Abstract
Histamine modulates several aspects of energy homeostasis. By activating histamine receptors in the hypothalamus the bioamine influences thermoregulation, its circadian rhythm, energy expenditure and feeding. These actions are brought about by activation of different histamine receptors and/or the recruitment of distinct neural pathways. In this review we describe the signaling mechanisms activated by histamine in the hypothalamus, the evidence for its role in modulating energy homeostasis as well as recent advances in the understanding of the cellular and neural network mechanisms involved.
Keywords: histamine, signaling, hypothalamus, energy homeostasis, H1 histamine receptor, H2 histamine receptor, H3 histamine receptor, H1R antagonist, feeding, thermoregulation
Introduction
Obesity is an important health problem because it is associated with an increased risk of metabolic and cardiovascular conditions such as diabetes mellitus, hypertension, and hyperlipidemia. Obesity results from an imbalance between energy intake and expenditure. The existent pharmacotherapy of obesity has very limited efficacy and therefore neurotransmitters and neuromodulators implicated in the regulation of energy metabolism represent opportunities for anti-obesity drug development. Several lines of evidence strongly suggest that histamine plays an important role in energy homeostasis by influencing both food intake and energy expenditure. Epidemiological evidence has revealed that use of antihistaminergic (H1 receptor antagonists/inverse agonists) medication, widely used against allergies, is associated with increased body weight and obesity. Moreover it has been proposed that other medications that have weight gain as a side effect (e.g. atypical antipsychotics) exert this action, at least in part, by acting also as antagonists at the H1 receptors (H1R). Transgenic animals in which histamine signaling is disrupted (H1R ko, H3R ko, HDC ko mouse) develop obesity and display increased food intake and decreased energy expenditure.
Histamine signaling in the brain
Histamine is synthesized in the tuberomamillary nucleus (TMN) neurons from histidine by the specific enzyme histidine decarboxylase (HDC). After release histamine is methylated by histamine N-methyl-transferase (which is located postsynaptically and in glia). The turnover of neuronal histamine is high, with its half-life being in the order of minutes (Dismukes and Snyder, 1974, Hough et al., 1984). Histaminergic fibers are especially dense in the cortex, hypothalamus, amygdala and striatum (reviewed in (Haas and Panula, 2003)). In the hypothalamus the histaminergic fibers are particularly dense in the anterior part (Wada, 1992). Another source of histamine, representing up to 50% of histamine contents in the brain, is represented by resident mast cells (Ulugol et al., 1996). Thus histamine released from mast cells induces wakefulness and plays a role in food seeking behavior during food deprivation (Chikahisa et al., 2013).
Four histamine receptors, which are GPCRs, have been cloned (H1-H4R). H1R, H2R and H3R are expressed in distinctive patterns in the brain (Arrang et al., 1995) and all three receptor types are highly expressed in the hypothalamus. The H1Rs mediate excitatory actions on central neurons usually by recruiting Gq/11 and PLC, which leads to the formation of the two second messengers, diacylglycerol (DAG) and inositol-1,4,5-triphosphate (Ins(1,4,5)P3) and Ca2+ release from internal stores. Several Ca2+-dependent processes have been reported to be influenced by histamine: first, the opening of a cationic nonselective conductance, which causes depolarization (Smith and Armstrong, 1993); second, the activation of the electrogenic Na+-Ca2+ exchanger in supraoptic neurons, which also causes depolarization (Smith and Armstrong, 1996); third, formation of nitric oxide and cyclic GMP (Richelson, 1978); fourth, hyperpolarization due to the opening of K+ channels (Weiger et al., 1997). In addition, blocking a leak potassium conductance through direct G-protein action, or through PLC, DAG and PKC, induces excitation in the thalamus (McCormick and Williamson, 1991), and in the striatum (Munakata and Akaike, 1994).
The H2Rs couple to Gs, adenylyl cyclase (AC) and PKA, which phosphorylates proteins and activates the transcription factor cyclic-AMP-response element (CRE)-binding protein (CREB). Usually H2R activation results in excitation or its enhancement. By activating H2Rs histamine reduces the small Ca2+-dependent K+ conductance (Haas and Konnerth, 1983) and reduces a long-lasting afterhyperpolarization thus affecting the accommodation of firing. Increased cyclic AMP concentration and PKA-mediated phosphorylation, shifts the activation of the inwardly rectifying Ih towards a more positive voltage and contributes to a depolarization that modifies the thalamic relay of sensory input (McCormick and Williamson, 1991).
H3Rs are present at histaminergic and other cell somata, dendrites and axons, where they provide negative feedback to restrict histamine synthesis and release. They also provide negative feedback on the release of other neurotransmitters, such as glutamate (Brown and Haas, 1999), acetylcholine and noradrenaline (Schlicker et al., 1992). H3Rs couple to Gi/o and inhibit high-voltage activated Ca2+ channels, a common mechanism for the regulation of exocytosis. Three functional splice variants of the H3R have been identified in the rat. In mouse, both RNAse protection assay experiments and PCR results revealed the presence of only one H3R isoform (Chen et al., 2003). H3Rs can also couple to the phospholipase A2 (PLA2) via the Gi/o proteins which results in production of arachidonic acid (Leurs et al., 1994).
In summary, H1Rs and H2Rs have mostly excitatory actions on neurons or potentiate excitatory inputs. By contrast, H3-receptor activation causes autoinhibition of TMN neurons and inhibition of neurotransmitter release. More recently morphological and physiological experiments demonstrate the presence of H3R also postsynaptically (Lazarov and Gratzl, 2006, Zhou et al., 2006, Lundius et al., 2010).
