffin-embedded tumor samples using the Illumina MiSeq platform (Illumina). Specific regions of 46 genes of the genome were amplified using the Illumina TruSeq Amplicon Cancer Hotspot panel, as described previously.19
Sanger sequencing
Prior to the availability of Clinical Laboratory Improvement Amendments (CLIA) certified NGS, mutation analysis by Sanger sequencing included selected regions of BRAF, KRAS, cKIT, EGFR, and PIK3CA genes and was performed using M13-linked PCR primers designed to amplify targeted sequences. PCR products were bidirectionally sequenced using the BigDye Terminator v1.1 chemistry and analyzed using the 3730 DNA Analyzer (Applied Biosystems). Sequence traces were analyzed using Mutation Surveyor software v3.25 (SoftGenetics).
Statistical analysis
The patient population and profiling data were characterized using standard descriptive statistics, performing Fisher’s exact test using GraphPad. The nonparametric Kaplan–Meier statistic was used to estimate the fraction of patients surviving in analyzed groups, to determine significance, using JMP software. When noted, subgroups were analyzed separately from the entire cohort.
Results
Analysis of biomarkers evaluated by immunohisto-chemistry
Expression of immunohistochemical markers relevant to sensitivity or resistance to the drugs in the standard therapeutic regimen for ACC, including etoposide, doxorubicin, cisplatin, and mitotane, were investigated (Table 2). In the adjuvant setting, mitotane is recommended despite toxicity and a low response rate of ~20% in metastatic ACC.20 Although its mechanism of action is unknown, recent evidence suggests that RRM1 gene expression may be relevant as a predictor of response to mitotane.15 In our cohort, RRM1 was expressed (≥2+ intensity and >50% staining) in 44 of 105 (42%) patients, indicating possible mitotane resistance in the 58% of ACC with loss of expression. TOPO2A was expressed (≥1+ intensity and ≥10% staining) in 45 of 107 samples, suggesting sensitivity to doxorubicin;17,21 however, PGP, a marker of resistance to doxorubicin,16 was expressed (≥1+ intensity and ≥10% staining) in 57 of 105 ACC tumors as well. A total of 50% of TOPO2A positive tumors also expressed PGP. ERCC122–26 and breast cancer resistance protein (BCRP),27,28 both markers of resistance to cisplatin, were elevated in 30 of 32 patients tested.
Table 2.
IHC markers of sensitivity and resistance to current ACC therapy.
| IHC MARKER | NUMBER POSITIVE | NUMBER INFORMATIVE | PERCENT | SIGNIFICANCE |
|---|---|---|---|---|
| TOPO2A | 45 | 107 | 42% | Sensitivity to doxorubicin17 |
| PGP* | 57 | 105 | 54% | Resistance to doxorubicin16 |
| PGP* and MRP1 | 23 | 55 | 58% | Resistance to etoposide44 |
| ERCC1* | 32 | 73 | 44% | Resistance to cisplatin22–26 |
| BCRP | 25 | 32 | 78% | Resistance to cisplatin27,28 |
| RRM1* | 44 | 105 | 42% | Sensitivity to mitotane15 |
Notes: The number positive indicates the number of cases where the protein expression was above the defined threshold, except for those cases where the expression of the biomarker below the threshold is considered predictive of response to therapy (denoted with an asterisk). The number informative is the total number of cases in which a valid result was obtained.
Abbreviations: TOPO2A, topoisomerase 2-alpha; PGP, P-glycoprotein; MRP1, multidrug resistance-associated protein 1; ERCC1, excision repair cross-complementation group 1; BCRP, breast cancer resistance protein; RRM1, ribonucleotide reductase large subunit 1.
