Structured Abstract
Purpose
To evaluate the relationship between mammographic breast density (MBD), background parenchymal enhancement (BPE), and fibroglandular tissue (FGT) in women with breast cancer (BC) and at high risk for developing BC.
Methods
Our institutional database was queried for patients who underwent mammography and MRI.
Results
403 (85%) had BC and 72 (15%) were at high risk. MBD (p=0.0005), BPE (p<0.0001), and FGT (p=0.02) were all higher in high risk women compared to the BC group.
Conclusions
Higher levels of MBD, BPE and FGT are seen in women at higher risk for developing BC when compared to women with BC.
Keywords: Breast cancer, Magnetic resonance imaging, Mammographic breast density, Fibroglandular tissue, Background parenchymal enhancement
1. Introduction
Mammographic breast density has been shown to be an independent risk factor for breast cancer [1–6]. While digital mammography has improved diagnostic accuracy in patients with dense breasts, sensitivity of mammography remains significantly lower in dense breasts, as low as 70% [7,8]. Decreased sensitivity of mammography is of particular concern to women at high-risk of developing breast cancer. There is well-established literature that supports the benefit of screening magnetic resonance imaging (MRI) in women at high-risk for breast cancer. Current screening recommendations for high-risk women may include the use of screening ultrasound and/or magnetic resonance imaging in addition to digital mammography. In its 2007 guidelines for breast cancer screening, the American Cancer Society recommended annual screening MRI as an adjunct to mammography for women at high-risk for breast cancer [9]. MRI has been shown to be an effective screening tool in this group, with sensitivity for cancer detection greater than that of mammography and of mammography and ultrasound combined [10–14].
With an increasing role of screening MRI, attention has turned to whether the amount and degree of enhancing breast tissue; including the proportion of fibroglandular tissue (FGT) and background parenchymal enhancement (BPE) is associated with a risk for breast cancer. FGT can be considered the MRI equivalent of mammographic breast density, which is a reflection of the stromal and epithelial tissue components of the breast tissue. Unlike breast density as depicted on mammography, MRI allows for a cross-sectional contiguous slice analysis of FGT [15]. BPE is thought to reflect the vascularity of the fibroglandular tissue and has been shown to be influenced by hormonal changes, including fluctuations in the menstrual cycle, menopausal status and hormone modifying medication [16–30].
Although BPE has been shown not to correlate directly with mammographic breast density [31], it similarly represents background noise on imaging, which may affect interpretation and detection accuracy [21, 32]. However, the association between BPE and breast cancer has not been as well established as it has for mammographic breast density. While a relationship between BPE and breast cancer risk has been suggested [15], other recent studies have demonstrated no increased incidence of cancer with increased BPE [21, 32]. There is very limited information regarding the relationship of fibroglandular tissue on contiguous MR images, breast density and BPE in a high-risk population.
Continuing investigation is needed to determine if these MRI imaging characteristics could be used as imaging biomarkers for cancer risk. The purpose of our study was to evaluate the relationship between mammographic breast density, and the MRI imaging characteristics of fibroglandular tissue and BPE in high-risk women compared with those undergoing evaluation after being diagnosed with breast cancer and prior to surgery.
2. Materials and methods
2.1 Study Population
The Breast Cancer Database was established in January 2010 and includes all patients undergoing definitive breast cancer surgery at our institution. The variables collected in this database include personal and family history, screening history, method of diagnosis, stage at diagnosis, details of treatment and outcomes. The High Risk Breast Cancer Consortium was established in January 2011 and includes all patients who do not have breast cancer, but are at an increased risk for developing the disease based on having a strong family history of breast cancer (at least 1 first degree relative) [33–34], BRCA1,2 mutation carriers [35], a history of atypical hyperplasia (AH) and/or lobular carcinoma in situ (LCIS) [36–39]. The variables collected in this database include family history, genetic testing results, screening history, risk reduction strategies, and outcomes. All clinical data are obtained from detailed questionnaires filled out by patients who consented to the database studies and medical chart review. Waiver of authorization and consent was granted by the institutional review board for this Health Insurance Portability and Accountability Act compliant retrospective study.
We queried both longitudinal databases to identify all women who underwent both mammography and breast MRI at our institution. Patients who had either a mammography and/or an MRI performed at an outside institution were excluded from the analysis, as well as patients who didn’t have an MRI within 6 months of having a mammogram. Both imaging modalities for the breast cancer patients were performed after diagnosis and before surgery as part of their pre-surgical workup. The imaging data collected for the high risk patients were taken from their routine screening protocols. Our screening protocol follows conventional practice and of alternating screening mammography and breast MRI every 6 months [40]. For subgroup analyses, three risk cohorts were formed based on the etiology of breast cancer risk. Group 1 included patients who have >20% lifetime risk with a strong family history of breast cancer and/or who were BRCA 1,2 mutation carriers [9]; Group 2 included patients with intermediate risk who had a history of AH and/or LCIS [9]; and Group 3 included patients who had familial and/or genetic risk (Group 1) as well as history of AH and/or LCIS (Group 2). These three risk groups are not mutually exclusive.
