Abstract
This study identifies the potential role in heat-stress mitigation of phytohormones and other secondary metabolites produced by the endophytic fungus Paecilomyces formosus LWL1 in japonica rice cultivar Dongjin. The japonica rice was grown in controlled chamber conditions with and without P. formosus LWL1 under no stress (NS) and prolonged heat stress (HS) conditions. Endophytic association under NS and HS conditions significantly improved plant growth attributes, such as plant height, fresh weight, dry weight, and chlorophyll content. Furthermore, P. formosus LWL1 protected the rice plants from HS compared with controls, indicated by the lower endogenous level of stress-signaling compounds such as abscisic acid (25.71%) and jasmonic acid (34.57%) and the increase in total protein content (18.76%–33.22%). Such fungal endophytes may be helpful for sustainable crop production under high environmental temperatures.
Keywords: Paecilomyces formosus LWL1, Plant-growth promotion, Heat-stress mitigation, Phytohormones, Organic acids, Endophytes
1. Introduction
Networks of the plant metabolic pathways are being modelled through the stoichiometric biochemical reaction. The metabolic network consists of characterized enzymes in specified tissues and organs. Plant network models are mediated by microorganisms such as bacteria and fungi, known as “plantmicrobe” interactions (Khan et al., 2012). These plant-microbe interactions can provide regulatory dynamics towards plant growth in biotic and abiotic stresses. One of the major, currently encountered abiotic stresses, is heat stress (Sgobba et al., 2015). Higher temperatures resulting from global climate change have already had drastic effects on crop growth, and global temperatures are predicted to rise by 2–5 °C by the end of this century. The occurrence of heat-shock waves will further affect agricultural production (Fragkostefanakis et al., 2014). Overall, heat stress interrupts plant growth by impairing important physiological and morphological processes such as reductions in seed germination, net assimilation rate-dependent relative growth rate, plant height, photosynthesis, and flowering, along with premature cell death (Hasanuzzaman et al., 2013; Sgobba et al., 2015). Rice is perhaps the most important staple food crop for the majority of the world’s population (Lin et al., 2014). Despite this, its production is also severely reduced by heat stress; even a brief increase in temperature at a crucial stage, such as flowering, may significantly reduce the yield (Folsom et al., 2014; Lin et al., 2014).
Crop plants cannot change location, and therefore they have to deploy decoys to mitigate severe environmental conditions including heat stress. Crop plants can adjust to the stressful heat environment by manipulating their metabolism to produce osmolytes, antioxidants, and phytohormones (Hasanuzzaman et al., 2013). The up- or down-regulation of phytohormones, mainly gibberellins (GAs), indole acetic acid (IAA), abscisic acid (ABA), and jasmonic acid (JA), has a central role in enhancing heat-stress resistance (Bita and Gerats, 2013; Hasanuzzaman et al., 2013). GAs and IAA play important roles in the plant development process, while ABA and JA mediate abiotic and biotic stress responses to heat, cold, drought, and pathogens, for example. Moreover, exogenous application of these phytohormones has been proven to alleviate heat stress (Bita and Gerats, 2013; Hasanuzzaman et al., 2013; Claeys et al., 2014). Plant association with phytohormones produced by endophytes has also been helpful in mitigating abiotic and biotic stresses (Khan et al., 2012; Waqas et al., 2014a; 2014b). Endophytes benefit host plants in a number of ways by improving uptake of nutrients and water, water-use efficiency, endogenous hormone levels, and survivability competition without causing any harm (Khan et al., 2012). This important plant-microbe association (Khan et al., 2012; Higgins et al., 2014; Waqas et al., 2014b) is the basis of our study. We hypothesized that secondary metabolites produced by fungal endophyte symbiosis will not only maintain but also promote rice plant growth under prolonged heat stress conditions.