Role of hypothalamic histamine in feeding behavior
Several excellent reviews on this subject have been published (Jorgensen et al., 2007, Masaki and Yoshimatsu, 2009, 2010, Passani et al., 2011), therefore here we will only summarize the main observations. Hypothalamic histamine suppresses feeding behavior (Ookuma et al., 1989, Ookuma et al., 1993), mainly by activating H1R (Masaki et al., 2004). Histamine exerts its anorectic action by inhibiting appetite rather than by being a satiety signal (Valdes et al., 2010, Passani et al., 2011). The sites of action appear to be the ventromedial hypothalamus (VMH) and the paraventricular nucleus (PVN) and not the preoptic area/anterior hypothalamus (PO/AH) (Ookuma et al., 1989, Ookuma et al., 1993). Furthermore, HDC−/− (Hegyi et al., 2004), the H1R−/− (Masaki et al., 2004) and H3R−/− mice (Takahashi et al., 2002) display increased feeding and develop leptin-resistant obesity, demonstrating that histamine signaling regulates energy metabolism downstream of leptin. Furthermore, the reduction in body fat induced by central infusion of leptin is decreased in H1R−/− mice, indicating that this pathway is downstream of leptin (Masaki et al., 2001). Similarly, H1R signaling was shown to be downstream of amylin (Davidowa, 2007, Seth et al., 2012).
Besides the hypothalamus an important role in the control of feeding behavior is played by nucleus tractus solitarius (NTS) in the hindbrain which receives vagally mediated gastrointestinal satiation signals as well as blood-borne energy-related hormonal and nutrient signals (Grill and Hayes, 2012). Co-administration of amylin and leptin results in increased H1R expression in the VMH, arcuate as well as in the NTS (Seth et al., 2012).
The H1R pathway is independent or downstream of the melanocortin system (Masaki et al., 2003).
Epidemiological evidence as well as preclinical studies indicate that H1R antagonists/inverse agonists, widely used as antiallergic medication, are associated with increased food intake, body weight and obesity (Masaki et al., 2004, Ratliff et al., 2010). Furthermore, also other medications (e.g. atypical antipsychotics) that increase food intake and weight as a side effect have been shown to cause these actions, at least in part, by acting as H1R antagonists (Kim et al., 2007). The relative potencies of these drugs as H1 antagonists correlate with their orexigenic potencies (Kroeze et al., 2003). Conversely, H1R agonists and H3R antagonists have beneficial effects in obesity and several such drugs are in clinical trials (Celanire et al., 2005, Bonaventure et al., 2006, Barak et al., 2008, Poyurovsky et al., 2013). Furthermore, H1R agonists reduce the weight gain associated with antipsychotic treatment in humans and animal models (Deng et al., 2012, Poyurovsky et al., 2013). A recent study has found a significant association between H1R variants and body mass index in patients on antipsychotic medication (Vehof et al., 2011).
Histamine signaling in the hypothalamus, therefore, is relevant for the treatment of obesity since it has a dual action as an appetite suppressant acting in the (VMH and PVN) and as an enhancer of energy expenditure, acting in the PO/AH (see below).
Central control of thermoregulation
Homeothermia, the ability to regulate the core body temperature (Tcore) within a narrow range is observed in mammals and birds. The key role played by the PO/AH in the regulation of Tcore was established more than a 100 years ago, based on experimental brain lesions, and selective cooling and heating of brain regions with chronically implanted thermodes (reviewed in (Simon, 2000)). Sustained or alternating PO/AH cooling and heating induce physiological or behavioral thermoregulatory responses, causing Tcore to change in the direction opposite to that of the hypothalamic temperature (Thy). Hammel and collaborators proposed that a particular net thermoregulatory response was proportional to (Thy –Tset), where Tset was represents a hypothetical set reference, a complex parameter related to the level of activity of PO/AH thermoreceptors (Hammel et al., 1963). The nature of such thermoreceptors and the physiological relevance of central thermosensitivity are controversial (Barker and Carpenter, 1970, Kobayashi and Takahashi, 1993, Boulant, 2006). Furthermore, the thermosensitivity of PO/AH neurons is a plastic property both in vivo and in vitro. Thus, it has been found that the thermosensitivity can change rapidly in the presence of the pyrogens PGE2 (Tabarean et al., 2004) or IL-1 (Vasilenko et al., 2000, Sanchez-Alavez et al., 2006). Slower shifts in thermosensitivity are observed in PO/AH neurons during NREM sleep (Alam et al., 1995).
The neuronal network controlling brown adipose tissue (BAT) thermogenesis and the fever response has been studied extensively. Thermal and chemical stimulation in the PO/AH combined with selective hypothalamic transections have shown that warm-sensitive PO/AH neurons send efferent signals to loci involved in the control of BAT thermogenesis) (Zhang et al., 1995, Chen et al., 1998). Studies using combined retrograde labeling and immunocytochemistry revealed that EP3 prostanoid receptor-positive GABAergic PO/AH neurons project to the sympathetic premotor neurons in the rostral raphe pallidus (rRPA). The projections are either direct or via the dorsomedial hypothalamus (DMH) (Nakamura et al., 2002, Nakamura et al., 2005). Bilateral microinjections of GABA-A receptor agonists or antagonists into the rRPa or DMH, blocked the fever induced by intra-PO/AH PGE2 applications. These studies clearly revealed a tonic GABAergic inhibition of the DMH and rRPA by the PO/AH as crucial for basal thermoregulation and hyperthermic responses. Recent studies have established also the existence of direct glutamatergic projections from the PO/AH (Lundius et al., 2010, Dimitrov et al., 2011) as well as from the lateral hypothalamus (Tupone et al., 2011) to the rRPA and their activation results in hyperthermia. A subpopulation of glutamatergic PO/AH neurons projecting to rRPa are peptidergic (Dimitrov et al., 2011).
