Abstract
Objective
The diagnostic accuracy of anorectal manometry (AM), which is necessary to diagnose functional defaecatory disorders (FDD), is unknown. Using blinded analysis and standardised reporting of diagnostic accuracy (STARD), we evaluated whether AM could discriminate between asymptomatic controls and patients with functional constipation (FC).
Design
Derived line-plots of anorectal pressure profiles during simulated defaecation were independently analysed in random order by 3 expert observers blinded to health status in 85 women with FC and 85 age-matched asymptomatic healthy volunteers (HV). Using accepted criteria, these pressure profiles were characterized as normal (i.e. increased rectal pressure coordinated with anal relaxation) or types I-IV dyssynergia. Inter-observer agreement and diagnostic accuracy were determined.
Results
Blinded consensus-based assessment disclosed a normal pattern in 16/170 (9%) of all participants and only 11/85 (13%) HV. The combined frequency of dyssynergic patterns (I-IV) was very similar in FC (80/85 [94%]) and HV (74/85 [87%]). Type I dyssynergia (‘paradoxical’ contraction) was less prevalent in FC (17/85 [20%] than HV (31/85 [36.5%], p=0.03). After statistical correction, only type IV dyssynergia was moderately useful for discriminating between FC (39/85 [46%] and HV 17/85 [20%], p=0.001, PPV=70.0%, positive LR=2.3). Inter-observer agreement was substantial or moderate for identifying a normal pattern, dyssynergia types I and IV, and FDD, and fair for types II and III.
Conclusions
While the interpretation of AM patterns is reproducible, nearly 90% of HV have a pattern that is currently regarded as “abnormal” by AM. Hence AM is of limited utility for distinguishing between FC and HV.
Keywords: Anorectal manometry, Functional constipation, Dyssynergic defaecation, Rectoanal gradient, STARD (standards for reporting diagnostic accuracy)
INTRODUCTION
Functional constipation (FC) is a common disorder with a pooled prevalence in the community of 14% and significant cost and health care utilization. [1] Disordered defaecation, which is diagnosed by anorectal tests, is common in patients with medically-refractory chronic constipation. [2–4] The Rome criteria for functional defaecation disorder (FDD) implicate disordered evacuation due to an inadequate rectoanal pressure gradient resulting from paradoxical contraction or inadequate relaxation of the pelvic floor muscles and/or to inadequate rectal propulsive forces during defaecation. [5–7] Thus to fulfil current (Rome III) diagnostic criteria for FDD, patients with FC must have evidence of two of the following criteria: (a) impaired evacuation; (b) inappropriate contraction of the pelvic floor muscles or <20% relaxation of basal resting pressure; (c) inadequate propulsive forces. [5] While impaired evacuation is usually assessed by balloon expulsion [8] or imaging [9, 10], criteria (b) and (c) are assessed by measuring rectal and anal pressures during simulated evacuation (“push” manoeuvre) with anorectal manometry (AM). [11]
Several expert reviews provide guidance on technical performance and interpretation of AM. [11, 12] Based on limited data in asymptomatic participants in which rectal and anal pressures were simultaneously measured during the push manoeuvre, [6, 13] a reduced rectoanal gradient during simulated evacuation is used to diagnose FDD. Four anal manometry (AM) patterns have been defined, all of which are characterised by paradoxical contraction or failure of anal relaxation i.e. dyssynergia. [14] These patterns are characterized by a paradoxical increase in anal pressure with (type I) or without (type II) adequate increase in rectal pressure; failure of reduction in anal pressure with (type III) or without (type IV) adequate increase in rectal pressure.
With the advent of high-resolution manometry, [15] the ability of AM to distinguish healthy asymptomatic individuals from those with defaecatory symptoms has been questioned [16] because contrary to conventional wisdom, the rectoanal gradient (i.e. the difference between rectal and anal pressure) during simulated evacuation was negative in a majority of asymptomatic women. [17] We therefore performed a prospective, blinded, assessment of anorectal pressure patterns in women with FC and asymptomatic women with high-resolution AM (HRAM). The conduct and reporting of this study applied STARD (standards for reporting diagnostic accuracy) criteria. [18] In the absence of a gold standard for diagnosis of dyssynergia (AM is the current standard), the specific aim was to evaluate the accuracy of AM (index test) in discriminating health from disease (this acting as a surrogate reference standard).
METHODS
Study population
Consecutive female patients referred for investigation of FC over a 6 month period (June – December 2013) to the Royal London Hospital (Barts Health NHS Trust) GI Physiology Unit were considered for study enrolment. Healthy asymptomatic female volunteers were recruited by advertisement at Barts and the London School of Medicine and Dentistry during the same period. Prior to arriving for investigation, all participants (FC and HV) completed a comprehensive symptom questionnaire incorporating the Cleveland Clinic Constipation score (CCCS). [19] A structured history was also undertaken (medical/surgical and obstetric). Inclusion criteria for FC patients were a diagnosis of functional constipation based on Rome III symptom criteria [20] and scoring ≥12 on the CCCS – an indication of severity. [21] HV were selected on the basis of exclusion of any significant GI disease, self-reported functional symptoms and CCCS <9 and St Marks Incontinence Score <5. [22] Other exclusions included pregnancy or lactation, history of diabetes, cardiovascular, renal or hepatic disease. Ethical approval was granted by the Queen Mary University Research Ethics Committee (ref QMREC 2010/74 and QMREC 2013/12), and written informed consent obtained. No specific exclusions were applied for either group that might affect how the test itself performs (limited challenge bias). [23] The majority of FC patients had undergone further specialist evaluations including radio-opaque marker transit studies and barium proctography. [9] All data were purposively collected before the index test.
Technical specifications (index test)
HRAM was performed in all participants using a solid-state catheter (UniTip: UniSensor AG, Switzerland), of external diameter 12 F, incorporating 12 microtransducers, each of which measured circumferential pressure by means of a unidirectional pressure sensor embedded within silicone gel. Ten of these sensors were spaced 0.8 cm apart, spanning 7.2 cm. The most proximal microtransducer was located within a non-latex balloon 3.3 cm proximal to these. The most distal sensor (located 2 cm below the most distal of the central 10 sensors) was used as an external reference. Before every study, the catheter was immersed in tepid water for at least 3 minutes to pre-wet the sensors. Sensors were then zeroed to atmospheric pressure. Data acquisition, online visualization and signal processing were performed using a commercially available manometric system (Solar GI HRM v9.1, Medical Measurement Systems (MMS), Enschede, Netherlands). Each participant was instructed to defaecate if required prior to investigation. No bowel preparation was given and all participants were studied in the left-lateral position with knees and hips flexed. Prior to catheter insertion, the ability of the participant to understand the commands “squeeze” and “push” were confirmed by digital rectal examination, the latter by asking the participant to “bear down as if to defaecate”. [24] All test manoeuvres were performed in accordance with published international minimum standards [11] using a previously published protocol. [24] The catheter was inserted into the anorectum with the distal 2 microtransducers visible (the second most distal being located immediately outside of the anal verge). Following a 3-minute run-in period for the purposes of familiarisation, manoeuvers were performed in a standard sequence with a 30 second recovery period between each. For examination of simulated defaecation the participant was asked to “push” as practised for 5 seconds; this manoeuvre was performed twice [11]. All tests were performed by one of three independent gastrointestinal physiology practitioners with experience of lower GI physiological testing (S.M.S, E.V.C. and U.G).