In this study, we used the previously reported (Waqas et al., 2014a) fungal endophyte Paecilomyces formosus LWL1 (national center for biotechnology information (NCBI) accession number JQ013813) isolated from the roots of cucumber. It produces several important secondary metabolites, such as bioactive GAs (GA1, (10.55±2.60) ng/ml; GA3, (8.49±1.50) ng/ml; GA4, (3.86±0.91) ng/ml; GA7, (6.65±1.60) ng/ml; GA9, (1.05±0.21) ng/ml); IAA, ((34.07±3.92) μg/ml), and organic acids including oxalic acid ((0.009±0.001) mg/L), quinic acid ((0.058±0.002) mg/L), tartaric acid ((0.047±0.003) mg/L), and malic acid ((12.92±0.26) mg/L). In the same reported study, P. formosus LWL1 promoted the growth of dwarf Waito-C (GAs mutant) and Dongjin (GAs normal) rice cultivars under normal environmental conditions. Because of these findings, we further hypothesized that the same new fungal strain will also improve and maintain Dongjin rice cultivar growth under prolonged heat stress. Other major reasons for choosing the Dongjin rice cultivar include its predominant cultivation in South Korea (where this study was conducted) and the fact that its endophyte-infected plants showed significant growth under cold, salt, and drought stresses (Redman et al., 2011). The current study further investigated this important benefit of P. formosus LWL1 in improving the abiotic stress (heat stress) resistance of Dongjin rice plants, which has not been previously reported for this cultivar and this fungal endophyte strain under prolonged heat-stress conditions.
2. Materials and methods
2.1. Plant materials and growth conditions
Seeds of Oryza sativa L. cv. Dongjin were procured from the National Institute of Crop Science, Rural Development Administration, South Korea. The rice seeds were thoroughly surface-sterilized in autoclaved pots with 2.5% (0.025 g/ml) sodium hypochlorite for 30 min, washed and soaked in autoclaved double-distilled water (DDW). After 3-d soaking in autoclaved DDW, germinated rice seedlings were transplanted to sterilized sand medium (moisture content 18%–23%, pH 4.5–5.5, electrical conductivity (EC) 2.0 ds/m, bulk density 0.7–1.0 mg/m3, grain size 125–250 μm, nitrogen 800–2500 mg/kg, and phosphorus 150–850 mg/kg; other components included zeolite (60.00%), diatomite (20.00%), and vermiculite (19.67%)) and placed in a growth chamber. During plant growth, the growth chamber (KGC-175 VH, KOENCON, Korea) conditions were adjusted to 12-h light (08:00–20:00, 30 °C, relative humidity 70%) and 12-h dark (20:00–08:00, 25 °C, relative humidity 70%). Yoshida solution was applied to the rice seedlings as a nutrient source for 2 weeks (Yoshida et al., 1959). The pH of the Yoshida solution was maintained at 5.0–5.3 during the growth period.
2.2. Fungal endophyte application and heat stress treatment
After 2 weeks, the germinated rice plants were transplanted to semi-hydroponic media in autoclaved pots (25 cm×20 cm×20 cm) composed of three-time autoclaved (at 121 °C for 15 min) horticultural soil (fungus-free bio-soil, Dongbu Farm Hannong, South Korea) with the following physico-chemical composition: peat moss (10%–15%), perlite (35%–40%), coco peat (45%–50%), and zeolite (6%–8%), and NH4 + about 0.09 mg/g, NO3 − about 0.205 mg/g, P2O5 about 0.35 mg/g, and K2O about 0.1 mg/g and DDW. At the start of the experiment, bioactive P. formosus LWL1 was inoculated into 3000 ml of Czapek broth medium (sucrose 30 g/L, sodium nitrate 3 g/L, dipotassium phosphate 1 g/L, magnesium sulphate 0.5 g/L, potassium chloride 0.5 g/L, ferrous sulphate 0.01 g/L, and pH 7.3 at 25 °C) and incubated for 10 d at 30 °C at 150 r/min. About 100 ml of culture broth with an adequate number of propagules was applied to the designated semi-hydroponic media in pots before transplanting of plants and thoroughly mixed with the semi-hydroponic media to ensure equal distribution in the root-zone area. Thereafter, freshly autoclaved DDW was applied as needed. Three days after transplanting, the plants with and without fungal endophytes were exposed to heat stress. During heat stress the same growth chamber conditions were maintained, except for temperature, which was adjusted to 37 °C/28 °C (day/night cycle).