Central histaminergic modulation of core body temperature
Thermoregulatory functions of histamine signaling in the CNS have been discovered in various organisms from invertebrates (Hong et al., 2006) to lower vertebrates (Leger and Mathieson, 1997) as well as mammals. Early studies in mammals have identified a role of hypothalamic histamine in the control of body temperature (Green et al., 1975). The PO/AH, region which contains thermoregulatory neurons was identified as the main locus in which histamine affects body temperature (Colboc et al., 1982). Histamine injected in the medial preoptic nucleus (MPON) increases Tcore. Similarly, intra-MPON injection of a histamine-N-methyltransferase inhibitor (resulting in a local increase of endogenous histamine concentration) also produces hyperthermia (Gatti and Gertner, 1984). Behavioral temperature selection experiments demonstrate that preoptic histamine signaling affects both the “setpoint” of the hypothalamic thermostat, as well as heat loss mechanisms (Bugajski and Zacny, 1981). Both H1Rs and H2Rs appear to be involved in these responses. Histamine has been implicated also in more subtle thermal effects such as the rise in body temperature during arousal (Valdes et al., 2010).
Some studies suggest a hyperthermic tone due to histamine signaling. Thus, premedication with a H2R antagonist before general anesthesia augments core body hypothermia during this procedure (Hirose et al., 1995). The H3R−/− transgenic mice display a lowered core body temperature suggesting that these receptors mediate a tonic hyperthermic action (Toyota et al., 2002). While most studies find that histamine has hyperthermic actions, in pathological conditions histamine appears to mediate hypothermic responses. Ionizing radiation induces hypothermia that can be blocked by H1R and H2R antagonists applied centrally (Kandasamy and Hunt, 1988). Exposure of the head to ionizing radiation stimulates histamine release from brain-resident mast-cells (Kandasamy and Hunt, 1988). Other studies point to a hypothermic action of histamine, mediated exclusively by H1Rs. Thus, anaphylaxis-induced hypothermia is absent in HDC(−/−) mice or in the presence of H1R antagonists (Kang et al., 1999). Also, IL-1β-induced thermogenesis is potentiated by depletion of hypothalamic histamine (Sakata et al., 1995). The locus where histamine exerts its hypothermic effects has not been determined so far.
Peripherally, histamine is involved in the rise of skin blood flow during whole body heating (Wong et al., 2004). Similarly, combined H1R and H2R antagonists diminish the alcohol-induced flushing in individuals of Oriental origin (Miller et al., 1988).
Our studies have established that in mice histamine induces hyperthermia of similar amplitude when administered in either the medial (MPON) or the median (MnPO) preoptic nuclei (Sethi et al., 2012). Endogenous concentration of histamine was raised in either nucleus by local injection of histamine N-methyl transferase inhibitor (Sethi et al., 2012). H1R and H3R specific agonists were equally potent in inducing a hyperthermia when infused in the MnPO (Lundius et al., 2010). In contrast, H2R specific agonists mimicked the histamine effect when administered intra-MPON, while H1R specific agonists had a smaller effect (Tabarean et al., 2012). Surprisingly, H3R specific agonists were without effect in this nucleus (Tabarean et al., 2012). Recent work shows that histamine activates H1Rs and increases the Tcore also when injected in the DMH, region that contains glutamatergic thermoregulatory neurons that project to rRPa (unpublished observations).
Our experiments have also revealed that histamine modulation of the activity of GABAergic MnPO neurons provides a mechanism for selective modulation of Tcore at the beginning of the active phase of the circadian cycle (Sethi et al., 2011). Thus, injection of a H3R antagonist in the MnPO induces a delay in the onset of the rise of the body temperature associated with the active phase of the circadian cycle (Sethi et al., 2011).
Several H1R and H2R antagonists are clinically used in the treatment of several diseases however, surprisingly, very few thermoregulatory side effects have been reported. H1R antagonists (e.g. diphenhydramine hydrochloride) are clinically used in the treatment of histamine-mediated allergic conditions while H2R antagonists (e.g. ranitidine and cimetidine) are used to reduce the secretion of gastric acid. These compounds have little penetration through the blood-brain barrier which may explain few thermoregulatory side-effects. Nevertheless, H1R antagonists have been reported to increase seizure susceptibility in patients with febrile seizures (Takano et al., 2010, Zolaly, 2012). These observations suggest that even at very low concentrations, these drugs can act centrally to influence body temperature and other centrally regulated functions. Therefore, H1R antagonists in most cases should not be prescribed to patients, particularly young infants, with febrile seizures and epilepsy. Drug-induced fever due to H2R blockers was also encountered, however this rare occurrence seems to be due to an allergic reaction to the drugs, characterized by a marked increase in IgE (Hiraide et al., 1990).
Increased plasma levels of histamine as well as increased H1R expression in peripheral tissue are commonly associated with the systemic inflammatory response syndrome (Matsuda et al., 2004). The use of H1R or H2R antagonists in these conditions however does not appear to have beneficial effects. A possible role of central histamine signaling in symptoms related to this syndrome has not been explored so far. Since brain mast cells play a role in the regulation of thermoregulatory responses and survival following sepsis induction (Nautiyal et al., 2009) histamine is a possible candidate for these actions. Furthermore, histamine acting at distinct hypothalamic sites might induce either hypothermia or hyperthermia, depending on ambient temperature, as observed in systemic inflammatory response syndrome (Wanner et al., 2013).