2.2 Mammography and MRI Assessments
2.2.1 Mammography Imaging Technique
All mammography was performed with digital technique using MAMMOMAT® Novation DR software (version V8.3, Siemens Healthcare).
2.2.2 MR Imaging Technique
MRI examinations were performed on commercially available systems at 1.5-T (Avanto, Siemens Medical Solutions) or 3.0-T (TIM Trio, Siemens Medical Solutions) using a dedicated surface breast coil (7-Channel Breast Biopsy Array, InVivo Research). Patients were imaged prone, using a standard imaging protocol that included a localizing sequence followed by a sagittal T2-weighted sequence (TR/TE, 7220/84); a sagittal T1-weighted non-fat-suppressed 3D fast spoiled gradient-recalled echo sequence (4.01/1.52; flip angle, 12°; matrix, 384 × 384; field of view, 270 mm; section thickness, 1 mm) followed by the same sagittal T1-weighted fat-suppressed 3D fast spoiled gradient-recalled echo sequence performed before and four times after a rapid bolus injection of 0.1 mmol/L of gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals) per kilogram of body weight at an injection rate of 2.0 mL/sec via an intravenous catheter. Image acquisition began immediately after administration of the contrast material and saline bolus. The first contrast-enhanced dynamic sequence was obtained at approximately 100 seconds, followed by four additional consecutive sequences (three sagittal followed by one delayed axial). At our institution, pre-menopausal women who undergo a screening breast MRI undergo their breast MRI on Days 7–14 of the menstrual cycle. Pre-menopausal women who are newly diagnosed with breast cancer undergo their breast MRI regardless of their menstrual cycle in an effort to minimize any delays in their breast surgery.
2.3 Image Interpretation
2.3.1 Mammographic density
Mammographic breast density was classified according to the American College of Radiology’s categories as almost entirely fatty, scattered fibroglandular, heterogeneously dense breasts, or extremely dense (Figure 1) [41]. Mammographic breast densities were evaluated on two separate occasions. They were obtained from the original radiology reports. In addition, the mammograms were randomized and a single fellowship-trained breast imaging radiologist with 13 years of experience reassessed the mammographic breast density.
Figure 1.
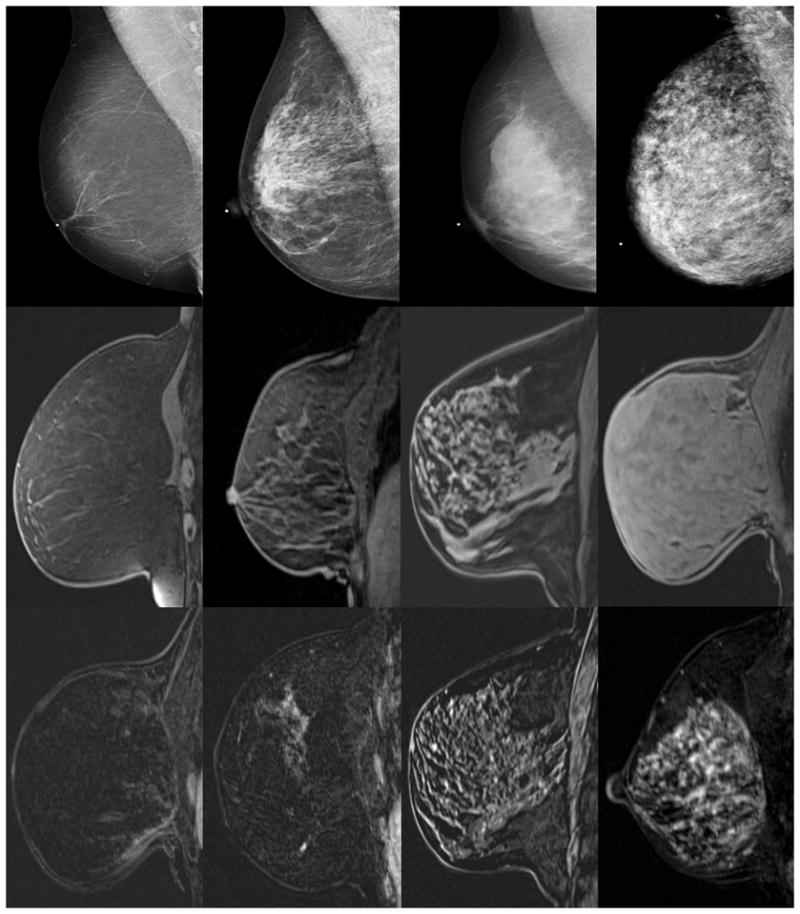
2.3.2 Fibroglandular tissue
FGT is defined as nonfat, non-cystic breast in relation to the total breast volume. The same experienced breast imaging radiologist assessed the amount of FGT parenchyma on contiguous nonfat- and fat-suppressed T1-weighted and T2-weighted images of both breasts. A four-point scale, similar to that used by the American College of Radiology to classify mammographic density, was used to classify the relative amount of FGT as almost entirely fat, scattered fibroglandular tissue, heterogeneous fibroglandular tissue and extreme fibroglandular tissue (Figure 1) [15]. Since FGT was not included in our reports, this assessment was performed by the radiologist.