Line-plot traces of rectal and anal pressure changes were extracted from individual HRAM pressure traces (approx. 3–6 sensors in the rectum, and approx. 4–7 spanning the anal canal depending on anal canal length) by an automated process, using the ‘e-sleeve’, or ‘area of interest’ function within the colour contour plot (HRM v9.1, Medical Measurement Systems). This is in accord with other recent HRAM methodological publications. [25] Rectal and anal line plots were automatically derived from the maximum pressure within each region at all recorded time points during the second push manoeuvre. This method was selected to avoid the implicit bias conferred by selecting what is often termed a ‘representative’ line plot. [26] However, since such automated selection might confer a performance bias, original HRAM colour contour plots were also retained for analysis.
Definition, cut-offs and categories of the results of the index test
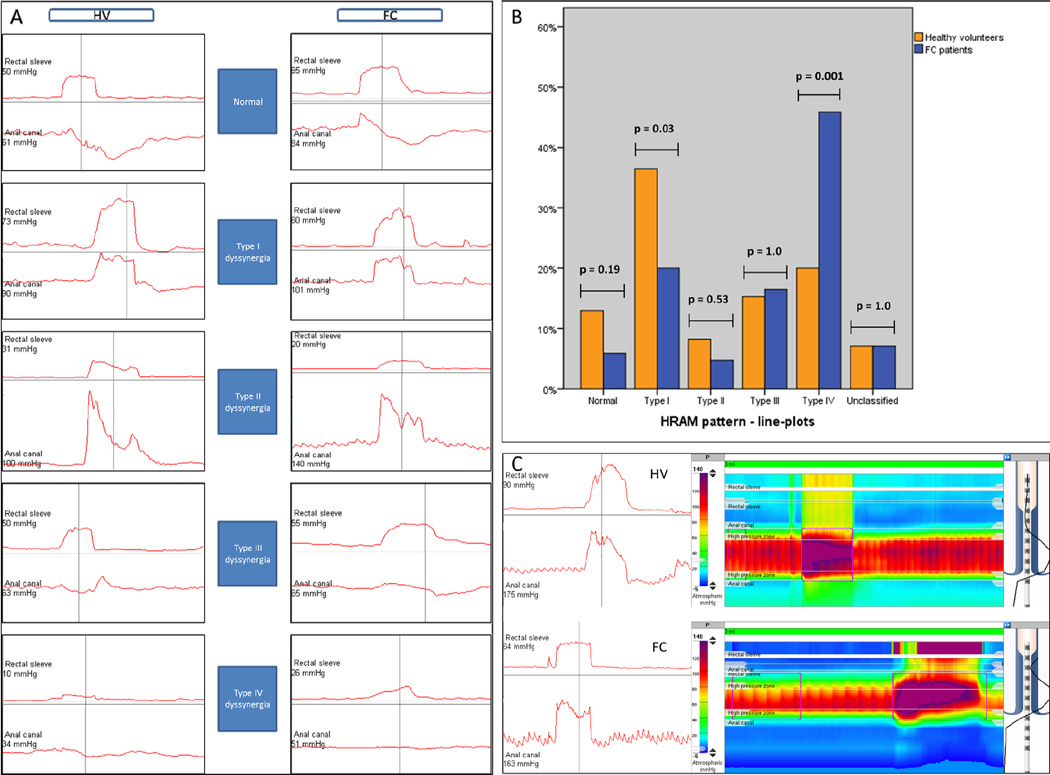
HRAM-derived line-plot images from both FC patients and HV were collated into a single database with all identifiers removed. Rectal and anal pressure changes [14] during the second “push” manoeuvre from each individual were presented electronically in a computer generated random order. Images were circulated to three observers (S.M.S, E.V.C. and A.E.B) who independently classified test results based on published criteria derived from standard manometry [14] and expert international guidance: [11]
normal – an adequate increase in rectal pressure (≥40mmHg) accompanied by a simultaneous reduction in anal pressure;
type I dyssynergia – an adequate increase in rectal pressure (≥40mmHg) accompanied by a paradoxical simultaneous increase in anal pressure;
type II dyssynergia – an inadequate increase in rectal pressure of (<40mmHg) (poor propulsive force) accompanied by a paradoxical simultaneous increase in anal pressure;
type III dyssynergia – an adequate increase in rectal pressure (≥40mmHg) accompanied by failure of reduction in anal pressure (≤20% baseline pressure);
type IV dyssynergia – an inadequate increase in rectal pressure of (<40mmHg) (poor propulsive force) accompanied by failure of reduction in anal pressure (≤20% baseline pressure).
Assimilation of the above derived two further diagnoses:
failed anal relaxation (FAR): any of 4 dyssynergia subtypes;
functional defaecation disorder (FDD): a combination of type II or type IV dyssynergia. In patients with FC, both subtypes are independently sufficient to fulfil a diagnosis of FDD without recourse to other tests. [5]
If changes in rectal and anal pressure were not consistent with any of the above recognised patterns these were reported as unclassified. To generate a final single result (for STARD analysis), disagreement between the 3 independent observers was resolved by consensus discussion mediated by the senior investigator (C.H.K.).
The same methods were used to classify tracings according to recent criteria derived from HRAM. [17] In this classification 2 phenotypes (“hybrid” and “low rectal”) closely resemble types II and IV dyssynergia, respectively. A third novel (“high anal”) pattern combined high anal pressures at rest and during evacuation (resembling the classical description of “anismus”). [27] This phenotype was therefore also studied using the published cut-off of >92 mmHg to define high resting pressure. [27]
Finally, original HRAM colour contour plots were reviewed and classified by the same blinded multi-observer methodology.
Statistical analysis
All data were analysed in accord with STARD guidance. [18] Inter-observer agreement was determined using kappa statistics. [28] Proportions of FC and HV participants with each finding were compared using Chi-square test with Bonferroni correction for multiple comparisons. Standard diagnostic accuracy metrics were calculated and presented with confidence intervals (CI): test sensitivity and specificity; positive and negative predictive values (PPV and NPV) and likelihood ratios (LRs). LRs were interpreted according to standard definitions. [29] Post-hoc analysis was performed using software functions to generate mean values for raw pressure data. These were analysed between groups as continuous variables using student t-tests; receiver operating characteristic (ROC) curves were used to explore diagnostic utility and optimal cut-offs. All data were analysed using Stata V10.0 (Stata Corporation, College Station, Texas, USA). Statistical significance was considered as p <0.05 (excepting Bonferroni correction).