Histaminergic control of energy expenditure
Maintenance of Tcore represents a major energy expenditure of a homeothermic organism. Uncoupling proteins (UCPs) are inner mitochondrial membrane transporters of free fatty acids, which dissipate the proton gradient by releasing stored energy as heat, without coupling to other energy consuming processes (Nicholls and Locke, 1984). UCP1 in brown adipose tissue (BAT) plays a central role in regulating energy expenditure and thermogenesis in rodents and neonates of larger mammalian species, including humans. UCP2 and UCP3 are not involved in adaptive thermogenesis, however their pharmacological activation in vivo can be significantly thermogenic (Brand and Esteves, 2005). The hypothalamus controls UCP1 and UCP3 expression in BAT and white adipose tissue (WAT) via the sympathetic neuron system. Infusion of histamine in the third ventricle or in the preoptic area (POA) produces similar increases in BAT sympathetic nerve activity (SNA) and in the UCP1 mRNA expression (Yasuda et al., 2004). In contrast similar injections in the lateral hypothalamus or the ventromedial hypothalamic nucleus were without effect (Yasuda et al., 2004), suggesting that the PO/AH is the principal locus of histaminergic modulation of thermogenesis. Histamine-deficient animals (HDC−/−) have an impaired ability to express UCP1 in BAT (Fulop et al., 2003) further suggesting a role of histamine signaling in the control of energy expenditure. Similarly, the upregulation of UCP1 mRNA expression induced by central infusion of leptin is markedly reduced in H1R−/− mice (Masaki et al., 2004) suggesting a role of this receptor subtype in mechanisms regulating energy expenditure. Increased hypothalamic histamine also results in a decreased respiratory quotient, which indicates increased lipid oxidation (Malmlof et al., 2005). In our study we have evaluated the effects of activation of histamine receptors in the MnPO and MPON by increasing the concentration of endogenous histamine or by local injection of specific agonists (Sethi et al., 2012). Both approaches induce an elevation of Tcore and decreased respiratory exchange ratio (RER). The hyperthermic effect is associated with a rapid increase in mRNA expression of UCPs in thermogenic tissues, the most robust being that of UCP1 in BAT. We have also found increased concentrations of UCP2 transcripts in white adipose tissue, observation that may explain the increased lipolysis reported previously (Tsuda et al., 2002). In diet-induced obese mice histamine had much diminished hyperthermic effects as well as reduced effect on RER. Similarly, the ability of preoptic histamine signaling to increase the expression of uncoupling proteins was abolished. We also found that the expression of mRNA encoding the H1R subtype in the preoptic area was significantly lower in obese animals (Sethi et al., 2012). Interestingly previous studies have reported reduced H1R binding in the hypothalamus of obese animals (Wu et al., 2013).
Additionally, histamine appears to be involved in the increased motor activity associated with arousal (Valdes et al., 2010), although its contribution to the total energy expenditure of an animal has not been estimated so far.
Cellular mechanisms involved in histamine induced hyperthermia
Extracellular recordings of rat PO/AH neurons have reported excitatory effects to bath-applied histamine, effect which was blocked by a H1R antagonist in most neurons while only in few neurons the excitation was blocked by H2R antagonist (Tsai et al., 1989). Our recent studies have revealed that histamine, applied locally, acts differentially on neurons of the MnPO and MPON respectively. The neurotransmitter reduced the spontaneous firing rate of thermoregulatory GABAergic MnPO neurons by activating H3Rs (Lundius et al., 2010). This effect involved a decrease in the level of phosphorylation of the extracellular signal-regulated kinase (ERK1/2) and was not dependent on synaptic activity. Single-cell reverse transcription/PCR analysis revealed the presence of H3R transcripts in the histamine-inhibited GABAergic MnPO neurons. H3R activation increased the A-type K+ current in these neurons and decreased their firing rate (Sethi et al., 2011). The Kv4.2 subunit is required for these actions since Kv4.2−/− preoptic GABAergic neurons are not affected by histamine or H3R agonists. Moreover, Kv4.2−/− mice display much decreased Tcore responses to histamine or H3R agonists.
Our experiments have also shown that a population of non-GABAergic MnPO preoptic neurons was depolarized, and their firing rate was enhanced by histamine acting at H1Rs (Lundius et al., 2010). As in previous reports, in MnPO neurons H1R couples to the PLC pathway and induces Ca2+ release from intracellular stores. The depolarization persisted in TTX and was not dependent on synaptic potentials, suggesting a postsynaptic action. Single-cell reverse transcription-PCR analysis revealed the presence of H1R transcripts in these cells. The inward current is activated in a Ca2+-dependent manner. It is important to note that the cationic inward current and the increase in intracellular Ca2+ concentration have both a transient component as well as a sustained one that lasts for tens of minutes after removal of the agonist. Similarly, histamine injected in the median preoptic nucleus (MnPO) induces long lasting hyperthermia in several mammalian species studied (Green et al., 1976, Colboc et al., 1982, Sethi et al., 2011), yet, in the brain histamine is quickly degraded by the activity of the histamine-N-methyltransferase. In the hypothalamus the neurotransmitter has a half-life in the order of minutes (Dismukes and Snyder, 1974, Hough et al., 1984) suggesting that its long lasting effects may reflect sustained changes in neuronal activity. The sustained component was blocked by TRPC channel blockers. Single-cell reverse transcription-PCR analysis of the cytoplasm harvested from recorded neurons revealed expression of TRPC1, TRPC5 and TRPC7 subunits in neurons excited by histamine. Similar experiments have also revealed the presence of transcripts for the glutamatergic marker Vglut2 and for the H1R in neurons excited by histamine. The histamine-induced inward current was reduced by intracellular application of antibodies directed against cytoplasmic sites of the TRPC1 or TRPC5 channel subunits (Tabarean, 2012). This data revealed a novel mechanism by which H1R activation induces persistent excitation and sustained intracellular Ca2+ elevation in glutamatergic MnPO neurons.