2.3.3 Background parenchymal enhancement
BPE is the amount of enhancing fibroglandular tissue. The level of global BPE was assessed using a combination of pre- and the initial post-contrast T1-weighted fat saturated and subtracted images. The volume and intensity enhancement was graded on a 4 point scale as minimal, mild, moderate, or marked in accordance with the new Breast Imaging-Reporting and Data System (BI-RADS) categories (Figure 1) [42]. Both intensity and volume of background enhancement were considered in the assessment. In women who were newly diagnosed with breast cancer, the assessment of the BPE and FGT was performed in the contralateral breast. Similar to the mammographic breast density, the BPE were obtained from the radiology reports. In addition a single radiologist retrospectively assessed the BPE.
Evaluation of the mammographic breast density, BPE and the amount of FGT was performed by a single radiologist who was blinded to the clinical history. All images were anonymized. The mammograms and breast MRIs were randomized so that the reader did not interpret the breast MRI with knowledge of the mammographic density. In cases where there was disagreement between the prospective and retrospective reading, assessment of the mammographic breast density or the BPE, two radiologists reviewed the images in consensus. Although FGT and BPE have been introduced in the 2013 edition of the BI-RADS lexicon, our institution, we have been routinely incorporating these findings in our standard breast MRI reports since 2010.
2.4 Statistical Analysis
Descriptive analyses were used to summarize the data and to see the distribution of the variables between the high risk and breast cancer patients. To test for an association between the variables of interest and breast cancer status, Pearson’s Chi Square Test was used with a significance level of α=0.05. When the expected value in at least one of the cells was less than 5, Pearson’s Chi Square was substituted with Fisher’s Exact Test. Variables of interest included age, body mass index (BMI), family history of breast cancer, BRCA status, AH, and LCIS, mammographic breast density, BPE, FGT. Additionally, since age is a potential confounder, logistic regression with a significance level of α=0.05 was used to examine the association of each of the following imaging characteristics: mammographic breast density, BPE, and FGT with breast cancer status, adjusting for age. All analyses were executed using SAS software, version 9.3 (SAS Institute, Cary, NC).
3. Results
Out of a total of 1419 patients enrolled in the Breast Cancer Database and 366 patients enrolled in the High Risk Breast Cancer Consortium during the study period, there was a total of 475 patients who had both mammography and MRI as part of their clinical care and were eligible for analysis. All 475 patients were referred by their physician for a mammogram and MRI and their imaging was completed at our institution. There was a total of 403 (28%) breast cancer patients and 72 (20%) high-risk patients. The median age of the study population for both groups was 52 years (range 22–87 years) (Table 1). With regards to use of exogenous hormonal therapy, there were 13 (3%) breast cancer patients who had a history of hormonal therapy before their current breast cancer diagnosis and 11 (15%) high risk patients who had a history of hormonal therapy at the time of enrollment into the high risk database. As expected, the patients enrolled in the High Risk Breast Cancer Consortium had a higher prevalence of known risk factors including a strong family history, BRCA mutations, and personal history of AH, LCIS (Table 1).
Table 1.
Clinical Characteristics
| Variables | Total (N=475) | % | BCD* (N=403) | % | HRBCC† (N=72) | % |
|---|---|---|---|---|---|---|
| Age (years) | median 52 (22–87) | median 53 (22–87) | median 51 (24–77) | |||
| Body mass index (kg/m2) | ||||||
| Underweight + Normal (<25) | 253 | 53 | 204 | 51 | 49 | 68 |
| Overweight + Obese (≥25) | 222 | 47 | 199 | 49 | 23 | 32 |
| Family history of breast cancer | ||||||
| No | 274 | 58 | 248 | 62 | 26 | 36 |
| Yes | 201 | 42 | 155 | 38 | 46 | 64 |
| History of atypical hyperplasia | ||||||
| No | 433 | 91 | 387 | 96 | 46 | 64 |
| Yes | 42 | 9 | 16 | 4 | 26 | 36 |
| History of lobular carcinoma in situ | ||||||
| No | 451 | 95 | 394 | 98 | 57 | 79 |
| Yes | 24 | 5 | 9 | 2 | 15 | 21 |
| Mammographic breast density | ||||||
| Entirely Fatty | 32 | 7 | 29 | 7 | 3 | 4 |
| Scattered Fibroglandular Tissue | 137 | 29 | 120 | 30 | 17 | 24 |
| Heterogeneously Dense | 223 | 47 | 196 | 49 | 27 | 37 |
| Extremely Dense | 83 | 17 | 58 | 14 | 25 | 35 |
| Background parenchymal enhancement | ||||||
| Minimal | 73 | 15 | 50 | 12 | 23 | 32 |
| Mild | 210 | 44 | 193 | 48 | 17 | 24 |
| Moderate | 128 | 27 | 107 | 27 | 21 | 29 |
| Marked | 64 | 14 | 53 | 13 | 11 | 15 |
| Fibroglandular tissue | ||||||
| Entirely Fatty | 108 | 23 | 94 | 23 | 14 | 19 |
| Scattered Fibroglandular Tissue | 141 | 30 | 129 | 32 | 12 | 17 |
| Heterogeneously Dense | 133 | 28 | 106 | 26 | 27 | 38 |
| Extremely Dense | 93 | 19 | 74 | 19 | 19 | 26 |
Breast Cancer Database
High Risk Breast Cancer Consortium
The majority of patients who enrolled in the Breast Cancer Database had invasive ductal carcinoma (60%), had early stage disease (stage 0,I) (73%), were estrogen receptor (ER) positive (79%), progesterone (PR) positive (68%), and Her2-neu negative (98%) (Table 2). Out of the 403 patients with breast cancer, 272 (67%) presented with non-palpable lesions and 131 (33%) presented with palpable lesions (Table 2).