2.3. Plant growth data
After 10 d, total plant (shoot and root) height, fresh weight (FW), dry weight (DW), and the leaf chlorophyll content (chlorophyll meter SPAD-502, Minolta, Japan) were collectively determined with thirty plant samples from each of the treatment groups, with and without heat stress and fungal endophyte infestation. After collection, samples were immediately frozen in liquid nitrogen and kept at −80 °C until freeze-dried (ISE Bondiro freeze dryer, Ilsin Bio Base, Yangju, South Korea).
2.4. Endogenous abscisic acid (ABA), jasmonic acid (JA), and total protein analyses
The freeze-dried plant samples (shoot and root) were used for ABA and JA analyses. We analyzed endogenous ABA content according to the methods of Qi et al. (1998) and Kamboj et al. (1999). Dried samples (0.1 g) were ground very finely with a grinder, extracted with 30 ml of extraction solution consisting of 95% isopropanol and 5% glacial acetic acid, and added to 200 ng of the internal standard [(±)-3,5,5,7,7,7-d6]-ABA. The extracted samples were dried and methylated by addition of diazomethane (10 μl). ABA contents were calculated by endogenous peak area/standard peak area×standard volume/sample weight.
For the determination of endogenous JA, freeze-dried plant samples (shoot and root; 0.1 g) were extracted with acetone and 50 mmol/L citric acid (70:30, v/v) and then 50 ng of the internal standard ([9,102H2]-9,10-dihydro-JA) was added to it. We followed the protocol as described by Waqas et al. (2014b). The extracts were then analyzed by gas chromatography-mass spectrometer (GC-MS, 6890 N GC system and the 5973 network mass selective detector, Agilent Technologies, USA). The endogenous JA contents were calculated according to the following formula: endo peak areas of JA/standard peak area of JA×volume of internal standard/sample weight.
Total protein content in P. formosus LWL1-inoculated and non-inoculated rice plants was determined according to the Bradford method (Bradford, 1976) using 100 mg fresh plant (shoot and root) samples from each treatment group.
2.5. Statistical analysis
The experiment was independently repeated three times in a completely randomized design under the same conditions. The data from each repetition of the same experiment were pooled together for statistical analysis. To identify significant differences between treatment means, Student’s t-tests were carried out using GraphPad Prism.
3. Results
3.1. Rice plant growth and heat stress adaptation response with and without fungal endophytes
The impact of P. formosus LWL1 on japonica Dongjin rice plant growth promotion was assessed under no stress (NS) and heat-stress (HS) conditions. Under normal growth conditions, P. formosus LWL1 application resulted in significantly increased plant height (17.02%), FW (51.65%), DW (34.97%), and chlorophyll content (13.98%) compared with controls (Table 1; Figs. 1a and 1b). The same growth-promoting effect of P. formosus LWL1 was also noted under prolonged heat stress, and plant height, FW, DW, and chlorophyll content were significantly increased by 17.97%, 50.32%, 47.52%, and 26.81%, respectively, compared with controls (Table 1; Figs. 1c and 1d).
Table 1.
Plant growth response of japonica rice (Oryza sativa L.) cv. Dongjin under no stress (NS) and prolonged heat stress (HS) with or without endophytic Paecilomyces formosus LWL1
| Treatment | Plant height (cm) | Fresh weight (g)1 | Dry weight (g)2 | Chlorophyll content (SPAD)3 |
| No stress (NS) | ||||
| Control | 21.85±1.00 | 26.89±1.24 | 1.43±0.08 | 31.61±1.63 |
| P. formosus LWL1 | 25.57±0.88* | 40.78±0.89* | 1.93±0.05* | 36.03±1.08* |
| Heat stress (HS) | ||||
| Control | 19.86±1.55 | 18.56±1.29 | 1.01±0.02 | 34.09±1.20 |
| P. formosus LWL1 | 23.43±1.48* | 27.90±1.03* | 1.49±0.03* | 43.23±0.89* |
Fresh weight per 30 plants (shoot and root).
Dry weight per 30 plants (shoot and root).
Soil-plant analysis development (SPAD) unit for measuring leaf chlorophyll content. The effects of P. formosus LWL1 on the growth of Dongjin (GAs normal) rice cultivars under normal environmental conditions (no stress) have been previously reported by Waqas et al. (2014).