In the limited number of patients for whom we had clinical data, we analyzed the association between protein expression levels and treatments correlated to potential benefit. Forty-nine of the patients with clinical outcomes were evaluated for TOPO2A protein expression; 20 of these patients overexpressed TOPO2A (≥1+ intensity and ≥10% staining); however, none were treated with the topoisomerase inhibitors doxorubicin or etoposide, perhaps because they had already been treated and they now exhibited refractory disease. Seven of the 29 patients with low TOPO2A were treated with doxorubicin, which is predicted to be associated with a lack of benefit. The average survival in those 7 patients was 38 months, compared to the 22 patients not treated with doxorubicin who had an average survival of 50 months (P-value = 0.574). PGP expression, a protein known to transport doxorubicin out of the cell, was not different between these two groups. Of 46 patients with RRM1 expression status who were treated with mitotane, 25 patients with low RRM1 levels survived an average of 9 months longer than 21 patients with high expression of RRM1 (P-value = 0.369). Notably, of 10 patients whose ERCC1 protein expression levels were tested and who with platinum therapy; four patients with low ERCC1 levels (<2+ intensity and <10% staining) had statistically significant survival benefit compared to six patients with normal ERCC1 expression (44 vs. 22 months; P-value = 0.0356).
An additional goal of the study was to assess whether there were subsequent treatment strategies suggested by biomarker aberrations, for patients for whom the standard therapies had failed. Several additional agents, including nab-paclitaxel, irinotecan, and aromatase inhibitors, were identified as possible options, based on the presence of their target markers in recurrent or metastatic subsets of these ACC samples (Table 3). These samples were also compared to the subset of cases which were documented to have primary disease only.
Table 3.
IHC markers for sensitivity to other agents, in recurrent and metastatic cases analyzed in this study.
| IHC MARKER | NUMBER POSITIVE | NUMBER INFORMATIVE | PERCENT | P-VALUE COMPARED TO CASES WITH PRIMARY ONLY | SIGNIFICANCE |
|---|---|---|---|---|---|
| TOPO1 | 35 | 61 | 57% | 0.009 | Sensitivity to irinotecan45 |
| SPARC | 42 | 79 | 53% | 0.13 | Sensitivity to nab-paclitaxel46,47 |
| PDGFR or cKIT* | 3 5 |
36 46 |
8% 11% |
0.018 | Sensitivity to multitargeted kinase inhibitors (imatinib, sunitinib)48 |
| AR | 8 | 74 | 11% | 0.15 | Sensitivity to androgen deprivation (Flutamide, leuprolide)49 |
| PR | 50 | 73 | 68% | 0.17 | Sensitivity to aromatase inhibitors (anastrozole)50,51 |
| ER | 2 | 74 | 3% | 1.0 | Sensitivity to aromatase inhibitors, (anastrozole) or antiestrogens (tamoxifen)51 |
Notes: P-value determined using Fisher’s exact test. The number positive indicates the number of cases where the protein expression was above the defined threshold. The number informative is the total number of cases in which a valid result was obtained.
Notably, the only cKIT mutation identified in the cohort was in a recurrent case.
Abbreviations: TOPO1, topoisomerase 1; SPARC, secreted protein acidic rich in cysteine; PR, progesterone receptor; ER, estrogen receptor; PDGFR, platelet-derived growth factor receptor; AR, androgen receptor.
Analysis of immune checkpoint biomarkers, PD-1 and PD-L1, identified PD-1 TILs in 20% of cases (9/46), overexpression of PD-L1 in 30% of cases (14/46), concurrence of PD-1/PD-L1 in 9% of cases (4/46), and occurrence of either PD-1 or PD-L1 in 41% of cases (19/46; Fig. 1).
Figure 1.
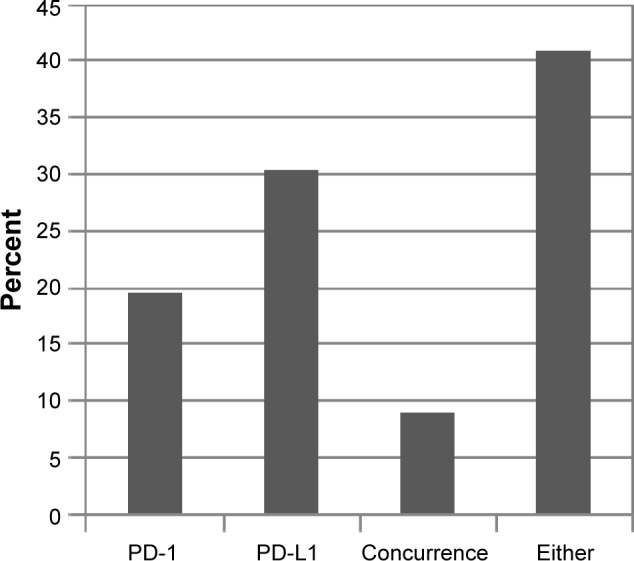
IHC analysis of PD-1 TILs and/or PD-L1 expression. Concurrence occurs when both an increase in PD-L1 and expression of PD-1 on the TILs were identified in a sample. Either indicates that either PD-1 or PD-L1 was identified in a sample.