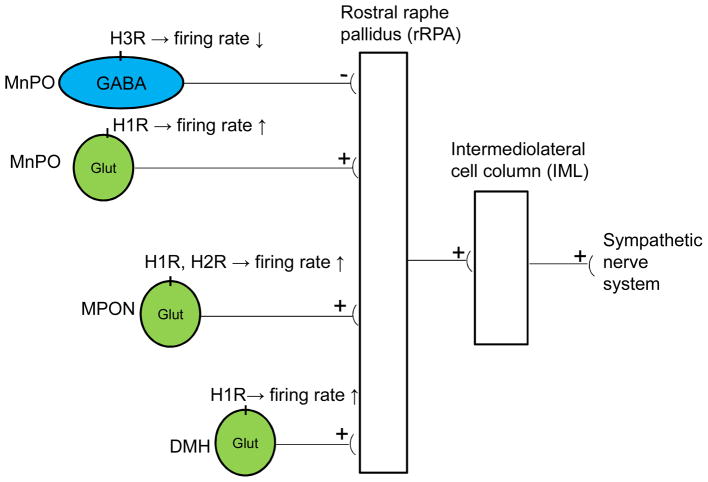
While histamine has a similar hyperthermic action when injected in the MPON, the mechanisms activated by histamine in the MPON are substantially different. Histamine activates both H1Rs and H2Rs, with the latter playing the predominant role (Tabarean et al., 2012) in MPON glutamatergic cells. In MPON the GABAergic neurons are not affectsd by histamine. A population of glutamatergic (Vglut2-positive) MPON neurons express H2Rs and are excited by H2R specific agonists. The agonists decreased the input resistance of the neuron and increased the depolarizing “sag” observed during hyperpolarizing current injections. H2R activation induced an inward current that was blocked by ZD7288, a specific blocker of the hyperpolarization activated cationic current (Ih). Increased Ih amplitude in response to hyperpolarizing voltage steps and a depolarizing shift in its voltage-dependent activation account for the inward current observed at voltages close to the resting membrane potential. Single-cell RT/PCR experiments revealed that the neurons excited by H2R agonists expressed the HCN1 and HCN2 channel subunits. Thus, taking in account also the kinetic properties, the Ih activated by histamine is likely conducted by heteromers formed by the HCN1 and HCN2 subunits. Overall, our data indicate that in the MPON histamine increases Tcore by potentiating the firing rate of glutamatergic neurons that express H2R or H1R (Tabarean et al., 2012). The specific mechanisms activated by H1Rs and a possible interaction between H1R and H2R signaling in these neurons have not been determined so far. Diagram 1 summarizes the cellular mechanisms and neural networks recruited by histaminergic modulation of thermogenesis.
Diagram 1.
The neural pathways and cellular mechanisms activated by histamine in the control of thermogenesis. The neural pathway scheme controlling thermogenesis was adapted from (Morrison, 2011). GABAergic neurons in the MnPOA tonically inhibit sympathetic premotor neurons in the rostral raphe pallidus. Histamine reduces the firing rates of GABAergic MnPO neurons. This results in stimulation of the sympathetic output system. Activation of H1 and/or H2 receptors expressed by glutamatergic MnPO, MPON, or DMH neurons, increase firing rates and stimulates the sympathetic neuron system.
Conclusions
Histamine has a complex influence on energy homeostasis and appears to be involved in numerous pathophysiological responses that involve changes in energy metabolism. At the level of the MnPO and MPON histamine induces potent hyperthermia and an increase in energy expenditure by activating several signaling pathways and neuronal networks. The site where histaminergic signaling results in hypothermia remains unknown. In spite of the potent modulation of both food intake and energy expenditure histamine signaling has not been successfully harnessed so far for the treatment of pathologies related to energy metabolism possibly due to peripheral side effects (H1R agonists) and actions on non-histaminergic signaling (H3R antagonists). The understanding of signaling downstream of histamine receptors and the unraveling of the neuronal networks involved in the various aspects of histaminergic modulation of food intake and energy expenditure may provide more specific targets for the pharmacotherapy of obesity.
Highlights.
Histamine acts in the hypothalamus, region that contains dense histaminergic projections, to decrease food intake and increase energy expenditure
Antagonistic activity at H1 receptors in the hypothalamus accounts, at least in part, for the weight gain associated with some anti-allergic and antipsychotic medications
Histamine receptors subtypes H1, H2 and H3 are expressed by distinct populations of hypothalamus neurons
Histamine exerts opposite effects and activates differential signaling mechanisms in distinct populations of hypothalamic neurons
Further studies of the neural networks involved in specific aspects of energy homeostasis are necessary
Acknowledgments
This work was supported by the National Institutes of Health Grants NS060799 and NS082255 (IVT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: thermosensitivity in rapid eye movement sleep. Am J Physiol. 1995;269:R1250–1257. doi: 10.1152/ajpregu.1995.269.5.R1250. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Drutel G, Garbarg M, Ruat M, Traiffort E, Schwartz JC. Molecular and functional diversity of histamine receptor subtypes. Ann N Y Acad Sci. 1995;757:314–323. doi: 10.1111/j.1749-6632.1995.tb17489.x. [DOI] [PubMed] [Google Scholar]
- Barak N, Greenway FL, Fujioka K, Aronne LJ, Kushner RF. Effect of histaminergic manipulation on weight in obese adults: a randomized placebo controlled trial. International journal of obesity. 2008;32:1559–1565. doi: 10.1038/ijo.2008.135. [DOI] [PubMed] [Google Scholar]
- Barker JL, Carpenter DO. Thermosensitivity of neurons in the sensorimotor cortex of the cat. Science. 1970;169:597–598. doi: 10.1126/science.169.3945.597. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Letavic M, Dugovic C, Wilson S, Aluisio L, Pudiak C, Lord B, Mazur C, Kamme F, Nishino S, Carruthers N, Lovenberg T. Histamine H(3) receptor antagonists: From target identification to drug leads. Biochem Pharmacol. 2006 doi: 10.1016/j.bcp.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Counterpoint: Heat-induced membrane depolarization of hypothalamic neurons: an unlikely mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1481–1484. doi: 10.1152/ajpregu.00655.2005. discussion R1484. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell metabolism. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brown RE, Haas HL. On the mechanism of histaminergic inhibition of glutamate release in the rat dentate gyrus. J Physiol. 1999;515 (Pt 3):777–786. doi: 10.1111/j.1469-7793.1999.777ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugajski J, Zacny E. The role of central histamine H1- and H2-receptors in hypothermia induced by histamine in the rat. Agents Actions. 1981;11:442–447. doi: 10.1007/BF02004704. [DOI] [PubMed] [Google Scholar]