Table 2.
Tumor Characteristics of the Breast Cancer Patients
| Variables | Total (N=403) | % |
|---|---|---|
| Palpability | ||
| Non-palpable | 272 | 67 |
| Palpable | 131 | 33 |
| Histology | ||
| Ductal carcinoma in situ | 106 | 26 |
| Invasive ductal carcinoma | 243 | 60 |
| Invasive lobular carcinoma | 37 | 9 |
| Other | 17 | 4 |
| Tumor Stage | ||
| 0 | 104 | 26 |
| I | 191 | 47 |
| IIA, IIB | 71 | 18 |
| IIIA, IIIB, IIIC | 28 | 7 |
| IV | 4 | 1 |
| Estrogen Receptor | ||
| Negative | 85 | 21 |
| Positive | 318 | 79 |
| Progesterone Receptor | ||
| Negative | 130 | 32 |
| Positive | 273 | 68 |
| Her-2 neu | ||
| Negative | 285 | 98 |
| Positive | 4 | 1 |
| Equivocal | 2 | 1 |
| Invasive Histological Grade | ||
| Well differentiated (Grade 1) | 45 | 15 |
| Moderately differentiated (Grade 2) | 150 | 51 |
| Poorly differentiated (Grade 3) | 102 | 34 |
For the high-risk patients, there were no statistically significant associations between the etiology of breast cancer risk and mammographic breast density (p=0.65), BPE (p=0.54), and FGT (p=0.16) (Table 3). Similarly in the breast cancer patients, there were no statistically significant associations between the etiology of breast cancer risk and breast density (p=0.78), BPE (p=0.38), and FGT (p=0.82) (Table 3).
Table 3.
Association of mammographic breast density (MBD), BPE, FGT and etiology of breast cancer risk factors in women who are at high risk for breast cancer and with breast cancer
| Total | % | MBD | BPE | FGT | |
|---|---|---|---|---|---|
| High Risk Breast Cancer Consortium (N=72) | |||||
| Family history, BRCA positive | 47 | 65 | p=0.65 | p=0.54 | p=0.16 |
| AH, LCIS | 35 | 49 | |||
| Family history, BRCA positive, AH, LCIS | 9 | 12 | |||
| Breast Cancer Database (N=403) | |||||
| Family history, BRCA positive | 160 | 40 | p=0.78 | p=0.38 | p=0.82 |
| AH, LCIS | 21 | 5 | |||
| Family history, BRCA positive, AH, LCIS | 13 | 3 | |||
However, when comparing the two cohorts, we found that women with breast cancer had significantly lower mammographic breast density (p=0.0005), lower BPE (p<0.0001), and lower FGT (p=0.02). Since mammographic density, FGT and BPE typically decrease with age, a regression model was used to assess age as a confounder. We found that FGT (p=0.15) was not significant, while mammographic breast density (p=0.02) and BPE (p<0.0001) were still significant, even after adjusting for the effects of age.
The groups were then stratified by menopausal status to assess for hormonal influence on mammographic breast density, BPE, and FGT. Postmenopausal status was associated with lower breast density, lower BPE and lower FGT. In the premenopausal women, there was no association of breast cancer with mammographic breast density (p=0.20), BPE (p=0.15) or FGT (p=0.48). However, among post-menopausal women, there was a statistically significant association between breast cancer and lower mammographic density (p=0.003), lower BPE (p<0.0001) and lower FGT (p=0.02) (Table 4).
Table 4.