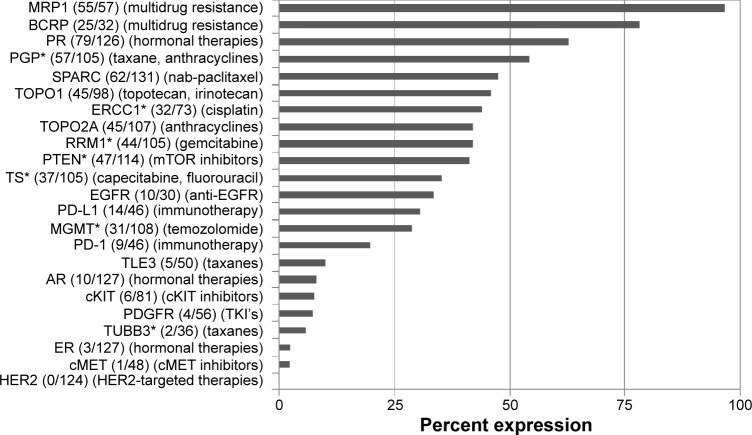
All markers tested by IHC are shown in Figure 2. Of note, PR was overexpressed in 63%, AR in 8%, and EGFR in 33% of cases. EGFR overexpression was seen in half as frequently as previously reported29; however, the published incidence was obtained using a different and less specific antibody. Loss of MGMT protein expression was identified in 71%, loss of PTEN in 59%, and low TS in 65% of cases. PTEN loss is noteworthy, as the PI3 kinase pathway (specifically overexpression of mammalian target of rapamycin [mTOR]) has been identified in the tumorigenesis of ACC, and recent phase I trials with mTOR inhibitors have shown promise.30,31 Therefore, treatment with everolimus or temsirolimus may provide benefit in those patients with loss of PTEN. Loss of MGMT has been associated with beneficial treatment utilizing temozolomide in other cancers; therefore, this treatment in ACC patients with low MGMT may be another area for exploration.
Figure 2.
Protein expression by IHC, reported as number positive (protein expression above defined threshold) of total cases tested in parentheses and shown as percent expression in graph. Associated therapies, based on current evidence, are indicated in the second parentheses.
Note: *Proteins for which either low expression or loss of expression is associated with therapeutic benefit of treatment listed in parentheses.
Analysis of biomarkers evaluated by in situ hybridization
The most commonly amplified gene was EGFR (5/45, 11.5%). Amplification of the cMET and HER2 genes were identified in single cases: cMET (1/38, 2.6%); HER2 (1/60, 1.7%). Together, EGFR protein overexpression or gene amplification of EGFR in 21% of cases suggests consideration of treatment targeting EGFR.
Analysis of biomarkers evaluated by mutation analysis
DNA sequencing
Key genes often mutated in cancer were tested for mutation status (Table 4). Most notably, two genes in the canonical Wnt signaling pathway were frequently mutated in the ACC cohort. CTNNB1 mutations were identified in 35% of cases, and a mutation in APC was identified in one case tested. In all, Wnt pathway mutations were identified in 38% of cases. CTNNB1 mutations have been reported in ACC at 25% (amino acid S45) and 31%.32–34 Five of the 10 CTNNB1 mutations identified in our cohort were specific to S45; all others were within 20 codons, in exon 3. Additionally, mutations in TP53 were identified in 47% of cases tested, which is higher than previously reported levels of 20%–27%35 and 33%,36 and may reflect the advanced disease stage of this study cohort, with a poorer prognosis. The majority of these were identified in highly conserved exons 5–8 and at high frequencies. Also, the median age of those with TP53 mutations was 46 years, compared to 49 years for non-TP53 mutated. Mutations of BRCA2, AKT1, ATM, cKIT, cMET, EGFR, ERBB4, JAK3, and KDR were all identified in single tumors in 32 cases tested.