- Celanire S, Wijtmans M, Talaga P, Leurs R, de Esch IJ. Keynote review: histamine H3 receptor antagonists reach out for the clinic. Drug Discov Today. 2005;10:1613–1627. doi: 10.1016/S1359-6446(05)03625-1. [DOI] [PubMed] [Google Scholar]
- Chen J, Liu C, Lovenberg TW. Molecular and pharmacological characterization of the mouse histamine H3 receptor. Eur J Pharmacol. 2003;467:57–65. doi: 10.1016/s0014-2999(03)01635-2. [DOI] [PubMed] [Google Scholar]
- Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512 (Pt 3):883–892. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikahisa S, Kodama T, Soya A, Sagawa Y, Ishimaru Y, Sei H, Nishino S. Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS One. 2013;8:e78434. doi: 10.1371/journal.pone.0078434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colboc O, Protais P, Costentin J. Histamine-induced rise in core temperature of chloral-anaesthetized rats: mediation by H2-receptors located in the preopticus area of hypothalamus. Neuropharmacology. 1982;21:45–50. doi: 10.1016/0028-3908(82)90209-x. [DOI] [PubMed] [Google Scholar]
- Davidowa H. Histamine H1-receptors differentially mediate the action of amylin on hypothalamic neurons in control and in overweight rats. Behav Brain Res. 2007;182:28–35. doi: 10.1016/j.bbr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Deng C, Lian J, Pai N, Huang XF. Reducing olanzapine-induced weight gain side effect by using betahistine: a study in the rat model. Journal of psychopharmacology. 2012;26:1271–1279. doi: 10.1177/0269881112449396. [DOI] [PubMed] [Google Scholar]
- Dimitrov E, Kim Y, Usdin TB. Tuberoinfundibular peptide of 39 residues modulates the mouse hypothalamic-pituitary-adrenal axis via paraventricular glutamatergic neurons. J Neurosci. 2011;518:4375–4394. doi: 10.1002/cne.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes K, Snyder SH. Histamine turnover in rat brain. Brain Res. 1974;78:467–481. doi: 10.1016/0006-8993(74)90929-9. [DOI] [PubMed] [Google Scholar]
- Fulop AK, Foldes A, Buzas E, Hegyi K, Miklos IH, Romics L, Kleiber M, Nagy A, Falus A, Kovacs KJ. Hyperleptinemia, visceral adiposity, and decreased glucose tolerance in mice with a targeted disruption of the histidine decarboxylase gene. Endocrinology. 2003;144:4306–4314. doi: 10.1210/en.2003-0222. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Gertner SB. The effect of intrahypothalamic injection of homodimaprit on blood pressure. Neuropharmacology. 1984;23:663–670. doi: 10.1016/0028-3908(84)90148-5. [DOI] [PubMed] [Google Scholar]
- Green MD, Cox B, Lomax P. Histamine H1- and H2-receptors in the central thermoregulatory pathways of the rat. J Neurosci Res. 1975;1:353–359. doi: 10.1002/jnr.490010504. [DOI] [PubMed] [Google Scholar]
- Green MD, Cox B, Lomax P. Sites and mechanisms of action of histamine in the central thermoregulatory pathways of the rat. Neuropharmacology. 1976;15:321–324. doi: 10.1016/0028-3908(76)90136-2. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell metabolism. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Haas HL, Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature. 1983;302:432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Hammel HT, Jackson DC, Stolwijk JA, Hardy JD, Stromme SB. Temperature Regulation by Hypothalamic Proportional Control with an Adjustable Set Point. J Appl Physiol. 1963;18:1146–1154. doi: 10.1152/jappl.1963.18.6.1146. [DOI] [PubMed] [Google Scholar]
- Hegyi K, Fulop KA, Kovacs KJ, Falus A, Toth S. High leptin level is accompanied with decreased long leptin receptor transcript in histamine deficient transgenic mice. Immunol Lett. 2004;92:193–197. doi: 10.1016/j.imlet.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Hiraide A, Yoshioka T, Ohshima S. IgE-mediated drug fever due to histamine H2-receptor blockers. Drug Saf. 1990;5:455–457. doi: 10.2165/00002018-199005060-00006. [DOI] [PubMed] [Google Scholar]
- Hirose M, Hara Y, Matsusaki M. Premedication with famotidine augments core hypothermia during general anesthesia. Anesthesiology. 1995;83:1179–1183. doi: 10.1097/00000542-199512000-00008. [DOI] [PubMed] [Google Scholar]
- Hong ST, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci. 2006;26:7245–7256. doi: 10.1523/JNEUROSCI.5426-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Khandelwal JK, Green JP. Histamine turnover in regions of rat brain. Brain Res. 1984;291:103–109. doi: 10.1016/0006-8993(84)90655-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen EA, Knigge U, Warberg J, Kjaer A. Histamine and the regulation of body weight. Neuroendocrinology. 2007;86:210–214. doi: 10.1159/000108341. [DOI] [PubMed] [Google Scholar]
- Kandasamy SB, Hunt WA. Involvement of histamine H1 and H2 receptors in hypothermia induced by ionizing radiation in guinea pigs. Life Sci. 1988;42:555–563. doi: 10.1016/0024-3205(88)90097-5. [DOI] [PubMed] [Google Scholar]
- Kang M, Yoshimatsu H, Kurokawa M, Ogawa R, Sakata T. Prostaglandin E2 mediates activation of hypothalamic histamine by interleukin-1beta in rats. Proc Soc Exp Biol Med. 1999;220:88–93. doi: 10.1046/j.1525-1373.1999.d01-14.x. [DOI] [PubMed] [Google Scholar]
- Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. From the Cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104:3456–3459. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Takahashi T. Whole-cell properties of temperature-sensitive neurons in rat hypothalamic slices. Proc Biol Sci. 1993;251:89–94. doi: 10.1098/rspb.1993.0013. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- Lazarov NE, Gratzl M. Selective expression of histamine receptors in rat mesencephalic trigeminal neurons. Neurosci Lett. 2006;404:67–71. doi: 10.1016/j.neulet.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Leger JP, Mathieson WB. Development of bombesin-like and histamine-like innervation in the bullfrog (Rana catesbeiana) central nervous system. Brain Behav Evol. 1997;49:63–77. doi: 10.1159/000112982. [DOI] [PubMed] [Google Scholar]