RESULTS
Study population
A total 85 patients with functional constipation (FC) and 85 healthy volunteers (HV) meeting selection criteria formed the study cohort. FC patients were slightly older than HV (mean age 46 vs. 42 years) and were more likely to be parous (82% vs. 59%). All FC patients had a CCCS ≥12 (median 17, interquartile range [IQR] 13–19) where as no HV had a CCCS >5 (median 1, IQR 0–2). The findings for individual CCCS symptom domains and Rome III criteria for FC are shown in Table 1. All FC patients had symptomatic difficulty in evacuating stool from the rectum [30] and 21% had no relaxation or paradoxical contraction of puborectalis on digital rectal examination. Barium proctography was performed in 81 FC patients (4 patients exceeded the equipment safety weight limit) of whom 59 (73%) had abnormal defaecatory function based on departmental control data (12 [15%] functional only, 33 [41%] dynamic structural only, 14 [17%] both; Suppl. table 1). [9] Balloon expulsion testing was not performed reflecting local practice. Radio-opaque marker transit studies had been performed in 42/85 patients of whom 18 (43%) had delayed transit. Of these, 17 had concomitant proctographic abnormalities (only one patient had generalised marker distribution and a normal proctogram).
Table 1.
Characteristics of the study population.
| Characteristics | HV N=85 |
FC N=85 |
|---|---|---|
| Age (median, range) | 41 (18–68) | 46 (15–78) |
| Parity (%) | 50 (58.8) | 70 (82.4) |
| Self-reported constipation | 0 (0) | 85 (100) |
| CCCS (median, IQR) | 1 (0–2) | 17 (13–19) |
| Frequency of bowel movement | 0 (0) | 1 (0–2) |
| Painful evacuation effort | 0 (0) | 3 (2–4) |
| Feeling incomplete evacuation | 0 (0–1) | 4 (3–4) |
| Abdominal pain | 0 (0–1) | 3 (2–4) |
| Minutes in lavatory per attempt | 0 (0) | 2 (1–3) |
| Assistance for defecation | 0 (0) | 1 (0–2) |
| Unsuccessful attempts per 24 hours | 0 (0) | 2 (2–3) |
| Duration of constipation in years | 0 (0) | 3 (1–4) |
| Rome III criteria for functional constipation (%) | 0 (0) | 85 (100) |
| Straining° | 0 (0) | 81 (95) |
| Lumpy or hard stool° | 0 (0) | 74 (87) |
| Feeling incomplete evacuation° | 5 (5.9) | 84 (99) |
| Feeling anorectal obstruction° | 0 (0) | 78 (92) |
| Manual manoeuvres° | 0 (0) | 42 (49) |
| < 3 defecations per week | 0 (0) | 62 (73) |
| Rare loose stool without laxatives | 0 (0) | 85 (100) |
| Insufficient criteria for IBS | 85 (100) | 85 (100) |
HV: healthy volunteers; FC: patients with functional constipation; IQR: interquartile range; CCCS: Cleveland Clinic constipation score.
Symptoms present in at least 25% of defecations.
Performance of the index test: inter-observer agreement
Inter-observer agreement between the 3 primary investigators was substantial for diagnosis of FDD (k = 0.63; 144 / 170 [84.7%] traces had agreement of all 3 observers without need for consensus), types I (k=0.71) and IV dyssynergia (k=0.61); moderate for normal pattern (k=0.47) and dyssynergic patterns (FAR; k = 0.50); and fair for type II (k=0.40) and type III dyssynergia (k=0.35) (Suppl. Table 2).
Performance of the index test against the reference standard: diagnostic accuracy (Figure 1)
Fig. 1.
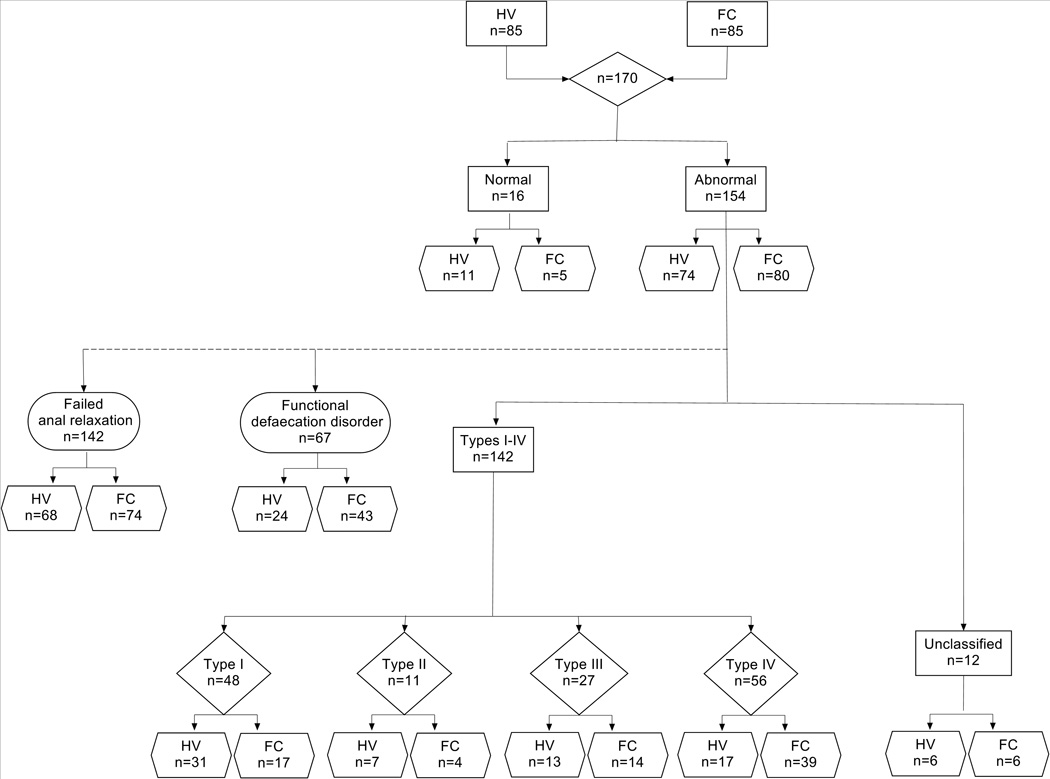
Standards for Reporting of Diagnostic Accuracy (STARD) flowchart detailing the study profile. HV: healthy volunteers; FC: patients with functional constipation.
Based on results of consensus, more than 90% of all participants showed an abnormal pattern of rectoanal coordination during attempted defaecation (Table 2; Figure 2A–B). A slightly higher proportion of patients with FC compared to HV (94 vs. 87%) had abnormal findings. The prevalence of type I dysynergia was more than 100% greater in HV than FC. The prevalence of types II and III dysynergia was comparable in HV and FC. Only type IV dyssynergia was found significantly more frequently in FC patients (46% FC patients vs. 20% HV, p=0.001). Based on synthesis of subtypes II and IV, 51% FC patients fulfilled AM criteria for FDD vs. 28% of HV (p=0.005). Seven percent of participants showed an inadequate increase in rectal pressure of <40mmHg (poor propulsive force), accompanied by a simultaneous reduction in anal pressure. Such changes are not consistent with any recognised patterns [14] deriving a fifth category. [31] This ‘unclassified’ pattern was equally encountered in FC patients and HV. These results were consistent regardless of individual observer (Suppl. Table 3).
Table 2.