Table 4.
Gene alterations.
| AKT1 | APC | ATM | BRCA2 | cKIT | cMET | CTNNB1 | EGFR | ERBB4 | JAK3 | KDR | TP53 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # positive | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 1 | 1 | 1 | 1 | 13 |
| Total tested | 28 | 29 | 28 | 6 | 34 | 29 | 28 | 31 | 29 | 28 | 28 | 28 |
| % total | 3.6 | 3.4 | 3.6 | 16.7 | 2.9 | 3.4 | 32.1 | 3.2 | 3.4 | 3.6 | 3.6 | 46.4 |
Notes: Gene alterations were considered to be pathogenic or presumed pathogenic. Mutations of unknown significance, or believed to be benign (synonymous or missense), based on the ACCMG descriptions, were not reported. The test is not designed to distinguish somatic versus germline origin of the alteration. Genes tested for which no alterations were identified included ABL1, ALK, BRAF, BRCA1, CDH1, CSF1R, ERBB2, FBXW7, FGFR1, FGFR2, FLT3, GNA11, GNAQ, GNAS, HNF1A, HRAS, IDH1, JAK2, KRAS, MLH1, MPL, NOTCH1, NPM1, NRAS, PDGFRA, PIK3CA, PTEN, PTPN11, RB1, RET, SMAD4, SMARCB1, SMO, STK11, and VHL.
Discussion
Rare and aggressive tumors such as ACC pose a particular challenge to patients and their treating physicians. In ACC, little data exist to guide treatment decisions if first-line treatment with EDP-M fails. The present study is novel because it is the first to examine a large number of ACCs from patients with primary, metastatic, or refractory disease for established markers of sensitivity or resistance to chemotherapeutic agents. In the absence of clinical trials to establish second-line treatment, the expression of drug targets in refractory ACC can offer guidance to the selection of a chemotherapeutic regimen for these patients. While this study demonstrated that markers of resistance were elevated in a majority of patients with recurrent or metastatic disease, it also identified expression of markers and relevant genes that suggested other potentially more efficacious chemotherapeutic agents. The results suggest that biomarker-driven clinical trials might provide insights into potential novel targeted therapies. Histology-based trials may also be useful for ACC, to further refine biomarker-associated differences in response to chemotherapies, as suggested by the limited outcomes seen in this cohort. This is also the first report establishing the presence of PD-1 and PD-L1 receptors in ACC, suggesting that further study of the potential role for this class of immune modifier drugs in the treatment of ACC is warranted. Ongoing immunotherapy-focused clinical trials including ACC patients may shed further light on their efficacy in ACC.
EDP-M is a rigorous chemotherapy regimen associated with substantial toxicity. As the response rate is only 23%,12 a method to predict whose cancer would be likely to respond could result in a higher quality of life for a significant number of patients. Patients who have biomarkers predictive of resistance to one or more components of EDP-M could be placed on another, potentially more effective regimen, which may reduce toxicity and increase the likelihood of clinical response.