Association between breast cancer and mammographic breast density (MBD), BPE, and FGT by menopausal status
| Menopausal Status | Total | % | MBD | BPE | FGT |
|---|---|---|---|---|---|
| Pre-menopausal (N=218) | |||||
| High Risk Breast Cancer Consortium | 35 | 16 | p=0.20 | p=0.15 | p=0.48 |
| Breast Cancer Database | 183 | 84 | |||
| Post-menopausal (N=257) | |||||
| High Risk Breast Cancer Consortium | 37 | 14 | p=0.003 | p<0.0001 | p=0.02 |
| Breast Cancer Database | 220 | 86 | |||
In our study, there was a significantly higher proportion of women with BMI≥25 in our breast cancer cohort when compared to the high-risk cohort (p=0.006) (Table 1). When we stratified by menopausal status, we found no association of breast cancer and BMI among the premenopausal women (p=0.44). However, there was a statistically significant association between breast cancer and overweight and obese women (BMI≥25) (p=0.003).
Overall, there was a strong reader agreement in the assessment of mammographic breast density for 399 of 475 cases (84%). There was a moderately strong reader agreement in the assessment of BPE for 361 of 475 cases (76%).
4. Disscussion
Our study demonstrated that patients with breast cancer had lower mammographic breast density, lower BPE and lower FGT compared to patients at high risk for developing breast cancer. Several studies have investigated the relationship of mammographic breast density, BPE, and FGT, but there is a dearth of information on these imaging characteristics in a population of women at higher risk for developing breast cancer.
Mammographic breast density is a reflection of the stromal and epithelial tissue content of the breast. Extremely dense breast tissue (ACR category 4) compared with almost entirely fatty breast tissue (category 1) is considered to be at 4–6 times increased risk of developing breast cancer, independent of other risk factors. Breast density is estimated to account for up to 30–40% of attributable risk among average risk population [3,4,6]. The mechanism by which breast density increases breast cancer risk is unclear. Although the exact causality is still under investigation, theories include local hormonal microenvironment and growth factors that may contribute to breast cancer incidence [43]. The BI-RADS assessment of mammographic density has been incorporated into several risk models including the Breast Cancer Surveillance Consortium’s 1-year and 5-year models, developed using over 1 million women [44, 45].
Breast density is routinely included in mammographic reports according to the BI-RADS lexicon. Density is determined by the interpreter’s estimation of the percentage of stromal and epithelial tissue as compared to the fat content of the breast. As a result, there is an inherent degree of subjectivity in the assignment of breast density based on mammogram. Reported rates of agreement have been variable. Several studies have demonstrated only moderate inter-observer agreement on the level of breast density, with lowest rates of agreement reported in dense breasts and distinguishing scattered fibroglandular from heterogeneously dense breasts [46–50]. Inconsistency in assigning these levels of breast density could have significant clinical consequences as risk assessment and screening recommendations are being modified based on breast density. Recently, automated systems to measure mammographic breast density have been commercially available. However, these systems use different techniques to quantify breast density and agreement between the different systems is unclear [51]. A recent report by Eng et al. concluded that fully-automated methods are valid alternatives to the labor-intensive “gold standard” Cumulus for quantifying density in FFDM [51].
While investigation of the inter-observer agreement of MRI imaging characteristics of BPE and FGT have been somewhat limited, higher inter-observer agreement has been demonstrated with MRI-depicted breast composition. Ikeda, et. al. demonstrated substantial overall agreement with k=0.63 [52]. Here too, however, the greatest degree of disagreement was found in the intermediate densities or scattered fibroglandular and heterogeneously dense (k=0.33 and 0.14, respectively). A recent study of 4 radiologists found that with training, inter-reader agreement increased from fair (κ=0.36) to moderate (κ=0.48). Improvement was sustained at three weeks after the training was completed (κ=0.45). Intra-reader agreement between times 2 and 3 (κ=0.79, range 0.56–0.98) was greater than between times 1 and 2 (κ=0.62, range 0.45–0.84), indicating readers learned and retained. Training consisted of a single two hour interactive presentation prepared by our fellowship trained breast radiologist with the most breast imaging (23 years) and breast MRI (12 years) experience at our institution in conjunction with examples from our institution and the published literature [53].
Currently, determination of BPE and FGT on MRI has been based on subjective estimation of percentage of fibroglandular tissue relative to the entire breast, similar to mammographic density assessment. However, MRI provides the possibility of a more quantitative determination of breast density because of its cross-section, 3 dimensional coverage of the breast tissue, and high contrast between fibroglandular and fatty tissue. Though not in common clinical practice, several studies have demonstrated the relative accuracy and utility in using 3D MRI for the estimation of both breast density [49, 55] and parenchymal patterns [56]. The MRI assessment of breast density may also be refined with segmentation techniques that can remove the fat and quantify the amount of fibroglandular tissue.
Parenchymal evaluation on MRI may also reflect the physiologic parameters of the tissue. MRI allows both quantitative analysis and physiological assessment of the breast parenchyma. BPE is affected by both the density of fibroglandular tissue and its vascularity. Several studies have suggested that BPE represents physiological hormonal enhancement, reflecting hormone-related changes in breast composition and vascularity. Fluctuations in BPE have been demonstrated throughout the menstrual cycle, with the highest levels of enhancement in the second half of the menstrual cycle during the luteal phase when breast cell proliferation is at its highest [20]. BPE has also been demonstrated to reflect variations in estrogen-mediated vascular permeability, with increased BPE seen in women taking estrogen replacement therapy, and decreased BPE with anti-estrogen medications and in postmenopausal patients [23–30]. The significant hormonal influence on parenchymal enhancement could explain our findings that a significant difference in BPE between the high-risk and breast cancer groups was found only in the postmenopausal group, where the hormonal influence is absent.