Significant differences between plants treated with and without P. formosus at the P<0.05 level by Student’s t-test. The experiment was independently repeated three times and values of respective treatments were pooled together to calculate the mean±SD. In each experimental repetition, 30 plants were randomly selected per treatment from three replicates
Fig. 1.
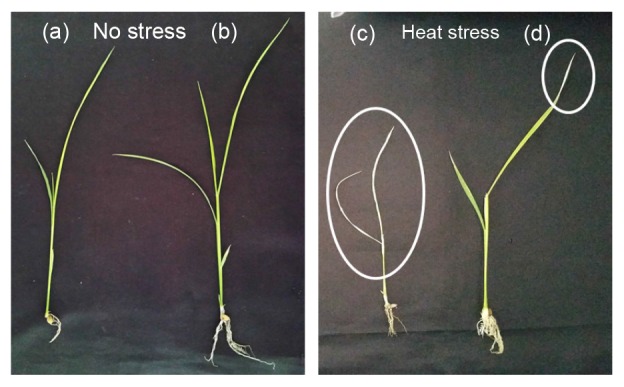
Plant growth promoting (a–d) and heat stress mitigation effect (c, d) of phytohormones and other secondary metabolites producing fungal endophyte Paecilomyces formosus LWL1 on japonica rice (Oryza sativa L.) cv. Dongjin under no stress (NS) and prolonged heat stress (HS)
Circle shows the deleterious effect of heat stress on endophytes free rice plant (c). However, the P. formosus LWL1 inoculation significantly reduced the deleterious effect as shown in (d)
3.2. P. formosus LWL1 regulation of endogenous ABA, JA, and total protein for prolonged heat-stress adaptation in japonica rice
The major abiotic stress modulating the phytohormone ABA (Khan et al., 2014) was analyzed in japonica Dongjin rice with and without P. formosus LWL1 under NS and HS (Fig. 2). In NS, the analysis revealed that there was no significant difference (P>0.05) in ABA levels between inoculated and non-inoculated plants. However, a significant difference was observed after exposure of plants inoculated with P. formosus and non-inoculated plants to HS. The inoculated rice plants had a significantly lower amount of ABA ((274.43±5.66) ng/g DW) compared with non-inoculated control plants ((369.83±12.84) ng/g DW) (Fig. 2).
Fig. 2.
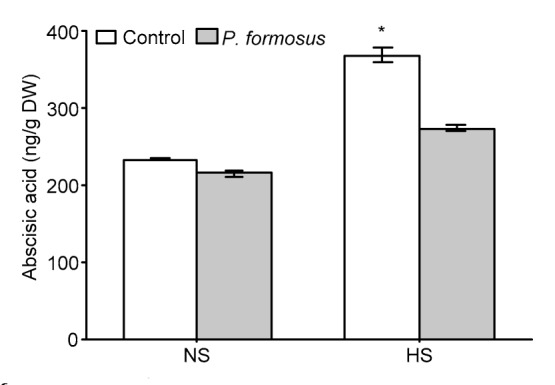
Major abiotic stress signaling phytohormone abscisic acid (ABA) in japonica rice (Oryza sativa L.) cv. Dongjin with or without endophytic Paecilomyces formosus LWL1 under no stress (NS) and prolonged heat stress (HS)
ABA was analyzed in freeze-dried samples of 30 randomly collected plants from each of three treatment replicates from three independently conducted experiments. Values of ABA analysis in respective treatments were pooled together to calculate the mean±SD. For each set of treatments, the asterisk on error bar indicates significant differences between plants treated with and without P. formosus at the P<0.05 level by Student’s t-test
The amount of JA was not significantly different in the inoculated and non-inoculated plants under NS (Fig. 3). Heat stress significantly increased the JA amount in non-inoculated plants ((275.46±6.75) ng/g DW) but the stress effect was mitigated by a lower JA amount in inoculated plants ((180.23±7.69) ng/g DW).
Fig. 3.