One agent of EDP-M, doxorubicin, is an anthracycline that binds to and intercalates with DNA and functions as a topoisomerase II inhibitor. Evidence outside of ACC suggests that this drug may be beneficial for patients whose tumors have elevated expression of TOPO2A.17 Unfortunately, in the limited cohort of patients for whom we have treatment and outcomes data, no patients with elevated expression of TOPO2A were subsequently treated with topoisomerase II inhibitors. However, we found decreased overall survival in low TOPO2A patients compared to the overall study population. Doxorubicin is a substrate for PGP, which binds and transports this drug out of the cell as part of the multidrug resistance mechanism.37,38 Patients with high levels of PGP have been shown to have higher levels of resistance to doxorubicin. We did not, however, observe any differences in survival between the high and low PGP cohorts treated with doxorubicin. Cisplatin, a platinum-based agent in EDP-M kills tumor cells by binding and cross-linking DNA strands, causing DNA damage and triggering cell death pathways. Tumor cells can offset the effects of platinum-based agents by implementing DNA repair mechanisms, particularly via the action of ERCC1. Platinum-based agents have been shown to be relatively ineffective in patients whose tumors harbor a high level of ERCC1 expression.16,17,22–24 In ACC, Ronchi et al showed that high ERCC1 expression was an indicator of poor responses in platinum-treated patients.26 Our findings are supportive of this conclusion, in that patients with high expression of ERCC1 who received platinum therapy had significantly shorter survival than patients with normal ERCC1 expression receiving platinum therapy. Mitotane (o’p’DDD) is a part of the EDP-M regimen but is also approved as monotherapy for ACC. Drug toxicity at therapeutic doses often limits efficacy.20 In vitro and in vivo data using gene expression support the use of RRM1 as a biomarker for prediction of response to adjuvant mitotane in ACC. Adjuvant mitotane resulted in improved progression-free survival in the presence of low expression levels of RRM1 by real time polymerase chain reaction (RT-PCR).15 There was no benefit among patients whose tumors expressed high levels of RRMI, nor was there improved overall survival in either study population. Our limited clinical data, which examined protein levels of RRM1 by IHC, trend in the same direction for patients with metastatic ACC.
There is evidence from in vitro studies that mitotane offsets the action of PGP;39 however, this has not been substantiated by in vivo studies.6 Continued work on PGP antagonists such as tariquidar has shown evidence of PGP inhibition in vivo in metastatic ACC.40 Our study demonstrated overexpression of PGP in 54% of tumors and provides further support for the pursuit of effective PGP antagonists. Our dataset was too small to evaluate the outcomes in patients with coincidence of overexpression of PGP and low expression of RRM1.
Our study suggests that other chemotherapeutic agents might have efficacy in selected patients with ACC. No protein or molecular marker suggesting efficacy was universally present, further indicating that an individualized approach to the selection of a treatment regimen for patients with refractory adrenal cancers is needed. Overall, we found that a majority of tumors in this cohort had high expression of markers indicative of drug resistance to one or more of the standard agents used to treat ACC, highlighting the need for the discovery of new options. Our study identified several biomarkers aberrantly expressed in ACC that have proven helpful in other cancers. For example, a recent case report41 profiled an ACC tumor that had low expression of TS and high expression of TOPO1, leading to a decision to treat with FOLinic acid, Fluorouracil, and IRInotecan (FOLFIRI). The patient responded and, at the time of report, had a continued response 346 days after profiling. In our patient cohort, 34 (31.5%) of 108 cases tested had both TOPO1 overexpression and loss of TS, suggesting that other refractory ACC patients may benefit from FOLFIRI.
Other targets were identified in the Wnt and PI3K pathways, as well as TP53. While no currently FDA approved drugs target aberrations in the Wnt pathway, the finding of mutations in the beta-catenin gene and/or APC in 38% of ACC suggests that Wnt inhibitors under development, such as PRI-174, should be studied in patients with ACC. Similarly, clinical trials targeting TP53 might be warranted, as ~50% of ACC patients in our cohort of refractory ACC, presented with TP53 mutations. Another area for clinical trials might be the PI3 kinase pathway; 57% of cases had a loss of PTEN and a single case had an AKT1 mutation, despite no mutations being found in either PTEN or PIK3CA.
Considerable interest has arisen in oncology, with the promising new agent inhibiting PD-L1 and programmed death 1 (PD-1).42 While PD-L1 and PD-1 are frequently found to be expressed at high levels in many cancers, there have been no prior reports characterizing the levels of expression in a large set of adrenal cancers. In our sample set, we found PD-L1 expression in 30% and PD-1 positive TILs in 20% of ACC cases tested. PD-L1 expression by tumors has been associated with favorable responses to target inhibition in clinical trials.43
Combining drugs that target PD-1/PD-L1 with cytotoxic chemotherapy is also gaining interest for tumors, including melanoma, non-small cell lung cancer, renal cell carcinoma, and other cancers, and may be an attractive option for further investigation in ACC patients with tumors overexpressing PD-1/PD-L1.