Distribution of dyssynergic patterns in the study population after consensus.
| Line-plot patterns | All N=170 |
HV N=85 |
FC N=85 |
p |
|---|---|---|---|---|
| Abnormal | 154 (91) | 74 (87) | 80 (94) | .19 |
| Type I dyssynergia | 48 (28) | 31 (37) | 17 (20) | .03 |
| Type II dyssynergia | 11 (6) | 7 (8) | 4 (5) | .53 |
| Type III dyssynergia | 27 (16) | 13 (15) | 14 (17) | 1 |
| Type IV dyssynergia | 56 (33) | 17 (20) | 39 (46) | .001* |
| Unclassified | 12 (7) | 6 (7) | 6 (7) | 1 |
| Types I–IV (FAR) | 142 (84) | 68 (80) | 74 (87) | .30 |
| Types II + IV (FDD) | 67 (39) | 24 (28) | 43 (51) | .005** |
HV: healthy volunteers; FC: patients with functional constipation; FAR: failed anal relaxation; FDD: functional defecation disorder. Values in parenthesis are percentages.
Bonferroni correction requires p≤0.008;
Bonferroni correction requires p≤0.003
Fig. 2.
(A) Examples of line-plots patterns in the study population after consensus. HV: healthy volunteers; FC: patients with functional constipation. (B) Bar chart showing frequency of abnormal high-resolution anorectal manometry (HRAM) patterns in the study population after consensus. (C) High anal phenotype in a FC patient and HV as line plots and raw colour contour trace.
The diagnostic accuracy for discriminating between FC and HV was poor (Table 3). Only type IV dyssynergia had a positive likelihood ratio of 2.3 indicative of a ‘small’ increase in the likelihood of disease. [29] Others suggested no (LR: 0.5–1.0) or minimal increase (LR: 1.0–2.0) in disease likelihood. These measures of diagnostic accuracy were comparable across observers (Suppl. Table 4).
Table 3.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and positive likelihood ration (LR+) of HRAM for diagnosis dyssynergic subtypes and FDD.
| Line-plot patterns | Sensitivity | Specificity | PPV | NPV | LR+ |
|---|---|---|---|---|---|
| Abnormal | 94.1 | 12.9 | 51.9 | 68.8 | 1.1 |
| Type I dyssynergia | 20.0 | 63.5 | 35.4 | 44.3 | 0.6 |
| Type II dyssynergia | 4.7 | 91.8 | 36.4 | 49.1 | 0.6 |
| Type III dyssynergia | 16.5 | 84.7 | 51.9 | 50.3 | 1.1 |
| Type IV dyssynergia | 45.9 | 80.0 | 69.6 | 59.6 | 2.3 |
| Types I–IV (FAR) | 87.1 | 20.0 | 52.1 | 60.1 | 1.1 |
| Types II + IV (FDD) | 50.6 | 71.8 | 64.2 | 59.2 | 1.8 |
FAR: failed anal relaxation; FDD: functional defecation disorder.
All values, except LR+, expressed as percentages.
Performance of the index test using new HRAM criteria [17] and post-hoc data analysis
Overall, 13% of participants had the “high anal” phenotype [17] (Figure 2C), which was more frequent in FC patients (14/85 [17%]) than in HV (8/85 [9%]). However, differences were not significant (p=0.25). Considering the poor diagnostic accuracy of HRAM using published pattern-based criteria, software functions were used to generate mean values for relevant variables: resting anal pressure; push rectal pressure, anal pressure change and rectoanal pressure gradient during push manoeuvres (Table 4).
Table 4.
Post-hoc analysis of raw software-derived data for defaecatory pressure variables
| Variable | HV Mean (SD) |
FC Mean (SD) |
p-value (t-test) |
ROC curves AUC (CI) |
|---|---|---|---|---|
| Mean resting pressure | 64.5 (21.1) | 62.7 (25.8) | 0.31 | 0.519 (0.43–0.61) |
| Push rectal pressure | 42.3 (19.0) | 30.3 (17.2) | 0.0001 | 0.675 (0.59–0.76) |
| Push anal pressure change | −9.2 (18.9) | −6.1 (15.5) | 0.88 | 0.425 (0.34–0.51) |
| Rectoanal pressure gradient | −13.4 (26.9) | −26.3 (24.3) | 0.0007 | 0.639 (0.56–0.72) |
HV: healthy volunteers; FC: patients with functional constipation; SD: standard deviation; ROC receiver operating characteristic; AUC: area under the curve; CI: confidence interval.
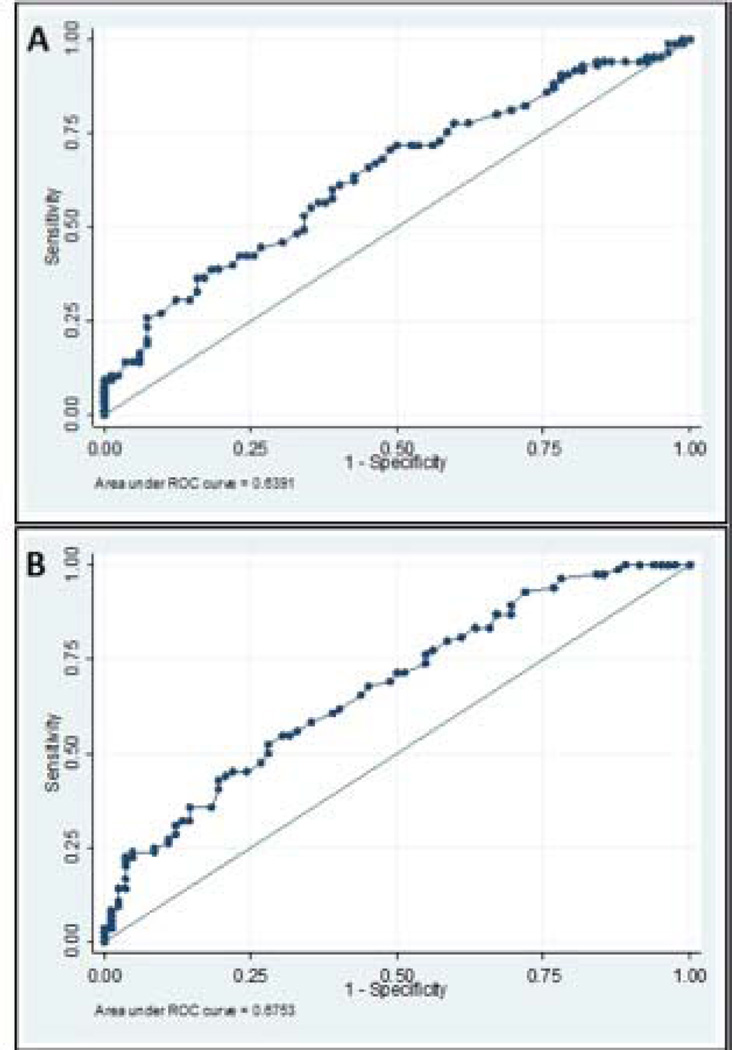
In keeping with earlier analyses, differences in anal sphincter relaxation during the push manoeuvre between healthy volunteers and patients were not significant (p=0.88). The rectoanal pressure gradient was found to be negative (i.e. <0 mmHg) for most (84%) participants regardless of health status (median, −18 mmHg; IQR, −38 to +1). However, a greater proportion of HV than FC patients (32/85 [38%] vs. 13/85 [15%]: OR 0.31 [CI 0.15–0.65]; p=0.002) had a gradient ≥0 mmHg. ROC curves of rectoanal pressure gradient and push rectal pressure had an area of 0.639 and 0.675 respectively for discriminating between FC and HV (Figure 3). For the push rectal pressure, cut-offs of less than 40mmHg [14] and ≤45mmHg [26] were most useful (i.e., sensitivity 53% and 43%; specificity 72% and 81%, respectively) for discriminating between FC and HV. Both parameters correctly identified 62% of patient’s health status. Adding other variables to push rectal pressure did not augment its utility for discriminating between FC and HV (data not shown).