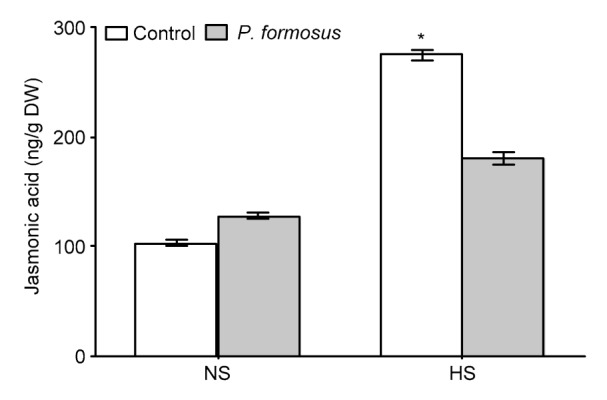
Phytohormone jasmonic acid (JA) in japonica rice (Oryza sativa L.) cv. Dongjin with or without endophytic Paecilomyces formosus LWL1 under no stress (NS) and prolonged heat stress (HS)
JA was analyzed in freeze-dried samples of 30 randomly collected plants from each of three treatments replicated in three independently conducted experiments. Values of JA analysis in respective treatments were pooled together to calculate the mean±SD. For each set of treatments, the asterisk on error bar indicates significant differences between plants treated with and without P. formosus at the P<0.05 level by Student’s t-test
P. formosus LWL1 inoculation extended its protective effect by increasing total protein content under NS and HS conditions (Fig. 4). The total protein content was significantly increased in inoculated plants compared with control plants under NS ((4.13±0.31) mg/g FW vs. (3.10±0.07) mg/g FW) and HS ((3.86±0.14) vs. (3.25±0.05) mg/g FW).
Fig. 4.
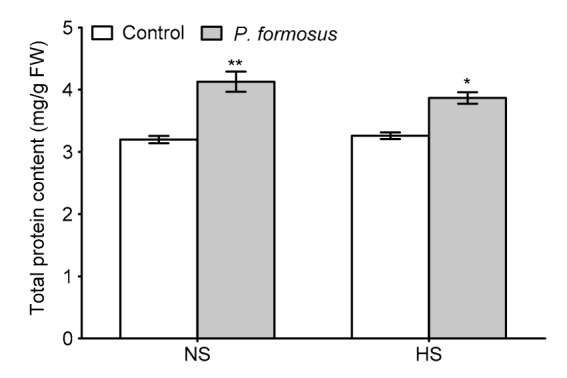
Total protein content in japonica rice (Oryza sativa L.) cv. Dongjin with or without endophytic Paecilomyces formosus LWL1 under no stress (NS) and prolonged heat stress (HS)
Total protein content was analyzed in fresh samples of 30 randomly collected plants from each of three treatment replicates from three independently conducted experiments. Values of total protein content in the respective treatments were pooled together to calculate the mean±SD. For each set of treatments, the asterisk on error bar indicates significant differences between plants treated with and without P. formosus at the * P<0.05 or ** P<0.01 level by Student’s t-test
4. Discussion
In the current study, P. formosus LWL1 improved plant growth of the japonica variety Dongjin rice under normal conditions and heat stress. Growth promotion may be attributed to the ability of P. formosus LWL1 to produce multiple important secondary metabolites (Khan et al., 2012; Waqas et al., 2014). Current results are in strong agreement with the findings of Khan et al. (2011), who reported the ability of the same fungus, P. formosus LHL10, to induce thermo-tolerance. In their experiment P. formosus LHL10 produced GAs and IAA but not in the same amount and types as that of P. formosus LWL1 used in the current study. However, the thermos-tolerance ability induced by several types of endophytes has been investigated in other crops or wild plants such as Capsicum annuum L., O. sativa L., and Dichanthelium lanuginosum (Redman et al., 2002; 2011; Khan et al., 2014).
IAA plays a role in plant development by regulating cell division and elongation. The inoculation of indole compounds beneficial to free-living plants (auxins) producing Trichoderma virens increased the biomass of wild-type Arabidopsis. The same fungal species stimulated lateral root formation and consequently increased the root surface area, which may enhance nutrient uptake for growth and development (Contreras-Cornejo et al., 2009). Expanding on the same idea, in another experiment Contreras-Cornejo et al. (2014) reported the salinity-stress tolerance induction potential of T. virens and T. atroviride in Arabidopsis seedlings. Plant-growth promotion under saline and normal conditions was related to an increase in endogenous IAA by fungal association that promotes a larger number of lateral roots and hairs. The Trichoderma spp. inoculation also enhanced the antioxidant and osmo-protectant status of Arabidopsis seedlings.