One limitation of our study was a lack of clinical data, as is it was not known which agents were chosen for 70% of the patients. Furthermore, this study cannot address the question of whether one should use adjuvant treatment with mitotane or another agent in resected high-risk adrenal cancer. Also, because these cases were tested on a commercial platform over several years, the availability of different tests changed, and not all cases had the same group of tests performed. And finally, the low frequency of actionable mutations identified suggests that Hotspot analysis of a small gene panel may have limited scope in ACCs. Furthermore, an evaluation of the percent of patients with germline versus somatic incidence of TP53 may be relevant, as germline TP53 mutations (Li–Fraumeni syndrome) are more common than somatic mutations in ACCs. The Illumina technology used was not designed to provide this distinction.
Overall, our data suggest that a multiplatform molecular profiling of refractory ACC tumors can offer insights to explain disease progression on standard chemotherapy and can also offer options for second-line therapy, to improve both outcomes and quality of life. Continuing evaluation is needed in the understanding of the molecular landscape of ACC in order to develop new and more effective treatments. Toward that end, this report provides insights at both the protein and genomic level, identifying clinically applicable known therapeutic targets in a large subset of ACC patients.
Acknowledgments
The authors acknowledge the significant contributions made by Nancy Doll, RN, CTR, for extensive review of clinical data.
Footnotes
ACADEMIC EDITOR: Barbara Guinn, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 2767 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Sherri Z. Millis is a former employee of Caris Life Sciences.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conception and design: MJD, SE, and SZM. Collection and assembly of data: SZM. Data analysis and interpretation: MJD, SE, and SZM. Manuscript writing: MJD, SE, and SZM. Jointly developed the structure and arguments for the paper: MJD, SE, and SZM. Made critical revisions and approved the final version: MJD, SE, and SZM. Final review and approval of the manuscript: MJD, SE, and SZM.
REFERENCES
- 1.United States National Cancer Institute Third National Cancer Survey: Incidence Data . National Cancer Institute Monograph. U.S. Dept. of Health, Education, and Welfare, Public Health Service, National Institutes of Health; Bethesda, MD: 1975. pp. i–x.pp. 1–454. [Google Scholar]
- 2.Bellantone R, Ferrante A, Boscherini M, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery. 1997;122:1212–1218. doi: 10.1016/s0039-6060(97)90229-4. [DOI] [PubMed] [Google Scholar]
- 3.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 4.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:963–970. discussion 70–1. [PubMed] [Google Scholar]
- 5.Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12:657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 6.Abraham J, Bakke S, Rutt A, et al. A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist. Cancer. 2002;94:2333–2343. doi: 10.1002/cncr.10487. [DOI] [PubMed] [Google Scholar]
- 7.Bonacci R, Gigliotti A, Baudin E, et al. Cytotoxic therapy with etoposide and cisplatin in advanced adrenocortical carcinoma. Br J Cancer. 1998;78:546–549. doi: 10.1038/bjc.1998.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukowski RM, Wolfe M, Levine HS, et al. Phase II trial of mitotane and cisplatin in patients with adrenal carcinoma: a Southwest Oncology Group study. J Clin Oncol. 1993;11:161–165. doi: 10.1200/JCO.1993.11.1.161. [DOI] [PubMed] [Google Scholar]
- 9.Decker RA, Elson P, Hogan TF, et al. Eastern Cooperative Oncology Group study 1879: mitotane and adriamycin in patients with advanced adrenocortical carcinoma. Surgery. 1991;110:1006–1013. [PubMed] [Google Scholar]
- 10.Khan TS, Imam H, Juhlin C, et al. Streptozocin and o,p’DDD in the treatment of adrenocortical cancer patients: long-term survival in its adjuvant use. Ann Oncol. 2000;11:1281–1287. doi: 10.1023/a:1008377915129. [DOI] [PubMed] [Google Scholar]