Fig. 3.
Receiver operating characteristic (ROC) curves for rectoanal pressure gradient (A) and mean rectal pressure (B) in HV and FC patients during simulated defaecation (area under the curve [AUC]: 0.639 and 0.675, respectively).
Using identical analytical methodology for HRAM colour contour plots yielded almost identical results (Suppl. Table 5).
DISCUSSION
There are four main observations in this study. First, among experienced practitioners, inter-observer reproducibility for interpreting anorectal pressure patterns during simulated evacuation was acceptable. Second, only 9% of all participants exhibited the accepted [13] ‘normal’ pattern of rectoanal coordination (i.e. an adequate increase in rectal pressure, accompanied by a simultaneous reduction in anal pressure) during simulated defaecation. Third, 94% of FC patients and 87% of HV had abnormal manometric patterns during simulated defaecation; this difference was not statistically significant. Four, some individual patterns discriminated FC from HV. Thus, the type IV pattern was modestly useful for discriminating FC patients from HV (i.e., PPV 70%, LR+ 2.3). Subtypes II and IV, which are both characterised by inadequate sphincter relaxation and poor propulsion were observed in 51% FC patients vs. 28% of HV with a LR+ of 1.8. The ‘high anal’ phenotype, which is only based on anal pressure, was also found more commonly in FC patients (17%) than HV (9%). Hence, measures that rely on the rectal pressure generated during the push manoeuvre were more useful than those that rely on anal pressure alone for discriminating between FC and HV. These findings have implications on the diagnosis and understanding of the pathogenesis of FDD.
Anal sphincter dyssynergia
The term dyssynergia originated in urology in the mid-1970s [32] and was first used in the context of defecation in 1992 [33]. Implicit in the term is the failure of coordinated changes in anal sphincter activity. The current study refutes the concept that either a failure of anal relaxation or paradoxical anal contraction, as measured by high resolution AM, are of pathophysiological significance: these findings were present in 87% of FC patients and 80% of HV and there was no difference in absolute pressure data between groups (p=0.88). This finding is not novel. Indeed, the specificity of ‘anismus’, [27], defined solely by recruitment of EMG activity, has been questioned by more recent studies. [34, 35] Nevertheless, accepting significant historical differences in methodology, dyssynergia identified by manometry is widely used to diagnose FDD [35–38] (Table 5). However, it is generally recognized that these studies included relatively small numbers of participants (particularly healthy) while others were uncontrolled. [26] Moreover, no previous study has performed blinded assessment of AM tracings or evaluated inter-observer reproducibility. Despite these limitations, rectoanal pressure patterns during evacuation are recommended to diagnose and classify FDD. [5, 14]
Table 5.
Prevalence of dyssynergic defaecation in healthy volunteers (HV) and patients with constipation (FC) based on manometric criteria.
| Authors, Year | HV/FC | Prevalence of dyssynergic defaecation (%) * |
|
|---|---|---|---|
| HV | FC | ||
| Barnes et al, 1988 [39] | 15/31 | 20 | 97 |
| Kerrigan et al, 1989 [36] | 29/16 | 12 | 73 |
| Wald et al, 1990 [37] | 12/36 | 8 | 31 |
| Roberts et al, 1992 [35] | 20/71 | 5 | 24 |
| Merkel et al, 1993 [38] | 17/18 | 12 | 50 |
| Voderholzer et al, 1997 [40] | 18/102 | 22 | 41 |
| Rao et al, 1998 [13] | 25/35 | 20 | 51 |
| Ratuapli et al, 2013 [17] | 62/295 | 82 | 92 |
| Present study | 85/85 | 80 | 87 |
Different criteria were used for diagnosis: paradoxical sphincter contraction or failed anal relaxation [36–40], inability to raise intrarectal pressure [35], negative rectoanal gradient [13, 17], during simulated evacuation. In one study [35] the diagnosis was based on the combination of electromyographic recruitment >50%, evidence of an adequate intrarectal pressure on straining (>50 cmH2O) and defective evacuation (either quantitatively or in terms of prolonged straining).
The recently described ‘high anal’ phenotype, [17] characterized by high anal pressures at rest and during evacuation, closely resembles classical ‘anismus’, [27] and might be useful for discriminating between HV and FC. The current study, which evaluated these parameters on an independent sample, showed that while the pattern was not common (13%), it was approximately twice as frequent in FC patients compared to HV. Interestingly, the upper 90% percentile of the current HV dataset was 91mmHg and therefore almost identical to the published anismus literature (92mmHg). [27] Comparison of these data with those obtained by modern techniques has obvious limitations, however this finding still lends support to the use of the 90th percentile cut-off which was used to define the ‘high anal’ phenotype in the Ratuapli study [17] and suggests that anal dyssynergia in the context of very high resting pressures may have some diagnostic utility.
Rectoanal pressure gradient
The rectoanal pressure gradient is a function of both rectal propulsive effort and anal relaxation. Half a century ago, Harris et al. [41] observed that the rectoanal gradient during Valsalva manoeuvre was negative (i.e. sphincter pressures exceeded rectal pressures) in each of 41 times this manoeuvre was performed in 15 healthy males. This finding was confirmed by Phillips et al. [42] who showed that sphincter-ampulla pressure gradient was sustained despite rising intra-abdominal pressure by bearing down in 39 healthy volunteers. More recently, studies using high-resolution methods [16, 17] have also demonstrated that the rectoanal pressure gradient was negative in 51/62 (82%) asymptomatic women regardless of age (≥ or <50 years) and that there was considerable overlap in gradient between asymptomatic participants and constipated patients with abnormal balloon expulsion times. The current study is in keeping with the latter findings with 79% of all participants showing a negative pressure gradient. Although this variable did significantly differ between FC and HV (p=0.0007), the relatively similar proportions of participants with a negative pressure gradient (FC patients: 85% vs. HV: 62%) would confer limited utility of this variable to distinguish health from disease in practice. This presents an obvious conundrum for the current understanding of defaecation. One explanation for this observation was recently provided by Sauter et al [25] who hypothesise that simulated defecation may drive the recording catheter against the wall of the anal canal producing a ‘contact pressure’ that may result in a negative rectoanal pressure gradient.