Several studies have found that higher body mass index (BMI ≥25) is associated with lower breast density and that post-menopausal obese women have a 31% increased risk of developing breast cancer [57–59]. In our study, there was a significantly higher proportion of post-menopausal women with a BMI≥25 in the breast cancer cohort. This may also contribute to the higher proportion of women with lower breast density and lower FGT in women with breast cancer compared to the women at high risk for developing the disease. However, there is a dearth of literature on the relationship of BMI and BPE.
Understanding of BPE and its associated risks is becoming more important in light of increased breast MRI utilization. In its 2007 guidelines for breast cancer screening, the American Cancer Society recommended annual screening MRI as an adjunct to mammography for certain high-risk women [9]. Since then, several additional organizations including the National Comprehensive Cancer Network, American College of Radiology, American College of Obstetricians and Gynecologists and The American Society of Breast Surgeons, have recommended screening MRI for this high-risk population.
The correlation between levels of BPE and association with breast cancer risk is an emerging topic that has been reported variably. Hambly et al. and DeMartini et al. [21,32] do not show an increased incidence of cancer with increased BPE, but King et al. [15] showed a significantly increased odds ratio for breast cancer with moderate or marked BPE. In our study, independent of age, patients with breast cancer showed lower levels of mammographic breast density, BPE, and FGT compared with those at higher risk for developing breast cancer. MRI may represent a more physiological parameter that is not available with conventional imaging.
Limitations of this study include its retrospective nature and small population size, which may affect some of the statistical results and p-values which largely depend on sample size. Ideally, a larger study that includes a control group of women at normal risk for breast cancer may further elucidate the associations we found in our study. Also, this study did not control for patient related issues such as menopausal status, weight, and details of hormonal therapies. In addition, although pre-menopausal women underwent a screening breast MRI during days 7–14 of the menstrual cycle, pre-menopausal women who were newly diagnosed with breast cancer underwent their breast MRI regardless of their menstrual cycle. In addition, the lack of automated evaluation of mammographic density limits the study as this density was interpreted qualitatively by a radiologist. We also acknowledge possible selection bias given that our study cohort was influenced by clinician referral patterns.
In conclusion, we found that patients with post-menopausal breast cancer had lower mammographic breast density, lower BPE and lower FGT compared to patients at high risk for developing breast cancer. Women who are at higher risk for developing breast cancer had more dense breasts and increased BPE and FGT. We also found a higher proportion of overweight and obese women with postmenopausal breast cancer compared to the high risk population. Currently, there is a dearth of literature on the relationship of BMI with BPE or FGT and further studies on this topic are needed. The results of this study indicate that breast density, BPE and FGT are imaging characteristics that may not be associated with breast cancer tumorigenesis. Further studies on understanding the relationship of breast density, BPE and FGT in breast carcinogenesis are warranted.
References
- 1.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–92. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe JN. Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol. 1976;126:1130–7. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- 3.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–5. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 4.Byrne C, Schairer C, Wolfe JN, Parekh N, Salane M, Brinton LA, et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87:1622–9. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Dite GS, Stone J, Gunasekara A, English DR, McCredie MR, et al. Heritability of mammographic density, as risk for breast cancer. N Engl J Med. 2002;347:886–94. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 6.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 7.Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–83. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 8.Kerlikowske K, Hubbard RA, Miglioretti DL, Geller BM, Yankaskas BC, Lehman CD, et al. Comparative Effectiveness of Digital Versus Film-Screen Mammography in Community Practice in the United States: A Cohort Study. Ann Intern Med. 2011;155:493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 10.Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–25. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 11.Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005 May 21–27;365(9473):1769–78. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 12.Rijnsburger AJ, Obdeijn IM, Kaas R, Tilanus-Linthorst MM, Boetes C, Loo CE, et al. BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC Screening Study. J Clin Oncol. 