Another possible factor for plant growth promotion under normal and heat stress conditions may be the production of various kinds of organic acids, i.e., oxalic acid, quinic acid, tartaric acid, and malic acid, detected in the culture filtrate of the P. formosus LWL1 (Waqas et al., 2014a). Organic acids, secreted by microorganisms, lower the pH by acidifying the surrounding soil. In low-pH soil, inorganic phosphorus (Pi) is released from mineral phosphate resources, and the resultant solubilization then makes it available for plant uptake (Rodrıguez and Fraga, 1999). Microorganisms utilize these small organic compounds for nutrition, and they enhance plant growth through multiple pathways. One of these is the secretion of organic acids by these beneficial microorganisms to solubilize major plant nutrients for greater uptake. In another mechanism, the beneficial microorganisms modify the plant’s secreted organic compounds, which are then taken up again by the plant (Rodrıguez and Fraga, 1999; Ahemad and Kibret, 2014; Waqas et al., 2014a). In the current investigation, the attraction of beneficial microorganisms does not seem to be involved as the experiment was performed in gnotobiotic conditions; nutrient solubilization seems to be the major factor involved in rice growth promotion.
Plants up-regulate ABA biosynthesis under abiotic stresses, i.e., drought and heat, to reduce the stomatal openings and minimize transpiration (Khan et al., 2012; 2014; Hasanuzzaman et al., 2013). This process was investigated by the exogenous application of ABA in chickpeas under varying temperatures (Kumar et al., 2012). The exogenous ABA (2.5 μmol/L) not only mitigated the heat stress but improved chickpea seedling growth under extreme temperature stress of 45 °C/40 °C during a day/night cycle of 12 h/12 h. The inhibition of ABA by fluridone aggravated the heat intolerance by lowering the endogenous ABA level. Larkindale et al. (2005) investigated the involvement of ABA along with salicylic and active oxygen species in heat-stress protection and mutant Arabidopsis plants were severely defective in thermos-tolerance. In another experiment, Larkindale and Knight (2002) identified a protective effect of ABA in wild-type Arabidopsis by comparing it with ABA-insensitive abi-1. Moreover, the ABA-insensitive plants were highly susceptible to heat stress. However, our results are contradictory in that P. formosus LWL1 inoculation affected growth and heat tolerance by reducing the endogenous ABA level. These findings are in agreement with those of Khan et al. (2012; 2014) and may be attributed to the prevention of abiotic stress by various mechanisms including improvement in moisture and nutrient uptake. Khan et al. (2014) further analyzed the expression pattern of important genes related to ABA biosynthesis in GA-producing endophytes in both treated and control pepper plants under normal and heat-stress conditions. The GA-producing endophyte application significantly reduced the expression patterns of ABA1, ABA2, NCED3, AAO3, and the ABA-responsive gene RAB18 as shown by reverse transcription-polymerase chain reaction (RT-PCR) analysis.
JA is one of main defense hormones against abiotic and biotic stresses, and analysis showed the same pattern as that of ABA. The non-significant increase of JA in P. formosus LWL1-inoculated plants under the NS condition is in agreement with the findings of Conrath et al. (2006) and Waqas et al. (2014b). The insignificant increase in the JA level in inoculated rice plants may be the result of a priming effect of endophytes to prepare plants for any kind of challenge (Conrath et al., 2006; Waqas et al., 2014b). This small increase in JA may keep the defense system active but not at the expense of growth retardation. A high level of JA may then reduce growth at the cost of defense, as reported in rice and Arabidopsis, by delaying DELLA protein degradation (Yang et al., 2012). A decrease in the JA level of inoculated plants under HS is in agreement with the findings of Navarro-Meléndez and Heil (2014), who reported that infection of Phaseolus lunatus with its naturally associated endophyte Bartalinia pondoensis significantly reduced the JA level in mechanically damaged leaves. Production of phytohormones (GAs and IAA) and organic acids by P. formosus LWL1 may promote growth under NS and HS by maintaining the delicate balance of endogenous JA, i.e., maintaining it at a low enough level to produce the priming effect but not reduce growth or resistance to heat stress. Application of JA in minute amounts has also been reported to promote plant growth (Li et al., 2012), as revealed by its presence in small amounts in inoculated rice plants under normal conditions (Fig. 3). It may act as another compound, in addition to endophyte phytohormones, to promote plant growth and development.