- 11.Williamson SK, Lew D, Miller GJ, et al. Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study. Cancer. 2000;88:1159–1165. [PubMed] [Google Scholar]
- 12.Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 13.Von Hoff DD, Stephenson JJ, Jr, Rosen P, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 14.Jameson GS, Petricoin EF, Sachdev J, et al. A pilot study utilizing multi-omic molecular profiling to find potential targets and select individualized treatments for patients with previously treated metastatic breast cancer. Breast Cancer Res Treat. 2014;147:579–588. doi: 10.1007/s10549-014-3117-1. [DOI] [PubMed] [Google Scholar]
- 15.Volante M, Terzolo M, Fassnacht M, et al. Ribonucleotide reductase large subunit (RRM1) gene expression may predict efficacy of adjuvant mitotane in adrenocortical cancer. Clin Cancer Res. 2012;18:3452–3461. doi: 10.1158/1078-0432.CCR-11-2692. [DOI] [PubMed] [Google Scholar]
- 16.Mechetner E, Kyshtoobayeva A, Zonis S, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res. 1998;4:389–398. [PubMed] [Google Scholar]
- 17.Durbecq V, Paesmans M, Cardoso F, et al. Topoisomerase-II alpha expression as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Mol Cancer Ther. 2004;3:1207–1214. [PubMed] [Google Scholar]
- 18.Tissier F, Aubert S, Leteurtre E, et al. Adrenocortical tumors: improving the practice of the Weiss system through virtual microscopy: a National Program of the French Network INCa-COMETE. Am J Surg Pathol. 2012;36:1194–1201. doi: 10.1097/PAS.0b013e31825a6308. [DOI] [PubMed] [Google Scholar]
- 19.Millis SZ, Bryant D, Basu G, et al. Molecular profiling of infiltrating urothelial carcinoma of bladder and nonbladder origin. Clin Genitourin Cancer. 2015;13:e37–e49. doi: 10.1016/j.clgc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Bapat AA, Demeure MJ, Bussey KJ. A fly in the ointment: reassessing mitotane’s role in the treatment of adrenocortical carcinoma. Pharmacogenomics. 2012;13:1207–1209. doi: 10.2217/pgs.12.90. [DOI] [PubMed] [Google Scholar]
- 21.Jain M, Zhang L, He M, et al. TOP2A is overexpressed and is a therapeutic target for adrenocortical carcinoma. Endocr Relat Cancer. 2013;20:361–370. doi: 10.1530/ERC-12-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cobo M, Isla D, Massuti B, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007;25:2747–2754. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 23.Kim MK, Cho KJ, Kwon GY, et al. ERCC1 predicting chemoradiation resistance and poor outcome in oesophageal cancer. Eur J Cancer. 2008;44:54–60. doi: 10.1016/j.ejca.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Kwon HC, Roh MS, Oh SY, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007;18:504–509. doi: 10.1093/annonc/mdl430. [DOI] [PubMed] [Google Scholar]
- 25.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 26.Ronchi CL, Sbiera S, Kraus L, et al. Expression of excision repair cross complementing group 1 and prognosis in adrenocortical carcinoma patients treated with platinum-based chemotherapy. Endocr Relat Cancer. 2009;16:907–918. doi: 10.1677/ERC-08-0224. [DOI] [PubMed] [Google Scholar]
- 27.Kim YH, Ishii G, Goto K, et al. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer. 2009;65:105–111. doi: 10.1016/j.lungcan.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Yoh K, Ishii G, Yokose T, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 29.Adam P, Hahner S, Hartmann M, et al. Epidermal growth factor receptor in adrenocortical tumors: analysis of gene sequence, protein expression and correlation with clinical outcome. Mod Pathol. 2010;23:1596–1604. doi: 10.1038/modpathol.2010.153. [DOI] [PubMed] [Google Scholar]