- Leurs R, Traiffort E, Arrang JM, Tardivel-Lacombe J, Ruat M, Schwartz JC. Guinea pig histamine H1 receptor. II. Stable expression in Chinese hamster ovary cells reveals the interaction with three major signal transduction pathways. J Neurochem. 1994;62:519–527. doi: 10.1046/j.1471-4159.1994.62020519.x. [DOI] [PubMed] [Google Scholar]
- Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci. 2010;30:4369–4381. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmlof K, Zaragoza F, Golozoubova V, Refsgaard HH, Cremers T, Raun K, Wulff BS, Johansen PB, Westerink B, Rimvall K. Influence of a selective histamine H3 receptor antagonist on hypothalamic neural activity, food intake and body weight. International journal of obesity. 2005;29:1402–1412. doi: 10.1038/sj.ijo.0803036. [DOI] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Yasuda T, Noguchi H, Kakuma T, Watanabe T, Sakata T, Yoshimatsu H. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004;53:2250–2260. doi: 10.2337/diabetes.53.9.2250. [DOI] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Yoshimichi G, Yasuda T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H. Neuronal histamine regulates food intake, adiposity, and uncoupling protein expression in agouti yellow (A(y)/a) obese mice. Endocrinology. 2003;144:2741–2748. doi: 10.1210/en.2003-0031. [DOI] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H. Molecular mechanisms of neuronal histamine and its receptors in obesity. Current molecular pharmacology. 2009;2:249–252. doi: 10.2174/1874467210902030249. [DOI] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H. Neuronal histamine and its receptors: implication of the pharmacological treatment of obesity. Current medicinal chemistry. 2010;17:4587–4592. doi: 10.2174/092986710794182944. [DOI] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H, Chiba S, Watanabe T, Sakata T. Targeted disruption of histamine H1-receptor attenuates regulatory effects of leptin on feeding, adiposity, and UCP family in mice. Diabetes. 2001;50:385–391. doi: 10.2337/diabetes.50.2.385. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Hattori Y, Takahashi Y, Nishihira J, Jesmin S, Kobayashi M, Gando S. Therapeutic effect of in vivo transfection of transcription factor decoy to NF-kappaB on septic lung in mice. American journal of physiology Lung cellular and molecular physiology. 2004;287:L1248–1255. doi: 10.1152/ajplung.00164.2004. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Williamson A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanisms of histaminergic control of arousal. J Neurosci. 1991;11:3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, Goodwin DW, Jones FC, Gabrielli WF, Pardo MP, Anand MM, Hall TB. Antihistamine blockade of alcohol-induced flushing in orientals. J Stud Alcohol. 1988;49:16–20. doi: 10.15288/jsa.1988.49.16. [DOI] [PubMed] [Google Scholar]
- Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–1149. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata M, Akaike N. Regulation of K+ conductance by histamine H1 and H2 receptors in neurones dissociated from rat neostriatum. J Physiol. 1994;480 (Pt 2):233–245. doi: 10.1113/jphysiol.1994.sp020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, McKellar H, Silverman AJ, Silver R. Mast cells are necessary for the hypothermic response to LPS-induced sepsis. Am J Physiol Regul Integr Comp Physiol. 2009;296:R595–602. doi: 10.1152/ajpregu.90888.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Ookuma K, Sakata T, Fukagawa K, Yoshimatsu H, Kurokawa M, Machidori H, Fujimoto K. Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res. 1993;628:235–242. doi: 10.1016/0006-8993(93)90960-u. [DOI] [PubMed] [Google Scholar]
- Ookuma K, Yoshimatsu H, Sakata T, Fujimoto K, Fukagawa F. Hypothalamic sites of neuronal histamine action on food intake by rats. Brain Res. 1989;490:268–275. doi: 10.1016/0006-8993(89)90244-8. [DOI] [PubMed] [Google Scholar]
- Passani MB, Blandina P, Torrealba F. The histamine H3 receptor and eating behavior. The Journal of pharmacology and experimental therapeutics. 2011;336:24–29. doi: 10.1124/jpet.110.171306. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Fuchs C, Pashinian A, Levi A, Weizman R, Weizman A. Reducing antipsychotic-induced weight gain in schizophrenia: a double-blind placebo-controlled study of reboxetine-betahistine combination. Psychopharmacology. 2013;226:615–622. doi: 10.1007/s00213-012-2935-2. [DOI] [PubMed] [Google Scholar]
- Ratliff JC, Barber JA, Palmese LB, Reutenauer EL, Tek C. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity. 2010;18:2398–2400. doi: 10.1038/oby.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E. Histamine H1 receptor-mediated guanosine 3′,5′-monophosphate formation by cultured mouse neuroblastoma cells. Science. 1978;201:69–71. doi: 10.1126/science.26974. [DOI] [PubMed] [Google Scholar]
- Sakata T, Kang M, Kurokawa M, Yoshimatsu H. Hypothalamic neuronal histamine modulates adaptive behavior and thermogenesis in response to endogenous pyrogen. Obes Res. 1995;3(Suppl 5):707S–712S. doi: 10.1002/j.1550-8528.1995.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1{beta} Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Behling A, Lummen G, Gothert M. Histamine H3A receptor-mediated inhibition of noradrenaline release in the mouse brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:489–493. doi: 10.1007/BF00176630. [DOI] [PubMed] [Google Scholar]
- Seth R, Terry DE, Parrish B, Bhatt R, Overton JM. Amylin-leptin coadministration stimulates central histaminergic signaling in rats. Brain Res. 2012;1442:15–24. doi: 10.1016/j.brainres.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Sethi J, Sanchez-Alavez M, Tabarean IV. Kv4.2 mediates histamine modulation of preoptic neuron activity and body temperature. PLoS One. 2011;6:e29134. doi: 10.1371/journal.pone.0029134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi J, Sanchez-Alavez M, Tabarean IV. Loss of histaminergic modulation of thermoregulation and energy homeostasis in obese mice. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. The enigma of deep-body thermosensory specificity. Int J Biometeorol. 2000;44:105–120. doi: 10.1007/s004840000060. [DOI] [PubMed] [Google Scholar]