Rectal propulsive force
The current study showed no differences in anal pressure changes between FC patients and HV but some differences in rectoanal pressure gradient. This can only be explained by differences in rectal pressure during simulated defecation (the term ‘rectal propulsive force’ is generally applied to this phenomenon although force and acceleration are not actually measured) and was confirmed by results (positive likelihood ratio for type IV dyssynergia) and post-hoc analysis of raw data (p=0.0001; AUC 0.673). This finding also agrees with the principal components analysis performed by Ratuapli et al. [17] in which a ‘low rectal’ phenotype was identified with close resemblance to type IV dyssynergia. Interestingly, ROC analysis of data from the current study also showed that the two cited cut-offs for type IV dyssynergia (40mmHg and 45mmHg) match exactly those from published diagnostic criteria for low rectal pressure. [14, 26]
The discrepancy between the current results and some previous studies (especially for sphincter dyssynergia) is hard to explain but could reflect anxiety in the laboratory setting, [43, 44] the challenge of replicating the process of defaecation in the left lateral position with an empty rectum, [6, 13] or variable equipment and protocols. [45] Rao et al. [43] evaluated rectal expulsion of balloon and a stool substitute with synchronous rectoanal pressures during evacuation in the left lateral and seated positions in 25 healthy participants. They showed that the rectoanal gradient during simulated defaecation and rectal pressures were higher in the seated than the left lateral position: 36% of asymptomatic participants had dyssynergia during traditional manometry in the recumbent position compared to 20% in the seated position (p<0.05). HRAM pressures during balloon expulsion performed in the seated and left lateral positions have also been compared in 220 women [46] Although rectoanal pressures were not evaluated in the seated position, the rectoanal gradient in participants with normal balloon expulsion in both positions was progressively more negative in those with abnormal balloon expulsion in recumbent only, seated only, and both positions. The current study only evaluated participants in the left lateral position and using the recommended minimum standard of 2 attempts at 5 second ‘push’. Although this is common current practice, [11, 24] the results emphasize that important test variables such as subject position and protocol (e.g. number and duration of push attempts) would benefit from international standardisation. The use of an automated ‘area of interest’ function was in accord with recent HRAM methodological publication [25] but is also a potential source of variation from user-selected ‘representative’ line plots. [13] To counter this potential criticism, we repeated all analyses using complete HRAM colour contour plots i.e. without restricting analysis to a single sensor-derived line trace. These results, based on a summative global impression of anal and rectal pressure profiles, however yielded identical conclusions.
Limitations
Despite attempts to reduce performance bias by study design and adherence to STARD guidance there were still some weaknesses in the current study. First, it must be acknowledged that health status as a reference standard can only be considered a surrogate of the notional concept of FDD. This approach had to be taken since anal manometry is (in current practice) the only tool to measure sphincter pressures without inclusion of more invasive (and themselves questionable) methods, e.g. needle EMG. [47] The FC patients studied all met symptomatic criteria for a defaecation disorder as defined by recent ACG guidance [30] and severity criteria based on Cleveland clinic score cut-off of 12 points. [21] The majority of those tested (73%) also had evidence of impaired evacuation on barium proctography. Further, only one patient had a generalised disturbance of colonic transit in the absence of abnormal proctography i.e. a probable primary disturbance of colonic motility. While the use of the balloon evacuation test would undoubtedly have provided further phenotypic information in the patient cohort as in other recent studies that show concordance between balloon expulsion time and dyssynergia, [48, 49] it must be recognised that the main driver of poor manometric discriminant ability was not the failure to ‘enrich’ or limit the FC population to those with perfectly-defined defaecatory dysfunction but rather the observation that a similar majority of HVs also had evidence of dyssynergic defaecation.
Secondly, the definition of reference standard was made before the index test; this is a weakness which makes the index test in effect retrospective but a necessary feature of design. It would be impractical to recruit participants of unknown health status for HRAM testing and use the test result to predict symptom status because only a minority would have constipation due to defaecatory dysfunction. Nevertheless, investigations and their interpretation were performed completely blind to health status by multiple observers who reached almost identical conclusions. Finally, while HRAM trace interpretation was completely blind to health status, all 3 observers were aware that the overall data set contained an equal mix of 85 FC patients and 85 HV participants. However, this equal split did not appear to influence the observers, one of whom defined nearly all presented traces (95%) as abnormal (Suppl. Table 2). It seems unlikely that the current study results are not purely a function of the new technology given the success of HRAM methods and their almost universal adoption in the study of oesophageal function [50] (an organ with much functional homology to the anorectum).
Clinical significance
The results of this study do not completely negate the value of AM in the diagnosis of FDD and subtypes. Rather, integration of the pattern classification systems proposed by Rao et al [14], the new physiological phenotypes proposed by Ratuapli et al [17] and the current data provides for potential modification of existing disease classification and guidance. In summary:
Anal sphincter dyssynergia is not a pathophysiological finding except in the relatively small proportion patients in which this is accompanied by high resting tone. With some systems, the cut-off of 92mmHg [27] to define high resting pressure appears valid.
Type IV dyssynergia [14] is useful for distinguishing disease from health. Either of the published cut-offs (<40 [14] or ≤45mmHg [26]) are valid for defining low rectal pressure. The dominant effect of low rectal pressure in the current study suggests that the previously proposed ‘low rectal’ phenotype [17] may be a more appropriate diagnostic term.
Further, the results do not negate the value of AM in the management of FC. AM is used with integrated balloon catheters to guide behavioural therapy using direct visual biofeedback with numerous trials attesting to the general success of this therapeutic approach [51–53] with associated increases in rectoanal pressure gradient, reflecting improved rectoanal coordination. [13] The current study did not evaluate this role for AM.
In conclusion, the present data obtained by blinded multi-observer assessment, and in a relatively large sample size, suggest that the interpretation of AM patterns is reproducible. However, nearly 90% of HV have a pattern that is currently regarded as “abnormal” by AM. Hence AM is of limited utility for distinguishing between FC and HV. Taken together with other recent studies, [16, 17] these findings reinforce the need to re-evaluate the role of AM with high resolution or high definition catheter systems [54] for diagnosing dyssynergic defaecation.
Supplementary Material
What is already known about this subject?
Most patients with functional constipation (FC) have symptoms characterised by unsatisfactory defaecation. These symptoms and objective findings of impaired evacuation, including measurement of anorectal pressure changes during simulated defaecation with anorectal manometry (AM), are used to diagnose FDD and characterize subtypes of FDD.
It is essential to accurately diagnose FDD because these conditions are more appropriately treated with pelvic floor biofeedback therapy than laxatives.
Recent studies using high-resolution manometry suggest that even healthy people have AM findings traditionally associated with FDD. The accuracy of AM for diagnosis of FDD is thus unclear.
What are the new findings?
Based on blinded evaluation of the anorectal pressure profile during simulated evacuation in 170 female participants (85 with significant FC and 85 age-matched asymptomatic controls) by 3 expert observers blinded to health status and using standardised reporting of diagnostic accuracy (STARD), approximately 90% of healthy volunteers and patients with FC had an abnormal anorectal pressure profile during simulated defaecation.
Among the patterns, only type IV dyssynergia was more prevalent (p=0.001) in FC patients than controls (46% vs. 20%) leading to a ‘small’ increase in the likelihood ratio. This pattern is characterized by an inadequate rectal propulsive force and impaired anal relaxation.
Inter-observer agreement was substantial or moderate for diagnosis of FDD, normal pattern and dyssynergia types I and IV and fair for types II and III.
How might it impact on clinical practice in the foreseeable future?