The endophytic association with host plants has been reported to positively alter primary and secondary metabolites (Khan and Lee, 2013) under abiotic stress. The significant increase of a major primary secondary metabolite, i.e., total protein content, in japonica rice by P. formosus LWL1 under NS and HS is in agreement with the findings of Khan and Lee (2013). This investigation revealed that application of Penicillium funiculosum protected soybean plant growth under cadmium stress and increased the total protein content with or without cadmium stress. They also found that amino acids were metabolized normally with or without stress in endophyte-infected plants. Free amino acids, particularly proline, glutamine, and leucine accumulated in endophyte-infested soybean plants. Among these, the osmo-protectant proline is important for growth restoration and tolerance under abiotic stress.
5. Conclusions
The present study comes to the conclusion that endophytic fungi used in the present experiments are capable of producing phytohormones and bioactive compounds that can promote plant growth, induce abiotic stress tolerance, and prevent stress damage. The current data support the decisive role of P. formosus LWL1, which significantly improved japonica rice plant growth under prolonged heat stress. Endophytic association mitigated the heat damage effect and prevented plant stress as shown by down-regulation of the stress-related signaling molecules, ABA and JA, and increased in the total protein content.
Footnotes
Project supported by the National Research Foundation of Korea (NRF), Ministry of Science, ICT and Future-Planning through Basic-Science Research Program (No. 2014R1A1A2A10058022)
Compliance with ethics guidelines: Muhammad WAQAS, Abdul Latif KHAN, Raheem SHAHZAD, Ihsan ULLAH, Abdur Rahim KHAN, and In-Jung LEE declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ-Sci. 2014;26(1):1–20. doi: 10.1016/j.jksus.2013.05.001. [DOI] [Google Scholar]
- 2.Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Claeys H, Bodt SD, Inzé D. Gibberellins and DELLAs: central nodes in growth regulatory networks. Trends Plant Sci. 2014;19(4):231–239. doi: 10.1016/j.tplants.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Conrath U, Beckers GJM, Flors V, et al. Priming: getting ready for battle. Mol Plant-Microbe Inter. 2006;19(10):1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- 6.Contreras-Cornejo HA, Macias-Rodriguez L, Cortes-Penagos C, et al. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis . Plant Physiol. 2009;149(3):1579–1592. doi: 10.1104/pp.108.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contreras-Cornejo HA, Macías-Rodríguez L, Alfaro-Cuevas R, et al. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol Plant-Microbe Inter. 2014;27(6):503–514. doi: 10.1094/MPMI-09-13-0265-R. [DOI] [PubMed] [Google Scholar]
- 8.Folsom JJ, Begcy K, Hao X, et al. Rice Fertilization Independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol. 2014;165(1):238–248. doi: 10.1104/pp.113.232413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fragkostefanakis S, Röth S, Schleiff E, et al. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ. 2014;38(9):1881–1895. doi: 10.1111/pce.12396. [DOI] [PubMed] [Google Scholar]
- 10.Hasanuzzaman M, Nahar K, Alam MM, et al. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14(5):9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins KL, Arnold AE, Coley PD, et al. Communities of fungal endophytes in tropical forest grasses: highly diverse host- and habitat generalists characterized by strong spatial structure. Fungal Ecol. 2014;8:1–11. doi: 10.1016/j.funeco.2013.12.005. [DOI] [Google Scholar]
- 12.Kamboj JS, Browning G, Blake PS, et al. GC-MS SIM analysis of abscisic acid and indole-3-acetic acid in shoot bark of apple root stocks. J Plant Growth Regul. 1999;28(1):21–27. doi: 10.1023/A:1006299414481. [DOI] [Google Scholar]