- 30.Behbakht K, Sill MW, Darcy KM, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;123:19–26. doi: 10.1016/j.ygyno.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naing A, Kurzrock R, Burger A, et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res. 2011;17:6052–6060. doi: 10.1158/1078-0432.CCR-10-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tissier F, Cavard C, Groussin L, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 33.Ragazzon B, Assie G, Bertherat J. Transcriptome analysis of adrenocortical cancers: from molecular classification to the identification of new treatments. Endocr Relat Cancer. 2011;18:R15–R27. doi: 10.1530/ERC-10-0220. [DOI] [PubMed] [Google Scholar]
- 34.Soon PS, McDonald KL, Robinson BG, et al. Molecular markers and the pathogenesis of adrenocortical cancer. Oncologist. 2008;13:548–561. doi: 10.1634/theoncologist.2007-0243. [DOI] [PubMed] [Google Scholar]
- 35.Reincke M, Karl M, Travis WH, et al. p53 mutations in human adrenocortical neoplasms: immunohistochemical and molecular studies. J Clin Endocrinol Metab. 1994;78:790–794. doi: 10.1210/jcem.78.3.8126158. [DOI] [PubMed] [Google Scholar]
- 36.Libe R, Groussin L, Tissier F, et al. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clin Cancer Res. 2007;13:844–850. doi: 10.1158/1078-0432.CCR-06-2085. [DOI] [PubMed] [Google Scholar]
- 37.Bao L, Haque A, Jackson K, et al. Increased expression of P-glycoprotein is associated with doxorubicin chemoresistance in the metastatic 4T1 breast cancer model. Am J Pathol. 2011;178:838–852. doi: 10.1016/j.ajpath.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen F, Chu S, Bence AK, et al. Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. J Pharmacol Exp Ther. 2008;324:95–102. doi: 10.1124/jpet.107.127704. [DOI] [PubMed] [Google Scholar]
- 39.Bates SE, Shieh CY, Mickley LA, et al. Mitotane enhances cytotoxicity of chemotherapy in cell lines expressing a multidrug resistance gene (mdr-1/P-glycoprotein) which is also expressed by adrenocortical carcinomas. J Clin Endocrinol Metab. 1991;73:18–29. doi: 10.1210/jcem-73-1-18. [DOI] [PubMed] [Google Scholar]
- 40.Menefee ME, Huang H, Edgerly M, et al. Effects of the P-glycoprotein (Pgp) antagonist tariquidar (XR-9576; TQD) on Pgp function as well as the toxicity and efficacy of combined chemotherapy in patients with metastatic adrenocortical cancer (mACC) J Clin Oncol. 2008;26:2543. [Google Scholar]
- 41.Dean A, Wallace R. Clinical application of molecular profiling in selecting treatment for advanced refractory and rare solid tumours: An Australian experience. Eur J Cancer. 2013;49(suppl 2):955. [Google Scholar]
- 42.McDermott J, Jimeno A. Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today (Barc) 1998;2015(51):7–20. doi: 10.1358/dot.2015.51.1.2250387. [DOI] [PubMed] [Google Scholar]
- 43.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeh JJ, Hsu NY, Hsu WH, et al. Comparison of chemotherapy response with P-glycoprotein, multidrug resistance-related protein-1, and lung resistance-related protein expression in untreated small cell lung cancer. Lung. 2005;183:177–183. doi: 10.1007/s00408-004-2532-1. [DOI] [PubMed] [Google Scholar]
- 45.Braun MS, Richman SD, Quirke P, et al. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol. 2008;26:2690–2698. doi: 10.1200/JCO.2007.15.5580. [DOI] [PubMed] [Google Scholar]
- 46.Demeure MJ, Stephan E, Sinari S, et al. Preclinical investigation of nanoparticle albumin-bound paclitaxel as a potential treatment for adrenocortical cancer. Ann Surg. 2012;255:140–146. doi: 10.1097/SLA.0b013e3182402d21. [DOI] [PubMed] [Google Scholar]
- 47.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 49.Culig Z, Klocker H, Bartsch G, et al. Androgen receptors in prostate cancer. Endocr Relat Cancer. 2002;9:155–170. doi: 10.1677/erc.0.0090155. [DOI] [PubMed] [Google Scholar]
- 50.Stendahl M, Ryden L, Nordenskjold B, et al. High progesterone receptor expression correlates to the effect of adjuvant tamoxifen in premenopausal breast cancer patients. Clin Cancer Res. 2006;12:4614–4618. doi: 10.1158/1078-0432.CCR-06-0248. [DOI] [PubMed] [Google Scholar]
- 51.Yamashita H, Yando Y, Nishio M, et al. Immunohistochemical evaluation of hormone receptor status for predicting response to endocrine therapy in metastatic breast cancer. Breast Cancer. 2006;13:74–83. doi: 10.2325/jbcs.13.74. [DOI] [PubMed] [Google Scholar]