These findings suggest that high resolution AM is of limited accuracy for discriminating between healthy people and patients with FC. The role of AM for diagnosing FDD merits further study.
Acknowledgments
Dr. Bharucha receives grant support and consulting income from Medspira Inc and has licensed intellectual property on an anorectal manometry device to Medspira Inc. Dr. Bharucha has also filed a patent application for a device related to anorectal manometry with Given Imaging. Dr. Bharucha is supported by NIH Grant R01 DK78924.
Abbreviations
- AM
anal manometry
- CCCS
Cleveland Clinic constipation score
- CI
confidence intervals
- EMG
electromyography
- FC
functional constipation
- FDD
functional defaecation disorder
- HRAM
high resolution anal manomertry
- HV
healthy volunteer(s)
- LR
likelihood ratio
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver operating characteristic
- SD
standard deviation
- STARD
standardised reporting of diagnostic accuracy
Footnotes
Competing interests:
Ugo Grossi: no competing interests declared
Emma Carrington: no competing interests declared
Emma Horrocks: no competing interests declared
Mark Scott: no competing interests declared
Charles Knowles: no competing interests declared
Contributor statement: All authors were responsible for the conception and design of the study. UG collected the data. UG and CHK performed the statistical analysis and wrote the manuscript. All authors participated in the analysis and interpretation of the results and critical revision of the manuscript.
REFERENCES
- 1.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. The American journal of gastroenterology. 2011;106(9):1582–1591. doi: 10.1038/ajg.2011.164. quiz 1, 92. Epub 2011/05/25. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, Pemberton JH, Locke GR., 3rd American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144(1):218–238. doi: 10.1053/j.gastro.2012.10.028. Epub 2012/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook IJ, Talley NJ, Benninga MA, Rao SS, Scott SM. Chronic constipation: overview and challenges. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21(Suppl 2):1–8. doi: 10.1111/j.1365-2982.2009.01399.x. Epub 2009/10/15. [DOI] [PubMed] [Google Scholar]
- 4.Ragg J, McDonald R, Hompes R, Jones OM, Cunningham C, Lindsey I. Isolated colonic inertia is not usually the cause of chronic constipation. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2011;13(11):1299–1302. doi: 10.1111/j.1463-1318.2010.02455.x. Epub 2010/10/21. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130(5):1510–1518. doi: 10.1053/j.gastro.2005.11.064. Epub 2006/05/09. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. The American journal of gastroenterology. 1999;94(3):773–783. doi: 10.1111/j.1572-0241.1999.00950.x. Epub 1999/03/23. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Croak AJ, Gebhart JB, Berglund LJ, Seide BM, Zinsmeister AR, et al. Comparison of rectoanal axial forces in health and functional defecatory disorders. American journal of physiology Gastrointestinal and liver physiology. 2006;290(6):G1164–G1169. doi: 10.1152/ajpgi.00487.2005. Epub 2006/02/04. [DOI] [PubMed] [Google Scholar]
- 8.Chiarioni G, Kim SM, Vantini I, Whitehead WE. Validation of the Balloon Evacuation Test: Reproducibility and Agreement With Findings From Anorectal Manometry and Electromyography. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014 doi: 10.1016/j.cgh.2014.03.013. Epub 2014/03/29. [DOI] [PubMed] [Google Scholar]
- 9.Palit S, Bhan C, Lunniss PJ, Boyle DJ, Gladman MA, Knowles CH, et al. Evacuation proctography: a reappraisal of normal variability. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2014;16(7):538–546. doi: 10.1111/codi.12595. [DOI] [PubMed] [Google Scholar]
- 10.Halligan S, Malouf A, Bartram CI, Marshall M, Hollings N, Kamm MA. Predictive value of impaired evacuation at proctography in diagnosing anismus. AJR American journal of roentgenology. 2001;177(3):633–636. doi: 10.2214/ajr.177.3.1770633. Epub 2001/08/23. [DOI] [PubMed] [Google Scholar]
- 11.Rao SS, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2002;14(5):553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 12.Scott SM, Gladman MA. Manometric, sensorimotor, and neurophysiologic evaluation of anorectal function. Gastroenterology clinics of North America. 2008;37(3):511–538. vii. doi: 10.1016/j.gtc.2008.06.010. Epub 2008/09/17. [DOI] [PubMed] [Google Scholar]
- 13.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. The American journal of gastroenterology. 1998;93(7):1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. Epub 1998/07/22. [DOI] [PubMed] [Google Scholar]
- 14.Rao SS. Dyssynergic defecation and biofeedback therapy. Gastroenterology clinics of North America. 2008;37(3):569–586. viii. doi: 10.1016/j.gtc.2008.06.011. Epub 2008/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrington EV, Brokjaer A, Craven H, Zarate N, Horrocks EJ, Palit S, et al. Traditional measures of normal anal sphincter function using high-resolution anorectal manometry (HRAM) in 115 healthy volunteers. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014 doi: 10.1111/nmo.12307. Epub 2014/03/19. [DOI] [PubMed] [Google Scholar]
- 16.Noelting J, Ratuapli SK, Bharucha AE, Harvey DM, Ravi K, Zinsmeister AR. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. The American journal of gastroenterology. 2012;107(10):1530–1536. doi: 10.1038/ajg.2012.221. Epub 2012/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratuapli SK, Bharucha AE, Noelting J, Harvey DM, Zinsmeister AR. Phenotypic identification and classification of functional defecatory disorders using high-resolution anorectal manometry. Gastroenterology. 2013;144(2):314–322. e2. doi: 10.1053/j.gastro.2012.10.049. Epub 2012/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Annals of internal medicine. 2003;138(1):W1–W12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. Epub 2003/01/07. [DOI] [PubMed] [Google Scholar]
- 19.Mohammed SD, Lunniss PJ, Zarate N, Farmer AD, Grahame R, Aziz Q, et al. Joint hypermobility and rectal evacuatory dysfunction: an etiological link in abnormal connective tissue? Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22(10):1085–e283. doi: 10.1111/j.1365-2982.2010.01562.x. Epub 2010/07/14. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. Epub 2006/05/09. [DOI] [PubMed] [Google Scholar]
- 21.Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Diseases of the colon and rectum. 1996;39(6):681–685. doi: 10.1007/BF02056950. Epub 1996/06/01. [DOI] [PubMed] [Google Scholar]
- 22.Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44(1):77–80. doi: 10.1136/gut.44.1.77. Epub 1998/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philbrick JT, Horwitz RI, Feinstein AR. Methodologic problems of exercise testing for coronary artery disease: groups, analysis and bias. The American journal of cardiology. 1980;46(5):807–812. doi: 10.1016/0002-9149(80)90432-4. Epub 1980/11/01. [DOI] [PubMed] [Google Scholar]