2010;28:5265–73. doi: 10.1200/JCO.2009.27.2294. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, Konig R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28:1450–7. doi: 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- 14.Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD, et al. ACR Appropriateness Criteria Breast Cancer Screening. J Am Coll Radiol. 2013;10:11–4. doi: 10.1016/j.jacr.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 15.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011;260:50–60. doi: 10.1148/radiol.11102156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J. 2005;11:236–41. doi: 10.1111/j.1075-122X.2005.21499.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203:137–44. doi: 10.1148/radiology.203.1.9122382. [DOI] [PubMed] [Google Scholar]
- 18.Hussain Z, Roberts N, Whitehouse GH, García-Fiñana M, Percy D. Estimation of breast volume and its variation during the menstrual cycle using MRI and stereology. Br J Radiology. 1999;72:236–45. doi: 10.1259/bjr.72.855.10396212. [DOI] [PubMed] [Google Scholar]
- 19.Graham SJ, Stanchev PL, Lloyd-Smith JO, Bronskill MJ, Plewes DB. Changes in fibroglandular volume and water content of breast tissue during the menstrual cycle observed by MR imaging at 1. 5 T. J Magn Reson Imaging. 1995;5:695–701. doi: 10.1002/jmri.1880050613. [DOI] [PubMed] [Google Scholar]
- 20.Amarosa AR, McKellop J, Klautau Leite AP, Moccaldi M, Clendenen TV, Babb JS, et al. Evaluation of the kinetic properties of background parenchymal enhancement throughout the phases of the menstrual cycle. Radiology. 2013;268:356–65. doi: 10.1148/radiol.13121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol. 2012;198(4):W373–380. doi: 10.2214/AJR.10.6272. [DOI] [PubMed] [Google Scholar]
- 22.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol. 2012;22:2641–7. doi: 10.1007/s00330-012-2553-8. [DOI] [PubMed] [Google Scholar]
- 23.Hegenscheid K, Schmidt CO, Seipel R, Laqua R, Ohlinger R, Hosten N, et al. Contrast enhancement kinetics of normal breast parenchyma in dynamic MR mammography: effects of menopausal status, oral contraceptives, and postmenopausal hormone therapy. Eur Radiol. 2012;22:2633–40. doi: 10.1007/s00330-012-2544-9. [DOI] [PubMed] [Google Scholar]
- 24.Eng-Wong J, Orzano-Birgani J, Chow CK, Venzon D, Yao J, Galbo CE, et al. Effect of raloxifene on mammographic density and breast magnetic resonance imaging in premenopausal women at increased risk for breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1696–701. doi: 10.1158/1055-9965.EPI-07-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King V, Kaplan J, Pike MC, Liberman L, David Dershaw D, Lee CH, et al. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J. 2012;18:527–34. doi: 10.1111/tbj.12002. [DOI] [PubMed] [Google Scholar]
- 26.Oksa S, Parkkola R, Luukkaala T, Mäenpää J. Breast magnetic resonance imaging findings in women treated with toremifene for premenstrual mastalgia. Acta Radiol. 2009;50:984–989. doi: 10.3109/02841850903168083. [DOI] [PubMed] [Google Scholar]
- 27.Mousa NA, Eiada R, Crystal P, Nayot D, Casper RF. The effect of acute aromatase inhibition on breast parenchymal enhancement in magnetic resonance imaging: a prospective pilot clinical trial. Menopause. 2012;19:420–5. doi: 10.1097/gme.0b013e31823772a8. [DOI] [PubMed] [Google Scholar]
- 28.King V, Goldfarb SB, Brooks JD, Sung JS, Nulsen BF, Jozefara JE, et al. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology. 2012;264:670–8. doi: 10.1148/radiol.12112669. [DOI] [PubMed] [Google Scholar]
- 29.Delille J-P, Slanetz PJ, Yeh ED, Kopans DB, Halpern EF, Garrido L. Hormone replacement therapy in postmenopausal women: breast tissue perfusion determined with MR imaging--initial observations. Radiology. 2005;235:36–41. doi: 10.1148/radiol.2351040012. [DOI] [PubMed] [Google Scholar]
- 30.Pfleiderer SO, Sachse S, Sauner D, Marx C, Malich A, Wurdinger S, et al. Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res. 2004;6:R232–8. doi: 10.1186/bcr779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cubuk R, Tasali N, Narin B, Keskiner F, Celik L, Guney S. Correlation between breast density in mammography and background enhancement in MR mammography. Radiol Med. 2010;115:434–41. doi: 10.1007/s11547-010-0513-4. [DOI] [PubMed] [Google Scholar]
- 32.Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA. Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol. 2011;196:218–224. doi: 10.2214/AJR.10.4550. [DOI] [PubMed] [Google Scholar]
- 33.Sattin RW, Rubin GL, Webster LA, Huezo CM, Wingo PA, Ory HW, et al. Family history and the risk of breast cancer. JAMA. 1985;253:1908–13. [PubMed] [Google Scholar]
- 34.Claus EB, Risch N, Thompson WD. The calculation of breast cancer risk for women with a first degree family history of ovarian cancer. Breast Cancer Res Treat. 1993;28:115–20. doi: 10.1007/BF00666424. [DOI] [PubMed] [Google Scholar]
- 35.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–6. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 36.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–51. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 37.Dupont WD, Page DL. Breast cancer risk associated with proliferative disease, age at first birth, and a family history of breast cancer. Am J Epidemiol. 1987;125:769–79. doi: 10.1093/oxfordjournals.aje.a114594. [DOI] [PubMed] [Google Scholar]