- Smith BN, Armstrong WE. Histamine enhances the depolarizing afterpotential of immunohistochemically identified vasopressin neurons in the rat supraoptic nucleus via H1-receptor activation. Neuroscience. 1993;53:855–864. doi: 10.1016/0306-4522(93)90630-x. [DOI] [PubMed] [Google Scholar]
- Smith BN, Armstrong WE. The ionic dependence of the histamine-induced depolarization of vasopressin neurones in the rat supraoptic nucleus. J Physiol. 1996;495 (Pt 2):465–478. doi: 10.1113/jphysiol.1996.sp021607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV. Persistent histamine excitation of glutamatergic preoptic neurons. PLoS One. 2012;7:e47700. doi: 10.1371/journal.pone.0047700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV, Behrens MM, Bartfai T, Korn H. Prostaglandin E2-increased thermosensitivity of anterior hypothalamic neurons is associated with depressed inhibition. Proc Natl Acad Sci U S A. 2004;101:2590–2595. doi: 10.1073/pnas.0308718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV, Sanchez-Alavez M, Sethi J. Mechanism of H2 histamine receptor dependent modulation of body temperature and neuronal activity in the medial preoptic nucleus. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.02.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Suwa H, Ishikawa T, Kotani H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. The Journal of clinical investigation. 2002;110:1791–1799. doi: 10.1172/JCI200215784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Sakaue Y, Sokoda T, Sawai C, Akabori S, Maruo Y, Taga T, Ohno M, Takeuchi Y. Seizure susceptibility due to antihistamines in febrile seizures. Pediatr Neurol. 2010;42:277–279. doi: 10.1016/j.pediatrneurol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, Wu Y, Lee DH, Yanai K, Sakurai E, Watanabe T, Liu C, Chen J, Barbier AJ, Turek FW, Fung-Leung WP, Lovenberg TW. Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol. 2002;62:389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- Tsai CL, Matsumura K, Nakayama T, Itowi N, Yamatodani A, Wada H. Effects of histamine on thermosensitive neurons in rat preoptic slice preparations. Neurosci Lett. 1989;102:297–302. doi: 10.1016/0304-3940(89)90095-5. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Yoshimatsu H, Niijima A, Chiba S, Okeda T, Sakata T. Hypothalamic histamine neurons activate lipolysis in rat adipose tissue. Experimental biology and medicine. 2002;227:208–213. doi: 10.1177/153537020222700309. [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulugol A, Karadag HC, Dokmeci D, Baldik Y, Dokmeci I. The role of histamine H1 receptors in the thermoregulatory effect of morphine in mice. Eur J Pharmacol. 1996;308:49–52. doi: 10.1016/0014-2999(96)00260-9. [DOI] [PubMed] [Google Scholar]
- Valdes JL, Sanchez C, Riveros ME, Blandina P, Contreras M, Farias P, Torrealba F. The histaminergic tuberomammillary nucleus is critical for motivated arousal. Eur J Neurosci. 2010;31:2073–2085. doi: 10.1111/j.1460-9568.2010.07241.x. [DOI] [PubMed] [Google Scholar]
- Vasilenko VY, Petruchuk TA, Gourine VN, Pierau FK. Interleukin-1beta reduces temperature sensitivity but elevates thermal thresholds in different populations of warm-sensitive hypothalamic neurons in rat brain slices. Neurosci Lett. 2000;292:207–210. doi: 10.1016/s0304-3940(00)01470-1. [DOI] [PubMed] [Google Scholar]
- Vehof J, Risselada AJ, Al Hadithy AF, Burger H, Snieder H, Wilffert B, Arends J, Wunderink L, Knegtering H, Wiersma D, Cohen D, Mulder H, Bruggeman R. Association of genetic variants of the histamine H1 and muscarinic M3 receptors with BMI and HbA1c values in patients on antipsychotic medication. Psychopharmacology. 2011;216:257–265. doi: 10.1007/s00213-011-2211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. From biochemistry to pharmacology: the histaminergic neuron system in the brain. Nippon Yakurigaku Zasshi. 1992;99:63–81. doi: 10.1254/fpj.99.63. [DOI] [PubMed] [Google Scholar]
- Wanner SP, Yoshida K, Kulchitsky VA, Ivanov AI, Kanosue K, Romanovsky AA. Lipopolysaccharide-induced neuronal activation in the paraventricular and dorsomedial hypothalamus depends on ambient temperature. PLoS One. 2013;8:e75733. doi: 10.1371/journal.pone.0075733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger T, Stevens DR, Wunder L, Haas HL. Histamine H1 receptors in C6 glial cells are coupled to calcium-dependent potassium channels via release of calcium from internal stores. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:559–565. doi: 10.1007/pl00004983. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol. 2004;560:941–948. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yu Y, Wu Y, Patch C, Szabo A, Huang XF. Reduction of histamine H1 receptor binding induced by high-fat diet can be prevented by DHA and dietary fiber in specific brain areas of male rats. Brain research bulletin. 2013;97:119–125. doi: 10.1016/j.brainresbull.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Masaki T, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine regulates sympathetic nerve activity and expression of uncoupling protein 1 mRNA in brown adipose tissue in rats. Neuroscience. 2004;125:535–540. doi: 10.1016/j.neuroscience.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? J Physiol. 1995;485 (Pt 1):195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Xu JJ, Zhao Y, LeDoux MS, Zhou FM. Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J Neurophysiol. 2006;96:1581–1591. doi: 10.1152/jn.00148.2006. [DOI] [PubMed] [Google Scholar]
- Zolaly MA. Histamine H1 antagonists and clinical characteristics of febrile seizures. Int J Gen Med. 2012;5:277–281. doi: 10.2147/IJGM.S29320. [DOI] [PMC free article] [PubMed] [Google Scholar]