- 24.Carrington EV, Brokjaer A, Craven H, Zarate N, Horrocks EJ, Palit S, et al. Traditional measures of normal anal sphincter function using high-resolution anorectal manometry (HRAM) in 115 healthy volunteers. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26(5):625–635. doi: 10.1111/nmo.12307. [DOI] [PubMed] [Google Scholar]
- 25.Sauter M, Heinrich H, Fox M, Misselwitz B, Halama M, Schwizer W, et al. Toward more accurate measurements of anorectal motor and sensory function in routine clinical practice: validation of high-resolution anorectal manometry and Rapid Barostat Bag measurements of rectal function. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26(5):685–695. doi: 10.1111/nmo.12317. [DOI] [PubMed] [Google Scholar]
- 26.Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus) Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(5):589–596. doi: 10.1111/j.1365-2982.2004.00526.x. Epub 2004/10/27. [DOI] [PubMed] [Google Scholar]
- 27.Preston DM, Lennard-Jones JE. Anismus in chronic constipation. Digestive diseases and sciences. 1985;30(5):413–418. doi: 10.1007/BF01318172. Epub 1985/05/01. [DOI] [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG. Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 29.McGee S. Simplifying likelihood ratios. Journal of general internal medicine. 2002;17(8):646–649. doi: 10.1046/j.1525-1497.2002.10750.x. Epub 2002/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. The American journal of gastroenterology. 2014;109(8):1141–1157. doi: 10.1038/ajg.2014.190. (Quiz) 058. Epub 2014/07/16. [DOI] [PubMed] [Google Scholar]
- 31.Bertiaux-Vandaele N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. The American journal of gastroenterology. 2011;106(12):2165–2173. doi: 10.1038/ajg.2011.257. Epub 2011/10/20. [DOI] [PubMed] [Google Scholar]
- 32.Andersen JT, Bradley WE. The syndrome of detrusor-sphincter dyssynergia. The Journal of urology. 1976;116(4):493–495. doi: 10.1016/s0022-5347(17)58875-8. Epub 1976/10/01. [DOI] [PubMed] [Google Scholar]
- 33.Merkel IS, Wald A. Training for straining: biofeedback for pelvic floor dyssynergia. The American journal of gastroenterology. 1992;87(9):1223–1224. Epub 1992/09/01. [PubMed] [Google Scholar]
- 34.Schouten WR, Briel JW, Auwerda JJ, van Dam JH, Gosselink MJ, Ginai AZ, et al. Anismus: fact or fiction? Diseases of the colon and rectum. 1997;40(9):1033–1041. doi: 10.1007/BF02050925. Epub 1997/09/18. [DOI] [PubMed] [Google Scholar]
- 35.Roberts JP, Womack NR, Hallan RI, Thorpe AC, Williams NS. Evidence from dynamic integrated proctography to redefine anismus. The British journal of surgery. 1992;79(11):1213–1215. doi: 10.1002/bjs.1800791140. Epub 1992/11/01. [DOI] [PubMed] [Google Scholar]
- 36.Kerrigan DD, Lucas MG, Sun WM, Donnelly TC, Read NW. Idiopathic constipation associated with impaired urethrovesical and sacral reflex function. The British journal of surgery. 1989;76(7):748–751. doi: 10.1002/bjs.1800760735. Epub 1989/07/01. [DOI] [PubMed] [Google Scholar]
- 37.Wald A, Caruana BJ, Freimanis MG, Bauman DH, Hinds JP. Contributions of evacuation proctography and anorectal manometry to evaluation of adults with constipation and defecatory difficulty. Digestive diseases and sciences. 1990;35(4):481–487. doi: 10.1007/BF01536923. Epub 1990/04/01. [DOI] [PubMed] [Google Scholar]
- 38.Merkel IS, Locher J, Burgio K, Towers A, Wald A. Physiologic and psychologic characteristics of an elderly population with chronic constipation. The American journal of gastroenterology. 1993;88(11):1854–1859. Epub 1993/11/01. [PubMed] [Google Scholar]
- 39.Barnes PR, Lennard-Jones JE. Function of the striated anal sphincter during straining in control subjects and constipated patients with a radiologically normal rectum or idiopathic megacolon. International journal of colorectal disease. 1988;3(4):207–209. doi: 10.1007/BF01660715. Epub 1988/11/01. [DOI] [PubMed] [Google Scholar]
- 40.Voderholzer WA, Neuhaus DA, Klauser AG, Tzavella K, Muller-Lissner SA, Schindlbeck NE. Paradoxical sphincter contraction is rarely indicative of anismus. Gut. 1997;41(2):258–262. doi: 10.1136/gut.41.2.258. Epub 1997/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris LD, Pope CE, 2nd, et al. "Squeeze" Vs. Resistance: An Evaluation of the Mechanism of Sphincter Competence. The Journal of clinical investigation. 1964;43:2272–2278. doi: 10.1172/JCI105101. Epub 1964/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips SF, Edwards DA. Some aspects of anal continence and defaecation. Gut. 1965;6(4):396–406. doi: 10.1136/gut.6.4.396. Epub 1965/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. The American journal of gastroenterology. 2006;101(12):2790–2796. doi: 10.1111/j.1572-0241.2006.00827.x. Epub 2006/10/10. [DOI] [PubMed] [Google Scholar]
- 44.Duthie GS, Bartolo DC. Anismus: the cause of constipation? Results of investigation and treatment. World journal of surgery. 1992;16(5):831–835. doi: 10.1007/BF02066978. Epub 1992/09/01. [DOI] [PubMed] [Google Scholar]
- 45.Carrington EV, Grossi U, Knowles CH, Scott SM. Normal values for high-resolution anorectal manometry: a time for consensus and collaboration. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26(9):1356–1357. doi: 10.1111/nmo.12364. Epub 2014/08/30. [DOI] [PubMed] [Google Scholar]
- 46.Ratuapli S, Bharucha AE, Harvey D, Zinsmeister AR. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(12):e813–e820. doi: 10.1111/nmo.12208. Epub 2013/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bharucha AE, Rao SS. An update on anorectal disorders for gastroenterologists. Gastroenterology. 2014;146(1):37–45. e2. doi: 10.1053/j.gastro.2013.10.062. Epub 2013/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minguez M, Herreros B, Sanchiz V, Hernandez V, Almela P, Anon R, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126(1):57–62. doi: 10.1053/j.gastro.2003.10.044. Epub 2003/12/31. [DOI] [PubMed] [Google Scholar]
- 49.Chiarioni G, Kim SM, Vantini I, Whitehead WE. Validation of the balloon evacuation test: reproducibility and agreement with findings from anorectal manometry and electromyography. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(12):2049–2054. doi: 10.1016/j.cgh.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Diseases of the colon and rectum. 2007;50(4):428–441. doi: 10.1007/s10350-006-0814-9. Epub 2007/02/13. [DOI] [PubMed] [Google Scholar]
- 52.Rao SS, Seaton K, Miller M, Brown K, Nygaard I, Stumbo P, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(3):331–338. doi: 10.1016/j.cgh.2006.12.023. Epub 2007/03/21. [DOI] [PubMed] [Google Scholar]
- 53.Rao SS, Valestin J, Brown CK, Zimmerman B, Schulze K. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. The American journal of gastroenterology. 2010;105(4):890–896. doi: 10.1038/ajg.2010.53. Epub 2010/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee YY, Erdogan A, Rao SS. High resolution and high definition anorectal manometry and pressure topography: diagnostic advance or a new kid on the block? Current gastroenterology reports. 2013;15(12):360. doi: 10.1007/s11894-013-0360-2. Epub 2013/11/26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.