- 38.Page DL, Kidd TE, Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22:1232–9. doi: 10.1016/0046-8177(91)90105-x. [DOI] [PubMed] [Google Scholar]
- 39.Hollingsworth AB, Singletary SE, Morrow M, Francescatti DS, O’Shaughnessy JA, Hartman A-R, et al. Current comprehensive management of women at increased risk for breast cancer. Am J Surg. 2004;187:349–62. doi: 10.1016/j.amjsurg.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Le-Petross HT, Whitman GJ, Atchley DP, Yuan Y, Gutierrez-Barrera A, Hortobagyi GN, et al. Effectiveness of alternating mammography and magnetic resonance imaging for screening women with deleterious BRCA mutations at high risk of breast cancer. Cancer. 2011;117:3900–7. doi: 10.1002/cncr.25971. [DOI] [PubMed] [Google Scholar]
- 41.American College of Radiology (ACR) Illustrated breast imaging reporting and data system (BI-RADS) Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 42.American College of Radiology (ACR) Illustrated breast imaging reporting and data system (BI-RADS) Reston, VA: American College of Radiology; 2014. [Google Scholar]
- 43.Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, Carney PA, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98:1204–14. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 44.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337–47. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230:29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 46.Redondo A, Comas M, Macià F, Ferrer F, Murta-Nascimento C, Maristany MT, et al. Inter- and intraradiologist variability in the BI-RADS assessment and breast density categories for screening mammograms. Br J Radiol. 2012;85:1465–70. doi: 10.1259/bjr/21256379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciatto S, Houssami N, Apruzzese A, Bassetti E, Brancato B, Carozzi F, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast. 2005;14:269–75. doi: 10.1016/j.breast.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Berg WA, Campassi C, Langenberg P, Sexton MJ. Breast Imaging Reporting and Data System: inter- and intraobserver variability in feature analysis and final assessment. AJR Am J Roentgenol. 2000;174:1769–77. doi: 10.2214/ajr.174.6.1741769. [DOI] [PubMed] [Google Scholar]
- 49.Kerlikowske K, Grady D, Barclay J, Frankel SD, Ominsky SH, Sickles EA, et al. Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System. J Natl Cancer Inst. 1998;90:1801–9. doi: 10.1093/jnci/90.23.1801. [DOI] [PubMed] [Google Scholar]
- 50.Ooms EA, Zonderland HM, Eijkemans MJ, Kriege M, Mahdavian Delavary B, Burger CW, et al. Mammography: interobserver variability in breast density assessment. Breast. 2007;16:568–76. doi: 10.1016/j.breast.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Eng A, Gallant Z, Shepard J, McCormack V, Li J, Dowsett M, et al. Digital mammographic density and breast cancer risk: a case–control study of six alternative density assessment methods. Breast Cancer Res. 2014;16:439. doi: 10.1186/s13058-014-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikeda DM, Hylton NM, Kinkel K, Hochman MG, Kuhl CK, Kaiser WA, et al. Development, standardization, and testing of a lexicon for reporting contrast-enhanced breast magnetic resonance imaging studies. J Magn Reson Imaging. 2001;13:889–95. doi: 10.1002/jmri.1127. [DOI] [PubMed] [Google Scholar]
- 53.Melsaether AN, McDermott M, Gupta D, Pysarenko K, Shaylor SD, Moy L. Inter- and intrareader agreement for categorization of background parenchymal enhancement at baseline and after training. AJR Am J Roentgenol. 2014;203:209–15. doi: 10.2214/AJR.13.10952. [DOI] [PubMed] [Google Scholar]
- 54.Klifa C, Carballido-Gamio J, Wilmes L, Laprie A, Shepherd J, Gibbs J, et al. Magnetic resonance imaging for secondary assessment of breast density in a high-risk cohort. Magn Reson Imaging. 2010;28:8–15. doi: 10.1016/j.mri.2009.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nie K, Chang D, Chen JH, Hsu CC, Nalcioglu O, Su MY. Quantitative analysis of breast parenchymal patterns using 3D fibroglandular tissues segmented based on MRI. Med Phys. 2010;37:217–26. doi: 10.1118/1.3271346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nie K, Chen JH, Chan S, Yu HJ, Bahri S, Tseng T, et al. Development of a quantitative method for analysis of breast density based on three-dimensional breast MRI. Med Phys. 2008;35:5253–62. doi: 10.1118/1.3002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baglietto L, Krishnan K, Stone J, Apicella C, Southey MC, English DR, et al. Associations of mammographic dense and nondense areas and body mass index with risk of breast cancer. Am J Epidemiol. 2014;179:475–83. doi: 10.1093/aje/kwt260. [DOI] [PubMed] [Google Scholar]
- 58.Razzaghi H, Troester MA, Gierach GL, Olshan AF, Yankaskas BC, Millikan RC. Mammographic density and breast cancer risk in White and African American women. Breast Cancer Res Treat. 2012;135:571–80. doi: 10.1007/s10549-012-2185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmichael AR. Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG. 2006;113:1160–6. doi: 10.1111/j.1471-0528.2006.01021.x. [DOI] [PubMed] [Google Scholar]