- 13.Khan AL, Lee IJ. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013;13:86. doi: 10.1186/1471-2229-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan AL, Hamayun M, Radhakrishnan R, et al. Mutualistic association of Paecilomyces formosus LHL10 offers thermotolerance to Cucumis sativus . Antonie van Leeuwenhoek. 2012;101(2):267–279. doi: 10.1007/s10482-011-9630-x. [DOI] [PubMed] [Google Scholar]
- 15.Khan AL, Waqas M, Lee IJ. Resilience of Penicillium resedanum LK6 and exogenous gibberellin in improving Capsicum annuum growth under abiotic stresses. J Plant Res. 2014;128(2):259–268. doi: 10.1007/s10265-014-0688-1. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Kaushal N, Nayyar H, et al. Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiol Plant. 2012;34(5):1651–1658. doi: 10.1007/s11738-012-0959-1. [DOI] [Google Scholar]
- 17.Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128(2):682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkindale J, Hall JD, Knight MR, et al. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138(2):882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li DM, Guo YK, Li Q, et al. The pretreatment of cucumber with methyl jasmonate regulates antioxidant enzyme activities and protects chloroplast and mitochondrial ultrastructure in chilling-stressed leaves. Sci Hortic. 2012;143:135–143. doi: 10.1016/j.scienta.2012.06.020. [DOI] [Google Scholar]
- 20.Lin MY, Chai KH, Ko SS, et al. A positive feedback loop between HEAT SHOCK PROTEIN101 and HEAT STRESS-ASSOCIATED 32-KD PROTEIN modulates long-term acquired thermotolerance illustrating diverse heat stress responses in rice varieties. Plant Physiol. 2014;164(4):2045–2053. doi: 10.1104/pp.113.229609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro-Meléndez AL, Heil M. Symptomless endophytic fungi suppress endogenous levels of salicylic acid and interact with jasmonate-dependent indirect defenses of their host, Lima bean (Phaseolus lunatus) J Chem Ecol. 2014;40(7):816–825. doi: 10.1007/s10886-014-0477-2. [DOI] [PubMed] [Google Scholar]
- 22.Qi QG, Rose PA, Abrams GD, et al. Abscisic acid metabolism, 3-ketoacyl-coenzyme a synthase gene expression and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol. 1998;117(3):979–987. doi: 10.1104/pp.117.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redman RS, Sheehan KB, Stout RG, et al. Thermotolerance conferred to plant host and fungal endophyte during mutualistic symbiosis. Science. 2002;298(5598):1581. doi: 10.1126/science.1078055. [DOI] [PubMed] [Google Scholar]
- 24.Redman RS, Kim YO, Woodward CJDA, et al. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PLoS ONE. 2011;6(7):14823. doi: 10.1371/journal.pone.0014823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrıguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17(4-5):319–339. doi: 10.1016/S0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 26.Sgobba A, Paradiso A, Dipierro S, et al. Changes in antioxidants are critical in determining cell responses to short- and long-term heat stress. Physiol Plant. 2015;153(1):68–78. doi: 10.1111/ppl.12220. [DOI] [PubMed] [Google Scholar]
- 27.Waqas M, Khan AL, Lee IJ. Bioactive chemical constituents produced by endophytes and effects on rice plant growth. J Plant Inter. 2014;9(1):478–487. doi: 10.1080/17429145.2013.860562. [DOI] [Google Scholar]
- 28.Waqas M, Khan AL, Kang SM, et al. Phytohormone-producing fungal endophytes and hardwood derived biochar interact to ameliorate heavy metal stress in soybeans. Biol Fert Soils. 2014;50(7):1155–1167. doi: 10.1007/s00374-014-0937-4. [DOI] [Google Scholar]
- 29.Yang DL, Yao J, Mei CS, et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. PNAS. 2012;109:1192–1200. doi: 10.1073/pnas.1201616109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida S, Ohnishi Y, Kitagishi K. Role of silicon in rice nutrition. Soil Plant Food. 1959;5(3):127–133. doi: 10.1080/00380768.1959.10430905. [DOI] [Google Scholar]


